Abstract
To ensure comparability among leaf fresh mass measurements it is important to handle the leaves in a standardized manner. In the present work constraints of a commonly used method to achieve full turgor, storage between damp paper towels, were investigated. After overnight rehydration in a saturated atmosphere, the fresh mass of leaves of 14 species was measured, and the leaves were stored between paper towels (two treatments: moist and wet) at 4 °C. Their mass was measured after 24, 48, and 72 h. Leaf fresh mass increased during the first 24 h of storage between moist paper towels by an average of 1.8%, between wet towels by 3.3%. Among the species, the increase of leaf fresh mass between moist towels correlated with the species' desiccation propensity, indicating that it was rehydration from water loss during initial handling. On the other hand, between wet towels the fresh mass increase was associated with the species' leaf tissue structure, and it continued to increase beyond 24 h, indicating that the increase was a result of water penetration into the leaf air spaces. It is concluded that storage between moist paper towels results in reliable values of leaf fresh mass, and that desiccated leaves rehydrate well between moist towels. However, care has to be taken to avoid too wet conditions as they may lead to erroneously high fresh mass values, especially in species with large air spaces. Furthermore, exposure to unsaturated atmospheric conditions during handling has to be minimized.
Keywords: Leaf dry matter content, leaf fresh mass, leaf rehydration, leaf tissue density, plant functional traits, water deficit, wetlands
Introduction
Leaf fresh mass is a commonly measured variable in ecological and ecophysiological research. It is used in the calculation of plant water status (Stocker, 1929; Barrs, 1968; Jones, 2007), for calculations of leaf dry matter content as an anatomical and ecological trait (Evans, 1972; Ryser and Lambers, 1995; Shipley, 1995; Wilson et al., 1999), to analyse element concentrations in intact tissues (Shane et al., 2004), for quantification of substances requiring extraction from fresh tissue (Becana et al., 1986), or to estimate thickness of laminar leaves (Vile et al., 2005). The measurement of fresh mass is not trivial, as leaf water content fluctuates diurnally (Romero and Botia, 2006), and leaves easily lose water after harvesting. As a base for the above-mentioned calculations the mass of fully turgid leaves is required (Stocker, 1929; Barrs, 1968; Garnier et al., 2001). Determination of full turgor can be difficult for species with elastic walls, and a standardization of the method used for attaining full turgor is required (Evans, 1972).
Stocker (1929) describes a procedure for rehydration of a plant in order to measure leaf mass at full turgor. After transportation from the field to a laboratory in an air-tight container, the leaves or small branches are cut under water and the cut ends placed in small containers filled with water in a saturated atmosphere to let them regain their turgor. This procedure, with the modification that the storage to attain full turgor happens at 4 °C, has been formalized as a protocol for the measurement of leaf fresh mass (Garnier et al., 2001). Another frequently used method of attaining full turgor is to float leaf discs on water for 3–24 h (Weatherley, 1950; Barrs, 1968). This method is widely used in studies of leaf water status (Vostral et al., 2002; Garcia et al., 2007; Levin et al., 2007). Full turgor can also be ensured by storing leaves between damp paper towels or filter paper, possibly overnight, in a refrigerator (Wilson et al., 1999; Ryser and Urbas, 2000; Vendramini et al., 2002; Prior et al., 2003; Vesk and Westoby, 2004; Güsewell, 2005; Poorter et al., 2006; Fresneau et al., 2007; Niinemets et al., 2007). Leaves have also been kept fully immersed in water (Galmès, 2007; Saura-Mas and Lloret, 2007). Vile et al. (2005) suggest that discrepancies between different datasets in meta-analyses may be caused by differences between the methods of rehydrating the leaves.
The aim of the present work was to investigate the constraints of the widely used, but not clearly defined damp-towel method. A rehydration of entire twigs or large leaves with cut ends in water-filled test-tubes or jars overnight in a saturated atmosphere, as the protocol by Garnier et al. (2001) requires, poses strong spatial constraints, especially if the rehydration is done at refrigerator temperatures and with large numbers of plants. This can be impractical, for example, for many wetland graminoids with leaves of well over a metre in length. Floating leaf discs on water is also impractical for many wetland graminoids with thick leaves and large air spaces. Rehydration between damp paper towels is easier, especially when only parts of large leaves are being collected. However, in contrast to the methods of Stocker (1929) and Weatherley (1950), constraints of the damp-towel method have not been investigated, and the method is usually not precisely described by its users. The leaves are left overnight between damp, wet or moist towels, sometimes in moistened plastic bags. The amount of moisture, and the duration of rehydration are usually not specified in detail. Barrs (1968) described, for the floating leaf disc method, the effect of time and partial collection of leaves on leaf fresh mass in great detail, and compared it with the original method described by Stocker (1929). The flotation method usually results in larger leaf fresh mass. The fresh mass of leaf discs increases with the time of floating, with a rapid increase during the first few hours, followed by a slow increase during the following days. The latter has been suggested to be due to penetration of water into the intercellular spaces (Ashby and Wolf, 1947: Barrs and Weatherley, 1962), which also might pose a problem for leaves stored between damp towels.
In order to understand the influence of the conditions in the damp-towel method on the leaf fresh mass obtained, as well as the constraints of this method, the following questions were investigated in the present study. (i) Is there an effect of the degree of dampness of the paper towels on leaf fresh mass? (ii) Is there an effect of time of storage between the damp paper towels on leaf fresh mass? (iii) Can leaves with a water deficit be reliably rehydrated using damp paper towels? (iv) Do answers to the above questions depend on species-specific leaf characteristics?
Furthermore, in a non-saturating atmosphere, as generally found in a research laboratory, fresh leaves continuously lose water, and a measurement of the fully turgid mass requires swift work under protection against desiccation. To quantify potential measuring errors occurring in the laboratory, compared to the effects of storage and rehydration, the change in leaf fresh mass occurring during 1 min on a balance was assessed.
Materials and methods
Species used, collection, and measurements
Material from 14 species was collected on 6 July 2004, in wetlands around Sudbury, Ontario, Canada, except for Betula papyrifera (birch) and Vaccinium angustifolia, which were collected on rocky sites (Table 1). Nomenclature follows Gleason and Cronquist (1991). Entire shoots were collected, or twigs in the case of birch. The cut surface was wrapped in dripping wet paper towels and put into small plastic bags. The entire shoots were wrapped in plastic bags and transported to the laboratory within the hour, cut again under water, and the cut ends placed in buckets filled with water. The shoots were covered with plastic bags for 12–18 h until used for further processing. The temperature in the room was 18 °C.
Table 1.
The 14 species used in this study
| Growth form | Abbreviation | |
| Alisma triviale Pursh. | M | Atr |
| Betula papyrifera Marshall. | W | Bpa |
| Carex lacustris Willd. | G | Cla |
| Carex utriculata F.Boott. | G | Cut |
| Carex stricta Lam. | G | Cst |
| Chamaedaphne calyculata (L.) Moench | W | Cca |
| Glyceria striata (Lam). A.Hitchc | G | Gst |
| Iris versicolor L. | G | Ive |
| Lythrum salicaria L. | H | Lsa |
| Phalaris arundinacea L. | G | Par |
| Phragmites australis (Cav.) Trin. | G | Pau |
| Sparganium chlorocarpum Rydb. | G | Sch |
| Typha latifolia L. | G | Tla |
| Vaccinium angustifolium Aiton. | W | Van |
The growth forms are non-graminoid monocots (M), graminoid monocots (G), herbaceous dicots (H), and woody dicots (W).
Next morning, 16 fully developed, young, undamaged leaves of each species were sampled, one leaf per shoot, except in the case of B. papyrifera, Chamaedaphne calyculata, and V. angustifolium with two leaves per shoot. For five species with short leaves entire leaves were measured (Alisma triviale, B. papyrifera, C. calyculata, Lythrum salicaria, and V. angustifolium). Of each of the leaves of the nine graminoid species, one 5–7 cm long piece was taken, at a distance of approximately one-third of the leaf length from the leaf base. Fresh mass of the leaves was measured with a PB 303-5 precision balance with an accuracy of 1 mg, or an MX5 microbalance with an accuracy 1 μg (both Mettler-Toledo, Greifensee, Switzerland), depending on leaf mass.
After measuring the initial fresh mass, the leaves and leaf parts were put in plastic boxes between paper towels of two degrees of moisture, which we call here the wet treatment and the moist treatment. The wet towels had been dipped in water and allowed to drip for about 30 s. The moist towels were allowed to drip for 10 min. The towels were prepared just prior to the first measurement of leaf fresh mass, and stored in sealed plastic boxes until use. The wet and moist towels had an initial water content of 452±23% and 271±2% (n=5), respectively, of the paper dry mass. The paper towel used was White Swan® Valu 8′′ Roll Towel (Scott Paper Ltd, Streetsville, Ontario) with specific mass of 30 g m−2 and thickness of 0.11 mm.
The leaves were placed between the towels in 16 plastic boxes with airtight lids (19 cm×14 cm×5 cm), each containing one of the eight replicate leaves of each species in each moisture treatment in a random order. Each box contained either wet or moist paper towels. A synthetic-fibre scouring pad was placed under the paper towels to allow free drainage of water from the lowermost paper towels.
Leaf fresh mass was measured after 24, 48, and 72 h. At each measurement, the leaves were superficially dried by gently pressing them between dry paper towels, weighed, and immediately placed back between the wet or moist paper towels. Between the measurements the boxes were kept in a refrigerator at 4 °C. After the final fresh mass measurement the leaves were dried at 75 °C for 24 h in a Thelco Precision mechanical (forced air) convection laboratory oven (Thermo Scientific, Waltham, MA, USA), and their dry mass measured after cooling for at least 1 h in a desiccator.
After 24 h the wet and moist towels contained 337±14% and 242±7% water, respectively (mean±SE; n=5). After 72 h these values were 233±23% and 159±5% (n=8).
In addition, leaf rehydration after partial drying was investigated. For four graminoid species with contrasting leaf structure, Glyceria striata, Phragmites australis, Sparganium chlorocarpum, and Carex utriculata, an additional set of eight leaves was left for 30 min on a laboratory counter. Their mass was measured before and after the procedure, after which they were handled like the other leaves.
Short-term change in fresh mass under laboratory conditions was investigated with eight of the species. The leaves were left on the balance with a closed hood for 1 min, during which readings of mass were taken after 0, 30, and 60 s. The temperature in the laboratory was 25 °C.
Leaf dry matter content (dry mass divided by the initial fresh mass) and fresh-mass-based leaf tissue density (fresh mass per volume) were determined as the species-specific leaf traits. The latter was measured on three to four separate leaves of each species at the day of the initial fresh mass measurement. For the density measurement a pycnometer (25 ml Erlenmeyer form Pyrex® specific gravity bottle) was used. Leaf density was calculated based on the difference in mass between a water-filled bottle and a water-filled bottle containing the leaf. As the volume of the bottle is fixed, the difference between these two measurements is the result of the density difference between the leaf and the water. Interspecific variation in tissue density is mainly driven by variation in leaf air space.
Results
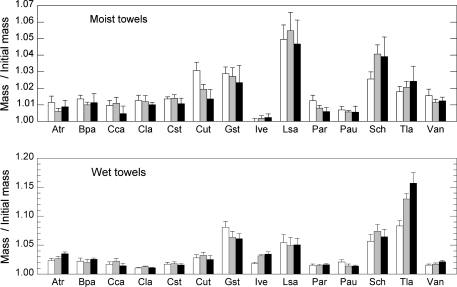
Compared with the initial fresh mass after overnight storage in a saturated atmosphere and standing in water, all species but Iris versicolor under moist conditions showed an increased leaf fresh mass after 24 h storage between wet or moist paper towels (Fig. 1; Table 2). However, even if the effect of storage was highly significant, it was very small. Stored between moist towels, the average increase during 24 h was 1.8% of the initial fresh mass, being over 2% in only four species. The increase in mass was dependent on species, and on moisture treatment. Lythrum salicaria showed the largest increase, 4.95%. Stored between wet towels, the increase was somewhat more than between moist towels, 3.3% in average. In the case of G. striata and Typha latifolia the increase between wet towels was about 8%.
Fig. 1.
Relative change of leaf fresh mass (Mass at the given time/Initial mass) after storage between moist and wet paper towels. The three bars for each species show the ratio after 24 (white), 48 (grey), and 72 h (black). For species abbreviations see Table 1. The error bars indicate one standard error. Note the different y-axes in the two graphs.
Table 2.
Statistics of the ANOVA with repeated measures to test the effect of 24 h storage between wet and moist paper towels on leaf fresh mass of 14 species
| df | Mean square | F | P | |
| Towel moisture (M) | 1 | 0.0106 | 0.01 | 0.758 |
| Species (Sp) | 13 | 36.6771 | 331 | <0.001 |
| M×Sp | 13 | 0.0744 | 0.67 | 0.790 |
| Residual | 191 | 0.1109 | ||
| Storage time (T) | 1 | 0.0668 | 585 | <0.001 |
| T×M | 1 | 0.0058 | 51.0 | <0.001 |
| T×Sp | 13 | 0.0020 | 17.7 | <0.001 |
| T×M×Sp | 13 | 0.0007 | 6.39 | <0.001 |
| Residual | 191 | 0.0001 |
Leaf fresh mass was the dependent variable (measured at 0 h and 24 h), towel moisture (moist, wet) and species (n=14) were the independent variables. The fresh mass values were log-arcsine-transformed to attain normality.
In general, there was no further significant increase in fresh mass between 24 h and 72 h, but the interactions with towel moisture and with species were significant (Fig. 1; Table 3). Under moist treatment, the effect of storage time was minor and there was no average increase in mass. Under the wet treatment leaf fresh mass increased by a further 0.5% on average, after 72 h being 3.8% above the initial fresh mass. A major increase over time was found for T. latifolia leaves, the increase during 72 h being 16%.
Table 3.
Statistics of the ANOVA with repeated measures to test the effect of storage time on leaf fresh mass of 14 species
| df | Mean square | F | P | |
| Towel moisture (M) | 1 | 4783 | 115.4 | <0.001 |
| Species (Sp) | 13 | 974 | 23.5 | <0.001 |
| M×Sp | 13 | 245.8 | 5.9 | <0.001 |
| Residual | 191 | 41.45 | ||
| Storage time (T) | 2 | 6.01 | 0.74 | 0.475 |
| T×M | 2 | 55.01 | 6.82 | 0.001 |
| T×Sp | 26 | 27.93 | 3.47 | <0.001 |
| T×M×Sp | 26 | 8.56 | 1.06 | 0.383 |
| Residual | 382 | 8.06 |
The ratio of the leaf fresh mass at the given time (24, 48, and 72 h) to the initial fresh mass (0 h) was the dependent variable, towel moisture, and species were the independent variables. The ratio was reciprocally transformed (y' = 1/(y–0.97)) to attain normality.
Under moist conditions, fresh mass increase (the average of all three measurements) did not correlate with either leaf dry matter content or fresh-mass-based leaf tissue density (Pearson correlation, P >0.35, n=14). However, under wet conditions, the average mass increase correlated significantly negatively with leaf dry matter content (r2=0.402, P=0.015, n=14) and with leaf tissue density (r2=0.372, P=0.020, n=14).
Drying for 30 min on the laboratory counter reduced leaf fresh mass of C. utriculata by 7.4±0.5%, G. striata by 17.4±0.9%, Phalaris arundinacea by 7.5±0.4%, and S. chlorocarpum by 5.6±0.6% (mean ±1SE; n=8). After 24 h storage between the moist or wet towels, the dried leaves had fresh mass values above the initial, predrying values, less than 2% lower than the fresh mass of the undried leaves stored under the same conditions (Table 4). Glyceria striata leaves, which had dried the most during the 30 min, had a slightly higher fresh mass than the undried leaves after 24 h storage. In an ANOVA with the ratio of leaf fresh mass after 24 h to initial leaf fresh mass as the dependent variable, the effects of desiccation treatment (P=0.003), towel moisture (P <0.001), species (P <0.001), and all the interactions were significant (P <0.01), indicating that species and towel moisture influenced the recovery from water deficit.
Table 4.
Increase of leaf fresh mass after 24 h rehydration as percentage (%) of the initial fresh mass, stored between wet or moist paper towels, with and without 30 min desiccation on the laboratory counter after the initial fresh mass measurement
Mean values ±1 SE.
| Species | Moist towels | Wet towels | ||
| Control | Dried | Control | Dried | |
| Carex utriculata | 3.07±0.50 | 1.02±0.21 | 2.79±0.56 | 2.28±0.48 |
| Glyceria striata | 2.88±0.41 | 4.46±0.82 | 8.06±1.05 | 10.51±1.75 |
| Phalaris arundinacea | 0.69±0.21 | 0.62±0.62 | 2.11±0.36 | 1.15±0.30 |
| Sparganium chlorocarpum | 2.56±0.43 | 0.63±0.91 | 5.65±1.20 | 4.55±0.83 |
Leaving the leaves on the balance for 30 s and 60 s led to a fresh mass loss of 0.5% and 1.2% in average, respectively (Table 5). The loss ranged from 0.15% (T. latifolia) to 1.4% (L. salicaria) after 30 s, and from 0.3% and 2.5% after 60 s. The effects of time, species (P <0.001), and the species×time interaction (P=0.002) were significant (repeated measures ANOVA). The relative loss of water during 60 s and the relative fresh mass gain during 24 h between moist towels correlated positively (Pearson correlation, r2=0.51, P=0.047, n=8), mainly driven by the two species with the highest water loss, on balance, having the highest gain during the storage.
Table 5.
Fresh mass loss as a percentage (%) of the initial fresh mass (0 s) on balance with a closed hood for 60 s
| Species | 30 s | 60 s |
| Alisma triviale | 0.29±0.05 | 0.51±0.05 |
| Betula papyrifera | 0.39±0.09 | 0.88±0.20 |
| Glyceria striata | 1.17±0.11 | 2.10±0.07 |
| Lythrum salicaria | 1.43±0.61 | 2.46±1.05 |
| Phalaris arundinacea | 0.66±0.08 | 1.05±0.13 |
| Phragmites australis | 0.42±0.05 | 0.78±0.06 |
| Typha latifolia | 0.15±0.01 | 0.32±0.04 |
| Vaccinium angustifolium | 0.75±0.13 | 1.22±0.15 |
Mean values ±1 SE.
Discussion
The sensitivity of leaf fresh mass to short-term fluctuations in environmental conditions and to the method of measurement necessitates a standardized approach to the measurement of this variable to ensure comparability of different datasets (Barrs, 1968; Garnier et al., 2001). The current data confirm that the measurement of leaf fresh mass is a sensitive variable, but also that, when care is taken to avoid water loss and water penetration to leaf air spaces, the measured values are reliable even after prolonged storage. Storage between moist towels over 24 h changes the fresh mass values by only a few per cent, compared with initial values obtained by placing cut ends of the leaves or twigs in water overnight in a saturated atmosphere. This is a very small effect compared with intra- or interspecific variation in traits calculated using leaf fresh mass. In the current dataset, for example, leaf dry matter content varied among the 14 species by a factor of three (0.139–0.420 g g−1). Many published datasets show similar or larger variation. Storage over 48 h or 72 h between moist towels did not result in significant changes in leaf fresh mass, compared with storage over 24 h.
The observed slight increase in mass after 24 h between moist towels may actually be a recovery from loss of water during initial handling. There is always some loss of water during handling of leaves in dry air, and the observed increase in fresh mass between moist towels was similar in amount to the loss in weight expected to happen during a 1–2 min exposure to laboratory air. Interspecific variation in mass increase during 24 h between moist towels correlated with mass loss on the balance. The two species with the highest measured water loss, L. salicaria and G. striata, had the highest increase in fresh mass of the species for which the loss was measured. The labile fresh mass of these species is probably associated with their leaf characteristics. Lythrum salicaria is known to have high transpiration rates (Fickbohm and Zhu, 2006), and as a species of moist to wet shady woods G. striata is not well protected against water loss (Dore and McNeill, 1980). On the other hand, Iris versicolor, the species with the least increase in mass during storage between moist towels, has a waxy water-repellent surface reducing water loss (Neinhuis and Barthlott, 1997). These results clearly show that a careful and swift measurement of fresh mass is essential for a correct determination of leaf fresh mass. Otherwise, systematic errors related to leaf structure may arise. The contribution of water loss to errors in measurement of fresh mass and volume of tree stems has previously been described by Yokoi and Kishida (1985).
The observed increase in fresh mass during storage can also be caused by water uptake beyond the turgid mass. This certainly seems to be the case for some species when stored between dripping wet towels, especially over longer periods, but does not seem to have posed a problem when the towels were merely moist. In that treatment, fresh mass did not increase further after the 24 h measurement. By contrast, in the wet treatment, the mass continued to increase after 24 h, the increase correlating with leaf dry matter content and leaf tissue density. The reason for the increased fresh mass of leaves stored between wet towels was most likely to be penetration of water into the intercellular air spaces of the leaf. Mass increase during storage was most pronounced in species with low tissue density, i.e. species with large intercellular air spaces, and in those with low leaf dry matter content. This phenomenon has been earlier described by Ashby and Wolf (1947), Barrs and Weatherley (1962), and Hewlett and Kramer (1963) for leaf discs rehydrated with the floating method. Ashby and Wolf (1947) also found the largest increase in the species with the largest aerenchyma.
The difference in species responses to the two levels of moisture is a clear indication of different mechanisms underlying the mass increase during storage: between moist towels the largest increase was found for species prone to water loss in a dry atmosphere, whereas between wet towels leaf mass increased the most for species with large air spaces.
Rehydration of severely desiccated leaves resulted in fresh mass values which were only slightly different from those of the non-desiccated leaves. The difference of about 2% after 24 h between moist towels was statistically highly significant, but comparatively small in amount. One also has to take into account that our desiccation treatment was quite severe compared with the expected effects in field-grown plants. The fresh mass values were consistently lower in dried plants, possibly as a result of changes in cell wall properties during desiccation (Milthorpe and Spencer, 1957; Weatherley, 1965). However, G. striata, the species with the most severe water loss during the 30 min, had, after rehydration, a higher water content in desiccated than in non-desiccated leaves, possibly indicating damage to cell walls and/or membranes.
Besides the fresh mass of a leaf, an accurate measurement of its dry mass is required for the calculation of leaf water status or leaf dry matter content. A correct protocol for obtaining the dry mass is as important as that for obtaining the fresh mass (Evans, 1972).
Conclusions
The strong significance of the observed minor differences in leaf fresh mass shows that storage between moist towels results in highly reliable values for leaf fresh mass. There is an indication that the small increase in weight during the first 24 h of storage was rehydration of leaves which were slightly desiccated during the initial handling. Moist towels also allowed a good recovery from a more substantial water loss. On the other hand, too high a water content of the towels may lead to water penetrating into the intercellular air spaces during storage resulting in erroneously high leaf fresh mass values, especially in species with large leaf air spaces. This can also be a potential problem when floating leaf discs on water, or storing leaves in jars filled with water. The results emphasize the extreme importance of minimizing the exposure of leaves to non-saturated air prior to the measurement of leaf fresh mass, and point to a potential bias in leaf fresh mass if air spaces are allowed to be filled with water.
Acknowledgments
We thank Jill O'Hara and Angie Charbonneau for help with the measurements. This project was supported by The Natural Sciences and Engineering Research Council of Canada (Grant numbers 249689-02 and 251515-02 to PR; Undergraduate Student Research Award to JB).
References
- Ashby E, Wolf R. A critical examination of the gravimetric method of determining suction force. Annals of Botany. 1947;11:261–268. [Google Scholar]
- Barrs HD. Determination of water deficits in plant tissues. In: Kozlowski TT, editor. Water deficits and plant growth. New York: Academic Press; 1968. pp. 235–368. [Google Scholar]
- Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Australian Journal of Biological Sciences. 1962;15:413–428. [Google Scholar]
- Becana M, Aparicio-Tejo P, Irigoyen JJ, Sanchez-Diaz M. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiology. 1986;82:1169–1171. doi: 10.1104/pp.82.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore WG, McNeill J. Canada: Research Branch, Agriculture Canada; 1980. Grasses of Ontario. Monograph 26. Ottawa, Ontario. [Google Scholar]
- Evans GC. The quantitative analysis of plant growth. Berkeley CA: University of California Press; 1972. [Google Scholar]
- Fickbohm SS, Zhu W-X. Exotic purple loosestrife invasion of native cattail freshwater wetlands: effects on organic matter distribution and nitrogen cycling. Applied Soil Ecology. 2006;32:123–131. [Google Scholar]
- Fresneau C, Ghashghaie J, Cornic G. Drought effect on nitrate reductase and sucrose-phosphate synthase activities in wheat (Triticum durum L.): role of leaf internal CO2. Journal of Experimental Botany. 2007;58:2983–2992. doi: 10.1093/jxb/erm150. [DOI] [PubMed] [Google Scholar]
- Galmés J, Anunciacíon A, Medrano H, Flexas J. Photosynthesis and photoprotection responses to water stress in the wild-extinct plant Lysimachia minoricensis. Environmental and Experimental Botany. 2007;60:308–317. [Google Scholar]
- Garcia AL, Madrid R, Nicolas N, Martinez V, Franco JA. Moderating water stress in tomato (Lycopersicon esculentum Mill.) plants by application of specific nitrogen doses. Journal of Horticultural Science and Biotechnology. 2007;82:664–670. [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology. 2001;15:688–695. [Google Scholar]
- Gleason HA, Cronquist A. Manual of vascular plants of northeastern United States and adjacent Canada. 2nd edn. New York: The New York Botanical Garden; 1991. [Google Scholar]
- Güsewell S. Responses of wetland graminoids to the relative supply of nitrogen and phosphorus. Plant Ecology. 2005;176:35–55. [Google Scholar]
- Hewlett JD, Kramer PJ. The measurement of water deficits in broadleaf plants. Protoplasma. 1963;57:381–391. [Google Scholar]
- Jones HG. Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance. Journal of Experimental Botany. 2007;58:119–130. doi: 10.1093/jxb/erl118. [DOI] [PubMed] [Google Scholar]
- Levin M, Lemcoff JH, Cohen S, Kapulnik Y. Low air humidity increases leaf-specific hydraulic conductance of Arabidopsis thaliana (L.) Heynh (Brassicaceae) Journal of Experimental Botany. 2007;58:3711–3718. doi: 10.1093/jxb/erm220. [DOI] [PubMed] [Google Scholar]
- Milthorpe FL, Spencer EJ. Experimental studies of the factors controlling transpiration. III. The interrelations between transpiration rate, stomatal movement, and leaf-water content. Journal of Experimental Botany. 1957;8:413–437. [Google Scholar]
- Neinhuis C, Barthlott W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Annals of Botany. 1997;79:667–677. [Google Scholar]
- Niinemets U, Porstmuth A, Tobias M. Leaf shape and venation pattern alter the support investments within leaf lamina in temperate species: a neglected source of leaf physiological differentiation. Functional Ecology. 2007;21:28–40. [Google Scholar]
- Poorter H, Pepin S, Rijkers T, de Jong Y, Evans JR, Körner C. Construction costs, chemical composition and payback time of high- and low-irradiance leaves. Journal of Experimental Botany. 2006;57:355–371. doi: 10.1093/jxb/erj002. [DOI] [PubMed] [Google Scholar]
- Prior LD, Eamus D, Bowman DMJS. Leaf attributes in the seasonally dry tropics: a comparison of four habitats in northern Australia. Functional Ecology. 2003;17:504–515. [Google Scholar]
- Romero P, Botia P. Daily and seasonal patterns of leaf water relations and gas exchange of regulated deficit-irrigated almond trees under semiarid conditions. Experimental and Environmental Botany. 2006;56:158–173. [Google Scholar]
- Ryser P, Lambers H. Root and leaf attributes accounting for the performance of fast- and slow-growing grasses at different nutrient supply. Plant and Soil. 1995;170:251–265. [Google Scholar]
- Ryser P, Urbas P. Ecological significance of leaf life span among Central European grass species. Oikos. 2000;91:41–50. [Google Scholar]
- Saura-Mas S, Lloret F. Leaf and shoot water content and leaf dry matter content of Mediterranean woody species with different post-fire regenerative strategies. Annals of Botany. 2007;99:545–554. doi: 10.1093/aob/mcl284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, McCully ME, Lambers H. Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae) Journal of Experimental Botany. 2004;55:1033–1044. doi: 10.1093/jxb/erh111. [DOI] [PubMed] [Google Scholar]
- Shipley B. Structured interspecific determinants of specific leaf area in 34 species of herbaceous angiosperms. Functional Ecology. 1995;9:312–319. [Google Scholar]
- Stocker O. Das Wasserdefizit von Gefässpflanzen in verschiedenen Klimazonen. Planta. 1929;7:382–387. [Google Scholar]
- Vendramini F, Diaz S, Gurvich DE, Wilson PJ, Thompson K, Hodgson JG. Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytologist. 2002;154:147–157. [Google Scholar]
- Vesk PA, Westoby M. Sprouting by plants: a modular organization. Functional Ecology. 2004;18:939–945. [Google Scholar]
- Vile D, Garnier E, Shipley B, et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Annals of Botany. 2005;96:1129–1136. doi: 10.1093/aob/mci264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vostral C, Boyce RL, Friedland AJ. Winter water relations of New England conifers and factors influencing their upper elevational limits. I. Measurements. Tree Physiology. 2002;22:793–800. doi: 10.1093/treephys/22.11.793. [DOI] [PubMed] [Google Scholar]
- Weatherley PE. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytologist. 1950;49:81–97. [Google Scholar]
- Weatherley PE. The state and movement of water in leaf. In: Fogg GE, editor. The state and movement of water in living organisms. Symposium of the Society of Experimental Biology. Vol. 19. New York: Academic Press; 1965. pp. 157–184. [PubMed] [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist. 1999;143:155–162. [Google Scholar]
- Yokoi Y, Kishida A. On the relationship between two indices (‘bulk density’ and ‘dry-matter content’) of dry-matter accumulation in plant organs. Botanical Magazine. 1985;98:335–345. [Google Scholar]