Abstract
The inhibition of β-galactosidase expression in a medium containing both glucose and lactose is a typical example of the glucose effect in Escherichia coli. We studied the glucose effect in the lacL8UV5 promoter mutant, which is independent of cAMP and cAMP receptor protein (CRP). A strong inhibition of β-galactosidase expression by glucose and a diauxic growth were observed when the lacL8UV5 cells were grown on a glucose–lactose medium. The addition of isopropyl β-d-thiogalactoside to the culture medium eliminated the glucose effect. Disruption of the crr gene or overproduction of LacY also eliminated the glucose effect. These results are fully consistent with our previous finding that the glucose effect in wild-type cells growing in a glucose–lactose medium is not due to the reduction of CRP–cAMP levels but is due to the inducer exclusion. We found that the glucose effect in the lacL8UV5 cells was no longer observed when either the crp or the cya gene was disrupted. Evidence suggested that CRP–cAMP may not enhance directly the lac repressor action in vivo. Northern blot analysis revealed that the mRNA for ptsG, a major glucose transporter gene, was markedly reduced in a Δcrp or Δcya background. The constitutive expression of the ptsG gene by the introduction of a multicopy plasmid restored the glucose effect in Δcya or Δcrp cells. We conclude that CRP–cAMP plays a crucial role in inducer exclusion, which is responsible for the glucose–lactose diauxie, by activating the expression of the ptsG gene.
In enteric bacteria, the synthesis of many catabolic enzymes is inhibited by the presence of glucose in the growth medium. Multiple mechanisms are involved in this phenomenon, referred to as “glucose effect” or “glucose repression” (1–5). Although glucose signaling may occur via different pathways, glucose ultimately would affect the transcription of catabolic operons by modulating transcription factor(s). In the lactose operon of Escherichia coli, the final targets of glucose are the lac repressor and the positive regulator, the complex of cAMP receptor protein (CRP) and cAMP. First, glucose prevents the entry of inducer into the cell, resulting in an increase in the concentration of the inducer-free lac repressor. The mechanism of this process, called “inducer exclusion,” is relatively well understood (3–5). The transport of glucose into the cell by the phosphoenolpyruvate-dependent carbohydrate phosphotransferase system (PTS) decreases the level of phosphorylation of enzyme IIAGlc, one of the enzymes involved in glucose transport. The dephosphorylated enzyme IIAGlc binds to and inactivates the lac permease, causing the inducer exclusion. Second, glucose lowers the level of CRP–cAMP by reducing the intracellular concentrations of both cAMP and CRP under certain conditions, for example, when added to cells growing on a poor carbon source such as glycerol or succinate (6, 7). Glucose is thought to reduce cAMP level by decreasing the phosphorylated form of enzyme IIAGlc, which is proposed to be involved in the activation of adenylate cyclase (3–5). Glucose also is known to reduce the CRP level through the autoregulation of the crp gene (7–10).
When E. coli finds both glucose and lactose in the medium, it preferentially uses the glucose, and the use of lactose is prevented until the glucose is used up, causing a biphasic growth (diauxie)(11, 12). The glucose–lactose diauxie is a prototype of the glucose effect. Concerning the mechanisms that lead to the inhibition of the lac operon expression, it widely has been believed that glucose inhibits lac expression by reducing the level of cAMP and therefore by depriving the lac operon of a transcriptional activator (CRP–cAMP) necessary for its expression.
Recently, we challenged this famous “cAMP model” and found that the level of CRP–cAMP in lactose-grown cells was essentially the same as that in glucose-grown cells (13). We also showed that disruption of the lacI gene completely abolished the glucose effect. These and other data have led us to conclude that the reduction in the CRP–cAMP level cannot be responsible for the glucose effect in the glucose–lactose system and that glucose prevents the expression of the lac operon by enhancing lac repressor activity (13).
The above finding does not exclude the possibility that CRP–cAMP plays any other role(s) in the diauxie, however. It is known that CRP–cAMP is involved in the expression of several PTS proteins, including those required for glucose uptake and phosphorylation (3, 4). It is possible that, in this way, the activity of the lac repressor is affected by glucose. Alternatively, CRP–cAMP might be involved in the glucose effect by directly enhancing the lac repressor action through cooperative binding at the lac promoter (14, 15). To test these possibilities, we investigated the glucose effect in the lacL8UV5 mutant in which the lac promoter is independent of CRP–cAMP (16). We found that both CRP and cAMP are required for the glucose effect. In addition, we showed that the expression of ptsG, a major glucose transporter gene, is under the control of CRP–cAMP. We conclude that CRP–cAMP plays a crucial role in the inducer exclusion, which is responsible for glucose–lactose diauxie, by activating the transcription of ptsG gene.
MATERIALS AND METHODS
Media and Growth Conditions.
Cells were grown aerobically at 37°C in M9 medium (17) supplemented with 0.001% thiamine or in Luria–Bertani medium (17). Antibiotics were used at the following concentrations: ampicillin (50 μg/ml), kanamycin (50 μg/ml), and tetracycline (15 μg/ml). Bacterial growth was monitored by determining the OD at 600 nm.
Bacterial Strains and Plasmids.
The E. coli K-12 strains and plasmids used in this study are listed in Table 1. KK8 was constructed by P1 transduction using HT28 as a donor strain. KK15 and KK17 were constructed by P1 transduction using IT1409. KK20 and KK21 were constructed by P1 transduction using IT1168 and IT1199, respectively. Construction of HT28, IT1409, IT1168, and IT1199 will be described elsewhere. The 3.6-kb BamHI–SalI fragment, containing the cya gene without its 5′ portion, derived from pIT228 (19), was inserted into the corresponding sites of pSTV28 (Takara Shuzo, Kyoto) to construct pIT298. The BamHI–XbaI fragment of pIT298 was cloned into the corresponding sites of pACYC184. Subsequently, the BamHI fragment containing the 5′ portion of cya under the control of the bla promoter was prepared from pSE3 (19) and inserted into the BamHI site of pIT298 to construct pIT302. The 4.8-kb HindIII–AccI fragment carrying the ptsH and ptsI genes prepared from Y. Kohara’s library (20) was cloned into pBR322 to construct pST51. The SalI–SalI DNA fragment containing the ptsG gene derived from Kohara’s library was cloned into the SalI site of pSTV28 to construct pIT499. The MluI site located 50 bp upstream of the ptsG start codon in pIT499 was changed to a HindIII site to construct pTH110. The 2.5-kb HindIII–EcoRI fragment of pTH110 carrying the entire structural gene for ptsG was cloned into the corresponding sites of pBR322 to construct pTH111. The 6-kb EcoRI–PstI fragment containing the lacYA genes was cloned into the corresponding sites of pBR322 to construct pIT539, in which the lacYA genes are expressed from the bla promoter.
Table 1.
Bacterial strains and plasmids used in this study
| Strain/plasmid | Relevant genotype and property | Source |
|---|---|---|
| Strain | ||
| W3110 | Wild-type | Laboratory stock |
| HT28 | W3110 Δcya::Kan | This study |
| IT1409 | W3110 Δcrp::Tet | This study |
| IT1168 | W3110 ptsG::Tn5 | This study |
| IT1199 | W3110 Δcrr::Kan | This study |
| PR158 | galK2 strR supo lacZYA21 F′plac+ lacZ+ | ref. 16 |
| PR166 | galK2 strR supo lacZYA21 F′placL8UV5lacZ+ | ref. 16 |
| KK8 | PR166 Δcya::Kan | This study |
| KK15 | PR158 Δcrp::Tet | This study |
| KK17 | PR166 Δcrp::Tet | This study |
| KK20 | PR166 ptsG::Tn5 | This study |
| KK21 | PR166 Δcrr::Kan | This study |
| Plasmid | ||
| pHA7 | Derivative of pBR322 containing the crp gene expressed from the bla promoter | ref. 18 |
| pIT302 | Derivative of pACYC184 containing the cya gene expressed from the bla promoter | This study |
| pTH111 | Derivative of pBR322 containing the ptsG gene expressed from bla promoter | This study |
| pST51 | Derivative of pBR322 containing the ptsHI genes expressed from the pts promoter | This study |
| pIT539 | Derivative of pBR322 containing the lacYA genes expressed from the bla promoter | This study |
Northern Blot Analysis.
Cells were grown on M9 media containing carbon sources, and total RNAs were extracted as described (21). The RNAs were resolved by agarose-gel electrophoresis in the presence of formamide and blotted onto Hybond-N+ membrane (Amersham) as described (22). The DNA probes were labeled with [α-32P] dCTP by random priming. The membranes were hybridized and washed, and the signals were visualized by autoradiography.
β-Galactosidase Assay.
β-Galactosidase activity was determined with permeabilized cells by the method of Miller (17).
RESULTS
Glucose Effect in the lacL8UV5 Strain.
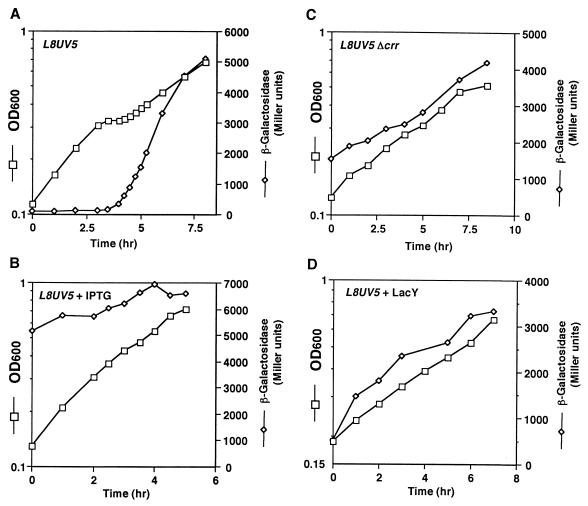
We first investigated the effect of glucose on the expression of β-galactosidase in strain PR166, which carries the lacL8UV5 variant on a F′ plasmid in a lacZYA deletion background (16). The L8 mutation is located in the CRP binding site in the lac promoter (23) and reduces the promoter activity by inhibiting CRP–cAMP binding (23) and/or by altering conformation of the CRP–DNA complex (24). The UV5 mutation, originally isolated as a suppressor of the L8 mutation (25), alters the −10 sequence such that it completely fits the consensus −10 sequence and enhances the promoter activity (23). Thus, the lacL8UV5 promoter has little ability to respond to CRP–cAMP (16). In fact, the β-galactosidase activity in PR166 cells growing on lactose medium was essentially the same as that in isogenic Δcrp cells (see Figs. 1A and 2A). If a reduction in cAMP level caused the glucose–lactose diauxie, the glucose effect would be abolished in the lacL8UV5 strain because the transcription of this promoter no longer requires CRP–cAMP. On the other hand, if the modulation of the lac repressor activity through the inducer exclusion was responsible for the glucose effect, one might expect that the L8UV5 mutation would not affect the glucose effect. We observed a typical diauxie and a strong repression of β-galactosidase activity in the lacL8UV5 mutant, as was the case in wild-type cells (Fig. 1A). In other words, the glucose effect was independent of the positive regulation of the lac operon by CRP–cAMP. The presence of isopropyl β-d-thiogalactoside in the growth medium completely eliminated the glucose effect (Fig. 1B). In addition, the disruption of the crr gene coding for IIAGlc (Fig. 1C) or the overproduction of Lac permease (Fig. 1D) essentially eliminated the glucose effect. These results are fully consistent with our claim that the inducer exclusion, mediated by IIAGlc, but not the reduction in cAMP levels is responsible for the glucose–lactose diauxie.
Figure 1.
Growth curve and β-galactosidase activity of lacL8UV5 cells growing on a glucose–lactose medium. Cells were grown in M9 medium containing 0.04% glucose and 0.2% lactose. The following strains and addition were used: (A) PR166; (B) PR166 plus 0.5 mM isopropyl β-d-thiogalactoside; (C) KK21; and (D) PR166 harboring pIT539. At the indicated time, samples were removed to determine the OD (squares) and β-galactosidase activity (diamonds).
CRP–cAMP Is Required for the Glucose Effect in the lacL8UV5 Strain.
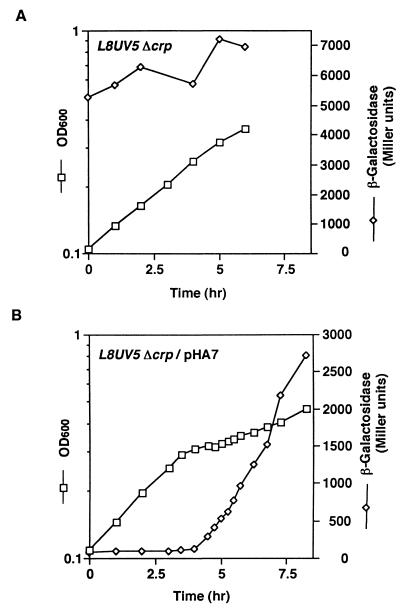
To examine whether CRP–cAMP plays any role(s) in the glucose effect, we disrupted the cya or crp gene in PR166. Interesting to note, the disruption of the crp gene completely eliminated the glucose effect (Fig. 2A). The diauxic growth and the strong repression of β-galactosidase activity by glucose in the lacL8UV5 Δcrp cells were restored by the introduction of pHA7, carrying the crp gene (Fig. 2B). The disruption of the cya gene also eliminated the glucose effect in the lacL8UV5 mutant, and the introduction of pIT302 carrying the cya gene restored the glucose effect (see Fig. 5B). These results clearly indicate that CRP–cAMP is required for the glucose effect.
Figure 2.
Glucose effect in lacL8UV5 Δcrp cells. KK17 (A) or KK17 harboring pHA7 (B) were grown in M9 medium containing 0.04% glucose and 0.2% lactose. At the indicated time, samples were removed to determine the OD (squares) and β-galactosidase activity (diamonds).
Figure 5.
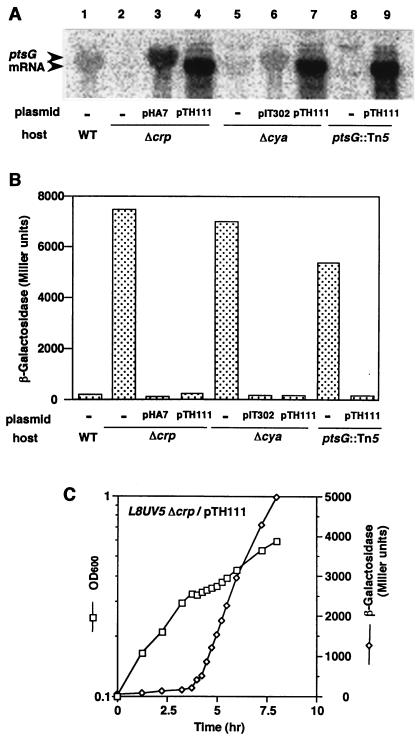
Glucose effect and the expression of ptsG gene in lacL8UV5 cells. (A) Northern blot analysis of the ptsG mRNA. Cells were grown in M9 medium containing 0.5% glucose and 0.5% lactose. Total RNAs, 50 μg (lanes 1, 2, 3, 5, 6 and 8) or 5 μg (lanes 4, 7 and 9), isolated from PR166 (lane 1), KK17 (lane 2), KK17 harboring pHA7 (lane 3), KK17 harboring pTH111 (lane 4), KK8 (lane 5), KK8 harboring pIT302 (lane 6), KK8 harboring pTH111 (lane 7), KK20 (lane 8), and KK20 harboring pTH111(lane 8) were subjected to Northern blot analysis. (B) Cells were grown in M9 medium containing 0.5% glucose and 0.5% lactose. β-Galactosidase activity was determined at OD600 of 0.6. Each value is the average of three experiments. (C) KK17 cells harboring pTH111 were grown in M9 medium containing 0.04% glucose and 0.2% lactose. At the indicated time, samples were removed to determine the OD (squares) and β-galactosidase activity (diamonds).
CRP–cAMP May Not Directly Enhance Repressor Action.
How does CRP–cAMP participate in the glucose effect? One attractive hypothesis is that CRP–cAMP would directly enhance lac repressor binding to the operator. In fact, it was reported that the ternary complex of CRP–cAMP and lac repressor bound to their respective binding sites is more stable than would be expected based on the affinities of independently bound proteins in vitro (14, 15). The crystallographic structure of the lac repressor–DNA complex also suggested that CRP–cAMP functions synergistically with the lac repressor and participates in the formation of a repression loop (26). If repressor binding to the operator were enhanced by CRP–cAMP in vivo, one might expect the expression of β-galactosidase in lacL8UV5Δcrp cells to be higher than that in isogenic crp+ cells. However, we found that this was not the case (Table 2). One could argue that the failure of CRP to affect the binding of repressor in L8UV5 is due to the mutation in the CRP binding site. Therefore, we determined the β-galactosidase activity in strains carrying the wild-type lactose operon. The β-galactosidase activity in the crp+ cells was rather higher than that in the isogenic Δcrp cells (Table 2). These data seem to be in conflict with the view that the presence of CRP–cAMP directly enhances lac repressor action in vivo.
Table 2.
Effect of CRP on the lac expression in the absence of inducer
| lac promoter | crp | Strain | β-Galactosidase activity |
|---|---|---|---|
| L8UV5 | + | PR166 | 55 |
| − | KK17 | 37 | |
| Wild-type | + | PR158 | 26 |
| − | KK15 | 6 |
Cells were grown at 37°C in Luria–Bertani medium to OD600 = 0.6. β-Galactosidase activity was determined and expressed in Miller units (17). Each value is the average of three experiments.
The Reduced Expression of ptsHI Is Not Responsible for the Failure of the Glucose Effect in Δcya or Δcrp Cells.
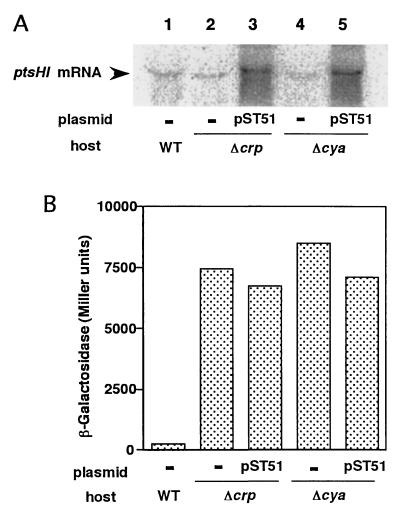
Another possible role of CRP–cAMP in the glucose effect is to enhance indirectly repressor action by modulating the PTS that is responsible for the inducer exclusion. Indeed, it is known that the expression of many PTS proteins is regulated by CRP–cAMP (3, 4). Several PTS proteins are involved in the uptake and phosphorylation of glucose. The major glucose transporter of E. coli consists of two components, cytoplasmic IIAGlc encoded by the crr gene and transmembrane IICBGlc encoded by the ptsG gene. In addition, the ptsH and ptsI genes for the general PTS proteins HPr and enzyme I, respectively, are required for the uptake and phosphorylation of glucose (3, 4). It has been reported that the transcription of the pts operon containing the ptsH, ptsI, and crr genes is activated severalfold by CRP–cAMP although the crr gene is predominantly transcribed from another constitutive promoter located within the 3′ end of ptsI (27). We examined the effect of CRP–cAMP on the expression of ptsHI by Northern blot analysis (Figs. 3A and 4A). The expression of ptsHI was reduced moderately by the disruption of the crp or cya gene as expected (Fig. 4A). To examine the role of expression of the ptsHI genes in the glucose effect, we introduced a multicopy plasmid pST51, carrying the ptsHI genes, into the Δcya or Δcrp strain. The introduction of pST51 overproduced the ptsHI RNA (Fig. 4A) but did not restore the glucose effect in lacL8UV5 cells that contained Δcya or Δcrp (Fig. 4B). The results indicate that the reduced expression of ptsHI is not responsible for the failure of the glucose effect in Δcya or Δcrp cells.
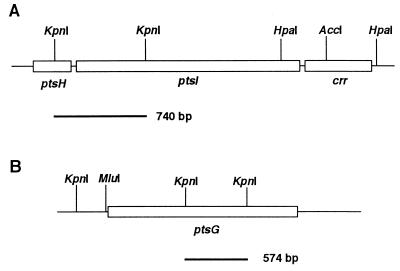
Figure 3.
DNA probes for Northern blotting experiment. (A) The restriction map of the DNA region around the ptsH, ptsI, and crr genes. (B) The restriction map of the DNA region around the ptsG gene. The data were taken from refs. 27 and 29, respectively. The open boxes indicate the coding region. Black lines beneath the map represent the DNA probes used for Northern blotting.
Figure 4.
Glucose effect and the expression of ptsHI genes in lacL8UV5 cells. (A) Northern blot analysis of the ptsHI mRNA. Cells were grown in M9 medium containing 0.5% glucose and 0.5% lactose. Total RNAs, 50 μg (lanes 1, 2, and 4) or 5 μg (lanes 3 and 5), isolated from PR166 (lane 1), KK17 (lane 2), KK17 harboring pST51 (lane 3), KK8 (lane 4), and KK8 harboring pST51 (lane 5) were subjected to Northern blot analysis. (B) Cells were grown in M9 medium containing 0.5% glucose and 0.5% lactose. β-Galactosidase activity was determined at OD600 = 0.6. Each value is the average of three experiments.
The Expression of ptsG Is Strongly Dependent on CRP–cAMP.
Concerning the regulation of the ptsG gene, it was reported that the activity of the glucose-specific enzyme II complex (IIAGlc + IICBGlc) was low in crp or cya mutants compared with the isogenic wild-type strain (28). This suggests that the expression of the ptsG gene is positively regulated by CRP–cAMP. However, no data are available on the transcriptional regulation of the ptsG gene by CRP–cAMP. We performed a Northern blotting experiment to investigate the regulation of ptsG expression by CRP–cAMP (Figs. 3B and 5A). When a DNA probe corresponding to a part of the structural gene of the ptsG (29) was used, a major mRNA specific for the ptsG was detected in a crp+ cya+ background (Fig. 5A). Interesting to note, little ptsG mRNA was visualized in a Δcrp or Δcya background. The presence of pHA7 or pIT302 restored the expression of ptsG mRNA in Δcrp and Δcya cells, respectively. These results strongly suggest that the transcription of ptsG is under the control of CRP–cAMP.
The Reduced Expression of ptsG Is Responsible for the Failure of the Glucose Effect in Δcya or Δcrp Cells.
We reasoned that the failure of the glucose effect in Δcrp or Δcya cells may be due to the reduction of the expression of ptsG. In fact, the disruption of the ptsG gene in crp+ cya+ background eliminated the glucose effect (Fig. 5B). To verify this conclusion, we introduced a multicopy plasmid pTH111, in which the ptsG is expressed constitutively under the bla promoter, into Δcrp or Δcya cells. Northern blot analysis revealed that the ptsG mRNA levels in Δcrp or Δcya cells carrying pTH111 were expressed highly(Fig. 5A). The size of ptsg mRNA derived from pTH111 is slightly shorter than that of the native ptsg mRNA due to the use of the bla promoter. Then, we investigated the effect of glucose on the β-galactosidase expression in Δcrp or Δcya cells carrying the plasmid in two conditions. First, the cells were grown in a M9 medium containing 0.5% glucose and 0.5% lactose, and the β-galactosidase activities were determined at OD600 = 0.6. As shown in Fig. 5B, the introduction of the ptsG plasmid completely restored the glucose effect in Δcrp or Δcya cells. Second, the cells were grown in a M9 medium containing 0.04% glucose and 0.2% lactose, and the β-galactosidase expression was monitored during cell growth (Fig. 5C). Diauxic growth and strong repression of β-galactosidase activity by glucose were observed. These results clearly indicate that the reduced expression of ptsG is responsible for the failure of the glucose effect in the absence of CRP–cAMP.
DISCUSSION
Transcription of the E. coli lac operon in a lactose-containing medium is strongly repressed by the presence of glucose, resulting in a diauxic growth curve. Because the lac operon is under both negative and positive transcriptional control by the lac repressor and CRP–cAMP, respectively (23, 30), glucose could inhibit lac transcription by increasing the level of unliganded repressor and/or by decreasing the level of CRP–cAMP in the cell. Previously, we presented evidence that the glucose effect in a glucose–lactose system is not due to a reduction in CRP–cAMP, as generally believed, but is due to the activation of lac repressor through inducer exclusion (13). In this paper, we addressed the question whether CRP–cAMP affects the repressor action and, if so, how it modulates the repressor activity.
First, we showed that glucose strongly inhibited lac expression, resulting in a typical diauxie in the lacL8UV5 strain, as was the case in a strain with the wild-type lac promoter. We also showed that either the disruption of the crr gene or the overproduction of LacY eliminated the glucose effect. These data are completely consistent with our previous conclusion that the glucose–lactose diauxie is not mediated by a decreased concentration of CRP–cAMP (13). Second, we found that the glucose effect in the lacL8UV5 strain was no longer observed in a Δcrp or Δcya background. Thus, CRP–cAMP was shown to be required for the glucose effect. Third, we found that the level of the ptsG mRNA is reduced markedly in Δcrp or Δcya cells and that the introduction of a ptsG plasmid restored the glucose effect.
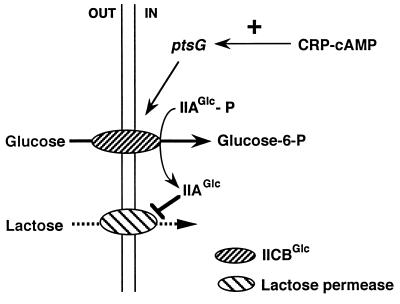
Based on these results, we concluded that the reduction in ptsG mRNA level is likely to be responsible for the failure of the glucose effect in Δcrp or Δcya cells and that an important role of CRP–cAMP in the glucose effect is to support inducer exclusion by activating the ptsG expression. A model explaining the role of CRP–cAMP in the inducer exclusion by glucose is presented in Fig. 6. The importance of the IICBGlc level in inducer exclusion has been shown previously (31, 32), namely, there is no inducer exclusion elicited by methyl α-glucoside below a certain level of IICBGlc. It is most likely that the ptsG gene belongs to the CRP–regulon. In fact, preliminary experiments indicate that the transcription of the ptsG gene is stimulated markedly by CRP–cAMP in vitro (unpublished data). It should be noted that cells lacking IICBGlc can still take up glucose, although less efficiently, through several other PTS proteins such as IIMan complexes (4). This means that glucose must be incorporated into cells through the IIAGlc/IICBGlc system to exhibit “the glucose effect.”
Figure 6.
A model explaining the role of CRP–cAMP in the glucose effect in the glucose–lactose system. The transport and phosphorylation of glucose by glucose PTS (IIAGlc + IICBGlc) increase the dephosphorylated IIAGlc that prevents the uptake of lactose by inhibiting the lac permease activity. An important role of CRP–cAMP in the glucose effect is to support inducer exclusion by activating the ptsG transcription. The reduction in ptsG transcription is responsible for the failure of the glucose effect in the absence of CRP–cAMP.
In addition to the regulation by CRP–cAMP, expression of the ptsG gene appears to be induced by glucose (29, 33). The transcription of the ptsHI operon also is known to be stimulated by both glucose and CRP–cAMP (27, 34). Because the levels of CRP and cAMP are reduced by glucose, the expression of the glucose PTS should be regulated not only by CRP–cAMP but also by other factors that mediate the effect of glucose. The mechanism by which glucose stimulates the expression of ptsG and ptsHI genes is largely unknown.
We also examined a possibility that CRP–cAMP might be involved in the glucose effect by directly enhancing lac repressor binding to its operator. Although our experiments suggest that CRP–cAMP may not directly enhance the repressor action in vivo, at least under our experimental conditions, we cannot rule out the possibility that cooperative interaction between CRP–cAMP and the lac repressor plays some other role in the regulation of the lac operon. For example, it has been proposed that the lac repressor–CRP cooperativity could act to sequester RNA polymerase at the lac promoter, in a transcriptionally repressed state, allowing rapid initiation of transcription once repressor is inactivated (14). In glucose–lactose medium, the lac transcription is induced quickly after the depletion of glucose. It is possible that CRP–cAMP may confer on the cell an effective way to switch from glucose to lactose utilization. Whether and how CRP–cAMP and lac repressor cooperate with each other in vivo remain to be determined.
Acknowledgments
We thank Max Gottesman for providing the strains PR166 and PR158. This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan. T.I. was supported by the Kato Memorial Bioscience Foundation.
ABBREVIATIONS
- CRP
cAMP receptor protein
- PTS
phosphoenolpyruvate-dependent carbohydrate phosphotransferase system
- IIAGlc
glucose-specific IIA protein
- IICBGlc
glucose-specific IICB protein
References
- 1.Magasanik B. In: The Lactose Operon. Beckwith J, Zipser D, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1970. pp. 189–220. [Google Scholar]
- 2.Ullmann A, Danchin A. Adv Cyclic Nucleotide Res. 1983;15:1–53. [Google Scholar]
- 3.Meadow N D, Fox D K, Roseman S. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- 4.Postma P W, Lengeler J W, Jacobson G R. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saier M L, Jr, Ramseier T M, Reizer J. In: Escherichia coli and Salmonella tryhimurium: Cellular and Molecular Biology. Neidhardt F C, Curtis R III, Ingraham J L, Lin E C C, Low K, et al., editors. Washington, DC: Am. Soc. Microbiol.; 1995. pp. 1325–1343. [Google Scholar]
- 6.Pastan I, Perlman R. Science. 1970;169:339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- 7.Ishizuka H, Hanamura A, Kunimura T, Aiba H. Mol Microbiol. 1993;10:341–350. doi: 10.1111/j.1365-2958.1993.tb01960.x. [DOI] [PubMed] [Google Scholar]
- 8.Hanamura A, Aiba H. Mol Microbiol. 1992;6:2489–2497. doi: 10.1111/j.1365-2958.1992.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 9.Ishizuka H, Hanamura A, Inada T, Aiba H. EMBO J. 1994;13:3077–3082. doi: 10.1002/j.1460-2075.1994.tb06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagami H, Inada T, Kunimura T, Aiba H. Mol Microbiol. 1995;17:251–258. doi: 10.1111/j.1365-2958.1995.mmi_17020251.x. [DOI] [PubMed] [Google Scholar]
- 11.Monod J. Growth. 1947;11:223–289. [Google Scholar]
- 12.Epstein W, Rothman-Denes L B, Hesse J. Proc Natl Acad Sci USA. 1975;72:2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inada T, Kimata K, Aiba H. Genes Cells. 1996;1:293–301. doi: 10.1046/j.1365-2443.1996.24025.x. [DOI] [PubMed] [Google Scholar]
- 14.Hudson J M, Fried M G. J Mol Biol. 1990;214:381–396. doi: 10.1016/0022-2836(90)90188-R. [DOI] [PubMed] [Google Scholar]
- 15.Vossen K M, Stickle D F, Fried M G. J Mol Biol. 1996;255:44–54. doi: 10.1006/jmbi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 16.Rockwell P, Gottesman M E. J Mol Biol. 1991;222:189–196. doi: 10.1016/0022-2836(91)90205-k. [DOI] [PubMed] [Google Scholar]
- 17.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 18.Aiba H, Fujimoto S, Ozaki N. Nucleic Acids Res. 1982;10:1345–1361. doi: 10.1093/nar/10.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inada T, Takahashi H, Mizuno T, Aiba H. Mol Gen Genet. 1996;253:198–204. doi: 10.1007/s004380050313. [DOI] [PubMed] [Google Scholar]
- 20.Kohara Y, Akiyama K, Isono K. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 21.Aiba H, Adhya S, de Crombrugghe B. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A laboratory manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 23.Reznikoff W S, Abelson J N. In: The Operon. Miller J H, Reznikoff W S, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1978. pp. 221–243. [Google Scholar]
- 24.Fried M G, Crothers D M. Nucleic Acids Res. 1983;11:141–158. doi: 10.1093/nar/11.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverstone A E, Arditti R R, Magasanik B. Proc Natl Acad Sci USA. 1970;66:773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis M, Chang G, Horton N C, Kercher M A, Pace H C, Schumacher M A, Brennan R G, Lu P. Science. 1996;271:1247–1254. doi: 10.1126/science.271.5253.1247. [DOI] [PubMed] [Google Scholar]
- 27.Reuse H De, Danchin A. J Bacteriol. 1988;170:3827–3837. doi: 10.1128/jb.170.9.3827-3837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rephaeli A D, Saier M H., Jr J Bacteriol. 1980;141:658–663. doi: 10.1128/jb.141.2.658-663.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erni B, Zanolari B. J Biol Chem. 1986;261:16398–16403. [PubMed] [Google Scholar]
- 30.Reznikoff W S. Mol Microbiol. 1992;6:2419–2422. doi: 10.1111/j.1365-2958.1992.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 31.Ruyter G J G, Postma P, van Dam K. J Bacteriol. 1991;173:6184–6191. doi: 10.1128/jb.173.19.6184-6191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Vlag J, Van’t Hof R, van Dam K, Postma P. Eur J Biochem. 1995;230:170–182. doi: 10.1111/j.1432-1033.1995.0170i.x. [DOI] [PubMed] [Google Scholar]
- 33.Stock J B, Waygood E B, Meadow N D, Postma P W, Roseman S. J Biol Chem. 1982;257:14543–14552. [PubMed] [Google Scholar]
- 34.Ryu S, Ramseier T M, Michotey V, Saier M H, Jr, Garges S. J Biol Chem. 1995;270:2489–2496. doi: 10.1074/jbc.270.6.2489. [DOI] [PubMed] [Google Scholar]