Abstract
Leishmaniaprotozoan parasites, the etiologic agent of leishmaniasis, are transmitted exclusively by phlebotomine sand flies of the genera Phlebotomus and Lutzomyia. In addition to parasites, the infectious bite inoculum contains arthropod salivary components. One well-characterized salivary component from Lutzomyia longipalpis is maxadilan (MAX), a vasodilator acting via the type I receptor for the pituitary cyclic AMP activating peptide (PACAP). MAX has been shown to elicit immunomodulatory effects potentially dictating immune responses to Leishmania parasites. When exposed to MAX, both resting and LPS-stimulated dendritic cells (DCs) show reduced CD80 and CD86 expression on most DCs in vitro. However, CD86 expression is increased significantly on a subpopulation of DCs. Furthermore, MAX treatment promoted secretion of type 2 cytokines (IL-6 and IL-10) while reducing production of type 1 cytokines (IL-12p40, TNFα, and IFN-γ) by LPS-stimulated DCs. A similar trend was observed in cultures of MAX-treated DCs containing naïve allogeneic CD4+ T cells: type 2 cytokines (IL-6, IL-13) increased while type 1 cytokines (TNFα and IFNγ) decreased. Additionally, the proinflammatory cytokine IL-1β was increased in cultures containing MAX-treated mature DCs. MAX treatment of LPS-stimulated DCs also prevented optimal surface expression of CCR7 in vitro. These MAX-dependent effects were evident in DCs from both Leishmania major (Lm)-susceptible (BALB/c) and -resistant (C3H/HeN) murine strains. These data suggest that modification of DC phenotype and function by MAX likely affects crucial cellular components that determine the pathological response to infection with Leishmania.
Introduction
The vector-borne disease leishmaniasis is re-emerging as a considerable world health issue. Parasitic protozoa of the genus Leishmania, the causative agent of leishmaniasis, are transmitted to mammalian hosts via phlebotomine sand flies of the genera Phlebotomus and Lutzomyia. The sand fly vector provides a niche supporting a stage of the parasite life cycle, facilitating an indirect lateral transfer between mammalian hosts. Completion of the parasitic life cycle depends on transfer to and successful infection of host phagocytic cells. The mechanism that determines successful transfer of Leishmania parasites into designated hosts is disputed. However, recent work suggests that the inoculum of infected sand flies contains at least two major components: metacyclic promastigotes and sand fly saliva. Vector salivary components have been demonstrated to have several properties associated with disease exacerbation, including increased parasite survival, parasite burden and pathology (1-4). Leishmania major (Lm) is one of 10 species of Leishmania considered medically significant. Infection with Lm results in old world cutaneous leishmaniasis (4). Disease progression by Lm infection is well understood in murine models (5) (6). Numerous studies have demonstrated that functional cell-mediated immunity in mammalian hosts is essential to mount effective resistance against infection: murine strains eliciting a Th1 cell-mediated response develop protective immunity to Lm, while strains promoting primarily a Th2 response experience exacerbation of the disease (6) (7). Resistant strains include C3H and C57BL/6, whereas BALB/c is susceptible. A considerable amount of knowledge has been acquired regarding the pathological and immunological host responses that are involved in either disease resolution or exacerbation. However, most studies have been implemented without consideration of the potential modulatory role of arthropod saliva. Studies have shown that salivary components exacerbate disease progression in Lm-infected animals: Mice subcutaneously co-inoculated with Lm and salivary gland homogenates (SGH) from Lutzomyia longipalpis or Phlebotomus papatasi developed significantly larger cutaneous lesions than those observed on mice injected solely with parasites (2) (3) (8) (9) (10). Furthermore, salivary components have been reported to affect inducible nitric oxide synthetase (iNOS) activity, the Th1–Th2 balance, and the chemotaxis and persistence of neutrophils and eosinophils at the site of Lm infection, thus affecting the ability of the host to mount an appropriate immune response against the parasite (11–16). Cases where mice have unsuccessfully resolved vector-borne or SGH-associated Lm infections have prompted investigation into the potential immunomodulatory mechanisms of vector salivary components.
One peptide derived from Lu. longipalpis saliva is maxadilan (MAX), a potent vasodilator important for sand fly feeding. MAX aids sand fly feeding by countering the vasoconstriction that occurs in response to biting. Indeed, Milleron et al. showed that sensitization to MAX significantly reduced the blood meal volume corresponding to a reduced number of eggs matured (17). In addition to its critical function of aiding sand fly feeding, MAX exhibits considerable immunosuppressive and anti-inflammatory effects, properties that have been attributed to its interaction with and subsequent signaling through the type 1 pituitary adenylate cyclase-activating peptide (PACAP) receptor (18–21). Morris et al. showed increased pathology and parasite burden in a resistant murine strain when MAX was co-inoculated with Lm. Furthermore, vaccination with MAX resulted in protection against infection (22). In vitro studies have also revealed increased secretion of IL-10 and IL-6 while generally inhibiting TNFα production in macrophages (21) (23) (24). Such altered cytokine secretion patterns are suggestive of development of a type 2 response shown to increase pathology of Lm infection (5).
Evidence suggests that salivary molecules have a profound impact on antigen presenting cells (APCs) since they have been shown to decrease antigen presentation, nitric oxide (NO) production, and killing of Lm (9) (24). During sand fly feeding, host APCs, including monocytes, macrophages and dendritic cells (DCs), are exposed to salivary components at the site of inoculation. In cutaneous leishmaniasis, inoculated promastigotes are rapidly taken up by dermal macrophages. Several investigators have suggested that epidermal Langerhans cells (LCs) or dermal DCs rather than macrophages are responsible for the initiation of anti-parasite immunity (25) (26) (27). Considering the crucial role of DCs in innate responses and subsequent translation to adaptive immunity, their exposure to immunomodulatory salivary molecules likely dictates the type of adaptive immune response mounted against Lm (27).
In this work we examined the ability of MAX to influence adaptive immune responses mediated by DCs using a well-defined murine leishmaniasis model. Here we analyzed and compared costimulatory molecule expression on DCs derived from BALB/c and C3H mice in the presence or absence of MAX. Murine bone marrow-derived DCs (BM-DCs) as well as ex vivo splenic DCs treated with MAX expressed altered levels of the costimulatory molecules CD80 and CD86 compared to untreated controls. This phenotype was more pronounced in MAX-treated DCs stimulated with LPS. In addition to inducing marked alterations of costimulatory phenotype, MAX promoted secretion of type 2 cytokines (IL-6 and IL-10) coupled with decreased secretion of type 1 cytokines (TNFα, IL-12p40, and IFN-γ) by LPS-stimulated DCs. The functional consequence of this altered costimulatory phenotype was analyzed by examining the ability of these DCs to stimulate allogeneic CD4+ T cell proliferation and by measuring the resulting cytokine production in these cultures. MAX treatment consistently reduced the extent of allogeneic CD4+ T cell proliferation induced by DCs; in addition, cytokine secretion profiles from allogeneic culture supernatants generally showed a considerable increase in cytokines directing Th2 responses (IL-6, IL-10, and IL-13) and a correlative decrease in those directing Th1 responses (TNF-α, IFNγ and IL-12p70). Increases in the proinflammatory cytokine IL-1β were also observed. Furthermore, MAX treatment considerably diminished the expression of CCR7 on LPS-stimulated DCs. These results suggest that MAX may alter DC effector function and delays their migration to draining lymph nodes, resulting in a phenotype that limits the ability of murine DCs to mount a protective type 1 adaptive immune response against parasitic infection.
Materials and Methods
Mice
Female BALB/c, C3H/HeN, and C57BL/6 mice, 4–6 weeks of age, were purchased from National Cancer Institute and used in all experiments at 6 and 12 weeks of age. Mice were maintained at the Laboratory Animal Resources facility at Colorado State University, Fort Collins, CO. Animal maintenance and care complied with National Institute of Health Guidelines (under pathogen-free conditions) for the human use of laboratory animals and institutional policies as described in the American Association of Laboratory Animal Care and Institutional Guidelines. All animal experiments were carried out using protocols approved by the Colorado State University Animal Care and Use Committee (ACUC). C3H/HeN mice used in this manuscript are designated “C3H” for simplicity.
Reagents
Dulbecco’s Modified Eagle Medium (DMEM) was used in culturing both DCs and responder T cells. DMEM was supplemented with 1% HEPES, 100 μg/ml of penicillin-streptomycin, 2 μM L-glutamine, 1 mM sodium pyruvate, 0.2 mM L-asparagine, 0.6 mM L-arginine and 10% FBS. This will be referred to as “complete DMEM”. Recombinant murine (rm) GM-CSF and rmIL-4 were purchased from PeproTech (Rocky Hill, NJ). The following antibodies were purchased from eBiosciences (San Diego, CA) and used for flow cytometry: FITC-conjugated anti-CD11c and PE-conjugated anti-CD80, -CD86, -CCR7 and -MHC class II (I-A/I-E). Fc receptor block was purchased from Miltenyi Biotec (Auburn, CA). AutoMACs™ (Miltenyi Biotec) magnetic cell isolation kits were used to purify CD11c+ cells. LPS from Escherichia coli 055:B5 was purchased from Sigma (St. Louis, MO). DCs were positively selected using anti-CD11c microbeads, whereas negative selection was used to isolate naïve CD4+ T cells in accordance to manufacturer’s recommendation. The MAX utilized in the experiments described herein was synthesized as a 63-amino acid polypeptide by Global Peptides (Fort Collins, CO) using a proprietary synthetic procedure. The synthetic MAX was prepared using the sequence from the Brazilian sibling species of Lu. longipalpis. The MAX found in saliva of sibling species varies in both quantity and potency: The preparation used in these experiments was synthesized using the sequence found in the sand fly colony originating from Lapinha Cave, which has been shown to be optimally active. (28) Synthetic MAX was highly pure, which was assessed by HPLC and gel electrophoresis. Western analysis using anti-MAX antisera identified one band at approx. 7 kDa. (Data not shown) Typically, DCs were treated with 10 ng/ml MAX diluted in 1X PBS, a concentration that was determined empirically from this study as well as others (5, 23).
Isolation of DCs from in vitro cultures of bone marrow cells
Cultures of bone marrow cells from BALB/c or C3H mice were established as described (29). Briefly, a single cell suspension was prepared from marrow obtained from femurs and tibias. Approximately 1×107 cells were added to each well of a 6-well plate in a volume of 2 mls of complete DMEM. Cells were incubated for 3 hrs at 37°C. Plates were then gently swirled and the medium containing non-adherent cells was removed and replaced with complete DMEM medium containing 50 ng/ml rmGM-CSF and 20 ng/ml rmIL-4. The cells were placed in culture for nine days and supplemented medium was replaced every three days. Cells were harvested and purified as CD11c+ using the magnetic bead isolation kit from Miltenyi™ Biotec. Inc. (Auburn, CA). This method typically yielded 95–98% pure CD11c+ cells.
Isolation of splenic DCs
Spleens were digested with collagenase D (Boehringer Mannheim, Germany) diluted to a final concentration of 2 mg/ml in collagenase buffer containing: 10mM HEPES (pH=7.0), 150 mM NaCl, 5 mM MgCl2 and 1.8 mM CaCl2. Collagenase was injected into the tissue using a 25-gauge needle fixed to 1 cc syringe. The tissue was minced into small sections and incubated in collagenase at 37°C for 30 minutes. The minced tissue was subsequently extruded through a 70-μm filter and washed through using MACs™ running buffer (Miltenyi Biotec.) prepared without azide. Magnetic separation was used to purify CD11c+ cells according to manufacturer’s instructions. Purified CD11c+ cells were washed in complete DMEM, counted and used in the treatment procedures.
Flow Cytometry
Cells were suspended in FACS staining buffer (PBS, 0.5% BSA and 0.01% azide) and treated with Fc receptor block (Miltenyi Biotec.). Cells were subsequently labeled with FITC- or PE-conjugated antibodies for 30 minutes at 4°C and washed with FACS buffer. Cells were analyzed by flow cytometry. All cytometry was carried out on a Coulter Epics XL-MCL flow cytometer. Live cells were gated on forward vs. side scatter characteristics and resolved based on geometric mean fluorescence intensity (M.F.I) emissions generated via excitation of PE- or FITC- conjugated antibodies using a 488nm laser.
Mixed leukocyte cultures
CD11c+ DCs were isolated from the cultures of BALB/c (H-2d) and C3H (H-2k) cells treated with or without MAX and subsequently stimulated with LPS or left as immature cells. The DCs were then irradiated with 32 Gy via a 6000 Ci nominal 137Cs source. Irradiated DCs were added to 96 well plates beginning with 1.0 × 105 DCs and doubling serial dilutions were performed. C57BL/6 (H-2b) splenic CD4+ T cells (5 × 105 per well) were isolated by negative magnetic sorting (Miltenyi Biotec, Auburn, CA) and added to each well. The cultures were incubated at 37°C for 48 hrs and then pulsed with 1 μCi [3H]-thymidine per well (Amersam, Boston, MA) and cultured for an additional 16 hrs before harvesting. Cells were harvested on glass fiber filters mats using a Tomtec cell harvester (Tomtec Inc., Hamden, CT). Inter-nuclear 3H incorporation was assessed by counting the isotopic emission (as counts per minute) from the mat using a Wallac 1450 Microbeta Plus (PerkinElmer Life Sciences, Boston, MA) liquid scintillation counter.
Cytokine Assays: Multiplex Analysis
Supernatants from resting, MAX-treated, LPS-stimulated, and MAX-treated + LPS stimulated cells were obtained from cultures of DCs (1×106 cells/ml) and submitted to Pierce Biotechnology, Inc. (Woburn, MA) for Searchlight™ Multiplex Sample Testing Service™. Analysis was performed to determine secreted concentrations of INFγ, IL-6, IL-10, IL-12p40, and TNFα in DC culture supernatants. Additionally, supernatants from MLR cultures were submitted for this analysis to determine concentrations of TNFα, IFNγ, IL-12p40, IL-6, IL-10, IL-13, and IL-1β.
Statistical Analysis
Comparison of the expression of costimulatory molecules on different populations and comparison of the cytokine levels in the presence or absence of MAX was performed by a nonparametric Wilcoxon test using Prism™ and InStat™ software (GraphPad Software, Inc., San Diego, CA). Differences were considered significant if P < 0.05.
Results
MAX treatment reduces surface expression of CD80 on CD11c+ DCs treated with LPS and results in a concomitant increase in CD86 expression on a subpopulation of these cells
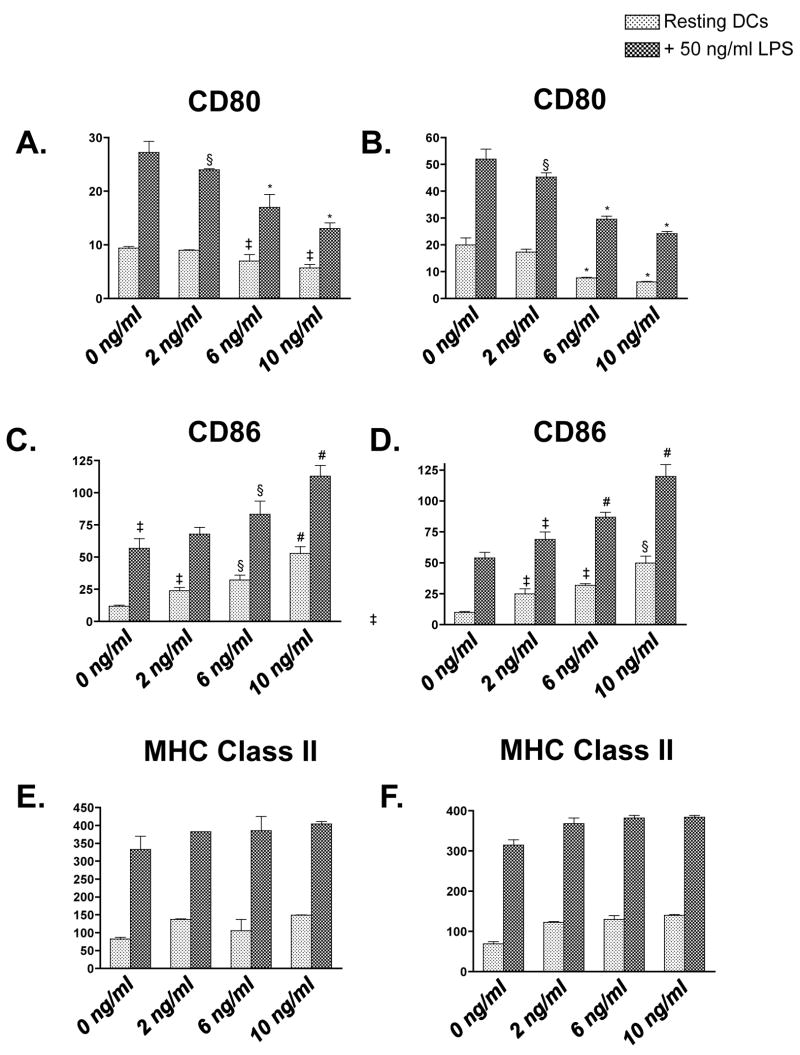
To determine whether MAX treatment resulted in altered expression of CD80 and CD86 costimulatory molecules on BM-DCs, purified CD11c+ cells from both BALB/c and C3H mice were treated with either vehicle (1X PBS) or 10 ng/ml MAX three hrs prior to stimulation with 50 ng/ml LPS for 30 hrs in vitro. Levels of CD80, CD86 and MHC class II were subsequently measured on BM-DCs via flow cytometric analysis. Figure 1 shows flow cytometry histograms illustrating altered surface expression of costimulatory molecules as a result of treatment of purified BM-DCs with MAX prior to LPS stimulation. Panels A and B show a considerable down regulation of CD80 on all cells from both BALB/c and C3H strains when treated with MAX (compare thick lines with shaded histograms). Panels C and D indicate a down regulation of CD86 on one subpopulation of DCs from both strains. However, a subpopulation of cells (>25%) shows a radical increase in CD86 expression (>10-fold). Differences were all statistically significant (P<0.001). No effect of MAX on MHC class II expression was observed on either strain as observed in other systems (Figure 2) (18).
Figure 1.
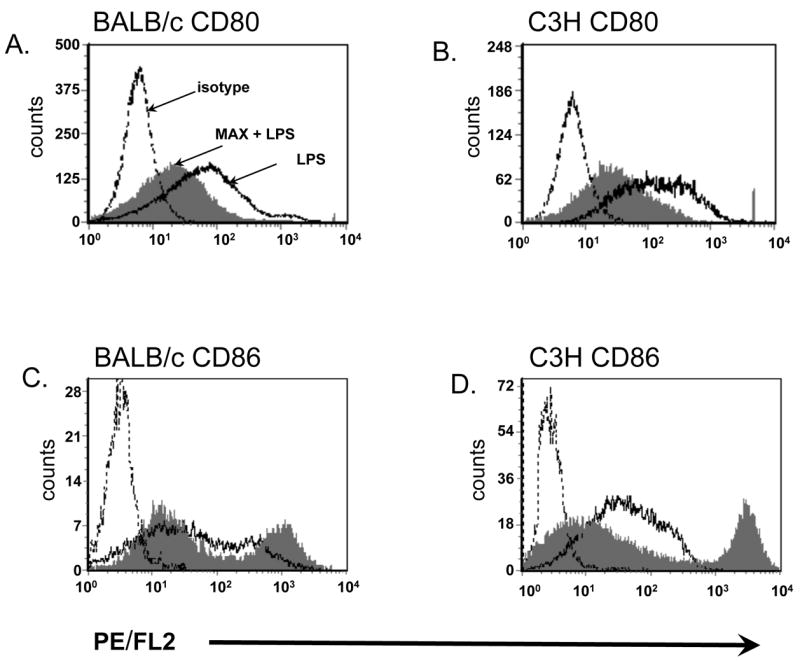
MAX treatment of BM-DCs from both BALB/c and C3H strains results in differential CD80 vs. CD86 expression. Phenotypic analysis of LPS-stimulated DCs (50 ng/ml LPS) was conducted in the absence (vehicle alone) or presence of 10 ng/ml MAX. Bone marrow progenitor cells were cultured for 9 days in rmGM-CSF and rmIL-4. CD11c+ cells were then purified by magnetic bead separation and were treated with vehicle alone (thick line histograms) or treated with MAX at 10 ng/ml (shaded histograms). Three hrs after MAX treatment, all cultures were stimulated with LPS for an additional 30 hrs. Expression levels of costimulatory molecules were assessed on purified CD11c+ cells by flow cytometry using PE-conjugated anti-CD80, or -CD86. CD80 cell surface expression was decreased on both BALB/c (panel A) and C3H (panel B) BM-DCs. Conversely, CD86 expression increased in a subpopulation of CD11c+ cells from both strains (C and D). Differences were all significant (p<0.001).
Figure 2.
MAX treatment of BM-DCs from both BALB/c (A, C and E) and C3H (B, D and F) strains results in differential CD80 (A and B) vs. CD86 (C and D) expression while showing little effect on MHC class II (E and F). Phenotypic analysis of both resting (0 ng/ml LPS [light stippled bars]) and stimulated (50 ng/ml LPS [dark stippled bars]) was conducted using 0, 2, 6, or 10 ng/ml MAX. Cells were cultured for 9 days with rmGM-CSF and rmIL-4. Purified CD11c+ cells were treated with MAX for 3 hrs prior to LPS stimulation, which lasted 33 hrs. Expression levels of costimulatory molecules were determined by flow cytometry using PE-conjugated anti-CD80, -CD86, and -Class II. Data are expressed as the average geometric mean fluorescence intensity (M.F.I) ±SEM of triplicate samples. MAX-treated DCs resulted in a dose-dependent decrease in CD80 expression on both BALB/c (A) and C3H (B) BM-DCs. Conversely, MAX treatment showed a dose-dependent increase in average CD86 expression on both resting and LPS-stimulated BALB/c (C) and C3H (D) BM-DCs. MHC class II expression, though significantly increased on cells treated with LPS, did not show extensive variance due to MAX treatment from either BALB/c (E) or C3H (F). Significant differences in M.F.I. between paired resting or LPS-stimulated DCs are indicated in the figure (comparing MAX-treated cells to untreated cells) as follows: *, P <0.001; §, P<0.01; ‡, P <0.05.
To determine a direct relationship between altered costimulatory expression and MAX treatment, we examined expression using graded doses of MAX. Figure 2 shows that the extent of altered costimulatory molecule expression on CD11c+ BM-DCs directly correlates to the concentration of MAX used in vitro. BM-DCs were pre-treated with vehicle, 2, 6, or 10 ng/ml MAX for 3 hrs prior to stimulation with 50 ng/ml LPS for 30 hrs. Figure 2 panels A and B show down regulation of CD80 with increasing concentrations of MAX on LPS-activated DCs from BALB/c (panel A) and C3H (panel B). Treatment with 10 ng/ml of MAX resulted in a 50% down-regulation of CD80 on both BALB/c and C3H LPS-stimulated BM-DCs. Conversely, MAX treatment of DCs resulted in a dose-dependent increase in overall average surface expression of CD86 in LPS-treated DCs that is due to the considerable intensity of CD86 up-regulation in a subset of DCs from either strain. (Figure 2, panels C and D, respectively). Treatment of resting DCs (Figure 2, light striped bars) with increasing concentrations of MAX marginally affected expression of these molecules. However, treatment of resting DCs with MAX showed an increase in CD86 expression in all CD11c+ cells. Similar results were obtained from DCs isolated ex vivo from spleen. (Data not shown.) Furthermore, we determined that the MAX effect is specific since antisera from MAX-immunized mice blocked the effect of MAX. In addition, treating DCs prior to MAX with the PACAP receptor antagonist PACAP6–38 completely abrogated these effects (Data not shown.) These data suggest that MAX modifies the costimulatory landscape of DCs in vitro, resulting in a phenotype that may influence the outcome of an ensuing immune response against parasitic infection.
MHC class II expression on resting and LPS-stimulated DCs is shown in Figure 2 panels E. and F. Though LPS-stimulated DCs from both strains showed significantly higher MHC class II expression than resting DCs as expected, MAX treatment induced no consistent variation regardless of the concentration used. The invariant MHC class II level from both the Lm-resistant and -susceptible strains indicates that MAX-treated DCs likely remain capable of delivering an antigen-specific signal to T cells. This result suggests that any MAX-dependent promotion of a Leishmania-susceptible rather than -resistant response is not dependent on variant MHC class II expression.
MAX treatment of either BALB/c- or C3H-derived DCs alters intrinsic cytokine secretion and generates a profile characteristic of a type 2 response
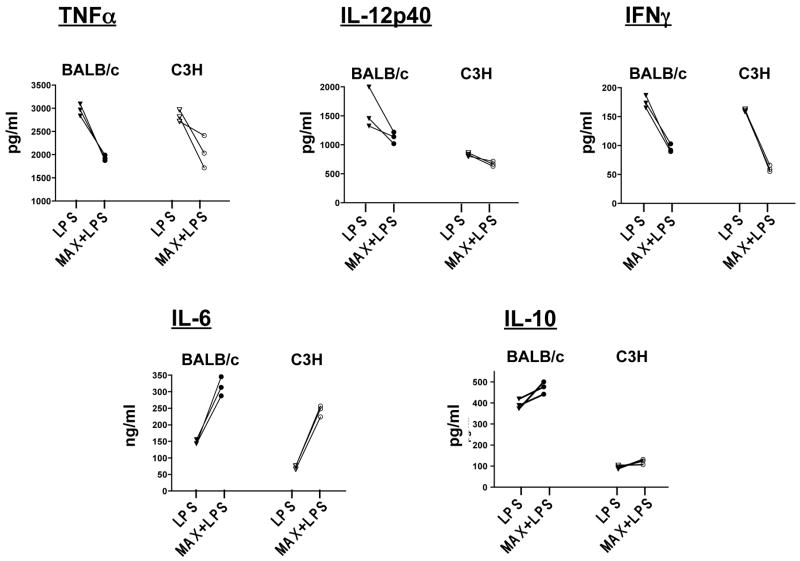
Figure 3 illustrates the trends in DC-specific cytokine secretion as a result of MAX treatment. MAX treatment resulted in decreased secretion of all three type 1 cytokines TNFα (P<0.001), IL-12p40 (P<0.05) and IFNγ (P<0.001) analyzed from LPS-stimulated DCs from both strains. Conversely, MAX treatment resulted in considerable increases of the type 2 cytokines IL-6 (P<0.001) and IL-10 (P<0.05) from both strains. Taken together, these data strongly suggest that MAX signals a redirection of cytokine secretion by activated DCs toward a type 2 response.
Figure 3.
MAX-treatment of BM-DC from either BALB/c or C3H mice alters intrinsic DC cytokine production by causing a decreased secretion of the type 1-associated cytokines (TNFα, IL-12p40 and INFγ) while serving to increase secretion of the type 2-associated cytokines (IL-6 and IL-10). BM-DCs were either untreated or treated with 10 ng/ml MAX for 3 hrs followed by maturation with 300 ng/ml LPS for 30 hrs. Culture supernatants were analyzed for TNFα, IL-12p40, INFγ, IL-10 and IL-6 using the SearchLight™ Multiplex Sample Testing service from Pierce Biotechnology. All differences between paired values of LPS vs. LPS+MAX were significant ( P<0.001) for all except secretion of IL-12p40 and IL-10 for C3H DCs (P<0.05).
Table 1 outlines the dose effect of MAX on cytokine secretion by DCs in culture. CD11c+ BM-DCs from BALB/c and C3H mice were either pretreated with 6 ng/ml or 10 ng/ml MAX for 3 hrs followed by 30 hrs of incubation with or without 300 ng/ml LPS. Additionally, untreated controls lacked MAX or LPS. Secretion of type 1 cytokines (IL-12p40, TNFα and IFNγ) and type 2 cytokines (IL-6 and IL-10) was assayed from culture supernatants and subsequently quantified using a Multiplex™ analysis service provided by Pierce Biotechnology, Inc. Significant differences in cytokine secretion between control and MAX-treated cultures were observed only when DCs were LPS-stimulated (Table 1), suggesting that MAX does not profoundly alter resting DC cytokine secretion.
Table 1.
Multiplex analysis: BALB/c C3H/HeN
| Cytokine analyzed from treated CD11c+ cells | MAX ng/ml | LPS ng/ml | % decrease(↓) or increase (↑) as compared to controls not treated with MAX | P-value | % decrease(↓) or increase(↑) as compared to controls not treated with MAX | P-value |
|---|---|---|---|---|---|---|
| IL-6 | 0
6 0 6 10 |
0
“ 300 “ “ |
NA†
NS‡ NA 47%↑ 114%↑ |
<0.001 <0.001 |
NA
NS NA 72.8%↑ 235.67%↑ |
NA
<0.001 <0.001 |
| IL-10 | 0
6 0 6 10 |
0
“ 300 “ “ |
NA
NS NA 9.8%↑ 20.43%↑ |
<0.05 <0.05 |
NA
NS NA 18.2%↑ 27.65%↑ |
NA
<0.05 <0.05 |
| IL-12p40 | 0
6 0 6 10 |
0
“ 300 “ “ |
NA
NS NA 22.5%↓ 29.21%↓ |
<0.01 <0.01 |
NA
NS NA 15.45%↓ 19.52%↓ |
NA
<0.05 <0.01 |
| TNFα | 0
6 0 6 10 |
0
“ 300 “ “ |
NA
NS NA 17.1%↓ 34.85%↓ |
<0.05 <0.002 |
NA
NS NA 19.1%↓ 27.45%↓ |
NA
<0.05 <0.01 |
| IFNγ | 0
6 0 6 10 |
0
“ 300 “ “ |
NA
NS NA 21.6%↓ 45.76%↓ |
<0.001 <0.001 |
NA
NS NA 21.55%↓ 62.67%↓ |
NA
<0.001 <0.001 |
Not applicable, marks the sample that percent changes were based upon: MAX-treated cells were compared to untreated cells and LPS and MAX treated cells were compared to LPS alone treated cells.
No significant change
MAX induces differential DC-mediated proliferation of allo-CD4+ T cells
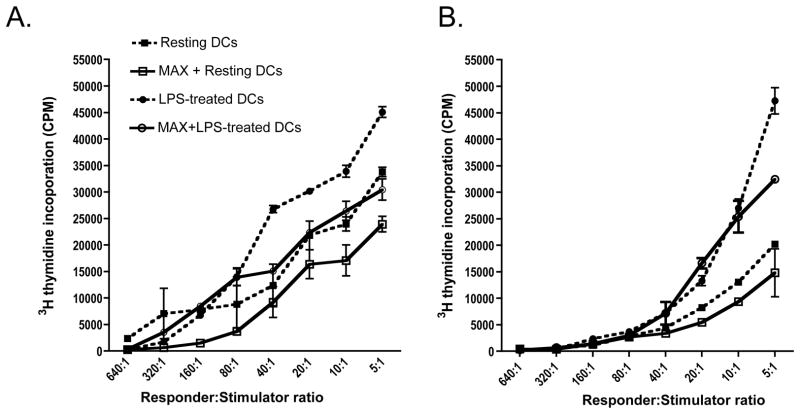
To ascertain whether the observed differential CD80/CD86 expression has functional consequences, we examined the effect of MAX on the ability of BM-DCs to stimulate proliferation of allogeneic CD4+ T cells. BM-DCs from BALB/c (H-2d) or C3H (H-2k) mice were utilized as stimulators for naïve C57BL/6 (H-2b) CD4+ T cell responders. DCs and T cells were co-cultured at responder:stimulator ratios ranging from 5:1 to 640:1 for 48 hrs followed by pulse labeling with 1 μCi [3H]-thymidine for an additional 16 hrs and proliferative activity was determined by radioactive incorporation into responder T cells. DCs activated with 50 ng/ml LPS for 36 hrs prior to MLR assembly induced significantly (P<0.05) increased proliferation when compared to immature (non-LPS treated) DCs (Figure 4, panel A and B: compare solid and open circles with solid and open squares). MAX treatment of BM-DCs from either BALB/c (Figure 4, panel A) or C3H (Figure 4, panel B) mice maintained substantial allogeneic T cell proliferative responses; however, we observed an overall modest yet repeatable and significant (P<0.01) reduction in the response that directly correlated to the concentration of MAX (0, 2, 6 or 10 ng/ml) (Data not shown.). MAX-treatment of DCs with or without LPS activation resulted in an abated proliferative response compared to untreated cells (Figure 4, compare solid lines to dashed lines). These results suggest that MAX-treated DCs remain capable of stimulating allogeneic CD4+ T cell proliferation but at a diminished level. As controls, T cells and DCs were each cultured alone. MAX treatment of responder T cells alone failed to show any significant proliferative response, suggesting that MAX does not affect intrinsic naïve T cell proliferation. Treatment of DCs alone with MAX showed no proliferation as well, which was expected since these cells were irradiated prior to culture (Data not shown).
Figure 4.
MAX-treated BM-DCs from either BALB/c or C3H mice induce proliferation of naïve allogeneic CD4+ T but requires greater stimulator input to achieve comparable proliferation as untreated controls. The proliferative activity of naïve allogeneic CD4+ T cells induced by either BALB/c (A) or C3H (B) BM-DCs was quantified by measuring [3H]-thymidine incorporation. BM-DCs were either untreated (dashed lines) or treated with 10 ng/ml MAX (solid lines) and left resting (squares) or activated with 50 ng/ml LPS (circles) for 36 hrs and used as stimulators. Prior to MLR culturing, DCs were irradiated using a 137Cs source (34 Gy). Graded amounts of DCs (starting with 1×105/well) from either BALB/c mice (A) or C3H mice (B) were incubated with 5×105 naïve CD4+ T cells from spleens of C56BL/6 mice. Culture wells were pulsed with 1μCi of [3H]-thymidine following 48 hrs of incubation and harvested 18 hrs afterwards. Differences were significant with P<0.01.
In order to determine whether the observed proliferative responses were a result of DC-dependent modulation rather than MAX directly acting on stimulated T cells, we examined various cultural conditions. In cultures of T cells supplemented with IL-2 and PHA, comparable levels of proliferation were observed with or without MAX (Data not shown). Additionally, similar proliferative results were obtained regardless of whether DCs were treated with MAX prior to MLR assembly or MAX was present throughout the duration of culture (Data not shown). In cases where MAX was absent throughout the MLR culture period, MAX-treated DCs were washed extensively to ensure that residual levels were removed. These data suggest the varying proliferative responses observed in the presence of MAX were the result of a modulation of T cell proliferation by MAX-treated DCs as opposed to a direct effect by MAX on T cells. This further suggests that the reduced proliferative response is due to altered abilities of DCs to stimulate T cell subsets rather than a toxic effect of MAX on the MLR cultures that may abrogate the ability of the T cells per se to proliferate. We also measured the ability of MAX-treated DCs to phagocytose FITC-labeled dextran beads and observed no alteration in this function regardless of the amount of MAX used in the analysis (Data not shown; WHW and KEP unpublished observation).
Cytokine secretion is altered in cultures of MAX-treated, LPS-stimulated BM-DCs and allogeneic (C56BL/6) CD4+ T cells
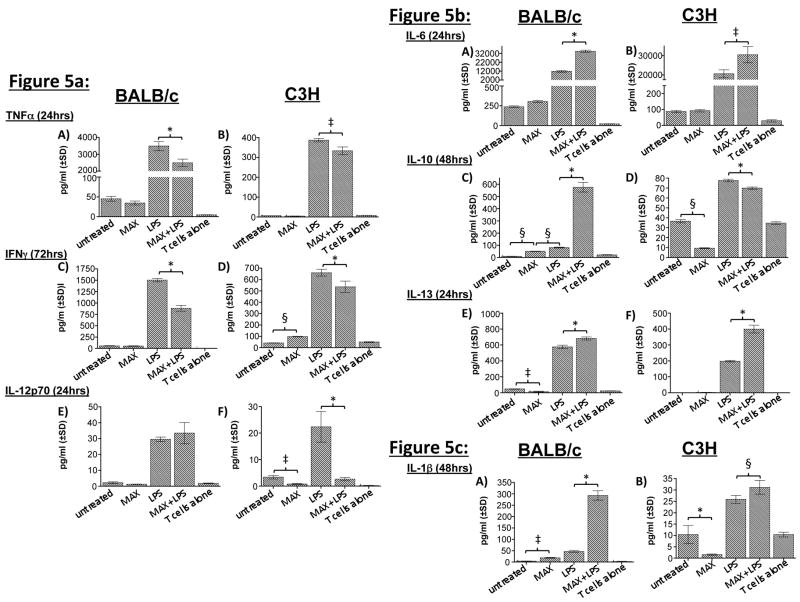
In order to determine whether MAX-treated DCs modulate cytokine secretion by allogeneic CD4+ T cells, we assayed the supernatants from MLR cultures for cytokines using the same analysis method employed in Figure 3 and Table 1. DCs were pretreated with 10 ng/ml MAX for 3 hrs followed by treatment with 100 ng/ml LPS for 24 hrs prior to MLR assembly. DCs were placed in culture with responder C57BL/6 CD4+ T cells at a 1:5 stimulator:responder ratio. Culture supernatants were assayed for: a) type 1 cytokines- TNFα, IFNγ and IL-12p70 (Figure 5a); b) type 2 cytokines- IL-6, IL-10 and IL-13 (Figure 5b) and c) type 1 and 2 cytokine IL-1β (Figure 5c). Cytokine secretion profiles were determined by harvesting triplicate culture wells after 24, 48 or 72 hrs of culturing. Analysis in Figure 5 was determined using the data from the peak time of secretion, which is indicated in parentheses. Figure 5a–c compares the patterns of cytokine secretion as a result of MAX treatment. Each cytokine assayed in Figure 5 is described in the following sections:
Figure 5.
MAX treatment of BM-DCs (10 ng/ml) from both BALB/c and C3H mice reprograms the cytokine secretion profiles in cultures containing allogeneic T cells. BM-DCs (1×106) either untreated, MAX-treated, LPS stimulated (300ng/ml) or treated with MAX+LPS from either BALB/c or C3H mice were cultured with 5×106 naïve responder T cells from C57BL/6 mice (1:5, stimulator-responder ratio). Generally, MAX treatment results in decreases in type 1 (TNFα, IFNγ, and IL-12p70) cytokine secretion while type 2 (IL-6, IL-10, and IL-13) secretion increases. There are, however, some strain-dependent effects. Additionally, IL-1β levels increase in both MLR cultures when MAX is present. a) Analysis of type 1-associated cytokines: TNFα, IFNγ, and IL-12p70. b) Analysis of type 2-associated cytokines: IL-6, IL-10 and IL-13. c) Analysis of IL-1β, which is neither strictly type 1 or 2. Times indicated in parentheses denote times of peak secretion. The concentration of secreted cytokines was determined by analysis of culture supernatants using the multiplex SearchLight™ analysis service from Pierce Biotechnology, Inc. Significant differences in cytokine secretion between DCs are indicated in the figure (comparing MAX-treated cells to untreated cells) as follows: *, P <0.001; §, P<0.01; ‡, P <0.05.
TNFα
TNFα is a type 1 inflammatory cytokine that is a member of a group of molecules that stimulate the acute-phase reaction. TNFα secretion has been strongly correlated with increased NO production in mouse macrophages, promoting killing of intracellular Lm (29). Figure 5a, panel A and B illustrates TNFα secretion profiles. MAX treatment of DCs from either strain without prior LPS stimulation failed to show a significant difference in TNFα secretion (P>0.05). After 24 hrs of culture, MAX-treated, LPS-stimulated DCs demonstrated a significant (P<0.001; BALB/c and P<0.05; C3H) decrease in TNFα secretion in MLR cultures when compared to DCs treated with LPS but without MAX. (Figure 5a, panel A and B). Overall TNFα secretion in BALB/c MLR cultures was 10-fold higher than that observed in the C3H (Figure 5a, panel A and B compare ordinates). Notably, MAX treatment resulted in significant TNFα reduction in DCs derived from either strain after 24 hrs.
IFNγ
DCs activated by IFNγ promote Th1 differentiation by up-regulating the transcription factor T-bet, the hallmark cytokine of Th1 cells. Extensive investigation has revealed that IFNγ release by T cells promotes the development of protective immunity against Lm (6). In 72 hrs post-LPS stimulation, MAX treatment of LPS-activated DCs from both strains resulted in a significant (P<0.001) reduction in IFNγ secretion when compared to LPS-treated cultures without MAX (Figure 5a, panels C and D). These results are suggestive of a diminishment of an important component of type 1 immunity.
IL-12p70
IL-12 is involved in the differentiation of naïve T cells into Th1 cells and is an important element in determining the balance of Th1 vs. Th2 immunity against leishmaniasis. Analysis of IL-12p70 release revealed strain-specific responses to MAX (Figure 5a, panels E and F). In the Lm-susceptible BALB/c strain we did not detect any significant differences in IL-12p70 secretion between MAX-treated or untreated LPS-stimulated DCs at 24 hrs. (Figure 5a, panel E) At later times, we detected a notable (4-fold) increase in release at 48 hrs, followed by a large decrease at 72 hrs (Data not shown.). In contrast, MAX treatment of DCs from the resistant C3H strain showed a significant (P<0.001) decrease in IL-12p70 secretion by LPS-activated DCs in MLR cultures at all three time points relative to LPS-stimulated controls (Figure 5a, panel F and data not shown). It is interesting to note that, unlike the inhibition of IL-12p40 secretion observed for BALB/c DCs (Figure 3), we did not observe this result in MLR cultures (Figure 5, panel E).
IL-6
Compared to other cytokines measured in the MLR supernatants, IL-6 was secreted in the greatest amount (Figure 5b, panel A and B) and was significantly (P<0.001 for BALB/c and P<0.05 for C3H) increased by MAX treatment of the DCs. Prominent secretion of IL-6 is thought to be counter to the type 1 response necessary to resolve leishmanial infection: IL-6 has been demonstrated to suppress TNFα activation in murine macrophages necessary for killing Leishmania amazonensis (30). Here we show that MLR culture supernatants containing LPS-treated DC stimulators derived from either strain secreted abundant amounts of IL-6. Levels are significantly (P<0.05) elevated when DCs from either strain are treated with MAX prior to LPS treatment (Figure 5b, panel A and B). This MAX-dependent increase in IL-6 secretion is suggestive of the elicitation of a mechanism that may counter the program of type 1 immunity, favoring a type 2 response.
IL-10
IL-10 has been associated with decreases in IL-12 and TNFα production, subsequently promoting the progression of leishmaniasis in BALB/c mice (31). It also has pleiotropic effects in regulating inflammation and functions to down-regulate expression of Th1 cytokines (32). Here we show that maximum IL-10 secretion by DCs in both strains occurs within 48 hrs after LPS treatment (Figure 5b, panels C and D). However, in MLR cultures, only BALB/c-derived DC stimulators showed any significant (P<0.001) increase in IL-10 secretion as a result of MAX treatment (Figure 5b, panel C). Instead, MAX+LPS-treated DCs from C3H mice showed a considerable (P<0.001) decrease in IL-10 compared to non-MAX treated cells (Figure 5b, panel D). This is in contrast to the IL-10 secretion profile observed in Figure 3 where secretion is assessed from DCs alone in which MAX-treatment resulted in increased secretion from both strains. Thus, these data are suggestive of a strain-dependent IL-10 response to MAX by DCs interacting with allogeneic CD4+ T cells.
IL-13
Recent data have shown (33) that lesions of localized cutaneous leishmaniasis express high levels of IL-13 and patients with visceral leishmaniasis have high levels of IL-13 in their serum. A possible regulatory role for IL-13 has been investigated in a series of experiments that has yielded data that supports the hypothesis that IL-13 is a significant susceptibility factor for Lm infection (34) (35) (36) (37) (38). Figure 5b panels E and F show that IL-13 secretion is significantly (P<0.001) increased in LPS-stimulated DCs treated with MAX from both BALB/c and C3H. These results suggest that MAX may promote a susceptibility scenario enabling parasites to initiate and establish a successful infection.
IL-1β
Work has shown that cells exposed to IL-1β following L. amazonensis infection accelerated helper T cell activation and disease progression (39). Additionally, treatment of Lm-infected mice with anti-IL-1 receptor antibodies inhibited the development of the cutaneous leishmaniasis lesion without affecting the number of parasites in the lesion (40). Figure 5c; Panels A and B show that MAX treatment of LPS-stimulated DCs from either strain results in a significant (P<0.001 for BALB/c and P<0.01 for C3H) increase in IL-1β secretion in MLR cultures containing DCs from either strain. Such alteration of IL-1β may prove to be an important immunomodulatory component of MAX signaling.
MAX treatment of LPS-stimulated DCs results in persistence of maximal secretion of IL-6, IL-13 and IL-1β over the course of 72 hours
Secretion of cytokines from the MLR cultures was monitored over a 72 hr period. In order to determine whether MAX affects the kinetics of cytokine secretion from MLR supernatants, analysis was performed after 24, 48 and 72 hrs of culturing. MAX treatment of DCs from either BALB/c or C3H mice resulted not only in overall increases in MLR cytokine secretion for IL-6, IL-13 and IL-1β but these maximal levels of secretion were sustained over the assay period (Figure 6, panels A, B and C). For IL-6, secretion by LPS-stimulated DCs that were not treated with MAX peaked 24 hrs after culturing followed by a steady decline by 48 hrs. MAX treatment resulted in not only a greater IL-6 secretion at 24 hrs but also in maintenance of peak levels of secretion for up to 72 hrs (Figure 6, panel A). IL-13 secretion by non-MAX treated DCs peaked at 24hrs followed by as rapid decline in 48 hrs for BALB/c DCs and a slow decline from comparatively lower levels for C3H DCs (Figure 6, panel B). MAX-treated DCs either sustained their elevated levels of IL-13 secretion throughout the experiment (C3H DCs) or continued to increase throughout the time course of the experiment (BALB/c DCs). When compared to untreated DCs, IL-13 levels increased from >15% to >52% (at 24 and 72 hrs, respectively) for BALB/c and, for C3H, was increased by >51% at 24 hrs followed by as sustained increase by >34% by 72 hrs. In mature non-MAX treated DCs from either strain, IL-1β secretion peaked between 24 and 48 hrs. By comparison, MAX treatment of DCs resulted in a significant (P<0.005) increase in secretion at 24hrs followed by a steady increase over time: From 24 to 72 hrs in culture, IL-1β increased by >18% to >49% in BALB/c DCs and by 22% to >39% in C3H DCs. These results suggest that cellular signaling via MAX functions to sustain the secretion of three cytokines that normally peaks between 24 and 48 hrs and wanes in times thereafter. Respectively, IL-6, IL-13 and IL-1β are involved in a) initiation and maintenance of Th2 immunity, b) increased susceptibility to Leishmania and c) the acceleration and amplification of the resulting Th2 immunity. The sustained time of maximal cytokine secretion may prove to be an important component in Leishmania susceptibility.
Figure 6.
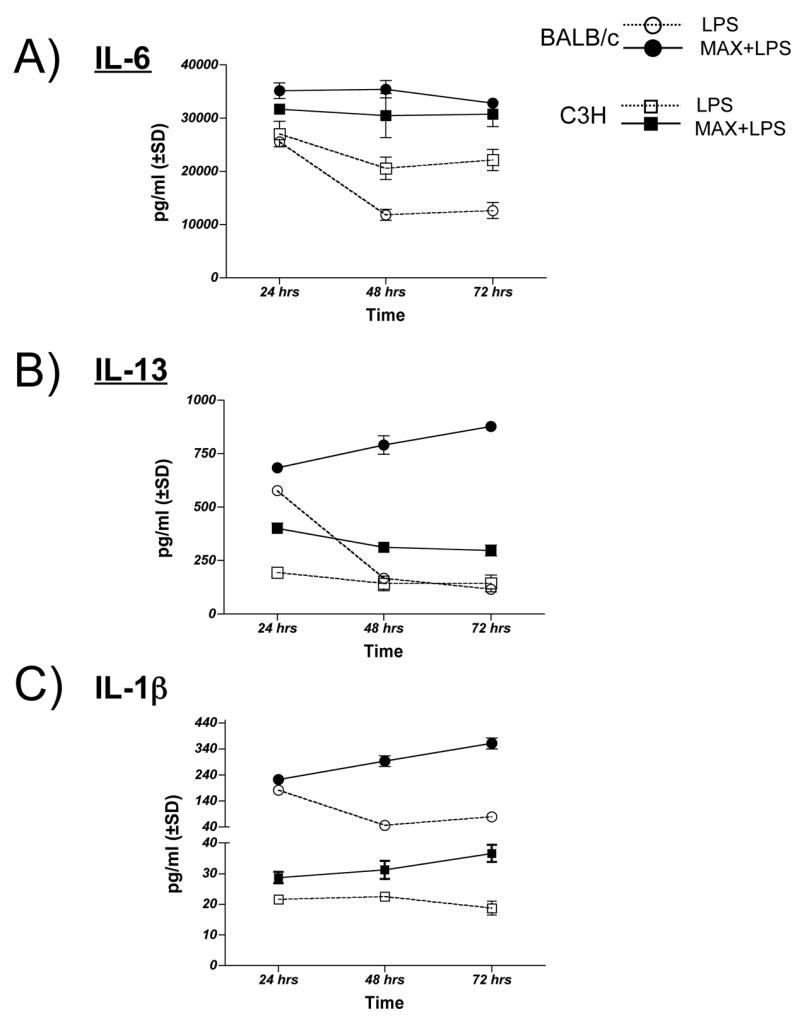
MAX treatment of BALB/c and C3H BM-DCs results in persistence of elevated levels of IL-6, IL-13 and IL-1β secretion up to 72 hrs in MLR cultures. BM-DCs (1×106) either LPS stimulated (300ng/ml) (open symbols) or treated with 10 ng/ml MAX+LPS (closed symbols) from either BALB/c (circles) or C3H (squares) mice were cultured for 24, 48 and 72 hrs with 5×106 naïve responder T cells from C57BL/6 mice. The concentration of secreted cytokines was determined by analysis of culture supernatants using the multiplex SearchLight™ analysis service from Pierce Biotechnology. P values were all <0.005 when comparing paired LPS and MAX+LPS results.
MAX treatment of DCs abrogates LPS-mediated up-regulation of CCR7
The results presented above indicate that optimal DC alteration of CD80/86 expression occurs between 12 and 30 hrs post-MAX treatment and LPS stimulation (KEP/WHW unpublished observation). Such kinetics suggest that DCs be exposed to MAX for a considerable length of time to facilitate a change in phenotype capable of conveying an alternative signal to T cells in draining lymph nodes. Typically, maturing peripheral DCs are able to migrate and arrive to their cognate draining lymph node in as little as 2 hrs post-antigen exposure (41). It is likely, during this time, that a considerable number of DCs emigrating into draining lymph nodes would have yet to manifest the full MAX effect. Considering the relatively long period of time required for MAX to facilitate a full and effective DC conversion, an additional function of MAX may be to impede or slow the ability of the DCs to migrate. Such slowing of migration would allow for a requisite time delay ensuring that migrating DCs have undergone optimal conversion due to prolonged exposure to MAX. We examined whether MAX modulates the expression of CCR7 because its expression is required for efficient DC migration from the site of antigen exposure to draining lymph nodes. Maturing DCs express high densities of CCR7 and down-regulate CCR1 and CCR5, facilitating migration to regional lymph nodes that constitutively and abundantly express the CCR7 ligands CCL19 and CCL21 (42) (43) (44) (45). In addition to delaying migration to ensure an optimal MAX effect, suppression of DC migration by salivary components would likely affect the immunological course of thwarting Lm infection. With this in mind, we sought to determine whether MAX negatively affects CCR7 expression on DCs. The combination of MAX and LPS treatments resulted in a 50% reduction of CCR7 expression on BALB/c DCs compared to cells stimulated with LPS alone (Figure 7). MAX treatment of LPS-stimulated C3H DCs resulted in a striking 65% down-regulation of CCR7. Interestingly, there were no considerable differences in CCR7 levels between control (immature DCs), MAX and MAX + LPS-treated C3H DCs (Data not shown). These results can be directly attributed to MAX considering that DCs treated with MAX that was pretreated with an anti-MAX antiserum resulted in proper up-regulation of CCR7 by LPS treatment (Data not shown). These data suggest that MAX has considerable effects on DC migratory capacity and likely serves to affect their immunologic competence by delaying their migration to regional lymph nodes and allowing the necessary time for MAX-mediated reprogramming, which may be a contributing factor in augmenting the disease process.
Figure 7.
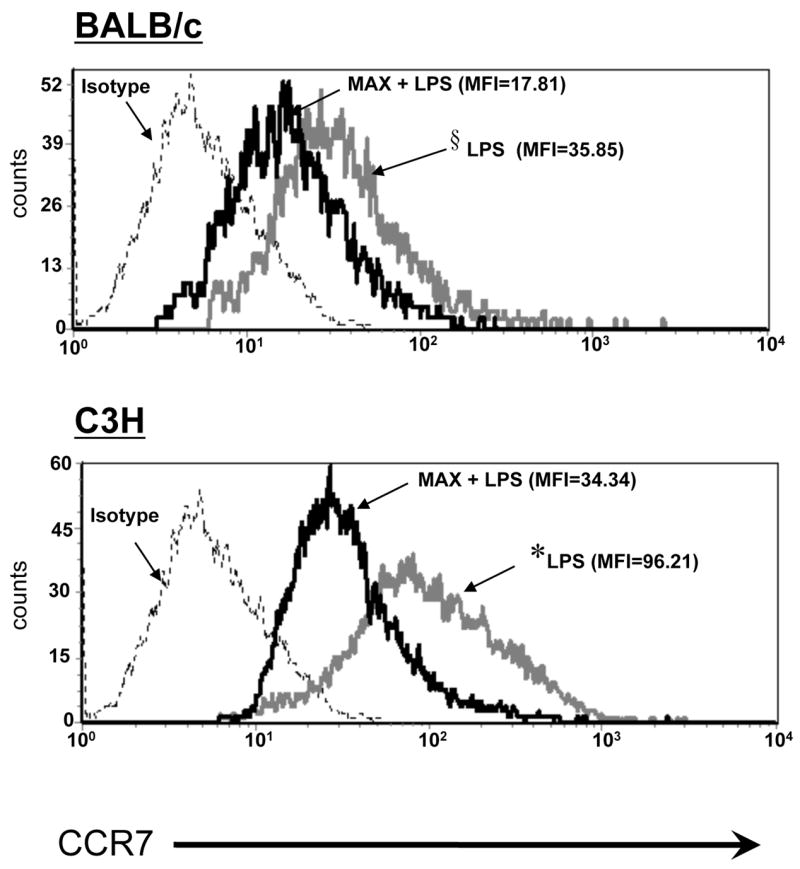
MAX prevents optimal LPS-mediated up-regulation of CCR7 on BM-DCs. BM progenitors from both BALB/c and C3H mice were cultured in GM-CSF and IL-4 for 9 days and CD11c+ cells were purified by magnetic bead separation. Cells were untreated (gray line) or treated (black line) with 6 ng/ml MAX for 3 hrs at 37°C prior to being treated with LPS at 100 ng/ml. LPS treatment was carried out for 30 hrs and DCs were harvested and labeled with PE-conjugated anti-CCR7 followed by analysis by flow cytometry. Histograms show the M.F.I. of CCR7+ (PE-positive) cells. P values are shown as: *, P <0.001; §, P<0.01.
Discussion
In this work we show that treating splenic- or BM-derived CD11c+ DCs with MAX resulted in considerable remodeling of cell surface expression of CD80 and CD86, but did not affect class II (I-A/I-E) expression (Figures 1 and 2). This is an important observation since it demonstrates that MAX is not affecting DCs simply because it is directly toxic for the cells. This observation agrees with a previous report in which we showed that sand fly saliva/MAX also did not affect MHC class II expression on macrophages (18). This effect of MAX on DC expression of CD80/86 was also shown to vary directly with the dose of MAX in DC cultures (Figure 2) when DCs from either BALB/c or C3H mice were used. The overall increase in the average CD86 expression was due to increased levels of the molecule on a subpopulation of DCs from both BALB/c and C3H mice (compare Figures 1 and 2). We have recently determined that this subset of DCs is primarily CD8−CD4− (Data not shown, KEP and WHW manuscript in preparation).
It has been suggested that the relative expression of CD80 and CD86 is an important determining factor for eliciting a type 1 vs. type 2 response (46) (47). Moreover, blockade of CD86 ameliorates Lm infection and down-regulates Th2 responses, allowing more CD80 engagement with CD28 on T cells, which leads to the activation of type 1 immunity (46) (48) (49). Therefore, it is possible that MAX exacerbates infection with Lm by phenotypically altering dermal DCs at the site of infection, allowing type 2 immunity to develop, in part, via preferential stimulation through CD86 rather than CD80.
Delgado et al. have shown results in line with those presented here using mammalian neuropeptides such as vasoactive intestinal peptide (VIP) and PACAP, which act through the same receptor system (VPAC1, VPAC2 and PAC1) as MAX (PAC1). These authors found that VIP/PACAP can induce the development of tolerogenic DCs (50). In addition, DCs treated with VIP or PACAP were CD11clowCD45RBhigh, failed to up-regulate CD80, CD86 and CD40 following LPS stimulation, and secreted high levels of IL-10. In earlier work this same group also showed that immature DCs treated with VIP/PACAP up-regulated CD86 expression enabling them to stimulate T cell proliferation and differentiation to Th2 effectors in vivo and in vitro (51). Additionally, they found that VIP/PACAP down-regulates both CD80 and CD86 expression on LPS-stimulated DCs. Delgado et al. were unable to detect PAC1 mRNA, a specific receptor for MAX, in BM-DCs by conventional PCR analysis and showed little effect of their maxadilan preparation on CD80/86 expression on these cells. There are several scenarios that may serve to explain the apparent discrepancy between our work and these authors. First, PAC1 is expressed in various isoforms, (fourteen, in all) which are represented by alternate mRNA splicing. (52) The distribution of the different isoforms is dependent on tissue type. Thus, an inability to detect PAC1 in BM-DCs via PCR may due to the presence of an isoform(s) present in these cells undetectable with one set of PCR primers. Second, Ushiyama et al. have shown that variants of PAC1 affect both the binding affinity of the ligands and the subsequent intracellular signaling downstream (53). Third, there is considerable variability in potency of MAX depending on the population of Lu. longipalpis from which it is obtained. Lanzaro et al. have found extensive amino acid variation (up to 23%) in MAX among different populations of Lu. longipalpis (28). Therefore, the inability to detect a response from a given preparation of MAX may be the result of a particular preparation having limited potency and/or potential to interact with and signal through PAC1 isoforms specific to BM-DCs. Importantly, we have recently shown that PAC1 is expressed on the surface of BM-DCs from both mouse strains albeit at low levels and not uniformly (Data not shown.) This low level of recognition may reflect the expression of various PAC1 isoforms on different DC subpopulations.
Since MAX modulated the expression of CD80/86 on DCs in a manner that would favor the development of type 2 immunity, we also assessed the effect of MAX on the production of cytokines by DCs to determine whether MAX promoted secretion of cytokines that would also lead to the development of type 2 immunity. Using DCs from either BALB/c or C3H mice, we found that MAX blocked secretion of the type 1 cytokines TNFα, IL-12p40 and IFNγ while stimulating the production of the type 2 cytokines IL-6 and IL-10 (Figure 3). We also show in Table 1 that these changes in cytokine secretion responded accordingly to variations in dosage of MAX yet, in the absence of LPS stimulation, we observed no significant changes. Such a switch in cytokine secretion may further enhance the ability of MAX-treated DCs to activate type 2 immunity, which would not protect against infection with Lm.
One of the most striking effects seen in Figure 3 is the ability of MAX to enhance secretion of IL-6. We have previously observed this effect of MAX on both human PBMCs (54) and mouse macrophages (24). IL-6 has been shown to inhibit the production of IFNγ from T cell cultures (30) and TNFα from mouse macrophages (24) and these cytokines are essential for killing Lm and L. amazonensis (55). It has also been shown that IL-6 induces a dose- and time-dependent suppression of cytokines required for the killing of Leishmania parasites by human macrophages (30). Additionally, IL-6 plays a central role in the final differentiation of B-cells into Ig-secreting cells, as well as inducing myeloma/plasmacytoma growth (56). These functions are directly opposed to the type 1 immunity required for optimal resolution of infection with Lm.
To determine whether the effect that MAX has on DC functions alters the ability of those DCs to activate T cells, we chose the MLR for our analysis. The MLR represents one of the most powerful DC-induced T cell activation methods known since it is driven by allogeneic differences between DCs and responding T cells and thus is a polyclonal reaction. As such, the MLR provides one of the most stringent tests of whether MAX affects the ability of DCs to activate T cells. We found that MAX-treated DCs were consistently less capable of inducing T cell proliferation (Figure 4), which demonstrates that MAX can alter the ability of DCs to activate T cells.
In addition to the effects of MAX on T cell proliferation, the cytokines released in the MLR cultures were altered (Figures 5 and 6). Typically, in MLR cultures, the type 1 reaction predominates (57); thus, the ability of MAX to shift the cytokine profile to type 2 is considered remarkable. MAX-treated DCs inhibited TNFα and IFNγ production, but stimulated IL-6, IL-13 and IL-1β in MLR cultures that utilized CD4+ T cells from C57BL/6 mice as responders. The results with TNFα, IFNγ and IL-6 are similar to the results we obtained with cultures of DCs alone (Figure 3). However, in MLR cultures, the effects of MAX on IL-10 and IL-12p70 secretion differed depending upon whether the stimulators (i.e. DCs) were from BALB/c or C3H mice, respectively. In the BALB/c MLR (Figure 5a), IL-10 production was dramatically enhanced, but this was not the case in the C3H MLR. On the other hand, in the C3H MLR (Figure 5b), IL-12 production was dramatically inhibited, but in the BALB/c MLR it was not. This suggests that in this more complex MLR assay system (which contains both DCs and T cells) that the ability of MAX to skew the immune response to type 2 immunity is more dependent upon increased IL-10 production in BALB/c cultures, but it was more dependent upon inhibition of IL-12 production in C3H cultures. Alternatively, the different genetic backgrounds of BALB/c and C3H mice may have also influenced the effects that MAX has on cytokine production.
MAX upregulated IL-1β secretion in both BALB/c and C3H MLR cultures (Figure 5c). This is likely important since recent work has shown that IL-1β enhances Th2 cell activation in C57BL/6 mice infected with L. amazonensis, and we have previously shown that blockade of IL-1 receptors by a monoclonal antibody in an Lm-infected mouse inhibited the development of cutaneous lesions, indicative of the important role of IL-1 in disease pathology (39) (40).
MAX also induced significantly higher levels of IL-13 in MLR cultures, which utilized either BALB/c or C3H stimulator DCs (Figure 5b). This is significant since IL-13 has been shown to rapidly inhibit the production of IL-12 by macrophages and to inhibit Lm parasite killing in vitro (35) (36). Furthermore, work has shown that IL-13 is a significant susceptibility factor for Lm infection in mice: Over-expression of IL-13 in transgenic C57BL/6 (Lm-resistant) mice results in susceptibility to infection with Lm, and, more significantly, this is independent of IL-4 expression. Moreover, it has been shown that BALB/c mice deficient in IL-13 can be resistant to Lm (35).
Moreover, we observed in Figure 6 that MAX not only increases secretion of IL-6, IL-13 and IL-1β but levels are maintained or continue to rise through at least 72 hrs of MLR culture. This is significant since it demonstrates a powerful and dominant effect that MAX has on altering these cytokine secretion profiles suggestive of a critical role for these cytokines in Leishmania infection permissiveness and disease pathogenesis.
Finally, we observed that MAX treatment of BALB/c and C3H DCs resulted in failure to fully up-regulate CCR7 expression in response to LPS stimulation (Figure 7). CCR7 expression is important for DC mobility, which allows the cells to migrate to draining lymph nodes where they stimulate the development of adaptive immunity. The effect of MAX on CCR7 expression is significant since a reagent capable of altering this crucial DC function would be important in determining the immunopathological response to Lm infection. This delay might also allow additional time to ensure that the MAX-induced DC phenotype is fully developed prior to the interactions of DCs with T cells.
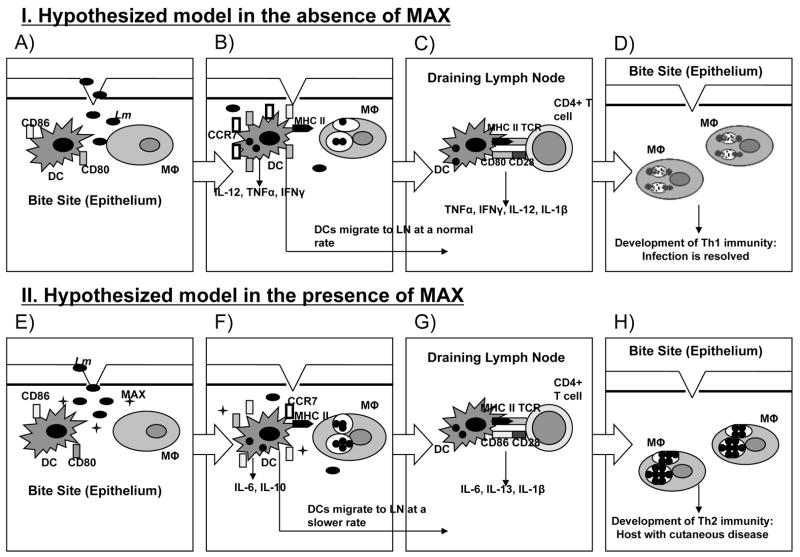
Figure 8 illustrates a possible mechanism through which MAX enhances infection with Lm. The model proposed in this figure outlines how an innately resistant C3H mouse responds to Lm infection in either the absence (Figure 8, group I) or presence (Figure 8, group II) of MAX. Figure 8 group I reflects the course of Lm disease progression in a resistant strain: Lm parasites are inoculated at the bite site (panel A), DCs become activated and up-regulate MHC class II, CD80, CD86, and CCR7 (panel B), permitting migration to draining lymph nodes where DCs promote Th1 differentiation (panel C). Consequently, a type 1 response required to resolve infection is established, and the host develops long-lasting immunity (panel D) (6). Figure 8 group II depicts the hypothesis that MAX, when co-inoculated with parasites, alters the relative expression of CD80 vs. CD86 on activated DCs favoring initiation of type 2 immunity (compare Figure 8 panels B and F). Exposure to MAX also increases secretion of IL-6 and IL-10 while decreasing secretion of TNFα, IL-12 and IFNγ (Figure 8, panel F). It has been demonstrated that TNFα and IL-12 secretion from macrophages is also reduced, which correlates with increased parasite burden (24). Dermal DCs are stalled in the periphery by reduced expression of CCR7, allowing complete expression of the MAX-induced phenotype (Figure 8, panel G). DCs eventually migrate and activate antigen-specific Th2 cells (Figure 8, panel G). The Th2 response is insufficient to clear parasites, allowing them to grow in macrophages, furthering disease pathogenesis. We are currently testing this proposed model. Whether altered expression of costimulatory molecules and modified secretion of cytokines are intrinsically linked remains to be determined and is the focus of further investigation in this laboratory.
Figure 8.
Model summarizing proposed mechanism of MAX-mediated disease exacerbation for the C3H resistant strain in both the absence (group I) and presence (group II) of MAX. Group I: A) Pathogen (Lm) is inoculated at the bite site. B) Activated DCs increase surface expression of MHC class II and CD80 and CD86. DCs secrete IL-12, TNFα, and INFγ. C.) Rapid up-regulation of CCR7 allows efficient migration to the draining lymph node, where DCs preferentially promote Th1 differentiation. Secretion of TNFα, IFNγ, IL-12, and IL-1β initiates a type 1 response. D.) Th1 cells activate macrophages (Mφ) at the site of infection, and parasites are eliminated. Group II: E) MAX in vector saliva is co-inoculated with pathogen (Lm). F) Activated DCs have decreased CD80 with relatively increased CD86 surface expression maintaining normal expression of Class II. Increased IL-6 and IL-10 permits parasite survival in dermal macrophages. G) Suboptimal CCR7 expression slows DC migration, ensuring prolonged exposure to MAX. Altered DCs harboring parasites eventually migrate to draining lymph nodes and signal differentiation of Th2 cells. H) Infection persists due to lack of Th1 immunity. Parasites remain and proliferate within dermal macrophages resulting in cutaneous disease.
An important issue to address when considering the model proposed above is that Lm is naturally transmitted by the Old World sand fly P. papatasi, not Lu. longipalpis. Hence, we are not working with the natural vector. It should first be noted that all results in this work were obtained using LPS, not Lm. In this work we are not strictly mimicking the natural infective process by pairing the appropriate sand fly/parasite combination, rather we are trying to elucidate a mechanism by which MAX affects DCs. Salivary components have been shown to increase parasite infectivity using the following vector/pathogen combinations in vivo: Lu. longipalpis and Lm (2), P. papatasi and Lm (11) (3) (58), Lu. longipalpis and L. donovani chagasi (10), and Lu. longipalpis and L. amazonensis (59). In each case exposure to vector salivary components altered the host immune response, suggesting that these mechanisms are seemingly general and conserved in nature. Consequently further studies are warranted regardless of whether the parasite/vector pair is natural. Though MAX is absent from P. papatasi saliva (the gene is not present), activities that are seemingly related to MAX have been attributed to a variety of molecules (13) (16). We have focused on MAX because it is best characterized, and it is generally beneficial to resolve the mechanistic components on the best-defined system.
Acknowledgments
We thank Terry Potter, Michele Falzone, Erik Arthun, Santiago Majia, Diana Alzate and Amanda Toot for help with experiments, valuable discussions, and with writing and critical review of the manuscript.
Footnotes
This research was supported by National Institutes of Health Grant RO1 AI65784 grant to R.G.T.
References
- 1.Titus RG, Ribeiro JM. The role of vector saliva in transmission of arthropod-borne disease. Parasitol Today. 1990;6:157–160. doi: 10.1016/0169-4758(90)90338-5. [DOI] [PubMed] [Google Scholar]
- 2.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J, Sacks DL. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. International Journal for Parasitology. 2007 doi: 10.1016/j.ijpar.2007.1004.1003. e Journal ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander J, Bryson K. T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunol Lett. 2005;99:17–23. doi: 10.1016/j.imlet.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 7.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 8.Theodos CM, Povinelli L, Molina R, Sherry B, Titus RG. Role of tumor necrosis factor in macrophage leishmanicidal activity in vitro and resistance to cutaneous leishmaniasis in vivo. Infect Immun. 1991;59:2839–2842. doi: 10.1128/iai.59.8.2839-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theodos CM, Titus RG. Salivary gland material from the sand fly Lutzomyia longipalpis has an inhibitory effect on macrophage function in vitro. Parasite Immunol. 1993;15:481–487. doi: 10.1111/j.1365-3024.1993.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 10.Warburg A, Saraiva E, Lanzaro GC, Titus RG, Neva F. Saliva of Lutzomyia longipalpis sibling species differs in its composition and capacity to enhance leishmaniasis. Philos Trans R Soc Lond B Biol Sci. 1994;345:223–230. doi: 10.1098/rstb.1994.0097. [DOI] [PubMed] [Google Scholar]
- 11.Mbow ML, Bleyenberg JA, Hall LR, Titus RG. Phlebotomus papatasi sand fly salivary gland lysate down-regulates a Th1, but up-regulates a Th2, response in mice infected with Leishmania major. J Immunol. 1998;161:5571–5577. [PubMed] [Google Scholar]
- 12.Donnelly KB, Lima HC, Titus RG. Histologic characterization of experimental cutaneous leishmaniasis in mice infected with Leishmania braziliensis in the presence or absence of sand fly vector salivary gland lysate. J Parasitol. 1998;84:97–103. [PubMed] [Google Scholar]
- 13.Katz O, Waitumbi JN, Zer R, Warburg A. Adenosine, AMP, and protein phosphatase activity in sandfly saliva. Am J Trop Med Hyg. 2000;62:145–150. doi: 10.4269/ajtmh.2000.62.145. [DOI] [PubMed] [Google Scholar]
- 14.Paranhos M, dos Santos WC, Sherlock I, Oliveira GG, de Carvalho LC. Development of eosinophilia in dogs intradermically inoculated with sand fly saliva and Leishmania (Leishmania) Chagasi stationary-phase promastigotes. Mem Inst Oswaldo Cruz. 1993;88:249–251. doi: 10.1590/s0074-02761993000200012. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro JM. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4:143–152. [PubMed] [Google Scholar]
- 16.Waitumbi J, Warburg A. Phlebotomus papatasi saliva inhibits protein phosphatase activity and nitric oxide production by murine macrophages. Infect Immun. 1998;66:1534–1537. doi: 10.1128/iai.66.4.1534-1537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milleron RS, Ribeiro JM, Elnaime D, Soong L, Lanzaro GC. Negative effect of antibodies against maxadilan on the fitness of the sand fly vector of American visceral leishmaniasis. Am J Trop Med Hyg. 2004;70:278–285. [PubMed] [Google Scholar]
- 18.Hall LR, Titus RG. Sand fly vector saliva selectively modulates macrophage functions that inhibit killing of Leishmania major and nitric oxide production. J Immunol. 1995;155:3501–3506. [PubMed] [Google Scholar]
- 19.Lerner EA, Ribeiro JM, Nelson RJ, Lerner MR. Isolation of maxadilan, a potent vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. J Biol Chem. 1991;266:11234–11236. [PubMed] [Google Scholar]
- 20.Lerner EA, Shoemaker CB. Maxadilan. Cloning and functional expression of the gene encoding this potent vasodilator peptide. J Biol Chem. 1992;267:1062–1066. [PubMed] [Google Scholar]
- 21.Soares MB, Titus RG, Shoemaker CB, David JR, Bozza M. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J Immunol. 1998;160:1811–1816. [PubMed] [Google Scholar]
- 22.Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001;167:5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- 23.Bozza M, Soares MB, Bozza PT, Satoskar AR, Diacovo TG, Brombacher F, Titus RG, Shoemaker CB, David JR. The PACAP-type I receptor agonist maxadilan from sand fly saliva protects mice against lethal endotoxemia by a mechanism partially dependent on IL-10. Eur J Immunol. 1998;28:3120–3127. doi: 10.1002/(SICI)1521-4141(199810)28:10<3120::AID-IMMU3120>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Brodie TM, Smith MC, Morris RV, Titus RG. Immunomodulatory Effects of the Lutzomyia longipalpis Salivary Gland Protein Maxadilan on Mouse Macrophages. Infect Immun. 2007;75:2359–2365. doi: 10.1128/IAI.01812-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flohe SB, Bauer C, Flohe S, Moll H. Antigen-pulsed epidermal Langerhans cells protect susceptible mice from infection with the intracellular parasite Leishmania major. Eur J Immunol. 1998;28:3800–3811. doi: 10.1002/(SICI)1521-4141(199811)28:11<3800::AID-IMMU3800>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 28.Lanzaro GC, Lopes AH, Ribeiro JM, Shoemaker CB, Warburg A, Soares M, Titus RG. Variation in the salivary peptide, maxadilan, from species in the Lutzomyia longipalpis complex. Insect Mol Biol. 1999;8:267–275. doi: 10.1046/j.1365-2583.1999.820267.x. [DOI] [PubMed] [Google Scholar]
- 29.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatzigeorgiou DE, He S, Sobel J, Grabstein KH, Hafner A, Ho JL. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J Immunol. 1993;151:3682–3692. [PubMed] [Google Scholar]
- 31.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 32.Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, Barnes PF. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourreau E, Prevot G, Pradinaud R, Launois P. Interleukin (IL)-13 is the predominant Th2 cytokine in localized cutaneous leishmaniasis lesions and renders specific CD4+ T cells unresponsive to IL-12. J Infect Dis. 2001;183:953–959. doi: 10.1086/319249. [DOI] [PubMed] [Google Scholar]
- 34.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann KH, Lefrang K, Humborg C, Ledermann B, Solbach W. IL-4-deficient Balb/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews DJ, Emson CL, McKenzie GJ, Jolin HE, Blackwell JM, McKenzie AN. IL-13 is a susceptibility factor for Leishmania major infection. J Immunol. 2000;164:1458–1462. doi: 10.4049/jimmunol.164.3.1458. [DOI] [PubMed] [Google Scholar]
- 36.Noben-Trauth N, Paul WE, Sacks DL. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J Immunol. 1999;162:6132–6140. [PubMed] [Google Scholar]
- 37.Mohrs M, Ledermann B, Kohler G, Dorfmuller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302–7308. [PubMed] [Google Scholar]
- 38.Dent AL, Doherty TM, Paul WE, Sher A, Staudt LM. BCL-6-deficient mice reveal an IL-4-independent, STAT6-dependent pathway that controls susceptibility to infection by Leishmania major. J Immunol. 1999;163:2098–2103. [PubMed] [Google Scholar]
- 39.Xin L, Li Y, Soong L. Role of interleukin-1beta in activating the CD11c(high) CD45RB- dendritic cell subset and priming Leishmania amazonensis-specific CD4+ T cells in vitro and in vivo. Infect Immun. 2007;75:5018–5026. doi: 10.1128/IAI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theodos CM, Shankar A, Glasebrook AL, Roeder WD, Titus RG. The effect of treating with anti-interleukin-1 receptor antibody on the course of experimental murine cutaneous leishmaniasis. Parasite Immunol. 1994;16:571–577. doi: 10.1111/j.1365-3024.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 41.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 43.Sozzani S. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev. 2005;16:581–592. doi: 10.1016/j.cytogfr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Gunn MD. Chemokine mediated control of dendritic cell migration and function. Semin Immunol. 2003;15:271–276. doi: 10.1016/j.smim.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Coelho AL, Hogaboam CM, Kunkel SL. Chemokines provide the sustained inflammatory bridge between innate and acquired immunity. Cytokine Growth Factor Rev. 2005;16:553–560. doi: 10.1016/j.cytogfr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 47.Freeman GJ, V, Boussiotis A, Anumanthan A, Bernstein GM, Ke XY, Rennert PD, Gray GS, Gribben JG, Nadler LM. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 48.Brown JA, Titus RG, Nabavi N, Glimcher LH. Blockade of CD86 ameliorates Leishmania major infection by down-regulating the Th2 response. J Infect Dis. 1996;174:1303–1308. doi: 10.1093/infdis/174.6.1303. [DOI] [PubMed] [Google Scholar]
- 49.Corry DB, Reiner SL, Linsley PS, Locksley RM. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- 50.Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175:7311–7324. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- 51.Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004;75:1122–1130. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- 52.Lutz EM, Ronaldson E, Shaw P, Johnson MS, Holland PJ, Mitchell R. Characterization of novel splice variants of the PAC1 receptor in human neuroblastoma cells: consequences for signaling by VIP and PACAP. Mol Cell Neurosci. 2006;31:193–209. doi: 10.1016/j.mcn.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Ushiyama M, Ikeda R, Sugawara H, Yoshida M, Mori K, Kangawa K, Inoue K, Yamada K, Miyata A. Differential intracellular signaling through PAC1 isoforms as a result of alternative splicing in the first extracellular domain and the third intracellular loop. Mol Pharmacol. 2007;72:103–111. doi: 10.1124/mol.107.035477. [DOI] [PubMed] [Google Scholar]
- 54.Rogers KA, Titus RG. Immunomodulatory effects of Maxadilan and Phlebotomus papatasi sand fly salivary gland lysates on human primary in vitro immune responses. Parasite Immunol. 2003;25:127–134. doi: 10.1046/j.1365-3024.2003.00623.x. [DOI] [PubMed] [Google Scholar]
- 55.Ritter U, Mattner J, Rocha JS, Bogdan C, Korner H. The control of Leishmania (Leishmania) major by TNF in vivo is dependent on the parasite strain. Microbes Infect. 2004;6:559–565. doi: 10.1016/j.micinf.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Lattanzio G, Libert C, Aquilina M, Cappelletti M, Ciliberto G, Musiani P, Poli V. Defective development of pristane-oil-induced plasmacytomas in interleukin-6-deficient BALB/c mice. Am J Pathol. 1997;151:689–696. [PMC free article] [PubMed] [Google Scholar]
- 57.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, Falo LD, Thomson AW. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–1523. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 58.Theodos CM, Ribeiro JM, Titus RG. Analysis of enhancing effect of sand fly saliva on Leishmania infection in mice. Infect Immun. 1991;59:1592–1598. doi: 10.1128/iai.59.5.1592-1598.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norsworthy NB, Sun J, Elnaiem D, Lanzaro G, Soong L. Sand fly saliva enhances Leishmania amazonensis infection by modulating interleukin-10 production. Infect Immun. 2004;72:1240–1247. doi: 10.1128/IAI.72.3.1240-1247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]