Abstract
Herpes simplex virus (HSV) infection of the cornea culminates in an immunopathological lesion (stromal keratitis - SK) that impairs vision. This report shows that HSV infection results in IL-23 up-regulation, but if this response fails to occur, as was noted in p19-/- mice, the severity of lesions, their incidence and the level of viral induced angiogenesis were significantly increased compared to wild-type (WT) animals (p < 0.05). The higher disease severity in p19-/- mice appeared to be the consequence of an increased IL-12 response that in turn led to the induction of higher numbers of IFN-γ producing CD4+ T cells, the principal orchestrators of SK. Our results indicate that the severity of HSV induced immunopathological lesions may be mainly the consequence of IL-12 driven Th1 T cell reactions rather than the action of IL-17 producing cells controlled by IL-23.
Keywords: Herpes simplex virus, Stromal keratitis, IL-23, IL-12, IFN-γ, IL-17
1. Introduction
Ocular infection with herpes simplex virus (HSV) culminates in immunoinflammatory lesions in the stroma (stromal keratitis - SK) that is a common cause of human blindness [1]. Understanding the pathogenesis of SK is expected to result in more effective control measures by targeting critical events. Studies in a mouse model of SK have revealed that many key events are set off by HSV infection. Among the early events are the production of several cytokines such as IL-6 and IL-12 [2, 3], chemokines such as MCP-1 and MIP-2 [4, 5] as well as several molecules that induce neovascularization of the normally avascular cornea [6]. Past studies by us and others have shown that IL-12 is upregulated by HSV infection [2, 7] and may be involved in generating IFN-γ producing CD4+ T cells that are thought to be the main orchestrators of SK lesions [8]. In support of this, animals unable to generate normal IL-12 responses develop diminished lesions [2]. Currently, the role of the structurally similar IL-23 molecule in the pathogenesis of SK is not understood. This topic is of interest since several autoinflammatory and some anti-microbial inflammatory reactions that were formerly thought to be mainly IL-12/IFN-γ mediated immunoinflammatory events were subsequently shown to be more the consequence of responses influenced by IL-23 that promotes the expansion and survival of pathogenic IL-17 producing T cells [9-11]. Although T cells that produce IL-17 have been demonstrated in human SK lesions [12] the respective role of Th1 and Th17 pathogenic T cells as orchestrators of SK is poorly understood. The present report evaluates the role of the IL-23/IL-17 axis in SK by comparing the outcome of infection in wild-type (WT) to those in mice unable to generate IL-23 because of p19 knockout. Our results show that HSV infection causes the upregulation of IL-23 but these results in only minimally detectible CD4/IL-17 producing responses in the eye. Furthermore lesions were significantly more severe in animals unable to produce IL-23 making it doubtful if the IL-23/IL-17 axis is mainly responsible for the ocular inflammatory lesions. In fact, in the absence of p19 more abundant IL-12 and Th1 responses were evident. We presume it is the IFN-γ producing Th1, rather than Th17 T cells, that are mainly responsible for mediating SK.
2. Materials and Methods
2.1. Mice
Female C57BL/6 mice, 6 to 7 weeks old, were purchased from Harlan Sprague-Dawley (Indianapolis, IN). P19 deficient mice were kindly provided by Dr. Nico Ghilardi (Genentech, Inc., CA). All investigations followed guidelines of the Committee on the Care of Laboratory Animals Resources, Commission of Life Sciences, National Research Council. The animal facilities of the University of Tennessee (Knoxville) are fully accredited by the American Association of Laboratory Animal Care.
2.2. Virus
HSV-l strain RE (kindly provided by Dr. Robert Lausch, University of Alabama, Mobile, AL) was used in all procedures. Virus was grown in Vero cell monolayers (American Type Culture Collection, Manassas, VA; Cat. no. CCL81), titrated, and stored in aliquots at -80°C until used.
2. 3. Corneal HSV infection
Corneal infections of all mouse groups were conducted under Avertin deep anesthesia. The mice were scarified lightly on their corneas with a 30-gauge needle, and a 2 μl drop containing different plaque-forming units (PFU) of HSV-1 RE was applied to the eye and gently massaged with the eyelids.
2. 4. Real-time quantitative PCR
The total cellular RNA was isolated from corneas and draining lymph nodes (DLNs) by using an RNeasy protect mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instruction. All samples were treated with RNase-free DNase (Qiagen). The extracted RNA was reverse transcribed using oligo (dt) primers and reverse transcriptase enzyme (Promega, Madison, WI) according to standard protocol. The cDNA obtained was used as a template for real-time quantitative PCR and real-time PCR was performed using a QuantiTect SYBR Green PCR kit (Qiagen). The primers used were murine p19 forward (TGCTGGATTGCAGAGCAGTAA) and reverse (GCATGCAGAGATTCCGAGAGA). Initially, mRNA levels were normalized to the GAPDH mRNA level. Briefly, a standard curve was generated using the PCR product of interested gene cloned into a pCR-XL-TOPO vector (Invitrogen, San Diego, CA). After spectrophotometric determination of the plasmid DNA concentration, the copy number was calculated using the following formula: (X g/μl DNA/(plasmid length in bp × 660) × 6.022 × 1023 = Y molecules/μl). Test samples were run in triplicate with a dilution series and the mean value of a particular dilution was used. Data are expressed as copy number per sample.
2. 5. Clinical observations
The eyes were examined on different days after infection for the development of clinical lesions by slit-lamp biomicroscopy (Kawa Co, Nagoya, Japan), and the clinical severity of keratitis of individually scored mice was recorded. The scoring system was as follows: 0, normal cornea; +1, mild corneal haze; +2, moderate corneal opacity or scarring; +3, severe corneal opacity but iris visible; +4, opaque cornea and corneal ulcer; +5, corneal rupture and necrotizing stromal keratitis. The severity of angiogenesis was recorded as described previously [6]. In reference to the angiogenic scoring system, the method relied on quantifying the degree of neovessel formation based on three primary parameters: 1) the circumferential extent of neovessels (as the angiogenic response is not uniformly circumferential in all cases); 2) the centripetal growth of the longest vessels in each quadrant of the circle; and 3) the longest neovessel in each quadrant was identified and graded between 0 (no neovessel) and 4 (neovessel in the corneal center) in increments of ∼0.4 mm (radius of the cornea is ∼1.5 mm). According to this system, a grade of 4 for a given quadrant of the circle represents a centripetal growth of 1.5 mm toward the corneal center. The score of the four quadrants of the eye were then summed to derive the neovessel index (range, 0 to 16) for each eye at a given time point.
2. 6. Cytokine assay
For cytokine (IFN-γ, IL-12p35, IL-12p40) assay, splenocytes from mice were suspended in 10% RPMI-1640, and 106 cells in 1 ml were stimulated in vitro with irradiated syngeneic-enriched naïve splenocytes pulsed with UV-inactivated HSV (MOI, 1.5 before UV inactivation). Similar number of cells was Con A-stimulated (5 μg/106 cells/ml) in 96-well plates. Plates were incubated at 37°C for 72 h. The supernatant fluid was collected and stored at -80°C until use. These supernatants were screened for the presence of individual cytokines by enzyme-linked immunosorbent assay (ELISA).
2. 7. Flow cytometric analysis
Single-cell suspensions were prepared from the SK corneas or lymphoid organs (spleen and DLNs). For the preparation of corneal samples, four corneas per group were dissected, pooled together, and digested with 60 U/ml Liberase (Roche Diagnostics, Alamenda, CA) for 60 min at 37°C in a humidified atmosphere of 5% CO2. After incubation, the corneas were disrupted by grinding with a syringe plunger on a mesh and a single-cell suspension was made in complete RPMI 1640 medium. Next, the single-cell suspensions obtained from corneal or lymphoid samples were stained for FACS. Briefly, single cells were first blocked with an unconjugated anti-CD32/CD16 mAb (BD Biosciences, San Jose, CA) for 15 min at 4°C in FACS buffer and stained by anti-CD4-APC, anti-CD8-PerCp, CD11c-PerCp, or CD11b-APC antibody (BD Biosciences) for 30 min. Cells were fixed and permeabilizied using Cytofix/Cytoperm (BD Biosciences) solution for approximately 30 minutes, followed by washing with perm washing solution. Intracellular staining was done with anti-IFN-γ-PE, anti-IL-12-PE, or anti-IL-17-PE antibody (BD Biosciences) for 30 minutes on ice and washed twice with perm wash. Finally, the cells were washed with FACS buffer and samples were acquired on a FACScan (BD Biosciences). The data were analyzed using the CellQuest 3.1 software (BD Biosciences).
2. 8. Protein quantification of corneal or lymphoid organs lysates by ELISA
The lysates from HSV infected cornea or lymphoid organs (spleen and DLNs) were used for the measurement of IFN-γ, IL-12, IL-23, or TGF-β by a standard sandwich ELISA protocol. For preparation of sample lysates, four corneas or lymphoid organs/time point (n = 4) were collected and minced with liquid nitrogen. Minced pieces were collected in 1 ml of DMEM without FCS and homogenized using an ultra sonicater (Heat systems-Ultrasonics, NY). The lysates were then clarified by centrifugation at 12,000 rpm for 5 min at 4°C. The supernatant was collected and stored at -80°C till further use. The ELISA plate was coated with anti-mouse capture antibody (100 μl/well of the capture antibody at various concentrations, BD Biosciences) and incubated at 4°C overnight. The plate was washed with 0.05% tween20/PBS and blocked with 3% BSA for 2 h at 37°C. After washing, serially diluted lysates were added to the plate and incubated at 4°C overnight. The plate was washed and then incubated with biotinylated detection antibody (BD Biosciences) for 2 h. Finally, peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratory, PA) was added. The color reaction was developed using ABTS (Sigma-Aldrich, St. Louis, MO) and measured with an ELISA reader (Spectramax 340; Molecular Devices, Sunnyvale, CA) at 405 nm. Quantification was performed with Spectramax ELISA reader software version 1.2.
2. 9. Virus recovery and titration
Swabs of the corneal surface were collected at various time points post infection (p.i.). The swabs were put into sterile tubes containing 600 μl serum free DMEM with 10 international units (IU) penicillin/ml and 100 μg streptomycin (Life Technologies, Grand Island, NY)/ml and were stored at -80°C. For detection and quantification of virus, the samples were thawed and vortexed. Individual subsamples (200 μl each sample) were diluted further, and viral titers were determined by a plaque assay performed on Vero cells as described elsewhere [13].
2. 10. Statistical analysis
Significant differences between groups were evaluated by using the Student’s t test. P < 0.05 was regarded as a significant difference between two groups. Results are expressed as mean ± standard error.
3. Results
Expression of IL-23 after HSV-1 infection
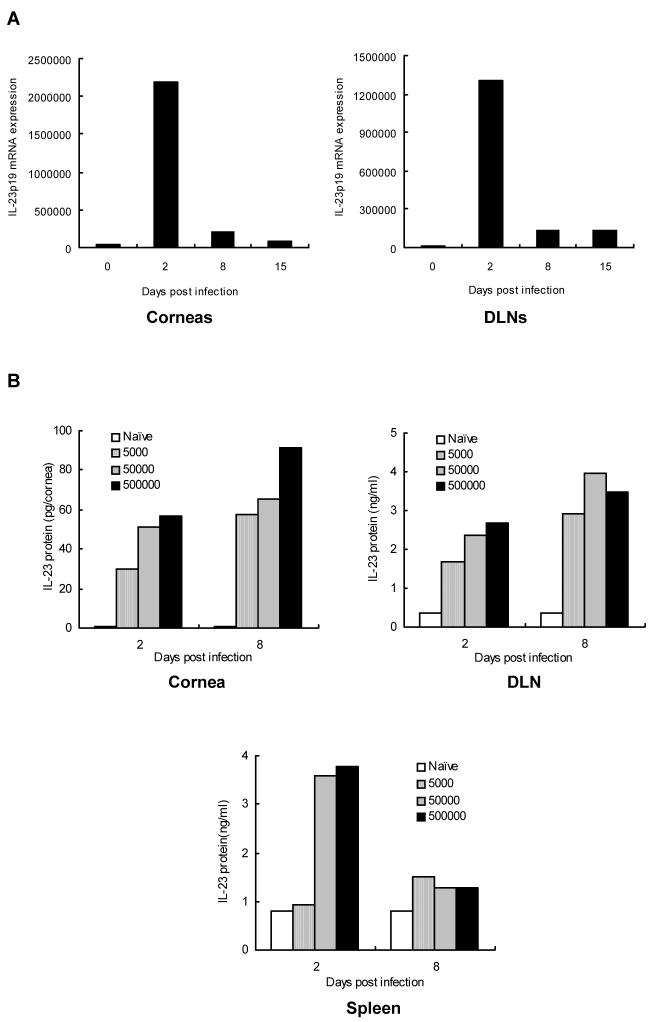
WT C57BL/6 mice were infected with HSV-1 (5 × 105 PFU) and at various times p.i. were killed to determine the levels of p19 mRNA by quantitative real-time PCR in both corneal and DLNs RNA samples. As is shown in Fig.1A, minimal or undetectable mRNA levels of p19 were present in naïve corneas or lymph nodes, but by day 2 p.i. abundant levels of IL-23 mRNA were detected. In other experiments, IL-23 protein levels were quantified in corneal extracts as well as extracts of lymphoid organs by ELISA in animals infected with different doses of virus (5 × 103, 5 × 104, 5 × 105 PFU). Peak levels were found with the highest dose used for infection (Fig. 1B). These results indicate that IL-23 is up-regulated by HSV infection and could perhaps participate in the immunopathogenesis of herpetic SK.
Figure 1. Expression of IL-23p19 mRNA and protein in the corneas and lymphoid organs after HSV-1 ocular infection.
(A) WT mice were infected with 5 × 105 PFU of HSV-1 on their scarified corneas. At different time points, mRNA was extracted from the corneas and DLNs, and reverse transcribed into cDNA. Kinetic expression of IL-23p19 mRNA was measured by real-time PCR. Test samples were run in triplicate with a dilution series and the mean value of a particular dilution was used. Data are expressed as copy number per sample. (B) WT mice (n = 3) were ocularly infected with different doses of virus (5 × 105, 5 × 104, 5 × 105 PFU) and levels of IL-23 protein were estimated from lysate supernatants of corneas, DLNs, or spleen of mice by an antibody capture ELISA as described by materials and methods.
The absence of IL-23 production results in more severe disease
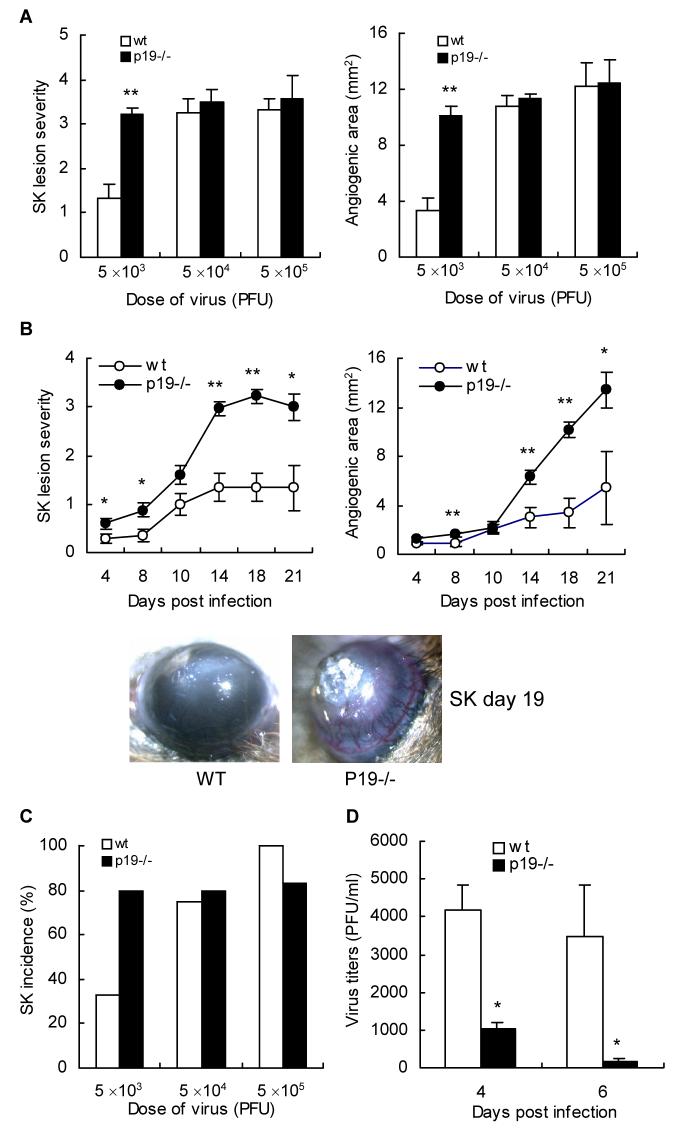
The expression of disease was compared in WT and p19-/- mice at various times p.i. upon infection with different doses of virus (5 × 103, 5 × 104, 5 × 105 PFU). At high doses of virus used for infection (5 × 104 and 5 × 105 PFU) disease severity in both WT and p19-/- animals was of similar magnitude (Fig. 2A). However, at a low dose of infection (5 × 103 PFU) highly significant differences were observed in the susceptibility of WT and p19-/- animals. In the latter, more eyes developed lesions and the mean severity scores of both angiogenesis and SK lesions were significantly more severe than in WT animals (P < 0.05, Fig. 2B). Lesions also occurred slightly earlier in p19-/- animals. Overall an average result of three separate experiments reveal that 80% of p19-/- developed SK of 3 or greater where as only 33% of WT animals developed such severity (Fig. 2C). Thus the failure to generate an IL-23 response results in more severe lesions at least at minimal doses of infection.
Figure 2. IL-23p19-/- mice have higher SK lesion severity, incidence, ocular angiogenesis, and enhanced viral clearance.
Corneas of IL-23p19-/- and WT mice (n = 4) were infected with various doses of HSV-1 (5 × 103, 5 × 104, 5 × 105 PFU), and the levels of SK severity and neovascularization were measured in the corneas by biomicroscopy in groups of p19-/- (●) and WT (○) mice. (A) SK lesion severity and angiogenesis scoring was conducted on day 18 p.i.. Results are representative of one of three independent experiments. Statistically significant differences in SK and angiogenic score were observed between the groups (** p < 0.01). (B) Stromal lesion severity and angiogenesis in IL-23p19-/- and WT mice (n = 4) infected with low dose of HSV-1 (5 × 103 PFU) were measured at different time intervals. Results are representative of three independent experiments. Statistically significant differences in SK and angiogenic score were observed between the groups (* p < 0.05, ** p < 0.01). Images were taken by stereomicroscopic imaging system at day 19 after virus infection (original magnification, × 40). (C) The incidence of clinical SK lesions was considered as over score 2 at day 14 p.i.. (D) Infectious virus titers were compared in the different mice groups (n = 4) following virus infection with 5 × 103 PFU. At days 4 and 6 p.i., swabs of the corneal surface were collected and were put into serum free DMEM with antibiotics. Individual samples were diluted further, and viral titers were determined by a plaque assay performed on Vero cells as described materials and methods. Statistically significant differences in number of virus on the cornea (* p < 0.05) were observed between the groups.
As the presence of replicating virus may contribute to lesion severity, we compared the levels of virus in the eye swabs collected from both groups of animals at different time points. As is evident in Fig. 2D, virus clearance occurred earlier in the corneas of p19-/- as compared to WT animals (p < 0.05). Thus although lesions were more severe in p19-/- animals virus was cleared from the epithelium earlier than in WT.
P19-/- animals develop more severe Th1 immune response
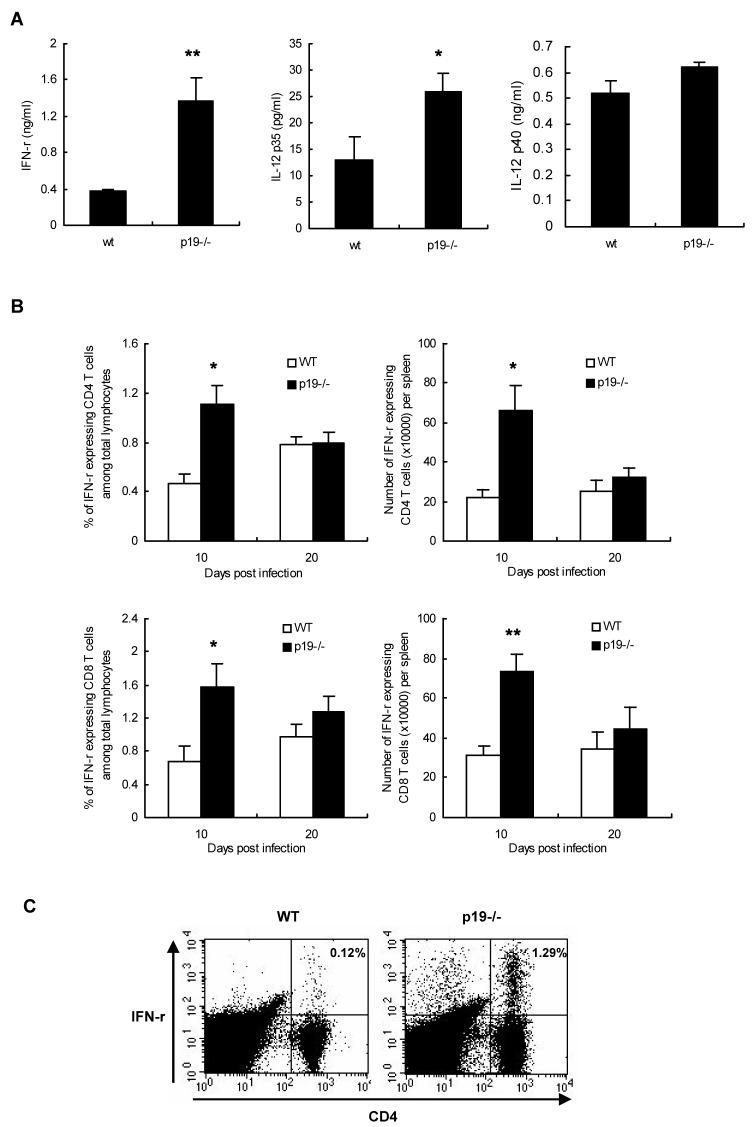
In an attempt to explain why p19-/- mice showed more severe responses than WT animals, we infected both groups with the low dose of virus (5 × 103 PFU) and lymphoid samples were collected at day 20 post infection to compare the magnitude of the HSV specific T cell responses. This was done by stimulating T cells with antigen and comparing the induced cytokine response by ELISA. As shown in Fig. 3A, splenocytes from p19-/- mice produced significantly higher (p < 0.05) levels of IFN-γ as compared to WT controls. Similarly, although no difference was observed in the production of p40 subunit, the IL-12p35 level was significantly higher in p19-/- as compared to WT (p < 0.05). These results may mean that in the absence of p19 the Th1 type of immune response is increased.
Figure 3. Increased Th1 type immune responses in the spleen of IL-23p19-/- mice after HSV-1 infection.
(A) At day 20 p.i. (5 × 103 PFU/eye), spleen cells of the different mice groups were used in antigen specific ELISA. For cytokine ELISA assay, splenocytes from mice were sampled, suspended, and stimulated with irradiated syngeneic-enriched naïve splenocytes pulsed with UV-inactivated HSV (MOI, 1.5) at 37°C for 72 h. Levels of individual cytokines were estimated from supernatant fluid by an antibody capture ELISA as outlined in materials and methods. Statistically significant differences in levels of individual cytokines (* p < 0.05, ** p < 0.01) were observed between the groups. At day 10 and 20 p.i., splenocytes of the different mice groups (n = 4) were used in intracellular cytokine staining assay. For intracellular cytokine staining assay, isolated spleen cells from both groups having SK lesions were stimulated with anti-CD3 and anti-CD28 (B) or stimulated with syngeneic naïve splenocytes pulsed with HSV (C) for 16 h. Single cells were extracellularly stained with anti-mouse CD4 (APC) and CD8 (PerCp) antibody and then intracellularly stained with anti-mouse IFN-γ PE antibody. Values shown in each graph reflect the percentage or number of spleen cells expressing IFN-γ. Statistically significant differences were observed between the groups (* p < 0.05, ** p < 0.01). Results are representative of two independent experiments.
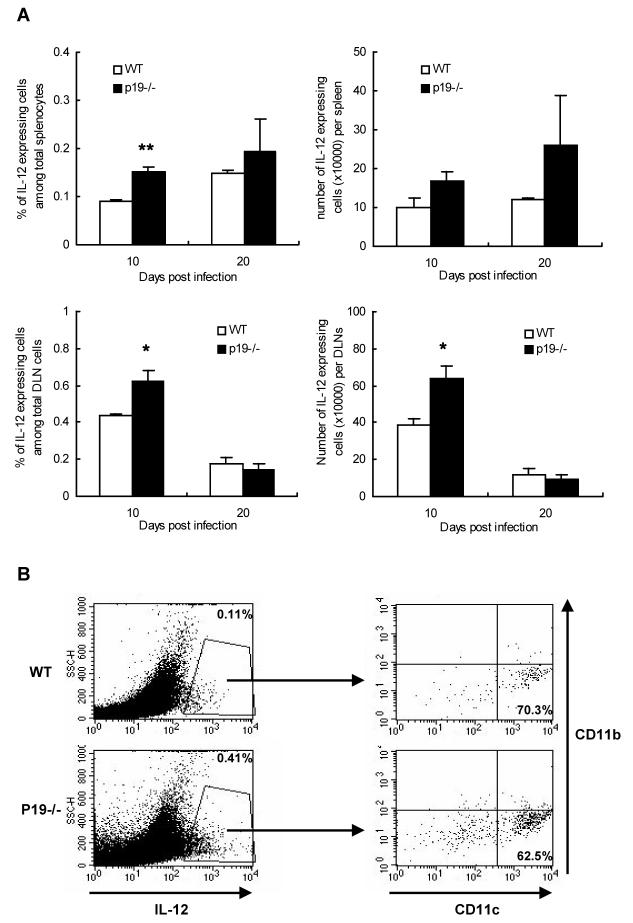
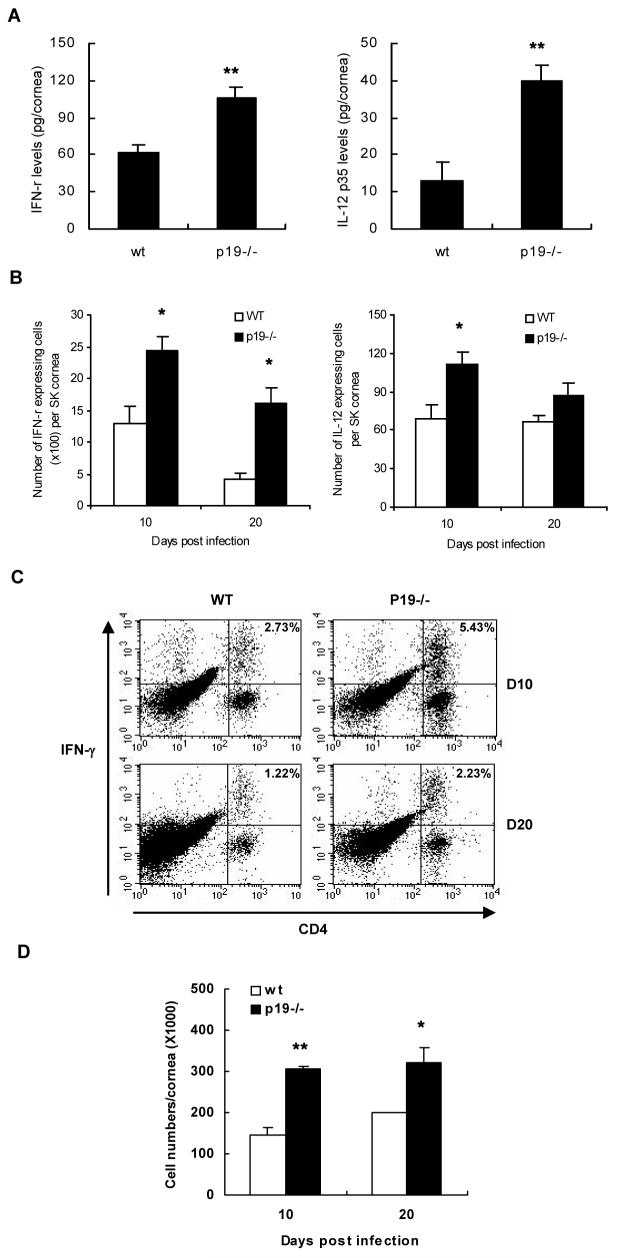
To determine which cell populations contribute to the Th1 type immune response against HSV, isolated splenocytes from both groups having SK lesions were stimulated with anti-CD3 and anti-CD28 to analyze cytokine production. As assessed by intracellular cytokine staining assay, the percentage and absolute number of IFN-γ expressing CD4+ T cells as well as IFN-γ expressing CD8+ T cells was significantly higher (p < 0.05) in p19-/- mice as compared to WT control animals (Fig. 3B). Similar results were also obtained when cells were stimulated with syngeneic naive splenocytes pulsed with HSV (Fig. 3C). In addition, we also compared the numbers of IL-12 producing cells in the infected DLNs and spleen in the two groups of animals. As shown in Fig. 4A, higher numbers of IL-12 expressing cells were noted in the p19-/- animals as compared to WT. The majority of cells producing IL-12 were found to be CD11c+ CD11b- (Fig. 4B). Total cell numbers in the DLNs and spleen of p19-/- were marginally increased as compared to WT animals (not shown) indicating that relative population of IFN-γ or IL-12 expressing cell types were greater in lymphoid organs of p19-/- animals. Thus these data indicate that p19-/- mice generated a greater Th1 type of immune response than did WT animals.
Figure 4. Increased population and number of IL-12 expressing cells in the lymphoid organs of p19-/- mice after HSV-1 infection.
At day 10 and 20 p.i., splenocytes or DLNs cells of the different mice groups (n = 4) were used in intracellular cytokine staining assay. Isolated splenocytes or DLNs cells from both groups having SK lesions were stimulated with anti-CD3 and anti-CD28 for 16 h. Single cells were extracellularly stained with anti-mouse CD11b (APC) and CD11c (PerCp) antibody and then intracellularly stained with anti-mouse IL-12 PE antibody (A and B). Values shown in each graph reflect the percentage or absolute number of spleen cells expressing IL-12.
The nature of the response in p19-/- and WT corneas was also measured using corneas isolated from the infected animals of both the groups. As shown in the Fig. 5A, the protein levels of IFN-γ and IL-12 were significantly higher in the lysates of p19-/- corneas as compared to WT at day 20 p.i. (p < 0.01). We next determined the number of IFN-γ expressing cells in the collagenase digested corneas of each group. As shown in Fig 5B, there was approximately two and four fold increase in the total number of IFN-γ producing cells in the p19-/- corneas after anti-CD3 and CD28 stimulation at day 10 and day 20 respectively as compared to WT. Such IFN-γ producing cell types in SK corneas were mainly CD4+ T cells confirming that pathogenesis of herpetic SK is contributed by Th1 type CD4+ T cells (Fig. 5C). Similarly, the numbers of IL-12 producing cells were also higher in p19-/- corneas as compared to WT controls (Fig. 5B). Total cell numbers in the SK cornea of p19-/- mice were also greater than those of WT mice at day 10 and 20 p.i. (Fig. 5D) suggesting both absolute numbers and frequencies of such cells in the corneas of p19-/- animals were higher as compared to WT animals. These data indicate that the p19-/- mice generated a more abundant Th1 type of immune response in the corneas than WT.
Figure 5. Increased Th1 type immune responses in the SK corneas of p19-/- mice.
At day 20 p.i., 6 corneas/group that were infected with 5 × 103 PFU HSV-1 were processed to measure the IFN-γ or IL-12 cytokine levels (A). Levels of individual cytokines were estimated from supernatants of corneal lysates of mice by an antibody capture ELISA as outlined in materials and methods. Statistically significant differences in levels of cytokines (** p < 0.01) were observed between the groups. Alternatively, isolated corneal cells from both groups having SK lesions (10 and 20 post infection) were stimulated with anti-CD3 and anti-CD28 for 16 h. Single cells were extracellularly stained with anti-mouse CD4 APC antibody and then intracellularly stained with anti-mouse IFN-γ or IL-12 PE antibody (B and C). Cells were gated on lymphocytes and values shown in each plot represent the percentage of CD4+ T cells expressing IFN-γ (C). At day 10 and 20 p.i., total cell numbers of collagenase digested cornea of the different mice groups (n = 6) were measured (D). Statistically significant differences in the absolute cell numbers of SK cornea (* p < 0.05, ** p < 0.01) were observed between the groups.
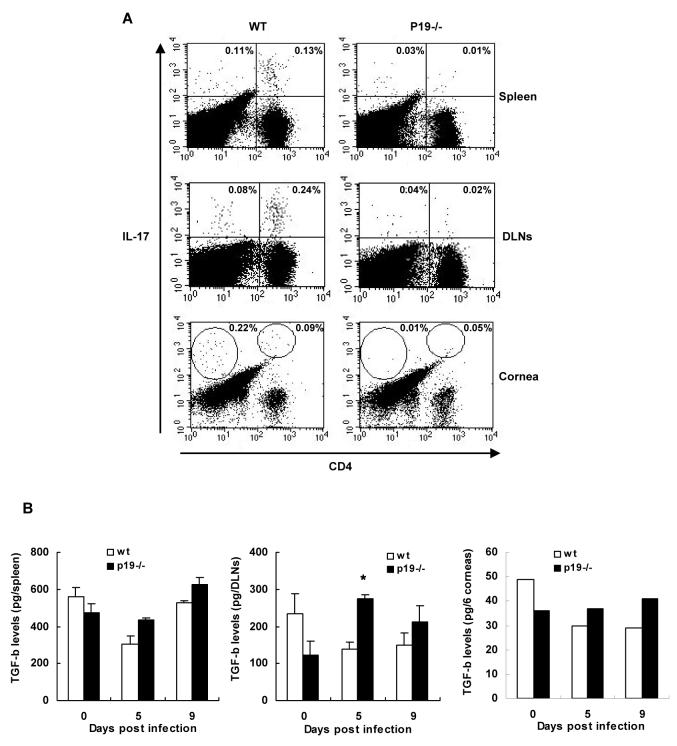
P19-/- mice develop less Th17 immune response
Previous studies showed that IL-23 expands a pathogenic CD4+ T cell population that produces IL-17 and contributes to inflammation [9]. To record such an effect, isolated splenocytes, DLN cells, and corneal cells from both groups having SK lesions were stimulated with anti-CD3 and anti-CD28 to analyze IL-17 production. As assessed by intracellular cytokine staining assay, comparable and little levels of IL-17-producing CD4+ T cells were observed in the spleen, DLNs, and corneas of both p19-/- and WT mice at day 10 post infection (data not shown). At day 20 post infection, however, higher levels of IL-17-producing CD4+ T cells were observed in the SK cornea, spleen, and DLNs of WT compared to p19-/- mice (Fig. 6A). Since TGF-β is critical for the induction of Th17 cells [14], we measured the protein levels of TGF-β in the corneas and lymphoid tissues of p19-/- and WT animals. We could not find significant differences in the TGF-β levels in the naïve and infected p19-/- animals corneas as compared to WT. Interestingly, significantly higher levels of TGF-β was found in the cervical DLNs but not the spleens of p19-/- animals at 5 days p.i. (Fig. 6B). These data indicate that IL-23 is needed for the maintenance of the Th17 cells post infection.
Figure 6. Decreased Th17 immune response in p19-/- mice after HSV-1 infection.
At day 20 p.i., splenocytes, DLN cells, and corneal cells from WT and p19-/- mice having SK lesions were stimulated with anti-CD3 and anti-CD28 for 16 h. Single cells were extracellularly stained with anti-mouse CD4 APC antibody and then intracellularly stained with anti-mouse IL-17 PE antibody. Cells were gated on lymphocytes and values shown in each plot represent the percentage of cells expressing IL-17 (A). Data are representative of two similar experiments. At day 5 and 9 p.i., DLNs, spleen, and 6 corneas/group that were infected with 5 × 103 PFU HSV-1 were processed to measure the TGF-β cytokine levels (B). Levels of TGF-β were estimated from supernatants of each tissue’s lysates of mice by an antibody capture ELISA as outlined in materials and methods.
4. Discussion
In many immunoinflammatory lesion events thought formerly to represent the consequences of IL-12 controlled IFN-γ producing Th1 type T effector cells were subsequently shown to be more the consequence of pathologic CD4+ T cells that produce IL-17 [11]. The differentiation and expansion of the latter was influenced by IL-23 rather than IL-12. We, and others, had shown the relevance and importance of IL-12 and IFN-γ producing CD4+ T cells in the orchestration of HSV induced inflammatory reactions in the cornea [1, 2, 8, 15, 16], but any role for Th17 mediated events had not been studied. The present investigation shows that ocular infection does result in IL-23 induction but the extent of the ocular Th17 response in the cornea was very limited. Furthermore, we observed that the severity of HSV induced SK lesions were in fact more severe than in WT animals if mice were unable to generate IL-23 because of p19 knockout. Thus in this viral-induced immunopathological reaction, SK lesions would appear to be mainly orchestrated by Th1 cells as advocated by several groups prior to our knowledge about the existence of the IL-23/IL-17 axis of inflammatory disease mediation.
The evidence that the IL-12/IFN-γ and IL-23/IL-17 IL represent separate mechanisms of cell mediated tissue damage appears now to be well accepted [9, 11, 17, 18]. In fact, based on the use of knockout animals, as well as approaches using in vivo cytokine neutralizing antibodies, the trend has usually been to show that lesions assumed to represent the consequence of IL-12/IFN-γ mediated events in fact primarily involved IL-23/IL-17 mediated mechanisms. This has been shown true for several autoimmunities and some inflammatory reactions against microbes [10, 11, 17]. Reasons why one type of inflammatory reaction prevails over another are usually not available. However, with some microbes and some purified microbial products more effective induction of IL-23 compared to IL-12 has been show to occur. This happens with Bordetella pertussis [19] as well as with stimulation by peptidoglycan from Staphylococcus aureus [20]. In addition, various toll-like receptor (TLR) ligands were shown to differentially stimulate IL-12 and IL-23 production from dendritic cells (DCs) [21, 22]. HSV has been shown to possess both TLR2 and 9 ligand activity [23] and in addition causes the release of endogenous TLR ligands such as hsp70 from infected cells [24, 25]. One or more of these TLR ligands could stimulate IL-12 and IL-23 production differentially in SK corneas. We are currently attempting to compare the relative IL-12/IL-23 inducing capacity of HSV, infected cell extracts and known TLR ligands in various cell types derived from the eye. We anticipate that HSV may be a better inducer of IL-12 than IL-23 and that the resultant IFN-γ response from innate cells, that includes neutrophils [26], may serve to suppress any concomitant Th17 responses. Such suppression of Th17 responses by IFN-γ has been shown to occur in vitro [27]. Moreover, HSV may also be a weak inducer of TGF-β which appears to act as a cofactor for Th17 development [28]. In our current study, there was no significant increase in the TGF-β levels in the WT corneas post infection. Additionally, we did not find any significant difference in the levels of TGF-β in the corneas of p19-/- animals as compared to WT animals. Taken together these events may explain the minimal Th17 response in the eye during HSV induced immunopathology, and such ideas are being further investigated.
Arguing forcefully against a major role for IL-23/Th17 induced tissue damage in the SK system was the observation that without the ability to produce IL-23 because of p19-/-, lesions were more severe than in WT. In preliminary results, we have also confirmed the previous observation of the Ghiasi group [2] that corneal scarring becomes less severe in animals unable to make IL-12 because of p35-/-. Moreover, animals unable to respond to IL-12 because of STAT4 knockout develop only mild lesions of SK [29]. The reasons for the enhanced pathology in p19-/- animals appeared to be associated with the increased IL-12/IFN-γ response that occurred in such animals. These enhanced responses were noted both in the spleen as well as in ocular inflammatory sites. The better IFN-γ-producing CD4+ T cell response likely accounted for the more severe SK lesions in p19-/- animals. Presumably in the absence of p19 more of the available p40 subunit may be able to dimerize with p35 subunit leading to more IL-12 production. Such a finding was also suggested to the observation of Becker et al [22] in an experimental colitis model wherein p19-/- animals were shown to develop more severe colitis than the intact animals. They also observed elevated levels of IL-12 production and the reversal of the phenotype by administration of blocking antibody to the p40 subunit.
In our model, p19-/- animals also cleared the infecting virus more promptly presumably because of the enhanced production of IFN-γ in p19-/- compared to WT animals. Furthermore, greater numbers of CD11c+CD11b+ cells as well as CD11c+CD11b- cells were also observed in the ocular tissues of p19-/- animals compared to WT (data not shown). Such cells may be myeloid and plasmacytoid DCs respectively, an abundant source of IFN-α which also might explain the more rapid clearance of virus from the eyes of p19-/- animals [30].
In summary, our results make the case that SK remains a disease whose mechanism of tissue damage appears to be mainly the consequence of an IL-12 influenced Th1 IFN-γ producing type of T cell rather than by a pathogenic Th17 producing mechanism. The latter likely plays only a minor role but this might synergize with the Th1 pathogenic response as has been suggested to occur in EAE model [31]. We are currently attempting to confirm our observations using adoptive T cell transfer approaches with in vitro generated Th1 and Th17 specific cells along with evaluating the effects of inhibition studies with specific anti-sera. Understanding the mechanisms is considered important as it could impact on measures used to control this important tissue damaging disease process.
5. Acknowledgments
We would like to thank Dr. Nico Ghilardi (Genentech, Inc., CA) for kindly providing p19-/- mice. We also thank Mr. Jason burchett for technical assistance. This research was supported by a research grant from the National Institutes of Health (RO1 EY05093). This paper was supported in part by research funds of Chonbuk National University in 2007 (NP-2007-12).
Abbreviations used in this paper
- HSV
herpes simplex virus
- SK
stromal keratitis
- IL-23
interleukine-23
- WT
wild-type
- IL-12
interleukine-12
- IFN-γ
interferon-γ
- IL-17
interleukine-17
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Deshpande SP, Zheng M, Lee S, Rouse BT. Mechanisms of pathogenesis in herpetic immunoinflammatory ocular lesions. Vet. Microbiol. 2002;86:17–26. doi: 10.1016/s0378-1135(01)00487-4. [DOI] [PubMed] [Google Scholar]
- [2].Osorio Y, Wechsler SL, Nesburn AB, Ghiasi H. Reduced severity of HSV-1-induced corneal scarring in IL-12-deficient mice. Virus Res. 2002;90:317–326. doi: 10.1016/s0168-1702(02)00249-6. [DOI] [PubMed] [Google Scholar]
- [3].Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest. Ophthalmol. Vis. Sci. 2002;43:737–743. [PubMed] [Google Scholar]
- [4].Thomas J, Kanangat S, Rouse BT. Herpes simplex virus replication-induced expression of chemokines and proinflammatory cytokines in the eye: implications in herpetic stromal keratitis. J. Interferon Cytokine Res. 1998;18:681–690. doi: 10.1089/jir.1998.18.681. [DOI] [PubMed] [Google Scholar]
- [5].Cook WJ, Kramer MF, Walker RM, Burwell TJ, Holman HA, Coen DM, Knipe DM. Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus 1 infection. Virol. J. 2004;1:5. doi: 10.1186/1743-422X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J. Virol. 2001;75:9828–9835. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kumaraguru U, Rouse BT. The IL-12 response to herpes simplex virus is mainly a paracrine response of reactive inflammatory cells. J. Leukoc. Biol. 2002;72:564–570. [PubMed] [Google Scholar]
- [8].Gangappa S, Deshpande SP, Rouse BT. Bystander activation of CD4(+) T cells can represent an exclusive means of immunopathology in a virus infection. Eur. J. Immunol. 1999;29:3674–3682. doi: 10.1002/(SICI)1521-4141(199911)29:11<3674::AID-IMMU3674>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [9].Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- [10].Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- [11].Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J. Immunol. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- [13].Babu JS, Thomas J, Kanangat S, Morrison LA, Knipe DM, Rouse BT. Viral replication is required for induction of ocular immunopathology by herpes simplex virus. J. Virol. 1996;70:101–107. doi: 10.1128/jvi.70.1.101-107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- [15].He J, Ichimura H, Iida T, Minami M, Kobayashi K, Kita M, Sotozono C, Tagawa YI, Iwakura Y, Imanishi J. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-1 infection. J. Interferon Cytokine Res. 1999;19:609–615. doi: 10.1089/107999099313749. [DOI] [PubMed] [Google Scholar]
- [16].Heiligenhaus A, Bauer D, Zheng M, Mrzyk S, Steuhl KP. CD4+ T-cell type 1 and type 2 cytokines in the HSV-1 infected cornea. Graefes Arch. Clin. Exp. Ophthalmol. 1999;237:399–406. doi: 10.1007/s004170050251. [DOI] [PubMed] [Google Scholar]
- [17].Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fedele G, Stefanelli P, Spensieri F, Fazio C, Mastrantonio P, Ausiello CM. Bordetella pertussis-infected human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization and interleukin-23 expression. Infect. Immun. 2005;73:1590–1597. doi: 10.1128/IAI.73.3.1590-1597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ma XT, Zhang XJ, Zhang B, Geng YQ, Lin YM, Li G, Wu KF. Expression and regulation of interleukin-23 subunits in human peripheral blood mononuclear cells and hematopoietic cell lines in response to various inducers. Cell Biol. Int. 2004;28:689–697. doi: 10.1016/j.cellbi.2004.07.002. [DOI] [PubMed] [Google Scholar]
- [21].Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur. J. Immunol. 2006;36:21–26. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- [22].Becker C, Dornhoff H, Neufert C, Fantini MC, Wirtz S, Huebner S, Nikolaev A, Lehr HA, Murphy AJ, Valenzuela DM, Yancopoulos GD, Galle PR, Karow M, Neurath MF. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J. Immunol. 2006;177:2760–2764. doi: 10.4049/jimmunol.177.5.2760. [DOI] [PubMed] [Google Scholar]
- [23].Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kobayashi K, Ohgitani E, Tanaka Y, Kita M, Imanishi J. Herpes simplex virus-induced expression of 70 kDa heat shock protein (HSP70) requires early protein synthesis but not viral DNA replication. Microbiol. Immunol. 1994;38:321–325. doi: 10.1111/j.1348-0421.1994.tb01785.x. [DOI] [PubMed] [Google Scholar]
- [25].Wang R, Town T, Gokarn V, Flavell RA, Chandawarkar RY. HSP70 enhances macrophage phagocytosis by interaction with lipid raft-associated TLR-7 and upregulating p38 MAPK and PI3K pathways. J. Surg. Res. 2006;136:58–69. doi: 10.1016/j.jss.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [26].Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J. Immunol. 2007;178:5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- [27].Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wahl SM. Transforming growth factor-beta: innately bipolar. Curr. Opin. Immunol. 2007;19:55–62. doi: 10.1016/j.coi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- [29].Banerjee K, Biswas PS, Rouse BT. Role of Stat4-mediated signal transduction events in the generation of aggressor CD4+ T cells in herpetic stromal keratitis pathogenesis. J. Interferon Cytokine Res. 2007;27:65–75. doi: 10.1089/jir.2007.0077. [DOI] [PubMed] [Google Scholar]
- [30].Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shriver L, Mann M, Dittel BN. Th17 cells alone are not sufficient to induce CNS autoimmunity, but can synergize with Th1 cells to induce EAE. J. Immunol. 2007;178:129–24. [Google Scholar]