Abstract
The MDR1 P-glycoprotein (Pgp), a member of the ATP-binding cassette family of transporters, is a transmembrane ATPase efflux pump for various lipophilic compounds, including many anti-cancer drugs. mAb UIC2, reactive with the extracellular moiety of Pgp, inhibits Pgp-mediated efflux. UIC2 reactivity with Pgp was increased by the addition of several Pgp-transported compounds or ATP-depleting agents, and by mutational inactivation of both nucleotide-binding domains (NBDs) of Pgp. UIC2 binding to Pgp mutated in both NBDs was unaffected in the presence of Pgp transport substrates or in ATP-depleted cells, whereas the reactivities of the wild-type Pgp and Pgps mutated in a single NBD were increased by these treatments to the level of the double mutant. These results indicate the existence of different Pgp conformations associated with different stages of transport-associated ATP hydrolysis and suggest trapping in a transient conformation as a mechanism for antibody-mediated inhibition of Pgp.
P-glycoprotein (Pgp), the product of the human MDR1 gene, acts as a broad specificity plasma membrane efflux pump for many hydrophobic compounds (1, 2) and recently was shown to function as a short chain lipid translocase (3). Pgp is a member of a superfamily of ATP-binding cassette (ABC) transporters, characterized by the presence of conserved ABC domains containing consensus nucleotide-binding domain (NBD) sequence motifs (4). ABC transporters of a subgroup that includes the MDR1 Pgp, a closely related MDR2 gene product that acts as a phospholipid translocase (5, 6), the yeast STE6 protein that transports the a pheromone (7), and the cystic fibrosis transmembrane conductance regulator (8), are characterized by a common architecture. These proteins are composed of two halves separated by a “linker” region; each half comprises a hydrophobic region with six predicted membrane-spanning segments and the ABC domain.
Expression of the MDR1 Pgp in tumor cells is associated with a clinically important phenotype of crossresistance to many structurally diverse anti-cancer drugs, which are pumped out by Pgp. Pgp was shown to bind its transport substrates (9), an event that most probably occurs in the lipid bilayer of the plasma membrane (10), and to hydrolyze ATP (11). The ATPase activity of Pgp is strongly stimulated by the addition of Pgp transport substrates (12). The stoichiometry, temporal sequence, and structural transitions linking the binding and transport of a Pgp substrate with the binding and hydrolysis of ATP are as yet unknown.
We previously have developed a mouse mAb UIC2, specific for the extracellular moiety of the human MDR1 Pgp (13). In contrast to several other mAbs that react with Pgp on the surface of intact cells, the addition of UIC2 to tissue culture media decreases the activity of Pgp toward all the tested Pgp transport substrates (13–16). The conformational epitope that is recognized by UIC2 is distinct from the epitopes of the other mAbs, because only UIC2 fails to react with a mutant Pgp that carries a deletion in the first extracellular loop (17). In an attempt to determine the mechanism of Pgp inhibition by UIC2, we have investigated the effect of different treatments and mutations that affect Pgp function on its reactivity with UIC2. Our results suggest a mechanism for Pgp inhibition by UIC2 and indicate the existence of different Pgp conformations associated with different stages of drug transport and ATP binding and hydrolysis.
MATERIALS AND METHODS
Cell Lines and mAbs.
The K562/i-S9 cell line was derived by infection of human K562 leukemia cells with a recombinant retrovirus carrying human MDR1 cDNA followed by subcloning (without cytotoxic selection) and immunostaining for Pgp (18, 19). The derivation of LMtk− murine fibroblasts transfected with the wild-type (KK), single-mutant (KM and MK), and double-mutant (MM) forms of human MDR1 cDNA will be described elsewhere (B.S., B.S.M., M. Polonskaia, E.B.M., and I.B.R., unpublished data). The mutant Pgps contain K433M and/or K1076M substitutions in the Walker A motifs; in addition, the cloning procedure resulted in a Q1280A substitution of the C-terminal amino acid in all of the transfected MDR1 cDNA sequences. All of the cell lines were maintained in DMEM supplemented with 10% fetal calf serum (HyClone), 100 units/ml of penicillin, 100 mg/ml of streptomycin, and 2 mM glutamine. All of the drugs used in this study were purchased from Sigma.
The derivation of murine mAbs MRK16 and UIC2, specific for the extracellular moiety of the human MDR1 Pgp, has been described elsewhere (13, 20). Both mAb preparations were >97% pure by SDS/PAGE. R-phycoerythrine (PE) conjugates of UIC2 and MRK16 were prepared by partial reduction of the mAb with 20 mM DTT, conjugation to maleimide-derivatized PE, and purification by size exclusion chromatography. A pool containing greater than 95% conjugates at a 1:1 ratio of mAb to PE was used. UIC2 also was labeled with fluorescein isothiocyanate (FITC) to give approximately four fluoresceins per antibody molecule. UPC10 (affinity-purified myeloma protein of the IgG2a isotype) was obtained from Sigma, and goat anti-mouse IgG2a PE conjugate was from Caltag (South San Francisco, CA).
UIC2 Reactivity Shift Assays.
Cells growing in suspension or trypsinized cells from monolayer cultures were washed twice with PBS at room temperature, 106 cells per tube in 1 ml of prewarmed Ca2+, Mg2+-free PBS (CMF-PBS) (GIBCO). The cells then were warmed up to 37°C for 10 min, mixed with 20-μl aliquots of drug preparations, and incubated at 37°C for another 10 min. Fifty-microliter aliquots of mAbs diluted 1:10 in PBS were added and, after mixing, incubated for another 20 min at 37°C. In indirect staining, cell samples were washed twice, stirred, incubated with secondary antibody in 100 μl of PBS for 20 min at 37°C. After incubation, the cells were washed twice with ice-cold PBS and transferred in 0.5 ml of ice-cold PBS with 1 μg/ml propidium iodide (PI) to exclude dead cells. All experimental conditions (incubation time, drug and antibody concentrations, sample volumes, etc.) have been determined in preliminary titration experiments for each of the cell lines. Two- or three-color cytofluorimetric analysis was performed by using a BDIS FACSort flow cytometer as described (21). Flow cytometric data were analyzed by using the BDIS CellQuest software.
In ATP depletion experiments, washed cells were preincubated with 20-μl aliquots of stock solutions containing oligomycin, sodium azide, or potassium cyanide for 15 min at 37°C and then treated with drugs, antibodies, and PI as above. Intracellular ATP was measured by the Bioluminescent Somatic Cell Assay Kit (Sigma). Light emission was measured by using AutoLumat LB953 Universal Luminometer (Berthold, Vildbad, Germany) at 8°C. The amount of ATP per cell was determined by using an internal ATP standard (added at the amount similar to that in the cell sample) and expressed as a percentage of ATP present in control cells (treated with PBS only).
Antibody affinity analysis was carried out by adding increasing amounts of mAbs UIC2 or MRK16 to a constant number of cells, generally 5 × 105 per reaction sample, suspended in 200 μl of PBS to which 0.1% of BSA was added (PBS/BSA). The reaction with antibody was for 30 min at 37°C. After two washes with ice-cold PBS/BSA the cells were stained with the secondary antibody (FITC-conjugated goat anti-mouse IgG2a) for 30 min on ice and processed for flow cytometry as above. In antibody competition assays, cells were incubated with UIC2 for 45 min at 37°C; after two washes, cells were stained with FITC-conjugated secondary antibody either immediately or after 30-min incubation at 37°C with 3 μg of UIC2 or MRK16. The median fluorescence of each sample was determined, and the data plotted as median fluorescence against the amount of mAb present in the reaction mixture. The maximum fluorescence at asymptotically high levels of antibody (Fmax) and the concentration of antibody (K1/2), giving half of the maximal fluorescence, were determined by using the SigmaPlot program by curve-fitting to an adsorption isotherm.
Immunoadsorbtion, Electrophoretic Analysis, and 8-azido-ATP Binding of Pgp.
To purify Pgp from LMtk− transfectants by immunoadsorbtion, cells were washed, dissociated with 20 mM EDTA in Ca2+, Mg2+-free PBS (CMF-PBS), and resuspended in CMF-PBS containing 1% BSA. After another wash, cells were resuspended in the same buffer at 107 cells/ml. Fifty micrograms of mAb MRK16 was added to 107 cells, and the mixture was gently rocked for 60 min at 4°C. After extensive washing, cells were lysed in 1 ml of the lysis buffer [50 mM Tris, pH 8.0 at 20°C/150 mM NaCl/10 mM EDTA/0.5% sodium deoxycholate/0.5% 3-[3-cholamidopropyl)dimethylammonio]-1-propane sulfonate/0.2 mM phenylmethylsulfonyl fluoride), incubated 15 min at 0°C and centrifuged. The supernatant was combined with 200 μl of a 10% suspension of rProtein A beads (Repligen) in the lysis buffer, and the mixture was rocked for 30–60 min at 4°C. After a brief centrifugation, the pelleted protein A beads were washed four times with a buffer used for 8-azido-ATP binding or ATPase assays. The immunoadsorbants were not frozen but used immediately for 8-azido-ATP binding or ATPase assays (freezing and thawing was found to lower the ATPase activity).
Metabolic protein labeling was carried out by culturing 4 × 106 cells for 19 hr with 0.25 mCi Trans35S-Label (ICN) in methionine-free culture media. Pgp from a total of 107 labeled cells was immunoadsorbed by the procedure similar to that in ref. 22, by using 20 μg of MRK16. Immunoadsorbed Pgp was heated to 50°C for 5 min before loading on 7.5% SDS/PAGE. After electrophoresis, autoradiography was carried out by using Fluoro-hance (RPI, Mount Prospect, IL). Cellular Pgp content was evaluated by the ratio of trichloroacetic acid-precipitable 35S in the immunoadsorbants to that in the total cell lysate. The amount of Pgp also was estimated by comparing the intensity of 35S-labeled or silver-stained Pgp bands with serial dilutions of a protein standard with similar size.
The procedure for photoaffinity labeling Pgp with 32P-8-azido-ATP (ICN) was developed by using recommendations of the manufacturer and in consultation with B.E. Haley (University of Kentucky). All of the steps involving 8-azido-ATP were carried out in dim light. Pgp immunoadsorbants were prepared from 5 × 106 cells; protein A beads were washed at the end five times with azido-ATP binding buffer (ABB; 15 mM Tris, pH 7.5 at 20°C/15 mM NH4Cl/0.1% 3-[3-cholamidopropyl)dimethylammonio]-1-propane sulfonate/2 mM MgCl2). After the final wash, each immunoadsorbant was separated into two equal portions and pelleted. Pellets were resuspended by gentle mixing in 30 μl of ABB containing 32P-8-azido-ATP at 2.5 μM and (in one of the two samples) unlabeled 8-azido-ATP at 0.5 mM (200-fold excess), preheated at 37°C. The samples were transferred onto a preheated piece of Parafilm at 37°C and, 1 min after resuspension, exposed to UV light (254 nm; 2–15W GE G15T8 germicidal lamps) at a distance of 8 cm for 10 sec. After labeling, the samples were placed on ice, pelleted by centrifugation, and analyzed by SDS/PAGE as described above. Gels were silver-stained, dried, and autoradiographed.
RESULTS
Pgp-Transported Compounds Increase UIC2 Reactivity.
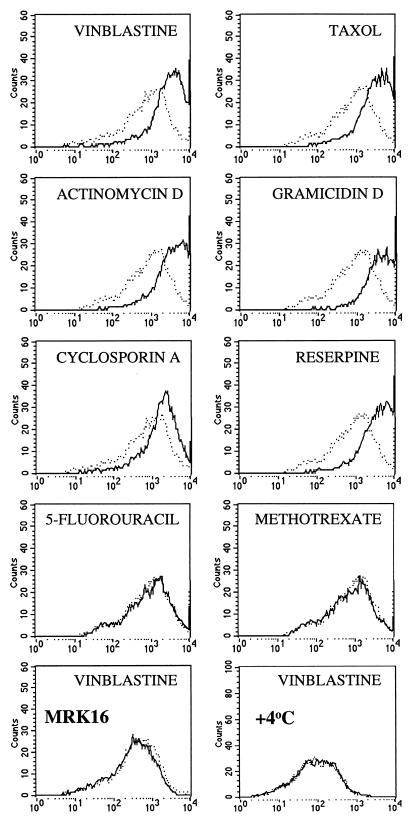
We have analyzed the effect of different Pgp-transported drugs on the reactivity of mAb UIC2 with a Pgp-expressing cell line K562/i-S9, which was derived without drug selection after infection of K562 leukemia cells with a MDR1-expressing retrovirus (18, 19). As shown in Fig. 1, UIC2 reactivity was increased in the presence of Pgp transport substrates, including vinblastine (at 1 μM or higher concentrations), vincristine (4.8 μM; not shown), taxol (4.8 μM), actinomycin D (3.2 μM), gramicidin D (2.2 μM), cyclosporin A (3.4 μM), reserpine (6.6 μM), and verapamil (9 μM; not shown). Three other Pgp substrates, colchicine (tested at concentrations up to 1.25 mM), etoposide (0.85 mM), and puromycin (1.075 mM), produced no detectable shift in UIC2 reactivity (data not shown). No change in UIC2 reactivity was observed in the presence of drugs to which our Pgp-expressing cell lines show no resistance under normal conditions, including 5-fluorouracil (up to 3.9 mM), methotrexate (1.125 mM) (Fig. 1), cisplatin (1.7 mM), carboplatin (1.375 mM), and azidothymidine (1.9 mM) (data not shown). Drug-induced increase in immunoreactivity was detectable by using UIC2, directly conjugated with PE or FITC, or indirectly labeled with a PE-conjugated secondary antibody (data not shown). Drugs had no effect on immunostaining with antibodies that do not react with K562/i-S9 cells (myeloma protein UPC10 of the IgG2a isotype, anti-glycophorin A, and anti-HLA-DR), with the antibodies that recognize other antigens on the surface of these cells (anti-CD71, anti-CD54, or anti-HLA-ABC) (data not shown), or with another anti-Pgp mAb, MRK16 (Fig. 1 and data not shown). The latter mAb recognizes a composite extracellular epitope of the human Pgp (20, 23), but does not show the same Pgp inhibitory effect as mAb UIC2 (13).
Figure 1.
Staining of K562/i-S9 cells with PE-conjugated mAb UIC2 in the presence of different drugs. Each graph shows the flow profiles of staining (on a log scale) carried out in parallel in the absence (dashed lines) and in the presence (solid lines) of vinblastine (25 μM), taxol (24 μM), actinomycin D (16 μM), gramicidin D (11 μM), cyclosporine A (17 μM), reserpine (33 μM), 5-fluorouracil (156 μM), and methotrexate (45 μM), at 37°C. The two bottom panels show staining with mAb MRK16 (at 37°C) and UIC2 (at 4°C), in the presence and in the absence of 25 μM of vinblastine.
Increased UIC2 reactivity in the presence of Pgp substrates also was observed with other Pgp-expressing cell types, including drug-selected derivatives of KB-3-1 and MCF7 cell lines, MDR1-transfected KB-3-1, LMtk−, NIH 3T3 and PA317 cells, Pgp-positive leukemia, lymphoma, and solid tumor samples, as well as normal lymphocytes and hematopoietic stem cells expressing Pgp. In general, cell lines with higher levels of Pgp required higher concentrations of Pgp substrates for maximal stimulation. The UIC2 reactivity was increased by Pgp substrates when the antibody was incubated with the cells at 37°C, but not at 4°C (Fig. 1), suggesting that Pgp function could be required for the altered reactivity.
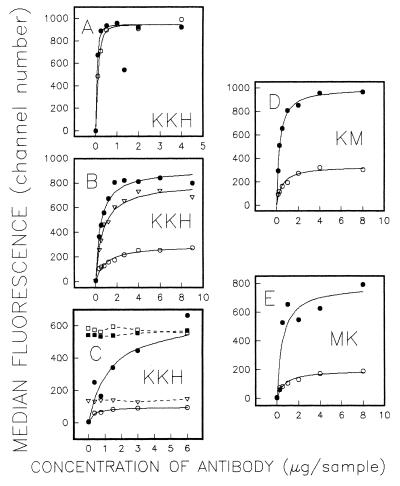
To determine if the increased UIC2 reactivity in the presence of drugs reflected an increased affinity of Pgp for the mAb or an increase in the number of UIC2-accessible Pgp molecules on the cell surface, we have carried out antibody affinity analysis by flow cytometry by using UIC2 and MRK16. Fig. 2 shows antibody affinity analysis carried out by using KK-H, a subline of LMtk− cells transfected with the human MDR1 gene (see below), in the presence and in the absence of 10 μM vinblastine. Vinblastine had no effect on the binding of MRK16 (Fig. 2A), but strongly increased the immunoreactivity of UIC2 (Fig. 2B). The elevated UIC2 reactivity was reflected by a decrease in K½ (the concentration of the antibody giving half of the maximal fluorescence), indicating increased affinity of Pgp binding, and by an increase in Fmax (median fluorescence at asymptotically high levels of the antibody), indicating an increased number of UIC2-binding sites on the cell surface. Different preparations of mAb UIC2 produced very similar Fmax values with the same cells in the presence of vinblastine, but the binding at 37°C in the absence of the drug varied for different antibody preparations and decreased over the time of storage for the same antibody preparation, resulting in increased fold difference in Fmax values (data not shown).
Figure 2.
Antibody affinity and competition assays. Binding of Pgp-specific mAbs was carried out for 30 min at 37°C and analyzed by indirect immunofluorescence. Each graph shows the median fluorescence for the cell population as a function of the amount of mAb added. (A) Binding of KK-H cells to mAb MRK16 in the absence (○) and in the presence (•) of 10 μM of vinblastine. (B) Binding of KK-H cells to mAb UIC2 in the absence of drugs (○), in the presence of vinblastine (•), and in the presence of 1 μM of oligomycin (▵). (C) Binding of KK-H cells to combinations of mAbs UIC2 and MRK16. Cells were preincubated 45 min at 37°C with the amounts of UIC2 indicated on the x axis, in the presence or in the absence of vinblastine. Cells then were washed and analyzed by indirect immunofluorescence either immediately (○ in the absence of vinblastine; • in the presence of vinblastine), or after incubation for 30 min at 37°C with 3 μg of MRK16 (□, no vinblastine; ▪, cells preincubated with vinblastine) or UIC2 (▵, no vinblastine). (D) Titration of KM-H with mAb UIC2 in the absence (○) and in the presence (•) of 10 μM of vinblastine. (E) Titration of MK-H with mAb UIC2 in the absence (○) and in the presence (•) of 10 μM of vinblastine.
Binding competition assays showed that, in the absence of vinblastine, a high proportion of Pgp molecules on the cell surface is accessible to MRK16, but not to UIC2, whereas in the presence of the drug UIC2 competed with MRK16 for the binding to all molecules. A representative experiment is shown in Fig. 2C. In this experiment, KK-H cells were preincubated at 37°C with antibody-free media or with increasing amounts of mAb UIC2, in the presence or absence of 10 μM vinblastine. The unbound UIC2 then was washed off, and the cells were stained with a FITC-conjugated secondary antibody either immediately or after further reaction with a saturating amount of MRK16. In the absence of vinblastine, incubation with MRK16 strongly increased the median fluorescence even in the cells that were preincubated with saturating concentrations of UIC2 (incubation with the same amount of UIC2 instead of MRK16 produced only a minor increase in fluorescence). In contrast, the Fmax achieved by preincubation with UIC2 in the presence of vinblastine was not increased any further by subsequent incubation with MRK16 (Fig. 2C). When binding competition analysis was carried out at 4°C in the absence of a drug, the immunoreactivity of both UIC2 and MRK16, directly conjugated with PE, was almost completely inhibited by 100-fold excess of either antibody, indicating that at low temperature both UIC2 and MRK16 were fully competitive with each other (data not shown).
Mutations of Nucleotide-Binding Sites Alter UIC2 Reactivity.
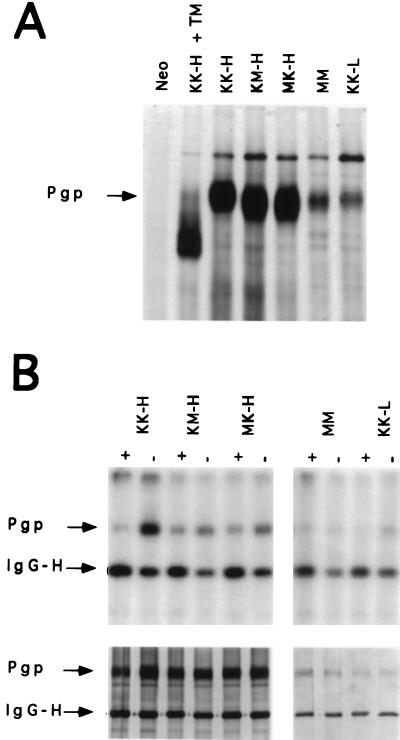
The temperature dependence of the UIC2 reactivity shift suggested that the UIC2-reactive Pgp conformation could be specific for functioning Pgp. To investigate the relationship between Pgp function and UIC2 reactivity, we have used mutant forms of Pgp, where either one or both NBD were functionally debilitated by a substitution of highly conserved lysine residues that are involved in nucleotide binding (24, 25). We have used site-directed mutagenesis to replace the K433 and K1076 lysines in the first Walker A fold of the N-terminal or C-terminal nucleotide-binding sites of Pgp with methionines. We have constructed expression vectors encoding the wild-type Pgp (designated KK), Pgp mutants with a single K→M substitution in either the N-terminal or the C-terminal NBD (designated MK and KM, respectively) and a double mutant with K→M substitutions in both NBDs (designated MM). These vectors were used to generate a series of mouse LMtk− transfectant cell lines expressing these four forms of Pgp, including two wild-type transfectant cell lines expressing different levels of Pgp and designated KK-L (low) and KK-H (high), single-mutant transfectants KM-H and MK-H and a double-mutant transfectant, MM. MRK16 immunoadsorbtion of 35S-labeled cellular protein (Fig. 3A) shows that the cell lines KK-H, MK-H, and KM-H contained similar amounts of Pgp, whereas the Pgp levels in cell lines KK-L and MM were 5–7 times lower. These cell lines also showed the same ratios of cell-surface Pgp, as measured by flow cytometric analysis of MRK16 binding to intact cells (data not shown), indicating that the efficiency of protein insertion into the plasma membrane was unaffected in mutant Pgps. The mutant Pgps also appear to be glycosylated, as indicated by a comparison of electrophoretic mobility between mutant Pgps and the wild-type Pgp isolated from untreated or tunicamycin-treated cells (Fig. 3A).
Figure 3.
Electrophoretic mobility and 8-azido-ATP photolabeling of wild-type and mutant Pgps isolated by MRK16 immunoadsorption from LMtk− transfectant cell lines. (A) Electrophoretic analysis of immunoadsorbants from 35S-methionine labeled cells. +TM: tunicamycin-treated cells. (B) (Upper) 32P-8-azido-ATP labeling of MRK16 immunoadsorbants from the indicated cell lines. + indicates the presence and − the absence of 200-fold excess of unlabeled 8-azido-ATP. (Left) Lanes KK-H, KM-H, and MK-H and (Right) lanes MM and KK-L correspond to different exposures of the autoradiogram. (Lower) Silver staining of the same gel.
Analysis of the ATPase activity of mutant Pgps, expressed in insect cell membranes and assayed in the presence or in the absence of Pgp transport substrates, revealed that both single and double mutants have lost detectable ATPase activity (26). We also have observed that Pgps, partially purified by immunoadsorption with MRK16 from MK-H, KM-H, or MM cells, showed little or no basal ATPase activity relative to the wild-type Pgp (data not shown). The lack of ATPase activity in the single mutants also was reported by others (27).
To determine if the K→M substitutions have affected nucleotide binding to mutant Pgps, we have tested immunoadsorbed complexes of MRK16 and Pgp for photolabeling with 32P-8-azido ATP, a photoactive ATP analog. To minimize nonspecific photolabeling, we used 32P-8-azido ATP at 2.5 μM (approximately 30-fold molar excess relative to Pgp), the lowest concentration that produced readily detectable Pgp labeling. In addition to Pgp, the Ig heavy chain (IgG-H) of MRK16 also was labeled with 32P-8-azido ATP, as previously observed by other investigators (28). The addition of unlabeled 8-azido-ATP at 200-fold excess relative to the labeled compound decreased the labeling of Pgp, but did not decrease (and even slightly increased) the labeling of IgG-H, indicating that IgG-H labeling was nonspecific. As illustrated in Fig. 3B, both wild-type Pgp from KK-H and KK-L cells and single-mutant Pgps from KM-H and MK-H cells show specific labeling with 32P-8-azido-ATP. The specific binding (defined as the difference in Pgp labeling in the absence and in the presence of the competitor) was lower for the KM-H or MK-H than for the KK-H cells, ranging from approximately 15% to 40% of the KK-H level in most experiments. In contrast, we could discern no decrease in the labeling of the double-mutant (MM) Pgp in the presence of the competitor, indicating loss of specific binding. Müller et al. (26) also have investigated 32P-8-azido ATP binding to the same mutant Pgps expressed in insect cell membranes by using a baculoviral vector. In this system, KK, MK and KM Pgps were photolabeled with 5 μM 32P-8-azido ATP, but MM showed no photolabeling under these conditions (though higher 32P-8-azido ATP concentrations led to the labeling of MM as well). Taken together, these results suggest that the K→M substitution was detrimental to the nucleotide-binding capacity of the NBDs.
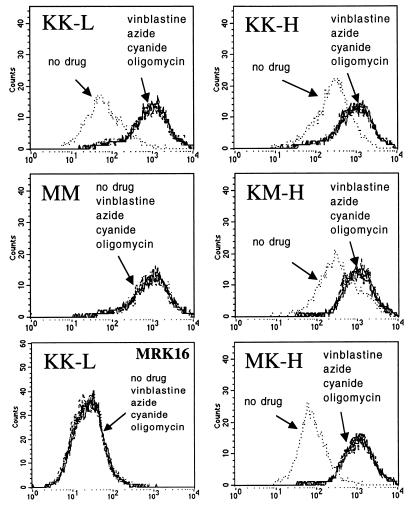
Flow cytometric analysis of UIC2 binding to cells expressing mutant Pgps showed that the NBD mutations altered the UIC2 reactivity at 37°C. When tested in the absence of drugs, the double-mutant MM cells showed increased binding of UIC2 relative to the wild-type KK-L transfectant, whereas MRK16 reactivity of these cell lines was almost identical (Fig. 4). In the presence of vinblastine, the UIC2 reactivity of MM cells was unaltered, but the reactivity of KK-L cells was increased to match that of MM (Fig. 4). The single mutant MK-H and KM-H transfectants and the wild-type KK-H cells were equally reactive with MRK16 (data not shown), but the UIC2 reactivity in the absence of drugs was strongly decreased in MK-H cells and slightly decreased in KM-H cells relative to the wild-type KK-H transfectants. Vinblastine increased the UIC2 reactivity of the KK-H, MK-H and KM-H cells, resulting in a very similar level of binding for all three cell lines (Fig. 4). UIC2 affinity analysis showed that Fmax of MK-H and KM-H cells was strongly increased in the presence of vinblastine (Fig. 2 D and E); UIC2/MRK16 binding competition assays with MK-H and KM-H lines produced essentially the same result as with the wild-type cells (not shown), indicating the existence of UIC2-reactive and nonreactive populations of single-mutant Pgp molecules. Taxol also increased UIC2 binding in KK-L, KK-H, MK-H, and KM-H cells (but not in MM), whereas incubation with etoposide had no effect on UIC2 reactivity (data not shown). MRK16 staining was unaffected by the drugs in any of the tested cell lines.
Figure 4.
UIC2 reactivity shift induced by ATP-depleting agents. Effects of vinblastine (25 μM) and ATP-depleting agents oligomycin (1 μM), sodium azide (6 μM), and potassium cyanide (4 μM) on the staining of KK-L, MM, KK-H, MK-H, and KM-H transfectants with PE-conjugated mAb UIC2. Under these experimental conditions, the level of ATP depletion caused by each of the three agents was 98–100%. (Bottom left) Staining of KK-L cells with mAb MRK16 under the same conditions.
ATP Depletion Increases UIC2 Reactivity.
Because the MM mutant with mutations in both nucleotide-binding sites has the highest UIC2 reactivity in the absence of substrates, we hypothesized that UIC2 binding reflects the conformation of Pgp that has no ATP molecules bound to it. We tested therefore to see if depletion of intracellular ATP would increase the UIC2 reactivity of Pgp. We found that agents inducing ATP depletion, including oligomycin (0.1–5 μM, causing 100% ATP depletion), sodium azide (0.4–6 μM, 63–95% ATP depletion), and potassium cyanide (1–4 μM, 83–95% ATP depletion) increased the reactivity of UIC2, but not MRK16, with KK-L and K562/i-S9 cells; higher concentrations of azide and cyanide caused significant cell death and abnormal morphology, hindering flow cytometric analysis (data not shown). All three agents increased the UIC2 reactivity of the wild-type Pgp in KK-L and KK-H cells to the same extent as vinblastine (Fig. 4). Affinity analysis (Fig. 2B) also showed that the effect of oligomycin on UIC2 binding to KK-H cells was very similar to that of vinblastine. All of the ATP depleting agents increased the binding of UIC2 binding to KK-L cells to the level of MM, while having no effect on MRK16 binding or on the MM cell reactivity. These agents also increased the reactivity of KK-H, MK-H, and KM-H cell lines to the same maximal levels (Fig. 4). Thus, the addition of Pgp transport substrates, mutagenesis of both nucleotide-binding sites, and ATP depletion all had the same effect on UIC2 reactivity.
DISCUSSION
The results of this study show that the reactivity of mAb UIC2 with cells expressing Pgp on their surface is increased at 37°C in the presence of Pgp transport substrates or agents that induce ATP depletion. This altered immunoreactivity of Pgp is unlikely to reflect the effect of these agents on the cell membrane, because the same increase in UIC2 reactivity is induced in the absence of these agents by mutations that inactivate both NBD of Pgp. Because Pgp substrates or ATP-depleting agents increased UIC2 reactivity at 37°C but not at 4°C, and because these treatments had no effect on the reactivity of the nonfunctional double mutant, the UIC2 reactivity shift is associated with conformational changes of functioning Pgp. The observed effect suggests that UIC2 preferentially reacts with a transient Pgp conformation that can be made permanent, however, by mutations of both NBD. Conformational transitions previously have been implicated in the function of other membrane transport proteins, including the calcium pump ATPase (where the conformation changes have been identified by differences in the crystal structure of the protein; ref. 29), the sodium/potassium pump ATPase (where the conformation changes have been less directly identified by changes in the intrinsic fluorescence of the protein; ref. 30), and the glucose transporter (where differential immunoreactivity has revealed such changes; ref. 31). In the case of Pgp, the existence of conformational changes in the presence of transport substrates or ATP has been indicated by infrared spectroscopy and fluorescence quenching studies (32, 33). In addition, interaction with several cyclosporine-related compounds was reported to increase the reactivity of Pgp with another mAb (MM4.17), but this effect is clearly different from the one observed in the present study, because it occurred at both 37°C and 0°C (34).
Antibody affinity analysis and binding competition assays carried out with UIC2 and MRK16 indicated that, in the absence of drugs, a substantial proportion of wild-type Pgp molecules does not react with UIC2 at 37°C, but all of the molecules become UIC2-reactive in the presence of transport substrates or ATP-depleting agents. This result indicates the existence of at least two distinct conformations of Pgp, one of which efficiently binds UIC2 but the other shows little or no binding at 37°C. The differences in UIC2 reactivity among different cell lines and under different conditions would reflect different ratios between the two Pgp conformations on the cell surface. These conformations may differ in the extent to which the Pgp molecule is exposed on the extracytoplasmic side of the membrane; it is interesting to note in this regard that Pgp was reported to exist in different transmembrane orientations, resulting in the exposure of different portions of the molecule on the outside of the cell (35). Alternatively, the two conformations may reflect different oligomeric states of Pgp, because several groups have reported that Pgp in the cell membrane may exist in the form of dimers or higher order oligomers (36–38).
All of the factors that increase UIC2 reactivity (Pgp transport substrates, ATP-depleting agents, and mutations of both nucleotide-binding sites of Pgp) induce essentially identical maximal shifts in the UIC2 immunofluorescence profiles. This finding suggests that all of these factors are likely to elicit the same UIC2-reactive Pgp conformation. Both ATP depletion and mutagenesis of both nucleotide-binding sites (discussed below) result in the loss of ATP binding to Pgp. Pgp-transported drugs also can induce such a loss by stimulating the Pgp ATPase activity. Thus, we hypothesize that the UIC2-reactive form of Pgp has no bound ATP, whereas the conformation that does not react with UIC2 represents Pgp with one or two bound ATP molecules. We also hypothesize that the increase in UIC2 reactivity in the presence of Pgp transport substrates results from substrate-stimulated ATP hydrolysis, though it is possible that some other aspects of Pgp interaction with its transport substrates may induce the same conformational change as ATP hydrolysis. Thus, the apparent inability of etoposide, colchicine, and puromycin to increase the UIC2 reactivity might be related to the relatively low ability of these drugs to stimulate the Pgp ATPase activity, as demonstrated for the first two drugs (26). On the other hand, these three compounds also differ from the other Pgp-transported drugs in some sterical aspect of their interaction with Pgp, because the ability of Pgp to transport these three drugs is selectively increased by a mutation (G185V) which, at the same time, decreases the Pgp activity toward those drugs that were found in the present study to increase UIC2 reactivity (36).
Pgp-transported drugs and ATP-depleting agents increase the UIC2 reactivity of both the wild-type (KK) and the single-mutant (MK and KM) Pgps. In contrast, UIC2 reactivity of the double mutant (MM) is equally high in the presence and in the absence of these agents. Biochemical analysis indicates that all three mutants (MM, KM, and MK) have lost discernible ATPase activity relative to the wild-type protein (26). The 8-azido-ATP photolabeling studies, however, demonstrate a difference in nucleotide binding between KK, MK, and KM, on one hand, and MM, on the other hand, because only the double mutant shows no specific photolabeling at low concentrations of 8-azido-ATP in immunoadsorbants (Fig. 3) or in insect cell membranes (26), even though MM in insect cell membranes can be photolabeled at higher concentrations of 8-azido-ATP (26). Although photoactivated crosslinking of 8-azido-ATP cannot be equated with physiological ATP binding, the observed debilitation of the nucleotide-binding capacity in the double mutant, together with the lack of effect of ATP depletion or Pgp substrates on its UIC2 reactivity, suggest that this protein does not properly bind ATP under physiological conditions.
Because we and others (26, 27) found that the single mutants (KM and MK) have no significant ATPase activity, the increase in UIC2 reactivity of these mutants is likely to result from disassociation rather than hydrolysis of bound ATP. The effect of Pgp transport substrates on the UIC2 reactivity of single mutants suggests that the substrates stimulate such disassociation. In the absence of drugs, MK and (to a lesser extent) KM showed lower UIC2 reactivity than the wild-type Pgp (though the maximum reactivity of these Pgps, stimulated by substrates or ATP depletion, is the same). This finding can be explained by suggesting that, in the absence of ATP hydrolysis, a higher proportion of single-mutant Pgps exists in the ATP-bound conformation with low UIC2 reactivity.
Our results also suggest an explanation for the inhibition of the Pgp transport function by mAb UIC2. According to our model, UIC2 “traps” Pgp in a transient conformation with no bound ATP and thereby blocks further ATP binding. In agreement with this hypothesis, UIC2 was found to inhibit the ATPase activity of Pgp expressed in insect cell membranes (B. Sarkadi, personal communication). The trapping of Pgp by UIC2 in a transient conformation also explains why the Pgp transport substrates increase cellular UIC2 reactivity to the same maximal level as ATP depletion or mutations of both NBD, despite the reversible nature of the Pgp-substrate interactions.
The results of the present study indicate that changes in UIC2 reactivity can be used as a sensitive assay to analyze conformational transitions associated with Pgp function; it appears likely that conformation-sensitive antibodies also may be developed against other transporters of the ABC family. The availability of an assay for detecting conformational changes associated with the function of Pgp provides a potentially useful diagnostic test for both the expression and the function of Pgp. Furthermore, the inhibition of Pgp through binding by conformation-specific reagents may be used for the development of new immunological and small-molecule agents for the reversal of Pgp-mediated drug resistance in cancer.
Acknowledgments
We are grateful to S. Park and M. Polonskaia for assistance with flow cytometry, B. Haley for advice on 32P-8-azido-ATP photolabeling, and A. Barcena, R. Garcia, G. Raghu, and S. Salov for help in different experiments. This work was supported in part by National Cancer Institute Grant R37 CA40333.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Pgp, P-glycoprotein; NBD, nucleotide-binding domain; ABC, ATP-binding cassette; PE, phycoerythrine; FITC, fluorescein isothiocyanate; PI, propidium iodide.
References
- 1.Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 2.Roninson I B, editor. Molecular and Cellular Biology of Multidrug Resistance in Tumor Cells. New York: Plenum; 1991. [Google Scholar]
- 3.Van Helvoort A, Smith A J, Sprong H, Fritzsche I, Schinkel A H, Borst P, van Meer G. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 4.Higgins C F. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 5.Smit J J, Schinkel A H, Oude Elferink R P, Groen A K, Wagenaar E, et al. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 6.Ruetz S, Gros P. Cell. 1994;77:1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 7.McGrath J P, Varshavsky A. Nature (London) 1989;340:400–504. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- 8.Riordan J R, Rommens J M, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J L. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 9.Cornwell M M, Safa A R, Felsted R L, Gottesman M M, Pastan I. Proc Natl Acad Sci USA. 1986;83:3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins C F, Gottesman M M. Trends Biochem Sci. 1992;17:18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- 11.Hamada H, Tsuruo T. J Biol Chem. 1988;263:1454–1458. [PubMed] [Google Scholar]
- 12.Sarkadi B, Price E, M, Boucher R C, Germann U A, Scarborough G A. J Biol Chem. 1992;267:4854–4558. [PubMed] [Google Scholar]
- 13.Mechetner E B, Roninson I B. Proc Natl Acad Sci USA. 1992;89:5824–5828. doi: 10.1073/pnas.89.13.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homolya L, Hollo Z, Germann U A, Pastan I, Gottesman M M, Sarkadi B. J Biol Chem. 1993;268:21493–21496. [PubMed] [Google Scholar]
- 15.Hollo Z, Homolya L, Davis C W, Sarkadi B. Biochim Biophys Acta. 1994;1191:384–388. doi: 10.1016/0005-2736(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 16.De Graaf D, Sharma R C, Mechetner E B, Schimke R T, Roninson I B. Proc Natl Acad Sci USA. 1996;93:1238–1242. doi: 10.1073/pnas.93.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schinkel A H, Arceci R J, Smit J J M, Wagenaar E, Baas F, Dolle M, Tsuruo T, Mechetner E B, Roninson I B, Borst P. Int J Cancer. 1993;55:478–484. doi: 10.1002/ijc.2910550326. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary P M, Roninson I B. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 19.Weisburg J H, Curcio M, Caron P C, Raghu G, Mechetner E B, Roepe P D, Scheinberg D A. J Exp Med. 1996;183:2699–2704. doi: 10.1084/jem.183.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamada H, Tsuruo T. Proc Natl Acad Sci USA. 1986;83:7785–7789. doi: 10.1073/pnas.83.20.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raghu G, Park S W, Roninson I B, Mechetner E B. Exp Hematol. 1996;24:1258–1264. [PubMed] [Google Scholar]
- 22.Hamada H, Tsuruo T. Cancer Res. 1988a;48:4926–4932. [PubMed] [Google Scholar]
- 23.Georges E, Tsuruo T, Ling V. J Biol Chem. 1993;268:1792–1798. [PubMed] [Google Scholar]
- 24.Fry D C, Kuby S A, Mildvan A S. Proc Natl Acad Sci USA. 1986;83:907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagaya M, Yagami T, Fukui T. J Biol Chem. 1987;262:8257–8261. [PubMed] [Google Scholar]
- 26.Müller M, Bakos E, Welker E, Varadi A, Germann U A, Gottesman M M, Morse B S, Roninson I B, Sarkadi B. J Biol Chem. 1996;271:1877–1883. doi: 10.1074/jbc.271.4.1877. [DOI] [PubMed] [Google Scholar]
- 27.Loo T W, Clarke D M. J Biol Chem. 1995;270:21449–21452. doi: 10.1074/jbc.270.37.21449. [DOI] [PubMed] [Google Scholar]
- 28.Georges E, Zhang J T, Ling V. J Cell Physiol. 1991;148:479–484. doi: 10.1002/jcp.1041480321. [DOI] [PubMed] [Google Scholar]
- 29.Martonosi A N. Biosci Rep. 1995;15:263–281. doi: 10.1007/BF01788359. [DOI] [PubMed] [Google Scholar]
- 30.Karlish S J. Methods Enzymol. 1988;156:271–277. doi: 10.1016/0076-6879(88)56027-5. [DOI] [PubMed] [Google Scholar]
- 31.Hebert D N, Carruthers A. J Biol Chem. 1992;267:23829–23838. [PubMed] [Google Scholar]
- 32.Sonveaux N, Shapiro A B, Goormaghtigh E, Ling V, Ruysschaert J-M. J Biol Chem. 1996;271:24617–24624. doi: 10.1074/jbc.271.40.24617. [DOI] [PubMed] [Google Scholar]
- 33.Liu R, Sharom F J. Biochemistry. 1996;35:11865–11873. doi: 10.1021/bi960823u. [DOI] [PubMed] [Google Scholar]
- 34.Jachez B, Cianfriglia M, Loor F. Anti-Cancer Drugs. 1994;5:655–665. doi: 10.1097/00001813-199412000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Wang G, Shapiro A, Zhang J T. Biochemistry. 1996;35:9728–9736. doi: 10.1021/bi960400s. [DOI] [PubMed] [Google Scholar]
- 36.Boscoboinik D, Debanne M T, Stafford A R, Yung C Y, Gupta R S, Epand R M. Biochim Biophys Acta. 1990;1027:225–228. doi: 10.1016/0005-2736(90)90311-b. [DOI] [PubMed] [Google Scholar]
- 37.Naito M, Tsuruo T. Biochem Biophys Res Commun. 1992;185:284–290. doi: 10.1016/s0006-291x(05)80988-x. [DOI] [PubMed] [Google Scholar]
- 38.Poruchinsky M S, Ling V. Biochemistry. 1994;33:4163–4174. doi: 10.1021/bi00180a009. [DOI] [PubMed] [Google Scholar]
- 39.Safa A R, Stern R K, Choi K, Agresti M, Tamai I, Mehta N D, Roninson I B. Proc Natl Acad Sci USA. 1990;87:7225–7229. doi: 10.1073/pnas.87.18.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]