Abstract
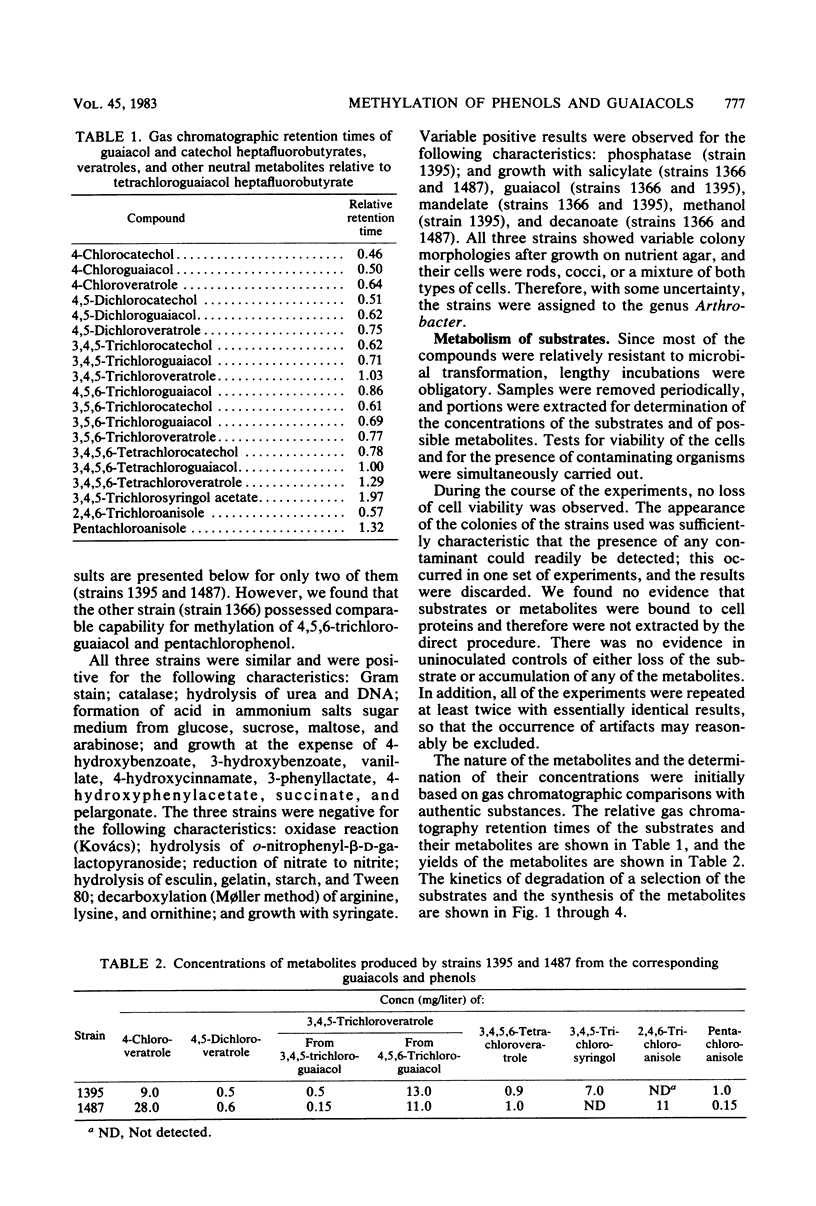
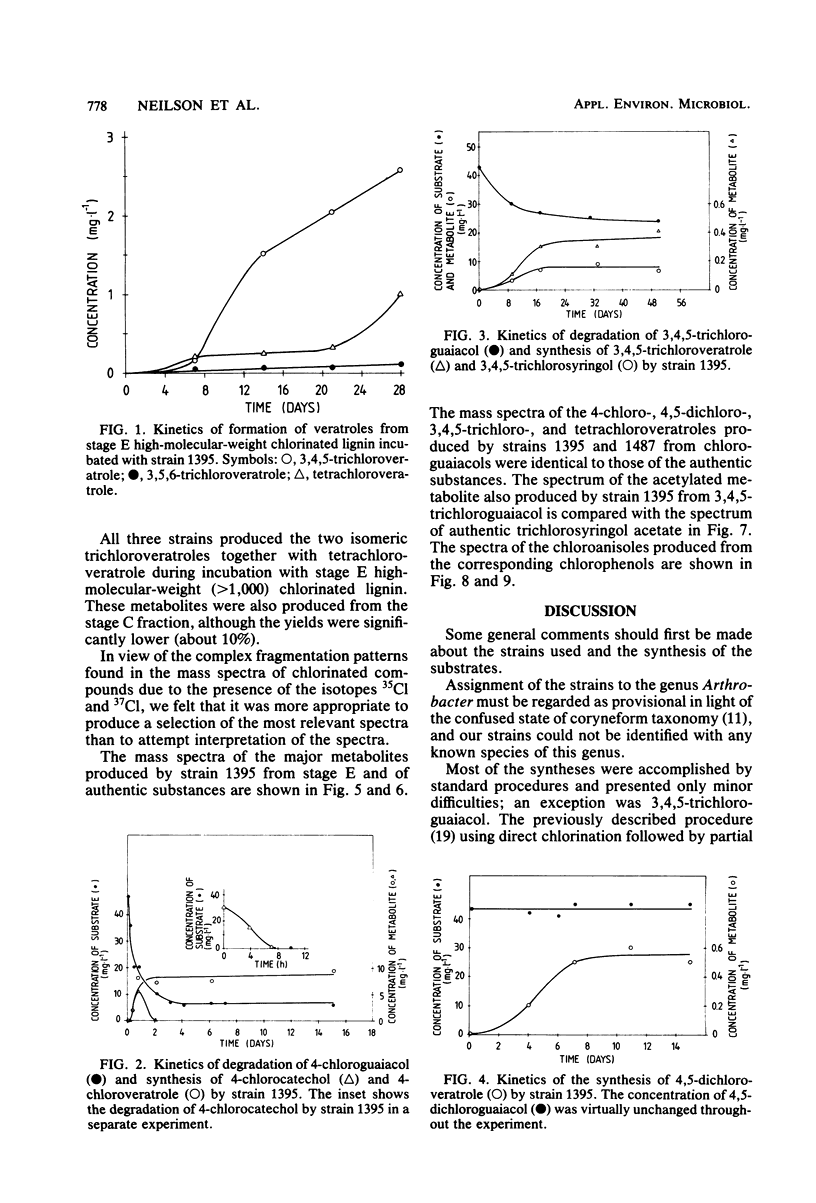
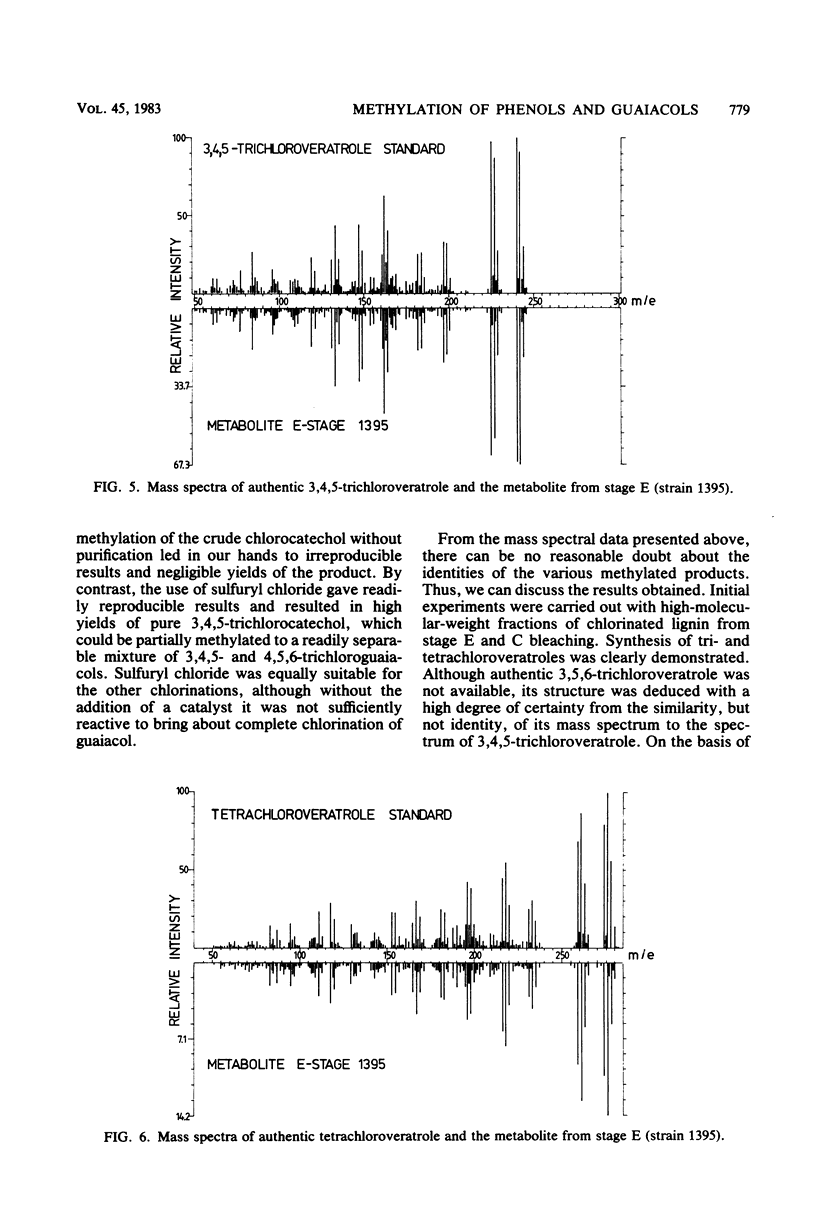
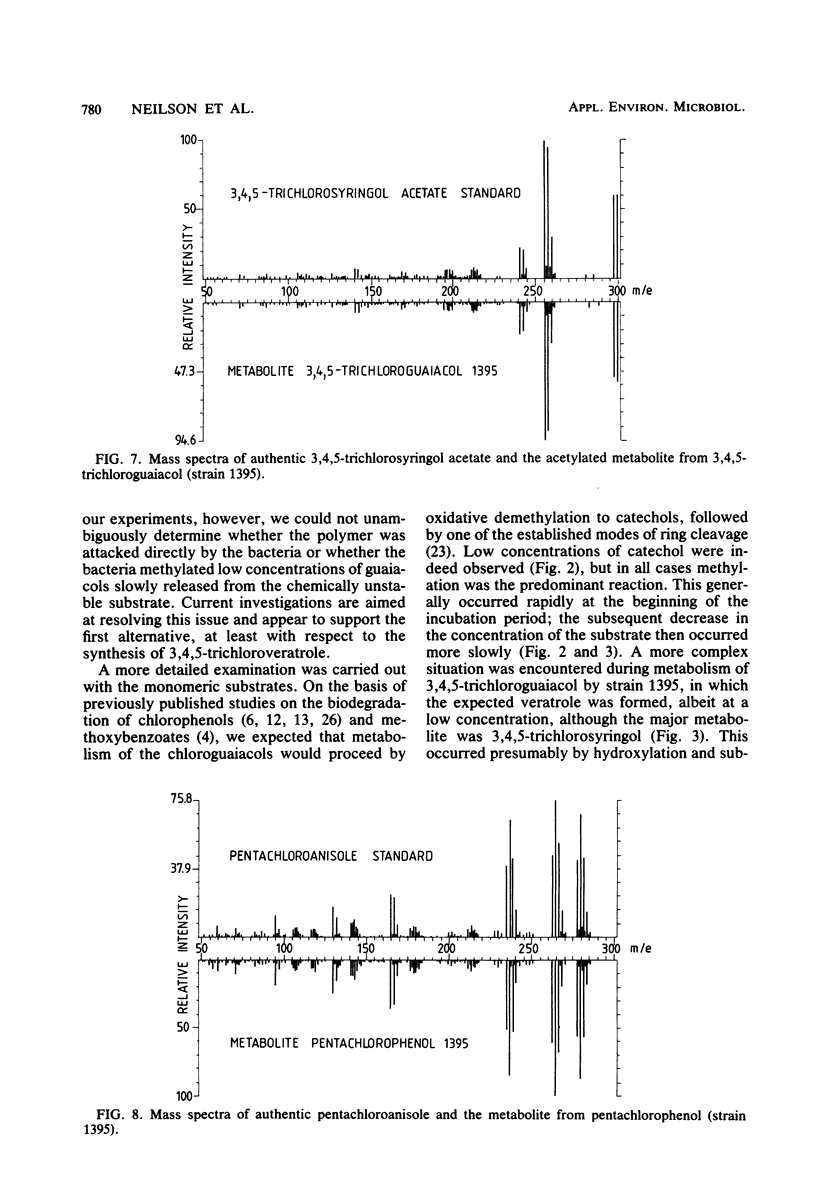
Two strains of bacteria, provisionally assigned to the genus Arthrobacter, were shown to metabolize mono-, di-, tri-, and tetrachloroguaiacols and pentachlorophenol to the corresponding O-methyl compounds. Hydroxylated intermediates were formed only transiently, except for the synthesis by one strain of 3,4,5-trichlorosyringol from 3,4,5-trichloroguaiacol. Two isomeric trichloroveratroles and tetrachloroveratrole were formed by three of the strains from a high-molecular-weight chlorinated lignin isolated from kraft pulp mill bleach plant. The concentrations of methylated metabolites varied widely and did not appear to be correlated with degradation. The possible environmental consequences resulting from synthesis of these highly lipophilic substances are discussed briefly.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cserjesi A. J., Johnson E. L. Methylation of pentachlorophenol by Trichoderma virgatum. Can J Microbiol. 1972 Jan;18(1):45–49. doi: 10.1139/m72-007. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Donnelly M. I., Dagley S. Bacterial degradation of 3,4,5-trimethoxycinnamic acid with production of methanol. J Bacteriol. 1981 Aug;147(2):471–476. doi: 10.1128/jb.147.2.471-476.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. R., Appleton J., Pemberton J. M. Isolation and characterization of the pesticide-degrading plasmid pJP1 from Alcaligenes paradoxus. J Bacteriol. 1978 Sep;135(3):798–804. doi: 10.1128/jb.135.3.798-804.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. M., Peel J. L. Metabolism of 2,3,4,6-tetrachlorophenol by micro-organisms from broiler house litter. J Gen Microbiol. 1974 Dec;85(2):237–243. doi: 10.1099/00221287-85-2-237. [DOI] [PubMed] [Google Scholar]
- Guldberg H. C., Marsden C. A. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev. 1975 Jun;27(2):135–206. [PubMed] [Google Scholar]
- Jones D. A numerical taxonomic study of coryneform and related bacteria. J Gen Microbiol. 1975 Mar;87(1):52–96. doi: 10.1099/00221287-87-1-52. [DOI] [PubMed] [Google Scholar]
- Knackmuss H. J., Hellwig M. Utilization and cooxidation of chlorinated phenols by Pseudomonas sp. B 13. Arch Microbiol. 1978 Apr 27;117(1):1–7. doi: 10.1007/BF00689343. [DOI] [PubMed] [Google Scholar]
- Müller-Enoch D., Thomas H., Streng W., Wildfeuer W., Haferkamp O. O-Methylierung von Adrenalin, 3.4-Dihydroxybenzoesäure und 6.7-Dihydroxycumarin in Sprosspilzen. Z Naturforsch C. 1976 Sep-Oct;31(9-10):509–513. [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokes J. R., Walker N. Chlorophenol and chlorobenzoic acid co-metabolism by different genera of soil bacteria. Arch Mikrobiol. 1974 Mar 4;96(2):125–134. doi: 10.1007/BF00590169. [DOI] [PubMed] [Google Scholar]
- Veser J., Geywitz P., Thomas H. Purification and properties of a catechol methyltransferase of the yeast Candida tropicalis. Z Naturforsch C. 1979 Sep-Oct;34(9-10):709–714. doi: 10.1515/znc-1979-9-1010. [DOI] [PubMed] [Google Scholar]
- van Veen W. L. Bacteriology of activated sludge, in particular the filamentous bacteria. Antonie Van Leeuwenhoek. 1973;39(2):189–205. doi: 10.1007/BF02578852. [DOI] [PubMed] [Google Scholar]


