Abstract
Rationale
Methamphetamine (METH) is typically characterized as a more potent psychostimulant than amphetamine (AMPH), but few studies have directly compared the effects of these drugs at low, behaviorally activating doses that tend not to produce focused stereotypy.
Objectives
To compare the effects of AMPH or METH treatment on locomotor activity in an open-field arena, focusing on their ability to produce conditioned locomotor activity, sensitization, and cross-sensitization.
Methods
Adult male rats were given AMPH or METH (0.5 or 1.0 mg/kg) for five days, with half of the rats presented with discrete, salient stimuli (S+) during the post-injection period. Following a three-day withdrawal, they were given three different injections on successive days: a saline challenge to assess conditioned responding, a drug challenge to assess sensitization, and a cross-sensitization test to the same dose of the drug with which they were not pre-treated.
Results
Except in certain conditions, AMPH and METH were equipotent at activating locomotor activity. The exceptions included when rats were presented with S+ on acute and drug challenge days, and in tests of cross-sensitization. There were no consistent differences in the magnitude of sensitization produced by AMPH or METH, and both drugs produced similar amounts of conditioned locomotion after a saline injection.
Conclusions
We have found specific conditions where METH is more potent than AMPH, but this study and others that used higher doses of these drugs are not consistent with the generalized characterization of METH as a more potent psychostimulant.
Introduction
Amphetamine (AMPH) and its N-methylated derivative methamphetamine (METH) are psychostimulants that share a nearly identical chemical structure and have similar pharmacokinetic properties (Melega et al. 1995), but have different rates of abuse. Epidemiological studies suggest the life-time incidence of non-medical METH use is 5.3%, whereas it is about 1.4% for AMPH (Colliver et al. 2006). Although this might be due in part to the greater availability of illicit METH (Romanelli and Smith 2006), it is commonly suggested that METH is relatively more potent than AMPH (Feldman et al. 1997; NIDA Research Report 2006). Empirical evidence for this assertion, however, is somewhat limited because the two drugs are typically not investigated within the context of the same study. When they are, it is often the case that AMPH and METH produce similar effects. For example, rats and rhesus monkeys trained to self-administer AMPH and METH take approximately equal amounts of the drugs (Balster and Schuster 1973; Yokel and Pickens 1973). Drug discrimination studies have shown that rats (Kuhn et al. 1974), much like humans (Lamb and Henningfield 1994), are unable to discriminate between the two drugs over a fairly wide dose range. Lastly, rats given either AMPH or METH at 1, 3 and 4 mg/kg, exhibit similar locomotor activation in an open-field (Shoblock et al. 2003; Milesi-Halle et al. 2007).
Studies showing a more potent effect of METH relative to AMPH have focused on the differential effects of these drugs on neurochemistry and behavior following a 2 mg/kg dose given acutely, or following binge-like exposure. Specifically, a single injection of 2 mg/kg METH is more effective than 2 mg/kg AMPH at releasing glutamate in the prefrontal cortex, although AMPH more potently increases dopamine in the prefrontal cortex. Furthermore, AMPH increases glutamate concentrations in the nucleus accumbens, whereas METH does not (Shoblock et al. 2003). In this study, lower photocell activity counts were seen in the METH- compared to the AMPH-treated group, which was likely due to METH-induced increases in focused stereotypies. This dose has also been shown to differentially alter 5-HT and norepinephrine levels in the caudate and hippocampus, respectively, in rats that spent more time in focused stereotypy after METH compared to AMPH (Kuczenski et al. 1995). In a binge-exposure model, where 2.5 or 4.0 mg/kg AMPH and equimolar doses of METH were first given in 15 single daily injections and later in “binge” treatments every 2 h, METH was shown to produce a prolonged “post-stereotypy” response compared to AMPH. Associated with these behaviors was a similar effect of both drugs on dopamine levels in the dorsal striatum and nucleus accumbens, but a relatively greater effect of METH on 5-HT levels in these brain regions (Segal and Kuczenski 1997).
Given that METH has only been shown to be more potent at relatively high, stereotypy-inducing doses, the present study was undertaken to compare the effects of AMPH and METH at relatively low doses (0.5 and 1.0 mg/kg) that activate behavior but tend not to induce stereotypy (Segal and Kuczenski 1987; Gentry et al. 2004). Another aim was to compare the magnitude of conditioned locomotor activity following a saline challenge and behavioral sensitization following a drug challenge, as well as to test for cross-sensitization between the two drugs, in groups exposed repeatedly to these drugs. In half of the groups, a discrete, salient stimulus was presented intermittently during the entire post-injection interval to assess if AMPH- or METH-induced locomotor activity was altered in a dose-specific manner relative to groups that behaved in the open-field without additional cues. For tests of cross-sensitization, rats pre-treated with AMPH were challenged with METH and those pre-treated with METH were challenged with AMPH.
Methods
Animals
Male Sprague-Dawley rats (n = 68), 2.5–3.5 months old, were obtained from Harlan (Indianapolis, IN) or bred in our animal facility from Harlan stock rats. They were kept on a 12:12h light/dark cycle, with experiments performed during the light cycle. Rats were housed individually in translucent home cages for an average of 25 days before the start of experiments, during which time they were handled for 15 min on five separate occasions. They remained individually housed for the experiment’s duration and were allowed free access to food and water. Experimental procedures were approved by the IACUC at the University of Illinois and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85–23).
Open-Field Activity
Activity was measured in open-field arenas consisting of an acrylic box (40.6 × 40.6 × 40.6 cm) fitted with two photobeam frames (16 beams/dimension; 2.5 cm between beams; Coulbourn Instruments; Allentown, PA): the lower frame (2.5 cm above the arena floor) recorded horizontal activity (i.e., locomotion), whereas the upper frame (15 cm above the floor) recorded rearing. Each chamber was in a sound-attenuating cubicle (76 × 80 × 63 cm) that had a 76 mm speaker mounted on the inside wall and a ceiling-mounted camera between two white lights (4 W each). Located just above the lower photobeam frame was a small lamp (lens diameter = 1.3 cm) containing a 28 V LED. The speaker and LED lamp were used to deliver stimuli in certain groups (see below). Each chamber was connected to a computer running software (TruScan, Coulbourn Instruments) that recorded beam breaks (100 ms sampling rate). Digital video was captured and stored to a computer for offline analysis of stereotyped behaviors.
On the first day of the experiment, rats were moved from the colony to the testing room, where they remained in their home cage for a 30-min acclimation period. They were then placed individually in the open-field chamber for 30 min, removed and injected with saline (1 ml/kg, i.p.), and returned to the open-field for 60 min. They were subsequently returned to the colony and randomly divided into AMPH (0.5 or 1.0 mg/kg, n = 16/dose), METH (0.5 or 1.0 mg/kg, n = 16/dose), or saline (n = 4) treatment groups.
On day 2, the procedure was repeated with the following exceptions. First, rats in drug treatment groups were injected with their assigned doses of AMPH or METH. Second, half of the rats in these groups (n = 8/drug/dose) were exposed to a stimulus (S+) during the entire 60 min post-injection period. The S+ consisted of a 55 dB tone and a white LED presented in a 5-s on/5-s off pattern (360 total presentations). The remaining rats (S− group) had no additional stimuli delivered. For the next four days (days 3–6), the procedure was repeated such that rats received five drug injections total. Following a three-day withdrawal period where they remained in the colony room, rats began three sessions of saline or drug challenges. For the first (day 10), all rats received saline injections. For the second (day 11), rats were given the same drug and dose that they received prior to withdrawal. The final challenge (day 12) was a cross-sensitization test: rats received the same dose of the alternative drug from their pre-withdrawal treatment. Thus, rats previously receiving METH were given AMPH, and those previously receiving AMPH were given METH.
A separate group (n = 4) was added to examine the effects of repeated exposure to the S+. These rats received six daily saline injections, three days without injections, and a final saline challenge. Their daily testing protocol was otherwise identical to other S+ groups.
Data Analysis
Locomotion was calculated as consecutive beam breaks in the lower frame (distance traveled, in cm); rearing was calculated as number of beam breaks in the upper frame. Activity was summed in 30- or 60-min bins. Behavior of rats given 1.0 mg/kg AMPH or METH was also scored for the existence of focused stereotypy and other qualitative aspects. Raters scored digital video of the 60 min post-injection by randomly selecting 30-s intervals every five min and assigning the following score: 1 = asleep/inactive; 2 = stationary sniffing; 3 = grooming; 4 = stationary activity, sniffing, head movement, and/or rearing; 5 = exploring; 6 = hyperactivity; 7 = hyperactivity with some repetitive movements; 8 = fast-patterned, mostly repetitive exploration; 9 = focused stereotypy, with repetitive head and/or body movements but no locomotion. Group averages of these scores were analyzed initially in successive 5-min bins. Because there were no consistent group differences at successive time periods and the time course curves were asymptotic after the 10-min time point, these data were subsequently averaged across the 60 min post-injection period for final analysis.
The influence of saline and drug treatment on activity was first analyzed by an overall ANOVA (group × stimulus × treatment day). Follow-up analyses included mixed, two-factor ANOVA (group × treatment, with treatment as the repeated measure) for comparison of the first saline and drug treatment days and two-factor ANOVAs (dose × stimulus condition) for analysis of drug effects in the presence (S+) or absence (S−) of stimuli. When comparisons were made between drug-induced activity on the first treatment day (i.e., day 2) and saline- or drug-challenge days (i.e., days 10, 11 or 12), a mixed-factor ANOVA (treatment day × stimulus condition) was performed; one exception was for the analysis of repeated saline treatment, where one-way ANOVA was used to compare day 1 (S− group), day 2 (S+ group), and challenge (S+ group). Analysis of difference scores (i.e., behavior on challenge − acute injection days) for sensitization and conditioning effects was done with two-factor ANOVAs (dose × stimulus condition). Whenever appropriate, post-hoc comparisons of specific groups were done with Tukey tests.
Drugs
d-Amphetamine sulfate and d-methamphetamine HCl (Sigma-Aldrich; St. Louis, MO) were dissolved in sterile saline at concentrations of 0.5 or 1.0 mg/ml and injected at a volume of 1 ml/kg. Dosages were calculated as the weight of the salt.
Results
AMPH and METH: Acute treatment and drug challenge following repeated exposure
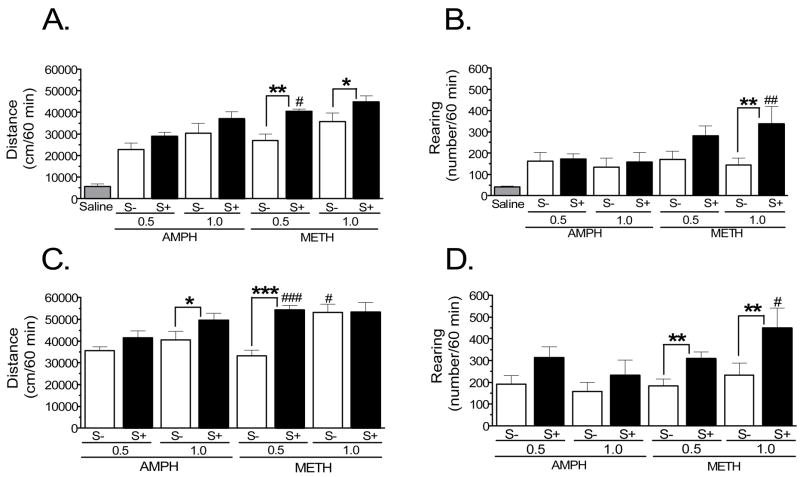
The overall ANOVA (group × stimulus × treatment day) on the locomotion data indicated all main effects and two-way interactions were statistically significant (F values > 3.25, p-values < 0.01), whereas the three-way interaction was not. For rearing, the main effects and the stimulus × treatment day interaction were significant (F values > 3.25, p-values < 0.01), whereas the remaining two-way and the three-way interactions were not. Follow-up analyses revealed that acute AMPH and METH (i.e., treatment day 2) significantly increased locomotor activity and rearing compared to behavior following saline on day 1 (Fig. 1). Mixed-factor ANOVA revealed significant main effects and interactions for locomotion (group: F7,56 = 5.43, p < 0.001; treatment day: F1,56 = 543, p < 0.001; group × treatment day: F7,56 = 4.66, p < 0.001); for rearing, there was a nonsignificant trend for the main effect of group (F7,56 = 2.36, p = 0.09), a significant main effect of treatment day (F1,56 = 86.3, p < 0.001), and a significant interaction (group × treatment day: F7,56 = 2.91, p < 0.05). Analysis of individual drug effects revealed a dose-dependent effect on locomotion (Fig. 1A) for both drugs (main effect of dose for AMPH: F1,28 = 5.35, p < 0.05; for METH: F1,28 = 5.20, p < 0.05), with the S+ significantly augmenting drug-induced locomotor activity for rats treated with METH (stimuli: F1,28 = 14.8, p < 0.001) and tending to do so in those given AMPH (stimuli: F1,28 = 3.93, p = 0.057). The only case where METH-induced locomotion was significantly greater than that induced by AMPH was at the 0.5 mg/kg dose given in the presence of the S+ (Fig. 1A). Both doses of AMPH and METH increased rearing to a similar extent, but the presence of the S+ augmented METH-induced rearing (stimuli: F1,28 = 8.27, p < 0.01). This was true at both doses, but was statistically significant at 1.0 mg/kg (Fig. 1B). METH-induced rearing was greater than that induced by AMPH in the groups given the drug with the S+, although this effect was statistically significant only at the 1.0 mg/kg dose (Fig. 1B).
Figure 1.
Cumulative (mean ± SEM) saline- or drug-induced locomotion (A, C) and rearing (B, D) for the 60-min period following injection (n = 8 rats/group). Shown in A and B are the data obtained on treatment day 1, when all rats received saline, and day 2, when they received AMPH or METH (0.5 or 1.0 mg/kg) in the presence (S+) or absence (S−) of a compound stimulus (flashing light and tone). ANOVA revealed no significant difference in the response to saline between groups, so these data were collapsed for presentation. Both doses of AMPH and METH significantly increased behavior over saline. * p < 0.05; ** p < 0.01; # p < 0.05, compared to 0.5 mg/kg AMPH, S+ group; ## p < 0.01, compared to 1.0 mg/kg AMPH, S+ group. Shown in C and D are data obtained after AMPH or METH challenge (treatment day 11). For distance: * p < 0.05; *** p < 0.001; # p < 0.05, compared to 1.0 mg/kg AMPH, S− group; ### p < 0.001, compared to 0.5 mg/kg AMPH, S+ group. For rearing: ** p < 0.01; # p < 0.05, compared to 1.0 mg/kg AMPH, S+ group.
Following repeated treatment and drug challenge, there were significant main effects of dose and stimulus for both AMPH- (F1,28 = 4.35, p < 0.05 and F1,28 = 6.04, p < 0.05, respectively) and METH-induced locomotion (F1,28 = 8.51, p < 0.01 and F1,28 = 11.1, p < 0.01, respectively), with a significant dose × stimulus interaction for METH (F1,28 = 10.4, p < 0.01). As shown in Fig. 1C, the presence of the S+ still augmented the locomotor response to METH at 0.5 mg/kg relative to the S− group; however, the same effect observed at the 1.0 mg/kg dose after acute treatment was not evident at METH challenge. At the 1.0 mg/kg AMPH dose, the enhanced locomotor activity seen in the S+ group after acute treatment was now statistically significant on AMPH challenge. In addition, METH was more potent at inducing locomotion compared to AMPH in the 0.5 mg/kg, S+ group and the 1.0 mg/kg, S− group. For rearing, the only statistically significant effect was a main effect of stimulus for rats given METH (F1,28 = 8.81, p < 0.01), with the S+ enhancing METH-induced rearing at both doses. Similar to the effect observed on the acute treatment day, METH more potently induced rearing in the 1.0 mg/kg, S+ group compared to AMPH (Fig. 1D).
Repeated treatment led to sensitization of AMPH- and METH-induced locomotor activity, as confirmed by repeated measures ANOVA comparing distance traveled following injection on the acute and challenge days (Fig. 2A). Specifically, there were significant main effects of group (F7,56 = 8.39, p < 0.001) and treatment day (F1,56 = 104, p < 0.001); the interaction was not significant. Post-hoc analysis indicated that nearly all groups significantly increased activity on the challenge day relative to the acute day (all p values < 0.01); the one exception was the 0.5 mg/kg METH, S− group, where the mean difference in locomotion showed a statistically non-significant trend (p = 0.061). Analysis of rearing data also indicated evidence of sensitization (group: F7,56 = 3.64, p < 0.01; treatment day: F1,56 = 10.8, p < 0.01 ), although this effect was variable: the only groups with significant increases in rearing on challenge compared to acute treatment were rats in the 0.5 mg/kg AMPH, S+ and the 1.0 mg/kg METH, S+ groups (Fig. 2B).
Figure 2.
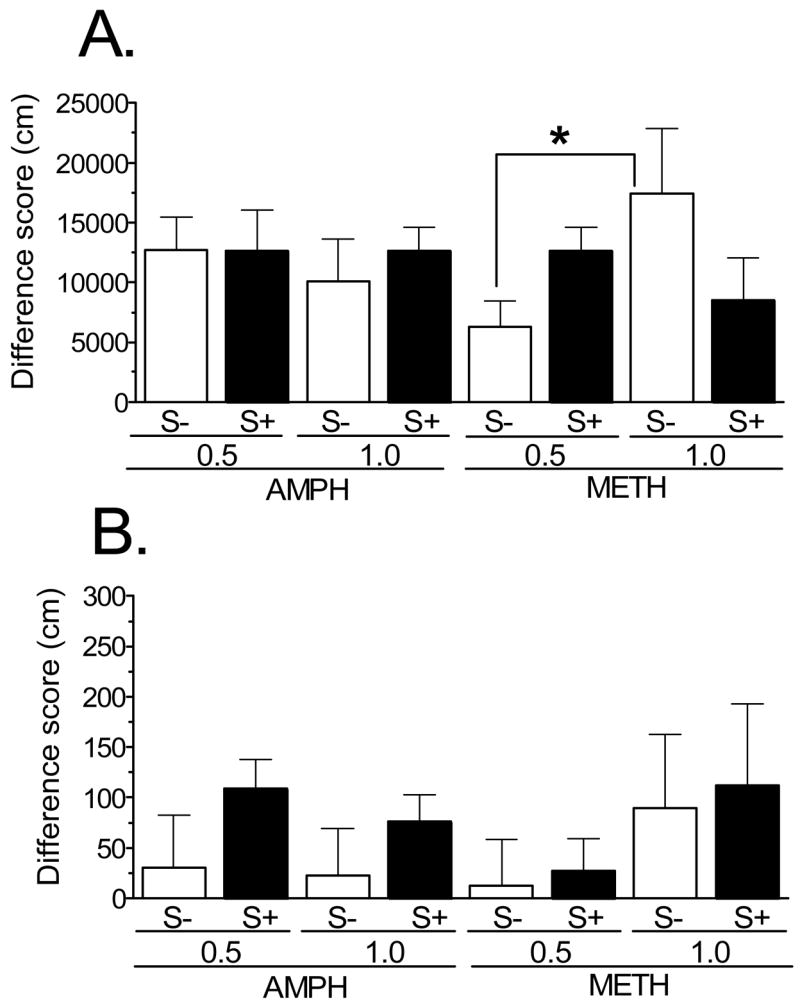
Sensitization to AMPH- and METH-induced locomotion (A) and rearing (B), represented as the difference between activity during the 60-min period following the challenge (day 11) and acute (day 2) injections (n = 8 rats/group). Statistical analysis, which was performed on the raw data from the two injection days (see Results), revealed statistically significant sensitization for locomotion in all groups except the 0.5 mg/kg METH, S− group; sensitization to drug-induced rearing was only evident in the 0.5 mg/kg AMPH, S+ and the 1.0 mg/kg METH, S+ groups. The only significant difference in the magnitude of sensitization was between the 0.5 and 1.0 mg/kg dose of METH in the absence of the stimuli (S−). * p < 0.05
Inspection of the difference scores for mean activity on challenge and acute treatment days (Fig. 2) revealed apparent group differences in the magnitude of sensitization for distance traveled and rearing. The only statistically significant difference, however, was for the relatively greater effect of 1.0 mg/kg METH compared to 0.5 mg/kg METH given in the S− groups. Thus, there were no significant differences in the magnitude of sensitization produced by AMPH and METH. The ratings of behavior captured on video revealed that at the 1.0 mg/kg dose, neither AMPH nor METH produced significant stereotypy following drug challenge. For the S− and S+ groups given AMPH, the mean (± SEM) scores for the post-injection period were 6.84 ± 0.31 and 7.17 ± 0.21, respectively; for the S− and S+ given METH, they were 7.26 ± 0.24 and 7.48 ± 0.11, respectively. These values, which were not statistically significant from each other, were closest to the “hyperactivity with some repetitive movements,” category.
Saline challenge following repeated AMPH or METH treatment
To determine whether conditioning occurred in response to repeated drug exposure, a saline challenge was performed on the first day following withdrawal (day 10). The presence of conditioning was defined as a statistically significant change in locomotion or rearing during the first 30 min after injection on the challenge relative to the first saline injection (day 1). A repeated measures ANOVA revealed significant main effects of group (distance: F7,56 = 3.00, p < 0.01; rearing: F7,56 = 3.01, p < 0.01) and treatment day (distance: F1,56 = 99.7, p < 0.001; rearing: F1,56 = 54.6, p < 0.001), but non-significant interactions. Post-hoc tests revealed significant conditioning effects (all p values < 0.01) for distance traveled in all groups except the 1.0 mg/kg AMPH, S− group. For rearing, both the S− and S+ groups given the 0.5 mg/kg dose of AMPH or METH, and the S+ group given 1.0 mg/kg METH, had statistically significant effects. As shown in Fig. 3A and B, which depict difference scores for mean activity on saline challenge and acute saline treatment days, the only statistically significant group differences in the magnitude of conditioned behavior were between S− groups pre-treated with AMPH. Also shown in Fig. 3 (panels C and D) are data from a separate group of rats given repeated treatments with saline along with the S+ and challenged with saline and the S+ after a three-day withdrawal period. They show that repeated exposure to the S+ produces no statistically significant change in behavior, although there was a trend for locomotion and rearing to be reduced on the saline challenge day. Day 1 data for the rats given saline repeatedly with the S+ are not shown because an equipment malfunction resulted in the loss of data for that test session; however, the results from our complete sample of rats show that saline-induced behavior on the first day in the chamber is similar across the population.
Figure 3.
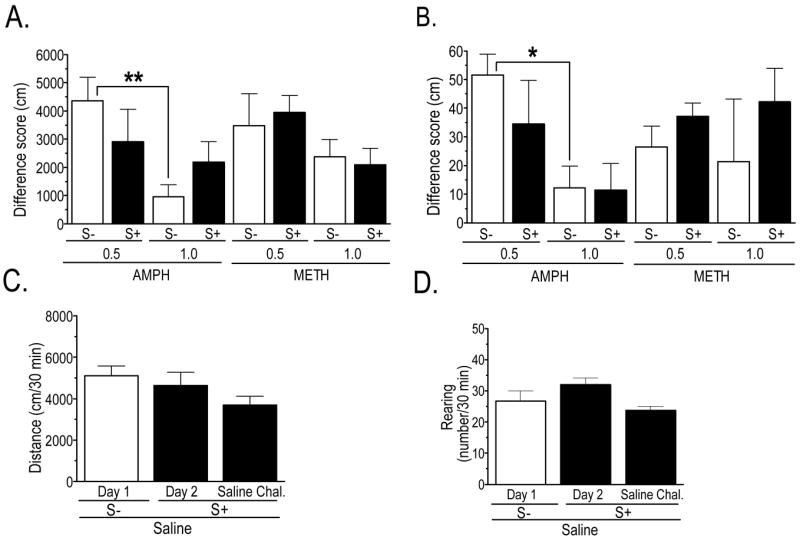
Locomotion and rearing following saline injection in rats treated repeatedly with AMPH or METH (A, B) or saline (C, D). A, B: Conditioned activity in the AMPH- or METH-exposed groups is represented as the difference between behavior following challenge (day 10) and acute (day 1) injections of saline, for the first 30 min following injection. Statistical analysis, which was performed on the raw data from the two injection days (see Results), revealed statistically significant conditioned locomotion in all groups except the 1.0 mg/kg AMPH, S− group; conditioned rearing was observed at the 0.5 mg/kg dose of both AMPH and METH, but only in the S+ group given METH at the 1.0 mg/kg dose. Due to an equipment malfunction, the 1.0 mg/kg AMPH, S− group is n = 7, while all other groups are n = 8. * p < 0.05; ** p < 0.01. C, D: Shown are data obtained from rats administered saline on Day 1 (n = 64) and a separate group (n = 4) given saline in the presence of stimuli (S+) on the second treatment day and again on a challenge day following repeated saline treatment.
In light of previous reports suggesting that the locomotor response to novelty (e.g., Hooks et al. 1992; Bevins and Peterson 2004) and the acute drug response (e.g., Sabeti et al. 2003) are predictive of chronic responses and conditioned locomotor effects, we performed a correlation analysis on measures of locomotion in all of the AMPH and METH treatment groups. As shown in Table 1, there were significant positive correlations between locomotion during the first 30 min that rats were in the open-field on day 1 (i.e., inescapable novelty response) and responses to the 1.0 mg/kg dose of AMPH on the acute and challenge days. This relationship was not present at 0.5 mg/kg AMPH or at either dose in METH-treated rats. Furthermore, novelty was generally not related to conditioned locomotion or to the magnitude of sensitization for either drug. Activity during the 60-min period following drug injection on day 2 (i.e., acute response) was not significantly correlated with conditioned locomotion for any of the groups, and the only statistically significant correlation with activity on drug challenge was for 1.0 mg/kg AMPH and 0.5 mg/kg METH. The most consistent relationship was between the acute drug response and sensitization. This was clearer when individual differences in initial sensitivity to drug-induced locomotion were controlled for by using a normalized sensitization score (the difference score for the challenge and acute treatment days divided by post-injection activity on the acute day). There were statistically significant, negative correlations for all groups except those getting 0.5 mg/kg AMPH with the S+. That is, as the initial response to AMPH and METH increased, the amount of sensitization produced after repeated treatment decreased. With only a few exceptions (e.g., the relationship between novelty and conditioning for the 0.5 mg/kg AMPH group), the addition of the S+ had no systematic effect on the observed correlations.
Table 1.
Pearson correlations (r) between locomotion during inescapable novelty or following the first drug injection and measures of locomotion after injection (saline or drug).
| 0.5 mg/kg AMPH | 1.0 mg/kg AMPH | 0.5 mg/kg METH | 1.0 mg/kg METH | |||||
|---|---|---|---|---|---|---|---|---|
| S− | S+ | S− | S+ | S− | S+ | S− | S+ | |
| Novelty | ||||||||
| Conditioning | −0.275 | −0.928** | 0.363 | 0.323 | 0.192 | −0.141 | −0.112 | 0.026 |
| Acute drug | 0.542 | −0.316 | 0.677 | 0.766* | −0.492 | −0.077 | −0.372 | 0.641 |
| Drug challenge | −0.024 | −0.489 | 0.717* | 0.805* | −0.282 | −0.339 | 0.415 | 0.551 |
| Sensitization (diff.) | −0.613 | −0.268 | −0.052 | 0.016 | 0.371 | −0.232 | 0.576 | 0.144 |
| Sensitization (norm.) | −0.735* | −0.216 | −0.319 | −0.434 | 0.269 | −0.227 | 0.276 | 0.003 |
| Acute Drug | ||||||||
| Conditioning | 0.496 | 0.447 | −0.047 | 0.293 | 0.281 | 0.481 | 0.227 | −0.095 |
| Drug challenge | 0.447 | 0.125 | 0.683 | 0.823* | 0.730* | −0.421 | 0.071 | 0.554 |
| Sensitization (diff.) | −0.804* | −0.438 | −0.518 | −0.354 | −0.564 | −0.714* | −0.704* | −0.139 |
| Sensitization (norm.) | −0.918* | −0.589 | −0.753* | −0.831* | −0.774* | −0.778* | −0.919* | −0.405 |
Cumulative locomotion during the 30 min following the first exposure to the open-field arena (Day 1 of testing) was used to define each rat’s novelty response. Conditioning was defined as the difference between locomotion during the 30-min period after saline injections on the acute and challenge days (Days 1 and 10, respectively). Cumulative locomotion during the 60-min period after AMPH or METH injection was used for measures of drug-induced behavior (acute and drug challenge). For sensitization, two measures were used: the difference (diff.) in locomotion for the 60-min period after injection on challenge and acute treatment days, and a normalized (norm.) measure that controlled for individual differences in the acute response to AMPH or METH by dividing the difference score by the post-injection activity measured after the first injection.
Cross-sensitization
The final challenge test was to determine the extent of cross-sensitization in the different treatment groups. Rats that were pre-treated with 0.5 or 1.0 mg/kg AMPH were given the same dose of METH, whereas those pre-treated with 0.5 or 1.0 mg/kg METH were given the same dose of AMPH. The presence of cross-sensitization was defined as a statistically significant increase in activity during the cross-exposure challenge relative to the activity measured in the group receiving the same drug and dose on their first drug exposure day. Compared to rats given an acute injection of AMPH, those pre-treated with the same doses of METH and challenged with AMPH exhibited enhanced locomotion, or cross-sensitization (Fig. 4A). ANOVA revealed a significant main effect of treatment group (0.5 mg/kg: F1,28 = 14.7, p < 0.001; 1.0 mg/kg: F1,28 = 24.5, p < 0.001), a significant main effect of stimuli (F1,28 = 15.3, p < 0.001) for the 0.5, but not the 1.0 mg/kg dose, and non-significant interactions for both doses. Post-hoc tests indicated the magnitude of cross-sensitization at 0.5 mg/kg was the greatest in rats pre-treated with METH and challenged with AMPH in the presence of the S+. At 1.0 mg/kg, both S− and S+ groups exhibited significant cross-sensitization, and this effect was to equivalent magnitudes (Fig. 4A). For rearing, only the main effect of treatment group at 1.0 mg/kg was significant (F1,28 = 5.63, p < 0.05). As shown in Fig. 4B, the only statistically significant cross-sensitization of rearing was in the 1.0 mg/kg group treated and challenged in the presence of the S+.
Figure 4.
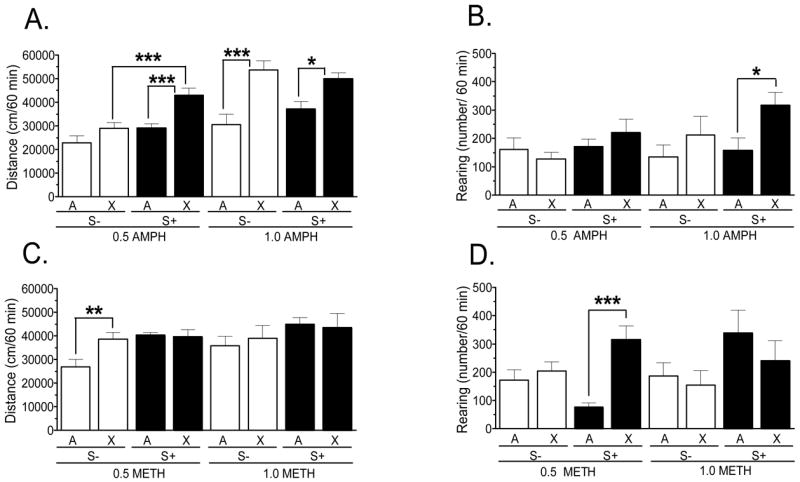
Locomotion and rearing in rats (n = 8/group) treated repeatedly with METH and challenged with AMPH (A, B) or treated repeatedly with AMPH and challenged with METH (C, D). Shown is the cumulative behavior for the 60-min following injection for “Acute” groups (i.e., those given the noted drug/dose for the first time) and “Challenge” groups (i.e., those given a challenge with the same dose, but different drug from that which they were treated repeatedly). Cross-sensitization was defined as a significant increase in behavior in the Challenge compared to the Acute group. * p < 0.05; ** p < 0.01; *** p < 0.01.
Rats pre-treated with AMPH and challenged with METH showed less evidence of cross-sensitization (Fig. 4C and D). For locomotion at 0.5 mg/kg, there were significant main effects of treatment group (F1,28 = 4.44, p < 0.05) and stimulus (F1,28 = 7.77, p < 0.01), and a significant interaction (F1,28 = 5.86, p < 0.05); for rearing at 0.5 mg/kg, there was a significant main effect of treatment group (F1,28 = 14.8, p < 0.05), a significant interaction (F1,28 = 8.47, p < 0.01), but a non-significant main effect of stimulus. Post-hoc analysis revealed these significant effects were due largely to the locomotion cross-sensitization in the S− group (Fig. 4C) and rearing cross-sensitization in the S+ group (Fig. 4D). For locomotion and rearing at 1.0 mg/kg, there were no statistically significant effects.
Discussion
Comparing AMPH- and METH-induced locomotor activation
After acute administration, the S+ enhanced drug-induced locomotion in both AMPH- and METH-treated rats, and enhanced rearing in those given METH. Between-drug comparisons revealed METH was more potent than AMPH at the 0.5 mg/kg dose for locomotion and the 1.0 mg/kg dose for rearing in the presence of the S+. The drugs were approximately equipotent in S− groups. Following repeated treatment and drug challenge, the S+ continued to enhance activity relative to S− groups. Furthermore, METH was still more potent than AMPH at the 0.5 and 1.0 mg/kg doses for locomotion and rearing, respectively. Unlike the acute treatment day, however, the 1.0 mg/kg dose of METH was more potent at inducing locomotion compared to AMPH in S− groups. Taken together, these results suggest that relatively low doses of METH are more potent than equivalent doses of AMPH at inducing locomotion and rearing in the open-field, but this effect is subject to the influence of contextual cues. This influence on METH-induced behavior is perhaps relevant for understanding human patterns of METH use and abuse. For example, it is intriguing to consider that a contributing factor to the worldwide rise in problem METH use (Rawson and Condon 2007) might be the documented shift to a route of administration (i.e., injection and smoking) that offers more opportunity for associations between drug effects and salient cues compared to the oral route of administration that is common for AMPH.
Sensitization after repeated treatment and withdrawal
Locomotor sensitization was observed for AMPH and METH at both doses and in both S+ and S− groups, with no consistent between-group differences. The presence of the light/tone stimulus did contribute, albeit in a non-systematic way, to rearing sensitization: the effect was only observed in S+ groups treated with 0.5 mg/kg AMPH or 1.0 mg/kg METH. Between-drug comparisons of locomotion and rearing sensitization revealed that AMPH and METH were approximately equipotent. Sensitization at these doses has been described before (e.g., Tilson and Rech 1973; Bevins and Peterson 2004), but a direct comparison between AMPH and METH has not. This is an important contribution of the present study because locomotor sensitization is influenced significantly by multiple procedural variables (Archer 1973; Walsh and Cummins 1976) and comparisons between studies can be difficult to interpret.
The finding that sensitization was not systematically influenced by the presence of the S+ was not surprising in light of previous studies showing that the salience of the environmental context where the drugs are administered is particularly robust (Badiani and Robinson 2004) and is difficult to influence by the addition of secondary cues (Panlilio and Schindler 1997; Crombag et al. 2000). It is not clear, however, why the S+ enhanced responses to both AMPH and METH. It might be the case that the addition of the S+ after the first drug treatment adds novelty to the environmental context, which results in a reduction in rats’ relative amount of habituation. This is important because the extent to which an animal has been previously habituated to a testing environment influences the magnitude of drug-induced behavior that is expressed (Carey and Damianopoulos 2006). By enhancing behavior on the first drug day, the baseline from which sensitization developed may have shifted, such that the overall magnitude of sensitization remains similar in S− and S+ groups.
There was a statistically significant, negative correlation between the acute drug response and sensitization. In other words, as the initial response to AMPH and METH increased, the amount of observed sensitization decreased. This relationship was strongest and most consistent across groups when the magnitude of sensitization was normalized to control for individual differences in the acute response. Initial response to AMPH has been shown to positively correlate with subsequent AMPH responses in previous studies (e.g., Bevins et al. 1997), and a similar relationship was shown recently for METH (Bevins and Peterson 2004). However, our results are similar to those reported previously for cocaine (Sabeti et al. 2003).
Conditioned activity following saline challenge
We observed conditioned locomotion in most groups pre-treated with 0.5 or 1.0 mg/kg AMPH or METH. Conditioned rearing was limited mostly to groups receiving the lower doses of AMPH or METH; repeated 1 mg/kg METH elicited conditioned rearing in the S+ group only. The only significant difference in the magnitude of conditioned activity was seen in AMPH-treated rats in S− groups: those given 0.5 mg/kg showed more activity on the saline challenge day than those given 1.0 mg/kg. Previous studies have also described conditioned locomotor activity in rats pre-treated with these doses of AMPH or METH (Tilson and Rech 1973; Bevins and Peterson 2004), but the present results demonstrate that the conditioned effects produced by the two drugs are approximately equal. Pairing repeated drug injections with the S+ had no consistent effects on conditioned responding. It was previously shown that repeatedly pairing an S+ with cocaine injections led to conditioned responding to the S+ alone (Panlilio and Schindler 1997; Hotsenpiller et al. 2001), but these studies used procedures that specifically enhanced conditioning to the S+ and prevented generalization to the environmental context. As noted above, the salience of the environmental context of the open-field arena in the present study was likely sufficient for the development of a conditioned association with the effects of AMPH and METH.
Cross-Sensitization
Cross-sensitization between AMPH and METH, which to our knowledge has not been described in the literature previously, occurred most robustly in groups given repeated treatments of METH and then challenged with AMPH. This was the case primarily for locomotion, which was evident at both doses and was largely uninfluenced by the S+. In rats given repeated AMPH and challenged with METH, however, evidence for cross-sensitization was only observed at the 0.5 mg/kg dose. Given that METH is rapidly metabolized to AMPH in the brain (e.g., Melega et al. 1995), it was not surprising that repeated METH treatment would produce cross-sensitization to AMPH. It was somewhat unexpected, however, to find that repeated AMPH failed to produce similar cross-sensitization to METH, especially because multiple previous studies, and several of the results of the current study, suggest these drugs have similar behavioral and pharmacological effects. A contributing factor for METH’s relative greater potency here might be related to its differential effects on neurochemistry in comparison to AMPH. This includes METH’s greater ability to induce 5-HT (Segal and Kuczenski 1997) and glutamate release (Shoblock et al. 2003) in key brain areas. It is also notable that methylphenidate, which is a psychostimulant like AMPH but has different pharmacodynamic properties, also does not cross-sensitize to METH (Kuczenski and Segal 2002).
Novelty as a predictor of drug-induced behavior
For both AMPH and METH, the locomotor response to inescapable novelty has been shown to be predictive of chronic drug responses and conditioned locomotor effects (e.g., Hooks et al. 1992; Bevins and Peterson 2004). Here, we found that novelty correlated significantly with acute and drug challenge in groups given 1.0 mg/kg AMPH, but not 0.5 mg/kg AMPH or either dose of METH. Novelty was not systematically correlated with measures of sensitization, however. The significant relationship at 1.0 mg/kg AMPH is consistent with previous studies (Hooks et al. 1991; Bevins et al. 1997), but the lack of correlation for the other dose of AMPH, both doses of METH, and sensitization in most groups, contrasts with others (Hooks et al. 1992; Bevins and Peterson, 2004). The source of these discrepancies between the present and previous studies is not clear, although it is likely not due to an influence of the S+ on the observed correlations. One contributing factor might be differences in the size of the open-field test apparatus, which can significantly influence the expression of drug-induced behavior (Walsh and Cummins 1976; Rebec and Bashore 1984). Regardless of its source, this inconsistency suggests that inescapable novelty is a predictor of AMPH- and METH-induced behavior only under particular circumstances.
Summary
We found that relatively low doses of AMPH and METH are equipotent at inducing locomotor activation after acute or challenge injection, and conditioned locomotion to saline after repeated treatment. In the presence of discrete, salient stimuli, however, the response to acute or challenge injections of METH, and to a lesser extent AMPH, was enhanced, and the overall magnitude of activity was higher in METH- compared to AMPH-treated rats. Furthermore, repeated treatment with METH produced robust cross-sensitization to AMPH, but repeated AMPH treatment resulted in minimal cross-sensitization to METH. These results, in addition to studies with higher doses of these drugs (Shoblock et al. 2003; Segal and Kuczenski 1997), suggest that there are certain conditions where METH is more potent than AMPH at stimulating behavior, but the common characterization of METH as a more potent psychostimulant is not consistent with the available experimental evidence.
Acknowledgments
The authors thank John P. Powers and Martin D. White for technical assistance. This work was funded by a grant from the National Institute on Drug Abuse (DA 01987).
References
- Archer J. Tests for emotionality in rats and mice: a review. Anim Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of plasticity. Behav Pharm. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. A comparison of d-amphetamine, l-amphetamine, and methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1973;1:67–71. doi: 10.1016/0091-3057(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Peterson JL. Individual differences in rats’ reactivity to novelty and the unconditioned and conditioned locomotor effects of methamphetamine. Pharmacol Biochem Behav. 2004;79:65–74. doi: 10.1016/j.pbb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Klebaur JE, Bardo MT. Individual differences in response to novelty, amphetamine-induced activity and drug discrimination in rats. Behav Pharm. 1997;8:113–123. [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN. Cocaine conditioning and sensitization: The habituation factor. Pharmacol Biochem and Behav. 2006;84:128–133. doi: 10.1016/j.pbb.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Colliver JD, Kroutil LA, Dai L, Gfroerer JC. Misuse of prescription drugs: Data from the 2002, 2003, and 2004 National Surveys on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2006. [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Feldman RS, Meyer JS, Quenzer LF. Stimulants: amphetamine and cocaine (Principles of Neuropsychopharmacology) Sinauer; Sunderland, MA: 1997. [Google Scholar]
- Gentry WB, Ghafoor AU, Wessinger WD, Laurenzana EM, Hendrickson HP, Owens SM. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacol Biochem Behav. 2004;79:751–760. doi: 10.1016/j.pbb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB. Individual differences in locomotor activity and sensitization. Pharmacol Biochem and Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Neill DB, Justice JB. Individual differences in amphetamine sensitization: Dose-dependent effects. Pharmacol Biochem and Behav. 1992;41:203–210. doi: 10.1016/0091-3057(92)90083-r. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal D, Cho A, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Appel JB, Greenberg I. An analysis of some discriminative properties of d-amphetamine. Pharmacologia. 1974;39:57–66. doi: 10.1007/BF00421458. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Henningfield JE. Human d-amphetamine drug discrimination: methamphetamine and hydromorphone. J Exp Anal Behav. 1994;61:169–180. doi: 10.1901/jeab.1994.61-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega W, Williams A, Schmitz D, DiStefano E, Cho A. Pharmacokinetic and pharmacodynamic analysis of the actions of D- amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–96. [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague–Dawley rats. Pharmacol Biochem Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA Research Report (2006) Methamphetamine Abuse and Addiction. NIH Publication No. 06-4210, pp. 1–8.
- Panlilio LV, Schindler CW. Conditioned locomotor-activating and reinforcing effects of discrete stimuli paired with intra-peritoneal cocaine. Behav Pharmacol. 1997;8:691–698. doi: 10.1097/00008877-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Condon TP. Why do we need an Addiction supplement focused on methamphetamine? Addiction. 2007;102:1–4. doi: 10.1111/j.1360-0443.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Bashore TR. Critical issues in assessing the behavioral effects of amphetamine. Neurosci Biobehav Rev. 1984;8:153–159. doi: 10.1016/0149-7634(84)90030-7. [DOI] [PubMed] [Google Scholar]
- Romanelli F, Smith KM. Clinical effects and management of methamphetamine abuse. Pharmacotherapy. 2006;26:1148–1156. doi: 10.1592/phco.26.8.1148. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual Differences in Cocaine-Induced Locomotor Sensitization in Low and High Cocaine Locomotor-Responding Rats Are Associated with Differential Inhibition of Dopamine Clearance in Nucleus Accumbens. J Pharmacol Exp Ther. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Repeated Binge Exposures to Amphetamine and Methamphetamine: Behavioral and Neurochemical Characterization. J Pharmacol Exp Ther. 1997;282:561–573. [PubMed] [Google Scholar]
- Segal D, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Shoblock J, Sullivan E, Maisonneuve I, Glick S. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology. 2003;165:369–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Rech RH. Conditioned drug effects and absence of tolerance to d-amphetamine induced motor activity. Pharmacol Biochem Behav. 1973;1:149–153. [Google Scholar]
- Walsh RN, Cummins RA. The open field test- a critical review. Psychol Bull. 1976;82:482–504. [PubMed] [Google Scholar]
- Yokel RA, Pickens R. Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther. 1973;187:27–33. [PubMed] [Google Scholar]