Abstract
It has been proposed that astrocytes should no longer be viewed purely as support cells for neurons, such as providing a constant environment and metabolic substrates, but that they should also be viewed as being involved in affecting synaptic activity in an active way and, therefore, an integral part of the information-processing properties of the brain. This essay discusses the possible differences between a support and an instructive role, and concludes that any distinction has to be blurred. In view of this, and a brief overview of the nature of the data, the new evidence seems insufficient to conclude that the physiological roles of mature astrocytes go beyond a general support role. I propose a model of mature protoplasmic astrocyte function that is drawn from the most recent data on their structure, the domain concept and their syncytial characteristics, of an independent rather than integrative functioning of the ends of each process where the activities that affect synaptic activity and blood vessel diameter will be concentrated.
Keywords: Homeostasis, information processing, voltage clamp, support roles, vesicular release
Arguably the major problem for astrocyte research is to find a guiding function for these cells. This seems surprising considering that, as the neuroglia, their distinctive morphologies have been recognized for over 100 years (Kettenmann and Ransom, 2005). Neurons were also first accurately described at the same time using the same technique of Golgi’s reazione nero by microscopists, such as the fabled Ramón y Cajal. However, although the general function of neurons is agreed on; namely that they underlie all the unique functions of the CNS based on neuronal excitability and organization of neurons into circuits, agreement on the functions of astrocytes remains clouded. This difference gives every study on neurons some type of hypothesis (i.e. question-to-be-answered), in terms of how a process influences synaptic transmission, neuronal excitability and its potentiation or inhibition, but presents a problem for focusing studies on astrocytes. Neurons were first found to be excitable based on pioneering electrophysiological work in 1902, although elucidation of the true nature of the action potential was determined during the period 1939–1955 (Katz, 1966). Starting in the 1960s sharp electrode recordings showed that the neuroglia are non-excitable (Nicholls et al., 2000).
Application of modern techniques has expanded on the early microscopic studies by showing more clearly the extent of the complex morphology of mature mammalian protoplasmic astrocytes; namely a small cell body with 1000–10 000 processes that connect at their ends with synapses and blood vessels, and communicate with the process of other astrocytes through gap junctions. Although this morphology is consistent with the role of maintaining the optimal functioning of the neuronal arrays by stabilizing the ECS (i.e. a support role), it has also been argued that the astrocytes might have an ancillary, instructive role in information-processing in the mammalian CNS and that the syncytium provides another information-processing pathway (Todd et al., 2006). The purpose of this essay is to critically present these two general viewpoints with the conclusion that the majority of the data to date is most consistent with a support role.
Like all scientists, I have to assume that the results published in peer-reviewed journals are correct as far as they go, but that differences with the authors in interpretation are quite admissible. Why, you might ask, since the interpretations were also presumably reviewed, are these to be questioned. Well, interpretations are never exclusionary and the data might fit into other schemes of what the cells do. Also, further data can always make a current interpretation no longer valid because of new data or newer, more correct ways of looking at the same data. How one distinguishes the ‘best’ interpretation is a judgment based on consistency and reasonableness. The only formal principle that is sometimes expressed is that of ‘Ockham’s razor’, which can be stated as ‘all other things being equal the simplest explanation is to be favored’ (Russell, 1989). This seems equivalent to saying that interpretations are often a best guess based on the evidence.
I recently wrote an article entitled ‘The problem of astrocyte identity’ (Kimelberg, 2004a) because the definition of what constitutes a mature astrocyte is also generally recognized as a major problem. In that article and the present one we are actually dealing with the same problem because understanding the functions of mature astrocytes will imply a classification. Here, I discuss only the mature protoplasmic astrocyte in gray matter (Fig. 1) and its physiological properties. Whether this belongs to a larger class of astrocytes and how they might differ within the class requires further study and discussion.
Fig. 1. Astrocytes at PND 21 (A–C) and at 1 month (D–F) from CA1.
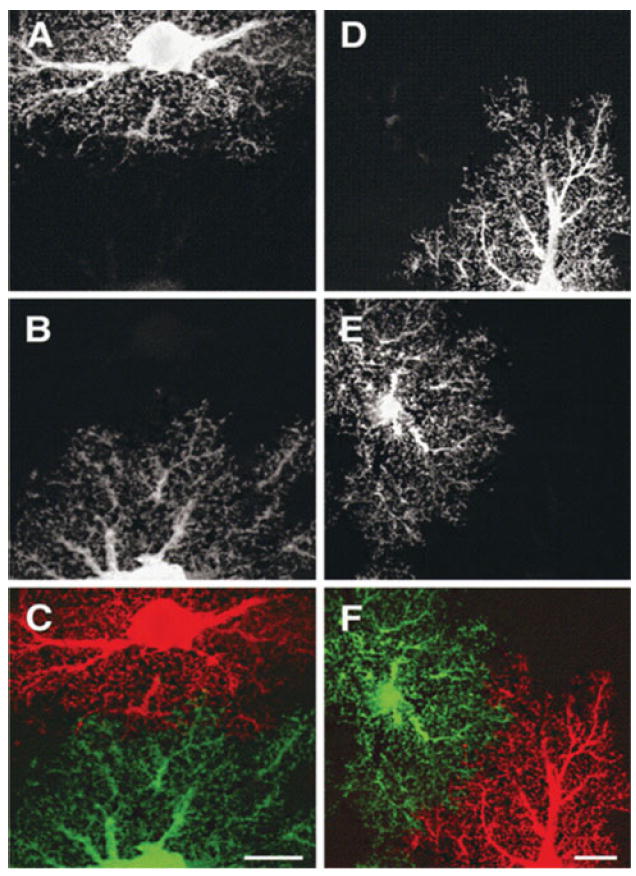
Images are maximum projections of dye-filled astrocytes in optical slices through 1 μm of tissue. Scale bars, 10 μm. Reproduced, with permission, from Bushong et al. (2004).
Evidence in support of supportive roles for the mature mammalian astrocyte
I now briefly summarize what I consider to be the properties that support the role of astrocytes as support cells.
Very dominant K+ conductance that gives a negative resting membrane potential that is very close to the K+ equilibrium potential and a linear I–V relationship. This, together with little or no anion conductance, led to a proposed role in the control of increased [K+]o by a current loop that was carried into and within the cell by K+ with the release of K+ distal to the region of increased of ECS [K+] (Orkand et al., 1966). An exclusive K+ conductance also maintains the negative membrane potential with minimal energy expenditure.
pH control by different transporters leading to control of ECS [H+] (Bourke et al., 1978; Kimelberg et al., 1979; Chesler and Kraig, 1987; Newman, 1999; Deitmer and Schneider, 2000).
Transmitter uptake and metabolism (especially glutamate). Inactivation of glutamate by conversion to glutamine by the astrocyte-specific, intracellular enzyme glutamine synthetase, which consumes ATP and ammonia (Berl et al., 1961; Martinez-Hernandez et al., 1977).
Water transport and aquaporins (AQPs), particularly AQP4 located perivascularily (Amiry-Moghaddam and Ottersen, 2003). This seems to assume water transport is rate-limiting relative to the fast transport of substrates such as glucose and K+, and is, presumably, needed to avoid increases in osmotic pressure inside the cell limiting the further transport of these substances (Kimelberg, 2004b). The processes described in (2)–(4) above can lead to swelling, and it has been proposed that astrocytes have volume-regulatory anion channels that are activated by such swelling to allow the efflux of Cl− with K+ efflux via the always-open K+ channels, to regulate volume (Mongin and Kimelberg, 2005). It has been hypothesized that, because such channels are also permeable to glutamate and that astrocytes swell markedly in pathological states, efflux of glutamate from astrocytes adds to the excitotoxic glutamate burden (Kimelberg, 2005).
Regulation of blood flow. Astrocytic end-feet exclusively surround all blood vessels in the CNS, including arterioles. They might, thus, act as transducers of increased neuronal activity, and increased Ca2+ in these end-feet correlates with either vasoconstriction or vasodilatation in vivo (Zonta et al., 2002; Mulligan and MacVicar, 2004; Takano et al., 2005; Iadecola and Nedergaard, 2007). Vasodilatation is caused by the production of prostenoids such as prostaglandin E2 (PGE2) by astrocytes (Takano et al., 2006). I interpret these effects on arteriolar diameter as a permissive/supportive role that provides neurons with adequate substrates for their increased energy metabolism.
Other supportive biochemical properties. Ammonia homeostasis, which is involved in glutamine synthesis has been mentioned in regard to (3). Astrocytes are a major site of antioxidants such as glutathione in the CNS (Aschner, 2000), and they supply substrate to neurons in the form of lactate as described in the lactate shuttle hypothesis (Magistretti et al., 1999).
Evidence in support of an instructional, information-processing role
In the past 15 years several authors have proposed that astrocytes might have a direct role in information-processing (Araque et al., 1999; Haydon, 2001; Volterra and Meldolesi, 2005; Perea and Araque, 2005a; Perea and Araque, 2006; Deitmer et al., 2006). Although the experiments reported in these papers are elegant, it is my view that the most reasonable and simplest conclusion from what we know about astrocytes is that they perform a supportive role. It is not impossible that astrocytes have an active role in information-processing but, as the well-regarded physicist Richard Feynman pointed out, the role of science is to determine what is less or more likely and not what is possible or impossible (Feynman, 1965).
Astrocytic syncytium
The linking of mature astrocytes into a syncytium that consists of up to hundreds of cells is a distinctive feature of mature astrocytes that was seen first in situ using the electron microscope to identify gap junctions (Massa and Mugnaini, 1982), and then by transfer of electrode-injected dyes (e.g. Fig. 2). This has recently been proposed to form an astrocyte-based information processing system that is additional to neuronal circuits and/or to integrate separated neuronal circuits (Pascual et al., 2005; Perea and Araque, 2005a; Volterra and Meldolesi, 2005; Todd et al., 2006), based mainly on the Ca2+ increases and flow through the cell to the processes and then to neighboring gap-junction-linked other astrocytes. A proposed function of this Ca2+ increase in releasing neuroactive agents at the ends of the processes is discussed later.
Fig. 2. Cells stained for GLAST.

Cells filled with biocytin through a patch pipette were immunohistochemically stained for GLAST and images encompassing the entire z dimension of the syncytium were acquired by confocal microscopy. (A) An example of a highly coupled GLAST(+), passive, recorded cell. This is a projection image acquired along the entire z axis of the biocytin-Cy2-Streptavidin channel. (B,C) The smaller panels on the right show portions of single z planes for Biocytin (B) and anti-GLAST (C) from the left image. Arrows point to the recorded and injected cell body. From a P7 rat. Scale bar, 20 μm. Reproduced, with permission, from Schools et al. (2006).
However, there is an alternative, simpler interpretation. That the function of the syncytium is to provide a large reservoir that buffers substances such as K+ and H+, which are taken up at particular sites where they locally increase because of increased neuronal activity. This was proposed initially for K+ spatial buffering (Kuffler and Potter, 1964; Kuffler et al., 1966). This intracellular K+ current-loop mechanism is based on K+-channel activity and no detectable Cl− conductances (Orkand et al., 1966).
Several either electrically neutral or electrogenic K+ or H+ transporters were identified originally in primary astrocyte cultures (Kimelberg et al., 1979) and then in astrocytes in situ (Deitmer and Schneider, 2000; Chesler, 2003). However, these transporters are essentially ubiquitous, so this is not definitive for astrocytes. It was hypothesized that they would also buffer K+ (such as the Na+K+2Cl co-transporter) and H+ (via Na/H and HCO3 transporting systems) (Kimelberg et al., 1979; Chesler, 2003). Uptake by either transporters or coupled cation plus anion uptake on separate channels might then be followed by diffusion and dilution within the syncytium.
Electrophysiological consequences of the syncytium
The syncytium might also be responsible for the diagnostic very low membrane (input) resistances of mature astrocytes with a fixed density of K+ channels because resistance is inversely proportional to area. The reversal potential in I–V plots, which is equivalent to the resting membrane potential, shows a close to K+ selective conductance. This can be explained by the K+-selective Rm being in series with the access resistance of the electrode tip (Ra) (Fig. 3A). The low resistance creates serious technical problems when using the most common technique for measuring the electrophysiological properties of small-cell soma, the continuous single electrode voltage clamp (cSEVC) because Ra can be as great as or greater than Rm (Sontheimer, 1995). Thus, a major drop of the voltage command (say 50–80%) will be across Ra, which contributes to a linear I–V curve instead of an outwardly rectifying one as expected from the Goldman-Huxley-Katz (GHK) constant field equations for purely ohmic channels (Hille, 1992) with the physiological K+ gradient as set up by the experimenter, as Ra is non-selective for ions. However, the relative contribution made by channels and/or the large increase in area due to the syncytium to the very low Rm resistance is unclear.
Fig. 3. Membrane tests.
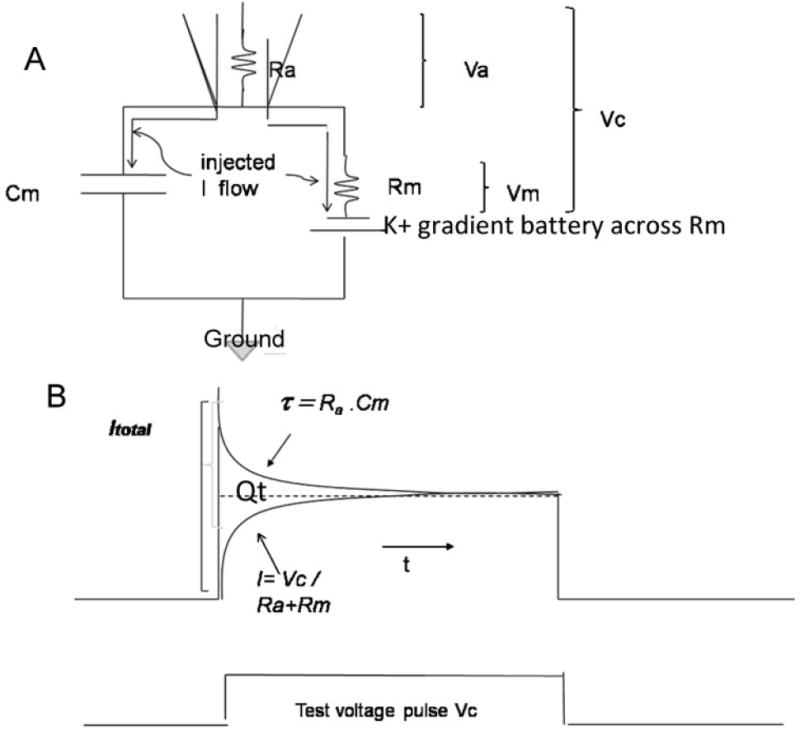
(A) Circuit and (B) pCLAMP9 membrane test. Ra = access resistance of the pipette, Rm = effective membrane resistance and Cm the effective capacitance. Effective means what is measured by the electrode. Vc is the command potential. Va is the voltage drop across Ra and Vm the voltage drop across Rm. Adapted from pCLAMP 9, 2003 Axon Instruments, Inc. See text for further details.
The latest pCLAMP 9 program used by a number of investigators for cSEVC has a membrane test feature that gives Ra from tau estimated for the small decline of the initial capacity transient corrected for the rapidly developing current flow from the amplifier through Ra+Rm (Fig. 3B). The total charging current not attributable to resistive current flow is shown as Qt. Qt gives the total capacitance from Cm = Qt/Vc, and Ra can be obtained from the standard relation tau = Ra.C. Finally, Rm can be obtained by subtraction from the total measured Rt, which is Rm+Ra. However, the values given by the program are of uncertain precision because of difficulties in accurately assessing tau, so we are left only with approximations.
With these caveats in mind, when we studied glial cells in hippocampal slices by cSEVC we got three different forms of I–V relations (Fig. 4). Other investigators have reported similar results (Steinhauser et al., 1992; D’Ambrosio et al., 1998). In a systematic developmental study we found progressive changes in the expression of these electrophysiological phenotypes, with the cells that give linear I–V curves representing ~90% of the recorded glia after 21 days (Zhou et al., 2006). Such linear (passive) I–V curves are now being used as diagnostic of mature astrocytes before studying other aspects (D’Ascenzo et al., 2007; Perea and Araque, 2007; Jourdain et al., 2007). Post-recording staining of smaller representative groups showed that these cells were all GLAST(+) astrocytes, whereas the two nonlinear phenotypes shown in Fig. 4 represented a mixture of GLAST(+) and NG2(+) cells whose relative proportions varied at different ages (see Zhou et al., 2006).
Fig. 4. Whole cell recordings from cells in situ.

(a–c) Whole cell recordings in response to the voltage steps shown in d for the three types of electrophysiological phenotypes seen for cells in situ. The insert (a) shows Na+ currents. Reproduced, with permission, from Zhou et al. (2006).
Vesicular transmitter release at synapses
An instructional role for astrocytes has been proposed, based on evidence indicating that the release of either glutamate or ATP from the ends of astrocytic processes at synapses occurs through exocytotic release from astrocytes and modulates synaptic activity. The original observations, which were interpreted as vesicular release, were made with primary astrocyte cultures (Parpura et al., 1994; Araque et al., 2000; Pasti et al., 2001; Montana et al., 2004). Later work presented evidence for vesicular release from astrocytes in situ (Pasti et al., 2001; Bezzi et al., 2004; Zhang et al., 2004; Jourdain et al., 2007; Perea and Araque, 2007). Increases in intracellular Ca2+ in astrocytes in response to neuronal stimulation have several effects on synaptic activity (Perea and Araque, 2007), and increased sensory input also results in increased intracellular Ca2+ (Perea and Araque, 2005b; Wang et al., 2006). The data to date provides convincing evidence that vesicular release of glutamate can be obtained under some conditions, leading to increased EPSC frequency in granule cell neurons in the dentate of the rat hippocampus (Jourdain et al., 2007) and induction of a slow inward current (SIC) that increases action potential firing in the target neuron in the nucleus accumbens (D’Ascenzo et al., 2007). The SIC has been attributed to glutamate acting on NR2B subunit-containing extrasynaptic NMDA receptors (Fellin et al., 2004; D’Ascenzo et al., 2007).
Recently, a genetic engineering approach has showed that inhibition of SNARE protein action that is considered targeted specifically to astrocytes via the GFAP promoter, alters long-term potentiation (LTP) (Pascual et al., 2005). Because astrocytes and neurons have many Ca2+-linked receptors in common, the other approach to ensure specific stimulation of Ca2+ responses in astrocytes is to uncage photosensitive Ca2+ complexes that have been injected into single astrocytes.
To prove this premise, however, studies must establish that the impaired fusion event only involves vesicles that release effective amounts of transmitters from astrocytes and not some other fusion-related event such as insertion of channels or other components into membranes that also leads to the release of transmitters or other substances that affect neurons. It must also be established that these vesicle fusion events occur physiologically to affect behavior. Vesicle density appears to be ten times less in astrocytes compared to presynaptic neuronal terminals (Jourdain et al., 2007), so the magnitude of the response is likely to be much less. Also Fiacco et al. (2007) produced transgenic mice that express a Gq-linked receptor only in astrocytes. Activation of this receptor was assumed to produce a more physiological increase in astrocytic Ca2+. This approach failed to produce SICs in neurons in the hippocampus, leading to the idea that changes seen using other means of increasing astrocytic Ca2+ are of a pathological rather than physiological nature. Also some of the astrocytic fusion events appear to involve lysosomes (Jaiswal et al., 2007; Zhang et al., 2007), which is unlikely to be physiological. The question also arises of what it means if there is bona fide vesicular release from astrocytes. By itself, this does not unambiguously indicate independent control of synaptic activity and a controlling role in information processing.
Are there widespread consequences of alterations in astrocyte support functions?
It is immensely difficult to distinguish between an essential supportive and a discretionary instructional role for astrocytes. For example, inhibiting a crucial SNARE fusion protein using genetic engineering has been shown to be associated with a change in the accepted information-processing linked property, LTP (Pascual et al., 2005). One really cannot say, however, that the alteration in activity of the fusion protein in the astrocyte directly caused the change in LTP. Even assuming a direct effect, how can we distinguish between astrocytes that affect the circuits involved in LTP in a manner that initiates LTP, or that allows neuronal circuits to generate LTP behavior? In the latter case involvement of astrocytes would seem to be permissive. This is a difficult distinction to make and, in the latter case, I would take this to mean that the astrocyte allows the process to take place but does not initiate it. The current assumption seems to be that an effect of altering fusion proteins in astrocytes on neuron-based electrophysiological activities does indicates modulation of synaptic activity in an initiating manner, but this does not seem to follow. If it did then, logically, the same reasoning would apply to changes in, for example, H+-transporting systems such as Cl−/HCO3− and Na+/H+ exchangers that are involved in maintaining [H+]o in the face of changes caused by neuronal activity (Newman, 1999; Chesler, 2003). Would we conclude from such observations that astrocytes have an instructional role in information processing via changes in [H +]o? Alternatively, astrocytes might release transmitter as a response to changed synaptic activity in order to produce a homeostatic response in the neuron and avoid some excessive change that might prevent the synapse doing what it had been instructed to do as part of a neuronal circuit.
The key point from the studies referred to above is that, when a transmitter is released from astrocytic processes by Ca2+-dependent fusion of vesicles that store the transmitter, it indicates crucial control of synaptic events that is equivalent to the control of information flow. However, we then add to the unknown complexity of neuronal information-processing a need to understand what controls the astrocyte control, and so on.
There is also much evidence for alternative pathways of transmitter release from astrocytes, with the necessary caveat of the experimental astrocyte model in which the tests (experiments) are done. Primary cultures of astrocytes, which provide a seductive experimental simplicity, are not reliable indicators of the properties of astrocytes and how they behave in situ (Kimelberg, 1995; Kimelberg et al., 1997; Kimelberg, 2001; Lovatt et al., 2007; Cahoy et al., 2008). These alternative paths are (1) release by reversal of excitatory amino acid (EAA) transporters, which is only likely to occur under pathological conditions, (2) swelling activated anion channels and also hemi-channels, and (3) P2X receptors for ATP. These routes, and others, are described in Deitmer et al. (2006). Clearly, these different pathways will have to be subject to the same criteria for establishing their functional relevance and, if astrocytes modulate synaptic activity in the normal course of events by transmitter release, why should these other routes not be involved to some degree? It would also seem that there should only be one main route, otherwise one might either expect a lot of noise in astrocytic control of synaptic activity or another level of regulation must be invoked, with no end in sight.
A general support role for astrocytes
What would a general support role for astrocytes look like? Bushong et al. (2002) emphasized in their combined immunohistochemical and dye-filling studies in the CA1 region of 1-month-old Sprague-Dawley rats, that the dye-filled astrocytes were morphologically homogenous and their highly bushy processes (1000–10 000 per cell body) occupied separate territories of ~70 000 μm3. This is an important concept. Note they cut 100-μm sections from brains of paraformaldehyde-perfused rats, presumably to avoid rapid, post-mortem, prefixation changes, so they did not see cell–cell coupling. In a later study (Bushong et al., 2004) the same group showed that these features develop with age and resemble the mature form at ≥postnatal day 21 (PN21). This is after the glutamate synaptic system matures (~PN14) and corresponds with the dominance of the purely electrophysiologically passive astrocyte in the CA1 (Fig. 5). Thus, their data are consistent with our finding that the electrophysiological diversity of astrocytes is a feature of the developing hippocampus, whereas in the mature brain a single astrocytic electrophysiological phenotype, characterized by a linear I–V relationship, is a characteristic of astrocyte maturation, be it the maturation of the syncytium or K+ channels, or something else.
Fig. 5. Electrophysiological phenotypes of glial cells.
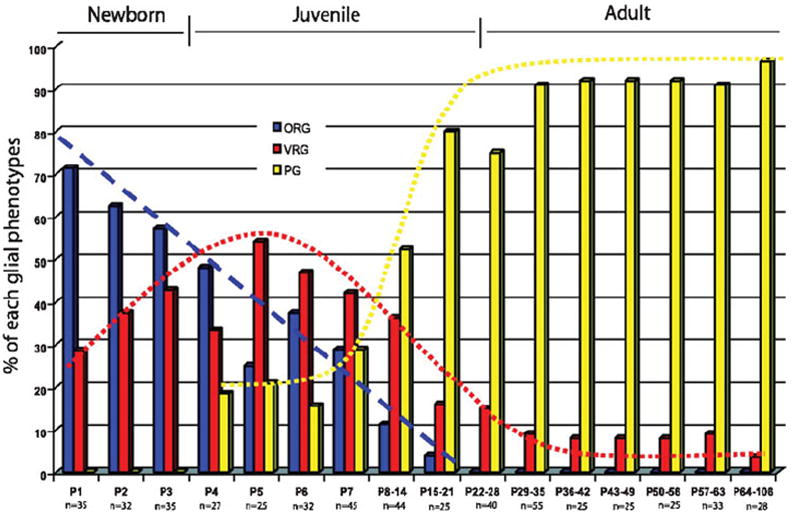
The percentage of glial cells, as first recognized by their morphology in differential interference contrast optics, in a living hippocampal slice that correspond to the three electrophysiological phenotypes shown in Fig. 4. The numbers on the x axis show the postnatal age or age range and n = number of cells recorded in newborn, juvenile and adult stages. Reproduced, with permission, from Zhou et al. (2006).
The large number of processes of mature astrocytes, that the processes of separate astrocytes overlap by only ~10%, and that gap junctional, electrophysiological, synaptic and domain maturity all occur at around the same time in the hippocampus (Bushong et al., 2002; Bushong et al., 2004; Zhou et al., 2006) can be incorporated into a general hypothesis of astrocyte function in the mature brain, or at least in the hippocampus (Fig. 6). This is that the vascular and synaptic end-feet function as autonomous units that respond to specific events at these loci as independent entities driven by local feedback signals. A proposal for independent domains for stimulation-induced Ca2+ increases was proposed a few years ago, and supported experimentally for the Bergmann glia lamellae and filipodia that enwrap the synapses that the Purkinje dendritic spines make with the parallel fibers (Grosche et al., 1999). In terms of either synchronizing or otherwise integrating neuronal activity, there has been no demonstration of any polarity in regard to these populous processes, so it would require a signal from one or several synapses to be transmitted randomly to all other synapses touched by processes of the same cell, limited by either the rate of diffusion of an intracellular messenger or the electrotonic decay of a membrane potential depolarization. However, it has also been the suggested that each astrocytic domain integrates all the synaptic activity within it and integrates them with vascular activity (Nedergaard et al., 2003). Tests to distinguish local autonomous control versus a more global integrative role are, unfortunately, difficult and as Galileo wrote in the Dialogue Concerning the Two ChiefWorld Systems ‘where the senses fail us, reason must step in’.
Fig. 6. Schematic of astrocyte processes interacting with synapses and one blood vessel (red circle).
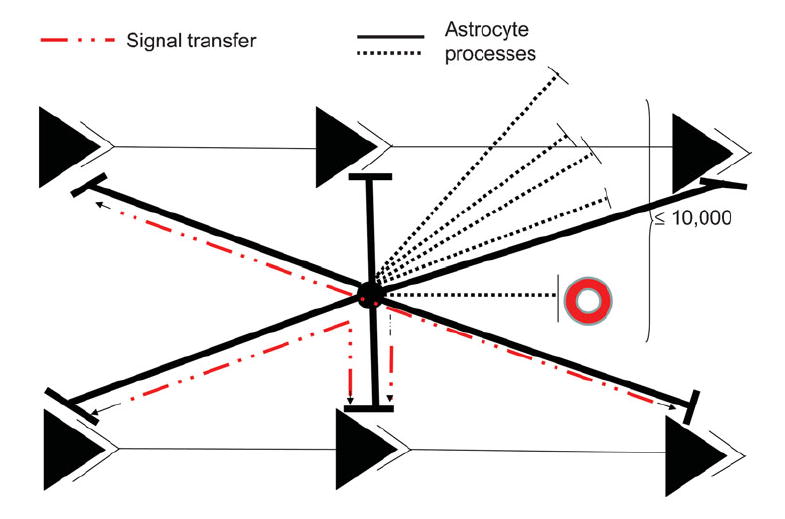
The black circle in the center represents an astrocyte cell body whose processes extend to many synapses (≤10 000). Shown are six synapses in two parallel neuronal circuits. Dashed black lines show members of part of the extensive astrocyte process domain in other planes. The postulated information pathway between astrocyte processes and some of the synapses of the same circuit, or between parallel synapses, is shown as a dashed red line. Astrocytes do not fire action potentials, so the signal is viewed as a spread of either intracellular Ca2+ or another intracellular messenger or messenger system. It is unlikely to be an electrotonic spread of a local depolarization based on calculations of the length constant of astrocytes in situ (Trachtenberg and Pollen, 1970). It is proposed that the perisynaptic astrocyte processes serve to maintain the local environment around the synapses for optimal functioning by localizing key proteins involved in uptake of, for example, glutamate and H+ and channels or transporters for K+ clearance released at the active synapse, and the activity of these fluxes is controlled by local feedback loops. These two possibilities (synaptic homeostasis based on feedback versus an instructional role based on initiating and integrative functions) are extremely difficult to distinguish either in situ or in vivo and, so far, there seem to be insufficient reasons to postulate the latter. See text for a fuller discussion.
The more limited support model of the mature astrocyte is well-grounded in cell and biological principles. Basic cell theory states that the autonomous ends of the many processes cannot exist independently of the cell nucleus, which is needed to replace proteins and synthesize and export to the processes either more of the same proteins or new ones induced by a change in conditions. Such transport needs a transporting system to carry proteins and remove them for degradation in soma lysozymes, or partly they might be degraded in lyzosomes present in the process ends. Because the ends of the perisynaptic processes (fine lamellae and filipodia that arise from the processes emanating from the astrocytic cell body) are too small to contain mitochondria, as emphasized by (Hertz et al., 2007), some of the ATP they need will diffuse from mitochondria in the soma and larger diameter processes that are more proximal to the soma. Otherwise the energy requirements at the process ends would have to be met by glycolysis, which is much less efficient. However, a recent paper in which GFAP promoter-driven GFP was used to identify small astrocyte perisynaptic end-feet found that the number of mitochondria in the end-feet was either as great or greater than other profiles in the surrounding neuropil (Lovatt et al., 2007). However, Chao et al. (2002) using electron microscopy and S100-immunor-eactivity to identify the processes have noted previously that the fine lamellae and filipodia are devoid of organelles, unlike the astrocytic cell body and processes. Therefore it remains an open question whether end-feet supply sufficient energy by oxidative metabolism to meet their needs, which would diminish the amount of lactate available for export to neurons, as envisaged in the neuronal–astroglial metabolic compartmentation theory of Magistretti and Pellerin (1999).
Another general biological principle is economy of space. The complex morphology of mature astrocytes, with their massive arborizations of processes, might have evolved, not because they participate actively in information-processing, but for the more prosaic task of conserving space in the fixed volume that complex brains occupy while still being able to carry out their supportive functions. 1000 to 10 000 synaptic targets and blood vessels can be served by one cell soma (Fig. 6), thus, conserving space, which is at a premium in mammalian brains. This constraint has also, for example, led to myelination to reduce axonal diameters to conserve space for a given action potential velocity (Katz, 1966; Hille, 1992; Chao et al., 2002). Thus, the domain concept for populous, process-bearing mature astrocytes is consistent with autonomous homeostatic end-feet functions rather than integrative activity for neuronal networks.
The next question is to determine the nature and location of different transporters and channels in the membranes so that we can better infer the precise ‘support’ function. Numerous techniques would then need to be applied to test these inferences (hypotheses) of function, according to the criteria of the scientific method.
Acknowledgments
I thank Drs Gary Schools and Min Zhou for many helpful discussions and help with and/or permission to use a number of our published Figures. I thank Charitable Leadership Foundation, Latham, NY for financial support.
References
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nature Reviews Neuroscience. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- Araque A, Li NZ, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. Journal of Neuroscience. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends in Neurosciences. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Aschner M. Neuron-astrocyte interactions: Implications for cellular energetics and antioxidant levels. NeuroToxicology. 2000;21:1101–1107. [PubMed] [Google Scholar]
- Berl S, Lajtha A, Waelsch H. Amino acid and protein metabolism–VI cerebral compartments of glutamic acid metabolism. Journal of Neurochemistry. 1961;7:186–197. doi: 10.1111/j.1471-4159.1959.tb12638.x. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nature Neuroscience. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bourke RS, Kimelberg HK, Daze MA. Effects of inhibitors and adenosine on (HCO3-/CO2)-stimulated swelling and Cl- uptake in brain slices and cultured astrocytes. Brain Research. 1978;154:196–202. doi: 10.1016/0006-8993(78)91072-7. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. International Journal of Developmental Neuroscience. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. Journal of Neuroscience. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. Journal of Neuroscience. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao TI, Rickmann M, Wolff JR. The synapse-astrocyte boundary: an anatomical basis for an integrative role of glia in synaptic transmission. In: Volterra A, Magistretti P, Haydon PG, editors. The Tripartite Synapse: glia in synaptic transmission. Oxford University Press; 2002. pp. 3–23. [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiological Reviews. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kraig RP. Intracellular pH of astrocytes increases rapidly with cortical stimulation. American Journal of Physiology. 1987;253:R666–R670. doi: 10.1152/ajpregu.1987.253.4.R666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM, Janigro D. Functional specialization and topographic segregation of hippocampal astrocytes. Journal of Neuroscience. 1998;18:4425–4438. doi: 10.1523/JNEUROSCI.18-12-04425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proceedings of the National Academy of Sciences of the U S A. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, McCarthy KD, Scemes E, Giaume C. Information processing and transmission in glia: calcium signaling and transmitter release. Glia. 2006;54:639–641. doi: 10.1002/glia.20428. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Schneider HP. Enhancement of glutamate uptake transport by CO2/bicarbonate in the leech giant glial cell. Glia. 2000;30:392–400. [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Feynman R. The Character of Physical Law. MIT Press; 1965. pp. 165–166. [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, et al. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nature Neuroscience. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nature Reviews Neuroscience. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. Journal of Cerebral Blood Flow and Metabolism. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sinauer; 1992. pp. 165–166. [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nature Neuroscience. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Fix M, Takano T, Nedergaard M, Simon SM. Resolving vesicle fusion from lysis to monitor calcium-triggered lysosomal exocytosis in astrocytes. Proceedings of the National Academy of Sciences of the U S A. 2007;104:14151–14156. doi: 10.1073/pnas.0704935104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nature Neuroscience. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Katz B. Nerve, muscle and synapse. McGraw-Hill; 1966. pp. 41–72. [Google Scholar]
- Kettenmann H, Ransom B. The concept of neuroglia: a historical perspective. In: Kettenmann H, Ransom B, editors. Neuroglia. 2. Oxford University Press; 2005. pp. 1–16. [Google Scholar]
- Kimelberg HK. Glia-neuronal culture models: do we need to change the paradigms? Trends in Neurosciences. 2001;24:205–206. doi: 10.1016/s0166-2236(00)01775-6. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. The problem of astrocyte identity. Neurochemistry International. 2004a;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience. 2004b;129:851–860. doi: 10.1016/j.neuroscience.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia. 2005;50:389–397. doi: 10.1002/glia.20174. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Biddlecome SM, Bourke RS. SITS-inhibitable Cl- transport and Na+-dependent H+ production in primary astroglial cultures. Brain Research. 1979;173:111–124. [PubMed] [Google Scholar]
- Kimelberg HK, Cai Z, Rastogi P, Charniga C, Goderie S, Dave V, et al. Transmitter-induced calcium responses differ in astrocytes acutely isolated from rat brain and in culture. Journal of Neurochemistry. 1997;68:1088–1098. doi: 10.1046/j.1471-4159.1997.68031088.x. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. Journal of Neurophysiology. 1966;29:768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Potter DD. Glia in the leech central nervous system: physiological properties and neuron-glia relationship. Journal of Neurophysiology. 1964;27:270–320. doi: 10.1152/jn.1964.27.2.290. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, et al. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. Journal of Neuroscience. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Astrocytes couple synaptic activity to glucose utilization in the brain. News in Physiological Sciences. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell K, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Massa PT, Mugnaini E. Cell junctions and intramembrane particles of astrocytes and oligodendrocytes: a freeze-fracture study. Neuroscience. 1982;7:523–538. doi: 10.1016/0306-4522(82)90285-8. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. Astrocytic swelling in neuropathology. In: Kettenmann HO, Ransom BR, editors. Neuroglia. Oxford University Press; 2005. pp. 550–562. [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. Journal of Neuroscience. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends in Neurosciences. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Newman EA. Sodium-bicarbonate cotransport in retinal astrocytes and Muller cells of the rat. Glia. 1999;26:302–308. doi: 10.1002/(sici)1098-1136(199906)26:4<302::aid-glia4>3.0.co;2-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls JG, Martin AR, Wallace BR, Fuchs PA. From Neuron to Brain. Sinauer Associates; 2000. pp. 133–154. [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. Journal of Neurophysiology. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. Journal of Neuroscience. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Glial calcium signaling and neuron-glia communication. Cell Calcium. 2005a;38:375–382. doi: 10.1016/j.ceca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. Journal of Neuroscience. 2005b;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Synaptic information processing by astrocytes. Journal of Physiology Paris. 2006;99:92–97. doi: 10.1016/j.jphysparis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Russell B. Wisdom of the West. Crescent Books; New York: 1989. pp. 162–163. [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. Journal of Neurophysiology. 2006;96:1383–1392. doi: 10.1152/jn.00449.2006. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. Whole-cell patch-clamp recordings. In: Boulton A, Baker GB, Walz W, editors. Patch-Clamp Applications and Protocols. Humana Press; 1995. pp. 37–74. [Google Scholar]
- Steinhauser C, Berger T, Frotscher M, Kettenmann H. Heterogeneity in the membrane current pattern of identified glial cells in the hippocampal slice. European Journal of Neuroscience. 1992;4:472–484. doi: 10.1111/j.1460-9568.1992.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nature Neuroscience. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Todd KJ, Serrano A, Lacaille JC, Robitaille R. Glial cells in synaptic plasticity. Journal of Physiology Paris. 2006;99:75–83. doi: 10.1016/j.jphysparis.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Trachtenberg MC, Pollen DA. Neuroglia: Biophysical properties and physiologic function. Science. 1970;167:1248–1251. doi: 10.1126/science.167.3922.1248. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nature Reviews Neuroscience. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, et al. Astrocytic Ca(2+) signaling evoked by sensory stimulation in vivo. Nature Neuroscience. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, et al. Fusion-related release of glutamate from astrocytes. Journal of Biological Chemistry. 2004;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, et al. Regulated ATP release from astrocytes through lysosome exocytosis. Nature Cell Biology. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. Journal of Neurophysiology. 2006;95:134–143. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neuroscience. 2002;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]


