Abstract
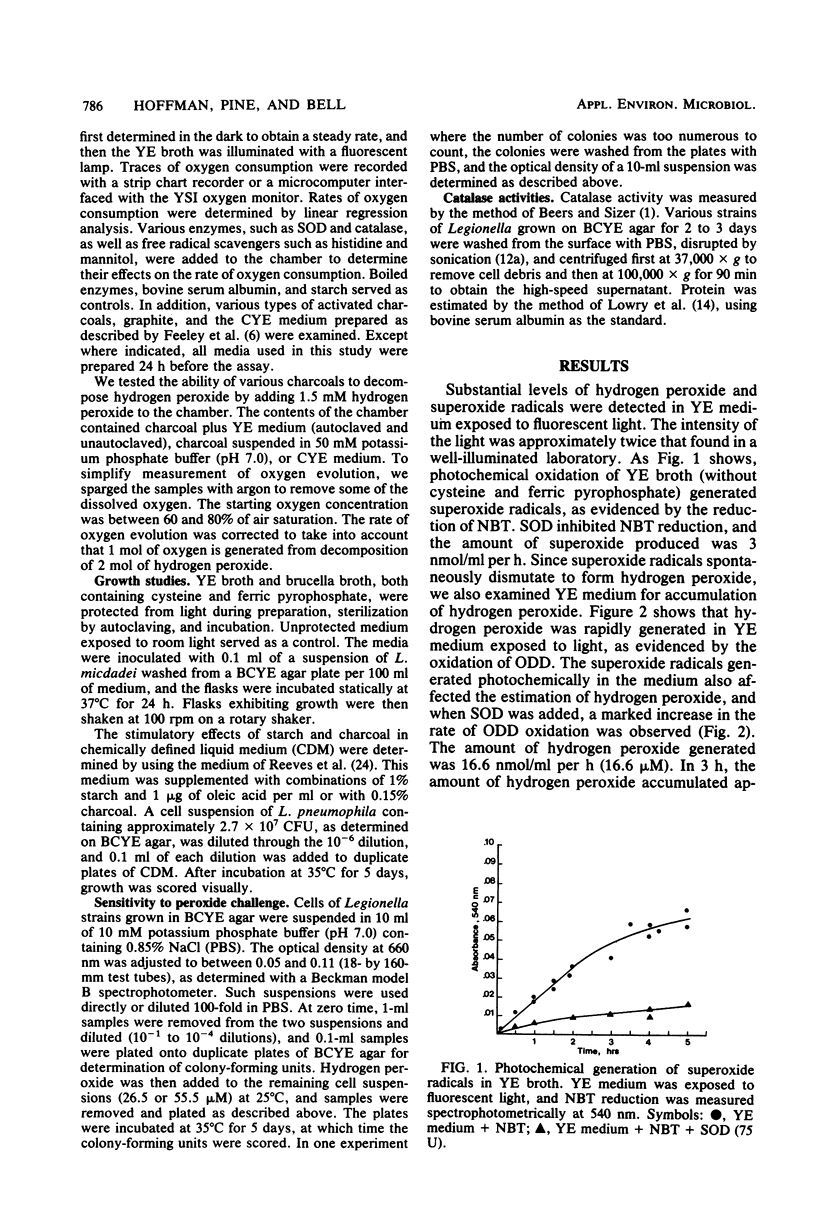
The difficulties associated with the growth of Legionella species in common laboratory media may be due to the sensitivity of these organisms to low levels of hydrogen peroxide and superoxide radicals. Exposure of yeast extract (YE) broth to fluorescent light generated superoxide radicals (3 microM/h) and hydrogen peroxide (16 microM/h). Autoclaved YE medium was more prone to photochemical oxidation than YE medium sterilized by filtration. Activated charcoals and, to a lesser extent, graphite, but not starch, prevented photochemical oxidation of YE medium, decomposed hydrogen peroxide and superoxide radicals, and prevented light-accelerated autooxidation of cysteine. Also, suspensions of charcoal in phosphate buffer and in charcoal yeast extract medium readily decomposed exogenous peroxide (17 and 23 nmol/ml per min, respectively). Combinations of bovine superoxide dismutase and catalase also decreased the rate of photooxidation of YE medium. Medium protected from light did not accumulate appreciable levels of hydrogen peroxide, and autoclaved YE medium protected from light supported good growth of Legionella micdadei. Various species of Legionella (10(4) cells per ml) exhibited sensitivity to relatively low levels of hydrogen peroxide (26.5 microM) in challenge experiments. The level of hydrogen peroxide that accumulated in YE medium over a period of several hours (greater than 50 microM) was in excess of the level tolerated by Legionella pneumophila, which contained no measurable catalase activity. Strains of L. micdadei, Legionella dumoffi, and Legionella bozmanii contained this enzyme, but the presence of catalase did not appear to confer appreciable tolerance to exogenously generated hydrogen peroxide.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Bey R. F., Johnson R. C. Protein-free and low-protein media for the cultivation of Leptospira. Infect Immun. 1978 Feb;19(2):562–569. doi: 10.1128/iai.19.2.562-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Granberg G. P., Nyberg G. K., Edlund M. B. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl Environ Microbiol. 1979 Mar;37(3):383–390. doi: 10.1128/aem.37.3.383-390.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Nyberg G., Wrethén J. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl Environ Microbiol. 1978 Aug;36(2):223–229. doi: 10.1128/aem.36.2.223-229.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H., Finegold S. M. Use of a semiselective medium to culture Legionella pneumophila from contaminated lung specimens. J Clin Microbiol. 1979 Aug;10(2):141–143. doi: 10.1128/jcm.10.2.141-143.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gorman G. W., Weaver R. E., Mackel D. C., Smith H. W. Primary isolation media for Legionnaires disease bacterium. J Clin Microbiol. 1978 Sep;8(3):320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. R., Pine L., Reeves M. W., Harrell W. K. Amino acid requirements of Legionella pneumophila. J Clin Microbiol. 1980 Mar;11(3):286–291. doi: 10.1128/jcm.11.3.286-291.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., George H. A., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979 Jan;25(1):8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Goodman T. G. Respiratory physiology and energy conservation efficiency of Campylobacter jejuni. J Bacteriol. 1982 Apr;150(1):319–326. doi: 10.1128/jb.150.1.319-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. R., Schalla W. O., Wong K. H., Perkins G. H. Simple, transparent medium for study of legionellae. J Clin Microbiol. 1982 Feb;15(2):342–344. doi: 10.1128/jcm.15.2.342-344.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUELLER J. H., MILLER P. A. Variable factors influencing the production of tetanus toxin. J Bacteriol. 1954 Mar;67(3):271–277. doi: 10.1128/jb.67.3.271-277.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. Superoxide dismutase: a photochemical augmentation assay. Arch Biochem Biophys. 1977 May;181(1):308–312. doi: 10.1016/0003-9861(77)90509-4. [DOI] [PubMed] [Google Scholar]
- Norris S. J. In vitro cultivation of Treponema pallidum: independent confirmation. Infect Immun. 1982 Apr;36(1):437–439. doi: 10.1128/iai.36.1.437-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrod E. P., Morse S. A. Presence of hydrogen peroxide in media used for cultivation of Neisseria gonorrhoeae. J Clin Microbiol. 1982 Jan;15(1):103–108. doi: 10.1128/jcm.15.1.103-108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett P. J., Cover W. H., Krieg N. R. The Microaerophile SPirillum volutans: Cultivation on Complex Liquid and Solid Media. Appl Environ Microbiol. 1982 Feb;43(2):469–477. doi: 10.1128/aem.43.2.469-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasculle A. W., Feeley J. C., Gibson R. J., Cordes L. G., Myerowitz R. L., Patton C. M., Gorman G. W., Carmack C. L., Ezzell J. W., Dowling J. N. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis. 1980 Jun;141(6):727–732. doi: 10.1093/infdis/141.6.727. [DOI] [PubMed] [Google Scholar]
- Pine L., George J. R., Reeves M. W., Harrell W. K. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1979 May;9(5):615–626. doi: 10.1128/jcm.9.5.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. W., Pine L., Hutner S. H., George J. R., Harrell W. K. Metal requirements of Legionella pneumophila. J Clin Microbiol. 1981 Apr;13(4):688–695. doi: 10.1128/jcm.13.4.688-695.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Allen R. G. Liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1980 Jan;11(1):19–21. doi: 10.1128/jcm.11.1.19-21.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Gowda S. Chemically defined medium for Legionella pneumophila growth. J Clin Microbiol. 1981 Jan;13(1):115–119. doi: 10.1128/jcm.13.1.115-119.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck J. L., Henneberry R. C., Cox C. D. Growth requirements of pathogenic Leptospira. Infect Immun. 1973 Jun;7(6):886–897. doi: 10.1128/iai.7.6.886-897.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W. J., Miller R. D. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J Clin Microbiol. 1979 Jul;10(1):50–55. doi: 10.1128/jcm.10.1.50-55.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth P. M. The action of light on culture media. J Clin Pathol. 1969 May;22(3):273–277. doi: 10.1136/jcp.22.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshpe-Purer Y., Henis Y. Factors affecting catalase level and sensitivity to hydrogen peroxide in Escherichia coli. Appl Environ Microbiol. 1976 Oct;32(4):465–469. doi: 10.1128/aem.32.4.465-469.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



