Abstract
OBJECTIVES
Although cigarette smoking is the most well-established environmental risk factor for pancreatic cancer, the interaction between smoking and other risk factors has not been assessed. We evaluated the independent effects of multiple risk factors for pancreatic cancer and determined whether the magnitude of cigarette smoking was modified by other risk factors in men and women.
METHODS
We conducted a hospital-based case-control study involving 808 patients with pathologically diagnosed pancreatic cancer and 808 healthy frequency-matched controls. Information on risk factors was collected by personal interview, and unconditional logistic regression was used to determine adjusted odds ratios (AORs) by the maximum-likelihood method.
RESULTS
Cigarette smoking, family history of pancreatic cancer, heavy alcohol consumption (>60 mL ethanol/day), diabetes mellitus, and history of pancreatitis were significant risk factors for pancreatic cancer. We found synergistic interactions between cigarette smoking and family history of pancreatic cancer (AOR 12.8, 95% confidence interval [CI] 1.6–108.9) and diabetes mellitus (AOR 9.3, 95% CI 2.0–44.1) in women, according to an additive model. Approximately 23%, 9%, 3%, and 5% of pancreatic cancer cases in this study were related to cigarette smoking, diabetes mellitus, heavy alcohol consumption, and family history of pancreatic cancer, respectively.
CONCLUSIONS
The significant synergy between these risk factors suggests a common pathway for carcinogenesis of the pancreas. Determining the underlying mechanisms for such synergies may lead to the development of pancreatic cancer prevention strategies for high-risk individuals.
INTRODUCTION
Pancreatic cancer incidence is highest in North America and Europe (11.8–12.5 cases per 100,000 people) and lowest in southern and eastern Asia and most of Africa (<3.5 cases per 100,000 people) (1). In the United States, pancreatic cancer ranks 10th in incidence of all cancers and second among the gastrointestinal (GI) cancers. At the time of this writing, it was estimated that approximately 33,730 new cases of pancreatic cancer would be diagnosed in 2006, constituting 2% of all cancer cases in the country (2). Pancreatic cancer is disproportionately deadly, however, ranking as the fourth most common cause of cancer death in both men and women in the United States. This relatively high death rate is due to the fact that cancer of the pancreas is often silent and is rarely diagnosed early; when it is detected, the cancer is usually surgically unresectable (3).
Several personal and environmental factors have been associated with pancreatic carcinogenesis. Cigarette smoking is the most well-established environmental risk factor for pancreatic cancer worldwide (4–12). However, it has been estimated that only approximately 25–30% of pancreatic cancer cases in the United States are related to cigarette smoking (6, 9). Other factors such as diabetes mellitus, alcohol consumption, and chronic pancreatitis have also been examined for a relationship to pancreatic cancer (5, 9, 13–28). However, to our knowledge, no research has been done to determine whether the associated risk of each factor varied by sex.
Because of the multifactorial nature of pancreatic carcinogenesis, possible interactions between risk factors may exist. Therefore, we embarked on the present large-scale case-control study to more precisely assess the potential influence of several factors on pancreatic cancer development and to evaluate possible synergism between these factors in elevating the risk for pancreatic cancer in men and women.
PATIENTS AND METHODS
Study Design and Population
The study design was hospital-based case-control in which cases and controls were prospectively ascertained. The study was approved by the institutional review board of The University of Texas M. D. Anderson Cancer Center. Written informed consent for an interview and for a biologic sample was obtained from each study participant. A total of 1,616 subjects (808 patients with pancreatic cancer and 808 healthy controls) were enrolled. This study is actively supported by NIH grants RO1 CA98380 and SPORE P20 101936.
Case patients were recruited from the population of patients with newly diagnosed pancreatic cancer who were evaluated and treated at the institution’s GI medical oncology outpatient clinics. The inclusion criteria were as follows: pathologically confirmed diagnosis of pancreatic ductal adenocarcinoma, U.S. residency, and ability to communicate in English. The exclusion criteria were presence of other types of pancreatic disease, such as neuroendocrine tumor, adenomas, cysts, or unknown primary tumors; concurrent cancer at another organ site; and past history of cancer. From January 2000 through May 2006, 1,239 patients with suspected pancreatic cancer were approached, 1,002 eligible patients were identified, and 808 patients were ascertained (Fig. 1). We failed to recruit 194 eligible patients (194/1,002 = 19.4%) for the following reasons: patient refusal (28.1%), physician refusal (3.6%), severity of the illness or sadness of the patient or family (16.2%), language barrier (5.7%), inadequate time to complete interview (8.9%), and change in the patient’s schedule (37.5%). The demographic features and medical histories of these missed patients were retrieved from their medical records and recorded in a database. Statistical analysis indicated that the missed patients did not differ from the recruited patients in terms of age, sex, educational level, state of residency, race/ethnicity, or stage of disease.
Figure 1.
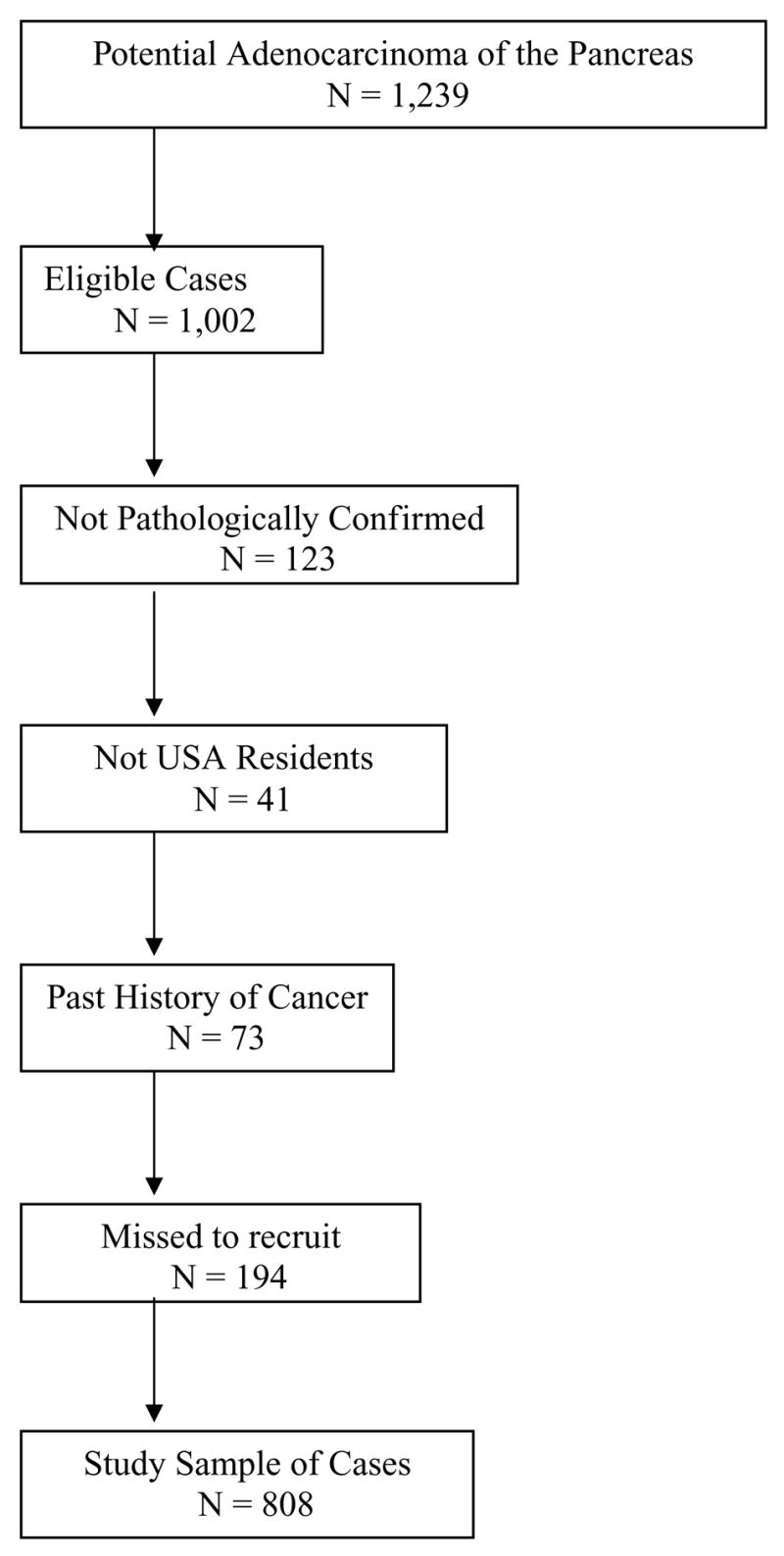
Ascertainment of patients with pancreatic cancer.
The control subjects were healthy friends and genetically unrelated family members (spouses and in-laws) of patients at our institution who had cancers other than pancreatic, GI, lung, or head and neck cancer (smoking-related cancers). The reason for excluding family members and spouses of patients with pancreatic cancer, as controls, is to avoid induction of selection bias. While first-degree family members may share the same genetic factors related to pancreatic cancer, spouses may share similar lifestyle factors as the cancer patients, especially regarding factors that are highly associated with cancer, like cigarette smoking and dietary habits. This may preclude us from determining the true relationship between pancreatic cancer development and family history of pancreatic cancer or some environmental risk factors. Moreover, we had excluded the spouses of patients with tobacco-related cancers such as upper aerodigestive and lung cancers. This approach was shown to be a successful strategy for reducing selection bias in case control study (29).
The eligibility criteria for controls were the same as those for patients, except for the cancer diagnosis. The patients and controls were frequency matched by age (±5 yr), race/ethnicity, and sex. Control subjects were recruited from the institution’s Central Diagnostic Radiology Clinics, where all cancer patients and their companions are sent for the initial cancer diagnosis or post-treatment follow-up examination. A short structured questionnaire was used to screen for potential controls on the basis of both eligibility criteria and matching criteria. Only those who met the eligibility and matching criteria were recruited into the study. Controls and patients were recruited simultaneously.
Data analysis of the answers received on the short questionnaire (the screen for control candidates) indicated that 83.6% agreed to participate in clinical research. There were no significant differences in age, sex, race/ethnicity, educational level, personal history of cancer, or accompanied patient’s type of cancer between those who were recruited as controls and those who refused to participate in the research.
A total of 808 controls were enrolled from January 2000 through May 2006. Controls were considered cancer-free at the time of enrollment and less likely to have a cancer, chronic illness, or previous exposure to tobacco and alcohol use than the hospitalized patients. Because the controls accompanied cancer patients, we sought to confirm the controls’ reasons for coming to the hospital; we found that the underlying causes for the controls’ companionship of the cancer patients were care and altruism. In a literature search, we found no evidence of an association between care or altruism and risk factors for pancreatic cancer. Moreover, all spouses reported that they would have chosen to be referred to the same hospital if they had been diagnosed with cancer during the same time period, probably because spouses tend to share the same family physician, have the same health insurance coverage, and live in the same geographic location. All the above-mentioned results indicated that the patients and controls had the same catchments, which further supports the idea that the study controls were representative of the hospital (M.D. Anderson Cancer Center) population from which the pancreatic cancer cases were selected (30–32).
Patients and controls were interviewed by well-trained interviewers who followed a written protocol to guide ascertainment and reduce surveillance, interviewer, and recall bias. No proxy interviews were conducted. The interviewers used a structured and a validated questionnaire to collect information on demographic features and potential risk factors, such as personal smoking history, alcohol use, medical history, occupational history, and family history of cancer.
Smokers were defined as subjects who had smoked ≥100 cigarettes during their lifetime. Smoking history was recorded in terms of pack-years, estimated by multiplying the number of years of smoking by the number of packs of cigarettes smoked per day, such that 1 pack-year = 1 pack of cigarettes per day for a year. Heavy smokers were defined as those who had >20 pack-years of smoking.
Ever-alcohol drinkers were defined as subjects who had consumed at least 4 alcoholic drinks of beer, wine, or hard liquor each month for 6 months in their lifetime. The starting age of drinking and (if applicable) the age at which drinking ended were recorded for each type of alcoholic beverage for all participants. Ever-drinkers were further classified according to the total lifetime volume of ethanol consumed in milliliters, which was computed according to the frequency of drinking, type of serving (glass, bottle, or can), number and size of each serving, and duration of consumption, summed over the whole period of alcohol use. Consumed-serving units were defined as 12.0 oz for beer, 4.0 oz for wine, and 1.5 oz for hard liquor, each of which is considered to be equivalent to approximately 12.0 mL of ethanol (33). Heavy alcohol consumption was defined as consumption of >60 mL of ethanol per day during the subject’s lifetime of alcohol drinking (34). This methodology of ethanol assessment was applied in our previous case-control study of hepatocellular carcinoma (35).
The questionnaire also was used to collect information about past history of several medical conditions (diabetes mellitus, colon polyps, thyroid diseases, pancreatitis, cholelithiasis [gallstones], and cholecystectomy), age at diagnosis, and duration of each condition. For diabetic patients, information about type of treatment (insulin or oral medications) was also collected.
Patients and controls were questioned about the history of cancer among their first- and second-degree relatives. Additional information was collected from those with a positive family history, including the number of family members with cancer, type(s) of cancer, age(s) at cancer diagnosis, and relationship(s) to the study subject.
Statistical Methods
The Microsoft Access program was used for data entry and data management and Stata software (Stata Corp, College Station, TX) was used for statistical analysis. χ2 tests were used to compare the distributions of categorical variables between patients with pancreatic cancer and control subjects where P values were estimated.
We performed univariable single-factor unconditional logistic regression analyses to assess the marginal effects of each factor on risk for pancreatic cancer, using maximum-likelihood estimation. We also performed multivariable unconditional logistic regression analyses using all variables significant at P < 0.05 in the single-factor analyses. For each factor, we calculated the adjusted odds ratio (AOR) and 95% confidence interval (CI), estimated using maximum likelihood estimation. All AORs were adjusted for age, sex, race/ethnicity, education, place of residency, and other significant risk factors using the likelihood ratio test. Finally, the population attributable risk percentage (PAR%) for each statistically significant risk factor was calculated using the AOR of that factor and its prevalence in the control group (Pe), such that
We also investigated possible interactions between risk factors using multiple logistic regression models. We chose to use additive models rather than multiplicative models because the former have a more appropriate scale for addressing biologic interactions and public health concerns. Moreover, the linear risk model in general cannot be validly estimated from case-control designs, unless the sampling fraction of cases and controls is known or can be estimated. Including an interaction term in the linear statistical model is a matter of effect measurement scale and that antagonism and synergy, which are biological phenomena, interactions do not depend on the measurement procedure and departure from additivity does not correspond to departure of a product term coefficient from zero (36, 37). For example, by crossing diabetes mellitus and cigarette smoking, a dummy variable was obtained for four categories: two for the presence of each risk factor alone, one for the presence of both risk factors, and one for the absence of both risk factors. The last of these categories was used as the reference category in the regression models. To assess deviation from the additive model (which assumes there is no interaction between variables), the synergism index (S = [OR11 − 1]/([OR01 + OR10] − 2), where OR11 = OR of the joint effect of two risk factors and OR10 and OR01 = OR of each risk factor in the absence of the other), and its 95% CI were calculated. A value of S equal to unity was interpreted as indicative of additivity, whereas a value greater than unity was indicative of superadditivity and synergism (38).
RESULTS
Patient Characteristics
Table 1 summarizes the demographic features of the pancreatic cancer patients and controls. The overall ratio of men to women was slightly greater than 1:1, with no statistically significant difference between patients and controls. The majority of study subjects were non-Hispanic whites, and the racial/ethnic distribution was similar between patients and controls for both men and women. Most patients and controls (53.0%) were from Texas, 13.8% were from neighboring states (Louisiana, Arkansas, Oklahoma, New Mexico), and the rest were from other U.S. states. Patients were slightly older than controls, with a mean difference in age of 1.70 ± 0.5 yr (P = 0.01). Patients also had a lower educational level than controls (P = 0.001). During statistical analyses, we choose to adjust for all demographic factors in the unconditional logistic regression models.
Table 1.
Characteristics of Study Subjects
| Patients (N = 808)
|
Controls (N = 808)
|
||||
|---|---|---|---|---|---|
| Study Variables | N | % | N | % | P Value |
| Sex | 0.2 | ||||
| Male | 449 | 55.6 | 478 | 59.2 | |
| Female | 359 | 44.4 | 330 | 40.8 | |
| Age, yr (mean ± SD) | 61.9 ± 10 | 60.2 ± 10.9 | 0.02 | ||
| <40 | 15 | 1.9 | 33 | 4.1 | |
| 41–50 | 96 | 11.9 | 120 | 14.9 | |
| 51–60 | 236 | 29.2 | 243 | 30.1 | |
| 61–70 | 288 | 35.7 | 261 | 32.3 | |
| >70 | 172 | 21.3 | 151 | 18.7 | |
| Race/ethnicity | 0.1 | ||||
| White | 698 | 86.4 | 721 | 89.2 | |
| African American | 53 | 6.6 | 29 | 3.6 | |
| Hispanic | 49 | 6.1 | 52 | 6.4 | |
| Asian | 8 | 1 | 6 | 0.7 | |
| Education level | 0.02 | ||||
| ≤Bachelor’s degree | 505 | 62.5 | 458 | 56.7 | |
| >Bachelor’s degree | 303 | 37.5 | 350 | 43.3 | |
| State of residency | 0.001 | ||||
| Texas or surrounding states | 502 | 62.1 | 585 | 72.4 | |
| Other state | 306 | 37.9 | 223 | 27.6 | |
Risk Factors for Pancreatic Cancer
DIABETES MELLITUS
Table 2 shows that patients with diabetes mellitus had a risk for pancreatic cancer 2.6 times greater than that of nondiabetic patients. However, 39.2% of pancreatic cancer patients and 27.8% of controls with diabetes mellitus were first diagnosed with diabetes at the time of cancer diagnosis or recruitment into the study. Thus, the estimated AOR for patients with a history of diabetes >1 yr was 2.4 (95% CI 1.7–3.4), while those with diabetes ≤1 yr had an AOR of 4.0 (95% CI 2.5–6.6). Despite this difference, we observed no significant correlation between estimated AOR and duration of diabetes; meaning that subjects with a long duration of diabetes (>10 yr, AOR = 1.9) are not at a significantly higher risk than those with a short-term duration of diabetes (2–5 yr, AOR = 2.7).
Table 2.
Effects of Risk Factors for Pancreatic Adenocarcinoma in Multivariable Logistic Regression Analysis
| Patients (N = 808)
|
Controls (N = 808)
|
||||||
|---|---|---|---|---|---|---|---|
| Study Variables | N | % | N | % | AOR (95% CI)* | ||
| Past history of diabetes mellitus | |||||||
| Never | 614 | 76.0 | 729 | 90.2 | 1 (reference) | ||
| Ever | 194 | 24.0 | 79 | 9.8 | 2.6 (1.9–3.4) | ||
| Duration of diabetes, yr | |||||||
| ≤1 | 76 | 9.4 | 22 | 2.7 | 4.0 (2.5–6.6) | ||
| >1 | 118 | 14.6 | 57 | 7.1 | 2.4 (1.7–3.4) | ||
| 2–5 | 55 | 6.8 | 25 | 3.1 | 2.7 (1.7–4.4) | ||
| 6–10 | 26 | 3.2 | 10 | 1.2 | 2.8 (1.3–5.9) | ||
| >10 | 37 | 4.6 | 22 | 2.7 | 1.9 (1.1–3.3) | ||
| Treatment of diabetes mellitus | |||||||
| Insulin only | 15 | 1.9 | 3 | 0.4 | 5.9 (1.7–21.1) | ||
| Oral only | 82 | 10.2 | 51 | 6.3 | 1.9 (1.3–2.8) | ||
| Both | 21 | 2.6 | 3 | 0.4 | 7.1 (2.1–24.4) | ||
| Cigarette smoking | |||||||
| Never | 323 | 40.0 | 415 | 51.4 | 1 (reference) | ||
| Ever | 485 | 60.0 | 393 | 48.6 | 1.6 (1.2–1.9) | ||
| Duration of smoking, pack-years | |||||||
| ≤20 | 199 | 24.6 | 203 | 25.1 | 1.4 (1.1–1.8) | ||
| >20 | 286 | 35.4 | 190 | 23.5 | 2.0 (1.6–2.6) | ||
| Alcohol consumption | |||||||
| Never | 371 | 45.9 | 364 | 45.0 | 1 (reference) | ||
| Ever | 437 | 54.1 | 444 | 55.0 | 1.0 (0.8–1.2) | ||
| Heavy alcohol drinking† | |||||||
| ≤60 mL ethanol/day | 355 | 43.9 | 386 | 47.8 | 0.9 (0.7–1.2) | ||
| >60 mL ethanol/day | 67 | 8.3 | 42 | 5.2 | 1.6 (1.1–2.5) | ||
AOR = odds ratio adjusted for age, sex, race/ethnicity, smoking history, diabetes history, heavy alcohol consumption, family history of pancreatic cancer, history of pancreatitis, education level, and state of residency.
Data on duration of drinking were missing for 15 patients and 16 controls.
Analysis of risk according to type of diabetes treatment determined that the estimated AOR was greater among those receiving both insulin and oral antiglycemic drugs (7.1, 95% CI 2.1–24.4) than among those receiving only insulin (5.9, 95% CI 1.7–21.1) or oral medications (1.9, 95% CI 1.3–2.8). The observed point estimate of association between diabetes and pancreatic cancer was higher in women (AOR = 4.2, 95% CI 2.2–8.1) than in men (AOR = 2, 95% CI 1.4–3.0).
ALCOHOL CONSUMPTION
Consumption of beer, wine, and hard liquor did not differ significantly between patients (76%, 65%, and 66%, respectively) and controls (80%, 63%, and 62%, respectively). However, pancreatic cancer patients (23.6%) were more likely than controls (15.6%) to be daily drinkers of hard liquor (P = 0.006). Moreover, patients had significantly more years of alcohol use (mean ± SD = 35.6 ± 11.8) than controls (29.4 ± 14.9) (P < 0.0001). The average lifetime intake of ethanol was 224,927 mL among pancreatic cancer patients and 159,403 mL among controls (P = 0.01). Overall, only heavy drinkers who consumed >60 mL ethanol/day had a greater risk (by 60%) of pancreatic cancer relative to nondrinkers (Table 2). However, the significant effect of heavy drinking on elevated risk for pancreatic cancer was observed in men only (P = 0.006, AOR = 2, 95% CI 1.2–3.3), not in women (P = 0.5, AOR = 1.2, 95% CI 0.5–3.5). In addition, among both patients and controls, men tended to have a significantly larger amount of lifetime ethanol consumption than women (P = 0.0001).
CIGARETTE SMOKING
Table 2 shows the significant relationship between cigarette smoking and pancreatic cancer, with a 60% greater risk among ever-smokers. The increase in risk was more pronounced among heavy smokers (>20 pack-years) than among mild or moderate smokers (≤20 pack-years) when both were compared with nonsmokers. The observed point estimate of association between heavy smoking and pancreatic cancer risk was higher in women (P < 0.001, AOR = 3.2, 95% CI 2.0–4.9) than in men (P = 0.04, AOR = 2, 95% CI 1.2–3.3).
CHRONIC MEDICAL CONDITIONS
Table 3 summarizes the prevalence of several diseases among patients and controls. Recent cholecystectomy was defined as surgical removal of the gallbladder ≤2 yr from the time of cancer diagnosis or control-group recruitment. All patients with recent cholecystectomy had their cholecystectomy during surgical removal of the pancreatic cancer, which may explain the observed and significant fourfold increase in pancreatic cancer risk among this group. However, we observed no significant association between cholecystectomy (>2 yr) and pancreatic cancer after controlling for the confounding effects of other significant risk factors (P = 0.2). This null association was observed for men (AOR = 1.3, 95% CI 0.7–2.2) and for women (AOR = 1.2, 95% CI 0.7–1.9).
Table 3.
Effects of Other Medical Conditions in Multivariable Logistic Regression Analysis
| Patients (N = 808)† |
Controls (N = 808)† |
||||
|---|---|---|---|---|---|
| History of medical conditions* | N | % | N | % | AOR (95% CI)‡ |
| Cholecystectomy, yr | 197 | 24.4 | 104 | 12.9 | 1.8 (1.4–2.4) |
| ≤2 | 95 | 11.8 | 25 | 3.1 | 3.9 (2.4–6.4) |
| >2 | 99 | 12.3 | 76 | 9.4 | 1.1 (0.9–1.8) |
| Pancreatitis, yr | 60 | 7.4 | 6 | 0.7 | 10.9 (4.3–27.7) |
| ≤2 | 50 | 6.2 | 1 | 0.1 | ——— |
| >2 | 10 | 1.2 | 3 | 0.4 | 4.3 (0.9–20.2) |
| Hypothyroidism, yr | 57 | 7.1 | 64 | 7.9 | 0.8 (0.5–1.2) |
| ≤10 | 23 | 2.8 | 35 | 4.3 | 0.6 (0.3–1.1) |
| >10 | 30 | 3.7 | 27 | 3.3 | 0.9 (0.6–1.5) |
| Hyperthyroidism, yr | 12 | 1.5 | 12 | 1.5 | 1.1 (0.5–2.7) |
| ≤10 | 4 | 0.5 | 5 | 0.6 | 0.8 (0.2–3.5) |
| >10 | 8 | 0.9 | 6 | 0.7 | 1.6 (0.5–4.6) |
| Colon polyps, yr | 186 | 23 | 155 | 19.2 | 1.2 (0.9–1.6) |
| ≤5 | 150 | 18.6 | 123 | 15.2 | 1.3 (0.9–1.8) |
| >5 | 36 | 4.5 | 29 | 3.6 | 1.1 (0.7–1.7) |
Cutoff point for disease duration = median duration among controls.
Data on duration of medical condition were missing for four patients and three controls.
AOR = odds ratio adjusted for age, sex, race/ethnicity, smoking history, history of diabetes, heavy alcohol consumption, family history of pancreatic cancer, history of pancreatitis, education level, and state of residency.
Sixty patients and six controls reported a history of clinical pancreatitis, which translated to an AOR of 10.9 (95% CI 4.3–27.7). The subjects were also analyzed according to their duration of pancreatitis: those who had pancreatitis for ≤2 yr and those with pancreatitis of >2 years’ duration. Most patients reported a history of pancreatitis within 2 yr of the cancer diagnosis (50/60 = 83.3%). The estimated AOR for subjects with a longer history of this risk factor was 4.3 (95% CI 0.9–20.2) after taking into consideration the confounding effect of several demographic and risk factors.
Moreover, recent-onset pancreatitis in cancer patients was not correlated with any other known risk factor. Conversely, longer-duration pancreatitis in cancer patients was highly correlated with long-term diabetes, heavy alcohol consumption, heavy cigarette smoking, or cholelithiasis and subsequent cholecystectomy (P = 0.0001).
We found no significant association between hypothyroidism or hyperthyroidism and pancreatic cancer development. Other thyroid conditions, such as goiter, Hashimoto’s thyroiditis, thyroid cyst, and Graves’ disease, were rarely reported, and the distributions did not differ significantly between patients and controls (P = 0.1). The same negative results were observed for short- and long-term history of colon polyps among men and women.
FAMILY HISTORY OF CANCER AND PANCREATIC CANCER
Table 4 shows that a family history of cancer in general and of pancreatic cancer in particular was associated with a significantly elevated risk for pancreatic cancer. The effect of a positive family history of pancreatic cancer was significant in women (AOR = 3.2, 95% CI 1.5–7.1) and in men (AOR = 2.3, 95% CI 1.3–4.2). The significant relationship was for both first-degree relatives (P < 0.001) and second-degree relatives (P = 0.03). Two patients but no controls reported more than one first-degree relative with pancreatic cancer. The distribution of other types of cancer among first-degree relatives was not statistically different between patients and controls. However, there were 60% and 80% greater risks of pancreatic cancer among subjects with positive family histories of breast cancer and colon cancer, respectively. Moreover, 11 patients (11/45 = 24.4%) and 2 controls (2/19 =10.5%) with a positive family history of pancreatic cancer had a positive family history of breast cancer among their first-degree relatives.
Table 4.
Effect of Family History of Cancer on Pancreatic Cancer (PC) Risk in Multivariable Logistic Regression Analysis
| Patients (N = 808)
|
Controls (N = 808)
|
||||
|---|---|---|---|---|---|
| Study Variables | N | % | N | % | AOR (95% CI)* |
| Family history of any cancer† | |||||
| Never | 187 | 23.1 | 245 | 30.3 | 1 (reference) |
| Ever of any cancer | 615 | 76.1 | 561 | 69.4 | 1.5 (1.1–1.8) |
| Pancreatic cancer | 69 | 8.5 | 30 | 3.7 | 2.7 (1.7–4.3) |
| Relationship of PC family member‡ | |||||
| First-degree relatives | 45 | 5.6 | 19 | 2.4 | 3.3 (1.8–6.1) |
| Second-degree relatives | 24 | 2.9 | 11 | 1.4 | 2.9 (1.3–6.3) |
| First-degree relatives with PC‡ | |||||
| Parents | 29 | 3.6 | 14 | 1.7 | 3.1 (1.5–6.3) |
| Siblings | 15 | 1.9 | 5 | 0.6 | 3.9 (1.4–11.5) |
| Offspring | 1 | 0.1 | 0 | 0 | ——— |
| Cancer sites among first-degree‡ | |||||
| Breast | 117 | 14.5 | 96 | 11.9 | 1.6 (1.1–2.3) |
| Colon | 83 | 10.3 | 64 | 7.9 | 1.8 (1.2–2.6) |
| Lung | 72 | 8.9 | 64 | 7.9 | 1.4 (0.9–2.1) |
| Prostate | 63 | 7.8 | 50 | 6.2 | 1.5 (0.9–2.3) |
| Melanoma | 22 | 2.7 | 19 | 2.4 | 1.3 (0.7–2.6) |
| Ovary | 20 | 2.5 | 15 | 1.9 | 1.9 (0.9–3.9) |
| Stomach | 22 | 2.7 | 25 | 3.1 | 1.1 (0.5–1.9) |
| Liver | 19 | 2.4 | 13 | 1.6 | 1.8 (0.8–3.9) |
AOR = odds ratio adjusted for age, sex, race/ethnicity, smoking history, history of diabetes, heavy alcohol consumption, history of pancreatitis, education level, and state of. residency.
Data on family history of cancer were missing for six patients and two controls.
OR was estimated compared to the reference subjects, without a family history of cancer.
INTERACTIONS BETWEEN RISK FACTORS
Table 5 shows the independent and joint effects of diabetes mellitus, cigarette smoking, and family history of pancreatic cancer on pancreatic cancer risk among the women and the men we studied. In particular, there was synergy between cigarette smoking and diabetes mellitus, as well as between cigarette smoking and family history of pancreatic cancer among women, after adjusting for the effect of other significant risk factors.
Table 5.
Synergistic Interaction Between Smoking, Diabetes, and Family History of Pancreatic Cancer (FHPC) Among Women
| Interaction Variables | Men AOR (95% CI)* | P | Women AOR (95% CI)* | P | |
|---|---|---|---|---|---|
| Smoking | Diabetes | ||||
| No | No | 1 (reference) | |||
| Yes | No | 1.4 (1.1–1.9) | 0.04 | 2.4 (1.7–3.4) | 0.0001 |
| No | Yes | 2.6 (1.3–5.2) | 0.006 | 4.2 (1.7–10.4) | 0.002 |
| Yes | Yes | 2.6 (1.6–4.4) | 0.0001 | 6.4 (2.3–17.5) | 0.0001 |
| Heavy smoking | Diabetes | ||||
| Yes | No | 1.5 (1.1–2.2) | 0.03 | 3.6 (2.2–5.7) | 0.0001 |
| No | Yes | 2.6 (1.3–5.3) | 0.008 | 4.1 (1.7–10.3) | 0.002 |
| Yes | Yes | 3.6 (1.8–7.2) | 0.0001 | 9.3 (2.0–44.1) | 0.005 |
| Smoking | FHPC | ||||
| No | No | 1 (reference) | |||
| Yes | No | 1.4 (1.1–1.9) | 0.01 | 2.2 (1.6–3.1) | 0.0001 |
| No | Yes | 4.6 (1.9–10.9) | 0.001 | 2.7 (1.1–6.7) | 0.03 |
| Yes | Yes | 2.4 (1.1–5.4) | 0.04 | 10.1 (2.2–45.9) | 0.003 |
| Heavy smoking | FHPC | ||||
| Yes | No | 1.6 (1.1–2.2) | 0.008 | 3.4 (2.1 –5.4) | 0.0001 |
| No | Yes | 4.7 (1.9–11.1) | 0.001 | 2.9 (1.2–7.5) | 0.02 |
| Yes | Yes | 2.2 (0.7–6.2) | 0.1 | 12.8 (1.6–108.9) | 0.02 |
AOR = odds ratio adjusted for age, sex, race/ethnicity, history of diabetes, family history of cancer, heavy alcohol consumption, education level, and state of residency.
S = synergy index described by Rothman (38) = (OR11 − 1)/(OR01 + OR10 − 2), where OR11 = odds ratio of the joint effect of two risk factors and OR01 and OR10 = OR of each risk factor in the absence of the other.
Both of these interactions fit the assumption of additive scales. Using the AOR as an estimate for the relative risk of disease development, the relative excess risk for patients with a history of cigarette smoking, particularly heavy smoking (>20 pack-years), along with diabetes mellitus or a family history of pancreatic cancer among women, exceeded the sum of the relative excess risks for each risk factor alone (for example, 6.4 – 1.0 > (2.4 –1.0) + (4.2 –1.0)). The estimated synergism index (S) among women was 1.2 (95% CI 0.2–2.6), 1.5 (95% CI 0.3–3.9), 3.1 (95% CI 1.5–4.7), and 2.7 (95% CI 1.2–4.3) for smoking/diabetes-, heavy smoking/diabetes-, smoking/family history of pancreatic cancer-, and heavy smoking/family history of pancreatic cancer-interaction, respectively. This may indicate that the joint effect of cigarette smoking and family history of pancreatic cancer is superadditive. No risk modification was observed among men, and no significant interaction was observed between other risk factors, such as heavy alcohol consumption with cigarette smoking, diabetes, or family history of cancer, in either men or women.
Using the prevalence of pancreatic risk factors in the study controls and the estimated AORs of these factors, we estimated that the PAR% values explained by the presence of diabetes mellitus, cigarette smoking, heavy alcohol consumption, and family history of pancreatic cancer in this study population were 9%, 23%, 3%, and 5%, respectively.
DISCUSSION
Our study is the largest case-control study of pancreatic cancer to date in which multiple risk factors were assessed simultaneously. We made three major findings: (a) we confirmed the significance of previously established risk factors for pancreatic cancer, such as diabetes mellitus, cigarette smoking and family history of pancreatic cancer in men and women; (b) we demonstrated the effects of heavy alcohol consumption on the risk for pancreatic cancer; and (c) we confirmed a significant synergistic interaction of cigarette smoking with diabetes mellitus and positive family history of pancreatic cancer. All estimated ORs were adjusted for the impact of confounding variables.
Our results suggest that diabetes mellitus is a significant risk factor for pancreatic cancer. Our estimated AOR is consistent with the pooled estimates from case-control and cohort studies presented by several meta-analyses (13–15). The hyperinsulinemia, greater blood glucose, and greater free fatty acids that occur in diabetes may promote the growth of pancreatic cancer (14, 39–41). Also, experimental evidence in hamsters indicated that the islet-cell proliferation associated with peripheral insulin resistance may enhance pancreatic carcinogenesis (42).
Most of our subjects with long-term diabetes were considered to have type II disease because of its late onset and because they were treated with oral hypoglycemic agents only. Because adult-onset diabetes is highly correlated with obesity and because obesity is also associated with risk for pancreatic cancer (43), the effect of obesity on risk among our patients cannot be excluded. The collection of obesity data was initiated late in this study, and information about obesity status was completed for only 232 patients and 345 controls. A restricted analysis in this subset of the population, with adjustment for a past history of obesity (defined as a body mass index [BMI] > 30) before the cancer diagnosis, did not alter the finding that diabetes was an independent risk factor for pancreatic cancer (data not shown). This finding is supported by Silverman et al. (24), who showed that diabetes and pancreatic cancer were associated in all BMI quartiles, with the strongest association in subjects at the lowest BMI quartile. Moreover, we observed high risks for pancreatic cancer among patients who were treated with insulin alone and, most notably, with both insulin and oral hypoglycemic agents. This observation is consistent with previous findings (8). While we have no information about why our patients with adult-onset diabetes were treated with insulin, one possible explanation is a failure of oral treatment to control severe disease; 75% of these patients had diabetes for >10 yr. It is possible that other mechanisms, such as oxidative stress and inflammation induced by severe hyperglycemia, explain the association between diabetes and pancreatic cancer (44).
At the same time, we observed no significant trend in pancreatic cancer risk magnitude with the duration of diabetes. This observation is consistent with the results of a meta-analysis of 17 case-control and 19 cohort studies (15). Since the latency period for pancreatic cancer cannot be determined and cancer development in general may take up to 10 yr, the lack of an increasing risk of pancreatic cancer with the long duration of diabetes may indicate that in some cases the diabetes was an epiphenomenon of the pancreatic cancer. In fact, diabetes was diagnosed concurrently with pancreatic cancer in 39% of our patients who had diabetes. Pancreatic cancer may cause diabetes by increasing peripheral insulin resistance (45), suppressing insulin secretion and impairing proinsulin conversion (46, 47), and causing chronic inflammation (48, 49). In summary, it is difficult to distinguish whether diabetes is a consequence or a risk factor of pancreatic cancer; both hypotheses are supported by experimental and epidemiologic evidence. In either case, and from the standpoint of public health, patients with diabetes mellitus should be considered at high risk for pancreatic cancer and may benefit from prevention and early detection programs (50).
Another key finding of our study is the significant increase in risk associated with heavy alcohol consumption. While our observations contradict those of other studies (5, 51), it is possible that the major reason for the observed lack of association shown by those studies was misidentification of alcohol exposure and underestimation of total ethanol consumption (52). This explanation is plausible because until now, there has been no standard definition of the alcohol content of a drink, and the type of beverage has not been taken into account when defining heavy drinking (34). In the current study, we used a validated and structured questionnaire and personally interviewed all subjects to ascertain their intake of all types of alcoholic beverages, the age at which they began drinking, the duration of intake, and the size and amount of intake according to the beverage type. Consistent with previous studies (9, 23), our results indicate that heavy ethanol consumption is the alcohol-related factor that contributes most to greater risk for pancreatic cancer. We previously used the same methodology of alcohol and ethanol assessment in alcohol-induced cancer (35).
The observed risk elevation of heavy alcohol consumption may be due to metabolic events that also have been linked to alcohol-associated chronic pancreatitis. It is generally accepted that ethanol metabolism alters the intracellular redox state, which may play the central role in the mechanisms underlying alcohol-induced chronic pancreatitis and pancreatic cancer (53–55). Ethanol metabolism through oxidation by alcohol dehydrogenases or through the microsomal oxidative system (cytochrome P450 E1) may generate toxic metabolites, such as acetaldehyde and reactive oxygen species. These metabolites can affect both the exocrine and endocrine pancreatic functions, activate pancreatic stellate cells to induce fibrosis, and cause the release of proinflammatory mediators (e.g., cytokines, NF-κB, COX-2), thereby inducing chronic pancreatitis. As a consequence, damage to the cellular organelles, DNA mutations, and genetic alterations may occur, all of which may contribute to pancreatic carcinogenesis.
Indeed, a past history of pancreatitis was significantly related to pancreatic cancer in our patients, but the magnitude of the AOR was correlated with only the duration of pancreatitis. This result may raise concerns about the difficulty of assessing the true risk elevation due to pancreatitis in a case-control setting. This difficulty might be reflected in three major biases: (a) recall bias during interviews with patients; (b) misclassification bias between chronic pancreatitis (irreversible tissue damage), acute pancreatitis (reversible tissue damage), and obstructive pancreatitis induced by a blockage or narrowing of the pancreatic duct by the pancreatic tumor (17, 18); and (c) bias due to the presence of risk factors for both pancreatic cancer and chronic pancreatitis (e.g., heavy alcohol consumption, cigarette smoking, diabetes mellitus, and gallstones). To overcome these biases, we separately analyzed subjects who had been diagnosed with pancreatitis >2 yr before their cancer diagnosis or enrollment in the study. We also reviewed the pathologic and radiologic records of the pancreatic cancer patients with possible chronic pancreatitis; all showed evidence of fibrosis, necrosis, calcification, or inflammation. Accordingly, we believe that pancreatitis is a major intermediate factor that could be related to major risk factors for pancreatic cancer (56, 57). In fact, we observed that all patients and controls with possible chronic pancreatitis had a history of heavy alcohol consumption (>60 mL of ethanol), long-term diabetes, gallstones, or heavy cigarette smoking (>20 pack-years). These factors may contribute to oxidative stress and chronic inflammation, leading to chronic pancreatitis. At the same time, some individuals may be genetically susceptible to oxidative stress and chronic inflammation induced by these factors, which may implicate the genetic contribution to pancreatic cancer.
Previous studies reported a greater risk of pancreatic cancer among individuals who had a prior history of cholecystectomy (24, 58). Such a greater risk could be related to increased release of cholecystokinin following cholecystectomy (59) or to the association between gallstones and chronic pancreatitis (60). In this study, we found no significant relationship between gallstones or prior cholecystectomy and pancreatic cancer after controlling for potential confounding variables as shown in Table 3. Our finding is consistent with previous studies (61, 62). It is possible that the observed positive association between cholecystectomy and pancreatic cancer in previous studies is confounded by other risk factors such as cigarette smoking, diabetes, chronic pancreatitis, heavy alcohol consumption, or family history of pancreatic cancer.
A significantly greater risk was demonstrated among subjects reporting a first-degree relative with pancreatic cancer (63–68). In this study, we observed a threefold greater risk for pancreatic cancer in subjects with a positive family history of pancreatic cancer. Having a first-degree or a second-degree relative with pancreatic cancer was a significant risk factor for the subject’s development of the disease. However, we believe that the accuracy of reporting pancreatic cancer is higher for first-degree relatives than for second-degree relatives. More than 50% of second-degree relatives were deceased grandparents, making it difficult to confirm the diagnosis. On the other hand, it is possible that our results were due to chance or were a function of a shared environmental exposure, such as cigarette smoke. However, we found no correlation between family history of pancreatic cancer and passive exposure to smoking during childhood or adulthood among either patients or controls (data not shown). In addition, all patients with pancreatic cancer were diagnosed after the fifth decade of life. Patients who had a positive family history of pancreatic cancer were later enrolled in a study of familial pancreatic cancer at this institution, during which the diagnosis of familial pancreatic cancer was confirmed (personal communication with the lead investigator).
The significantly elevated risk for pancreatic cancer extended to participants with a family history of breast or colon cancer after adjustment for all confounding risk factors. The same results were reported previously (24), and they are consistent with several inherited cancer syndromes in which genetic mutations are associated with both pancreatic cancer and cancers of other types, such as familial atypical multiple mole melanoma (p16 mutations) (69), breast cancer (BRCA2 mutations) (70), hereditary nonpolyposis colorectal cancer, and familial adenomatous polyposis (24, 71, 72).
The most notable finding of the current study was the interaction between risk factors. We showed a synergy (excess over additivity) between cigarette smoking and a positive family history of pancreatic cancer and between cigarette smoking and diabetes mellitus, independent of each other’s effects, in the etiology of pancreatic cancer among women.
The mechanisms by which cigarette smoking enhances the pancreatic cancer risk associated with diabetes mellitus are unknown. However, it is possible that, in some people with these risk factors, smoking-induced oxidative stress increases susceptibility to chronic inflammation, DNA damage, and pancreatic cancer development. Lowenfels et al. reported that, among patients with hereditary pancreatitis, pancreatic cancer developed 20 yr earlier in smokers than in nonsmokers (57). The synergistic effects of a positive family history and cigarette smoking on pancreatic cancer may be attributable to gene–environment interaction. Consistent with previous studies (4, 6, 7), we showed that the point estimate of association between smoking and pancreatic cancer was higher in women than in men. Moreover, our previous molecular epidemiology study of pancreatic cancer indicated that women with certain polymorphisms of carcinogen-metabolizing genes and dietary exposure to heterocyclic aromatic amines are at higher risk for pancreatic cancer than men (73). The risk modification of heavy smoking among patients with a positive family history for pancreatic cancer was previously suggested by Silverman et al. (24) and Schenk et al. (68), but neither of these groups evaluated the significance of the interaction in additive or multiplicative models.
Although our study is the largest to date, it has some limitations due to the source of pancreatic cancer patients. However, because of the high fatality of pancreatic cancer, a hospital-based design is more appropriate to ascertain newly diagnosed patients with pathologically confirmed adenocarcinoma of the pancreas. Moreover, we believe that our control selection was appropriate and representative of our study base, as elaborated in the Methods section. Another limitation may be that some suspected risk factors for pancreatic cancer were not included in our analysis, such as hormonal and menstrual factors (74, 75), use of aspirin (76, 77), physical activity (78), prior history of allergies (79), and occupational factors (80, 81). Questions related to these factors were only recently added to our questionnaire, and so information about these factors is not available for all subjects. Nevertheless, evidence for the significance of these risk factors has been inconclusive so far and may be confounded by a lack of adjustment for better established or known major risk factors (e.g., diabetes mellitus, heavy alcohol consumption, and family history of pancreatic cancer). Additionally, our study was specifically designed to minimize ascertainment or selection biases related to misdiagnosis of the cases. All our patients had pathologically confirmed adenocarcinoma of the pancreas, and both patients and controls were prospectively ascertained simultaneously and personally interviewed at M. D. Anderson.
In summary, our results suggested synergistic interaction between smoking and diabetes, and positive family history of pancreatic cancer among women after controlling for the confounding effects of other major risk factors. However, more assessment of such interaction is warranted in other large-scale epidemiological studies of different populations, in men and women separately.
If cigarette smoking, past history of diabetes mellitus, heavy alcohol consumption, and positive family history of pancreatic cancer are associated with pancreatic cancer independently from each other, we estimated that each factor contributed to 23%, 9%, 3%, and 5% of the pancreatic cancer cases in this study population, respectively. This finding is consistent with those from previous population-based studies (6, 9).
We support the establishment of an international consortium for pancreatic cancer to assist in the development of a risk model for pancreatic cancer in which estimated weighted scores are assigned to the major risk factors and the interactions between these factors. Such a model may help physicians detect pancreatic cancer earlier and may prompt the study of pancreatic cancer prevention strategies among high-risk individuals.
STUDY HIGHLIGHTS
What Is Current Knowledge
Cigarette smoking, diabetes mellitus, and family history of pancreatic cancer are the most established risk factors for pancreatic cancer.
What Is New Here
The magnitude of risk for smoking and diabetes is higher among women.
Heavy alcohol consumption (>60 mL ethanol/day) is an independent risk factor for pancreatic cancer.
Synergistic interactions between cigarette smoking, diabetes mellitus, and family history of pancreatic cancer are notable for pancreatic cancer development among women.
Acknowledgments
Financial support: The study was supported by NIH grants RO1 CA98380 (Li) and SPORE P20 101936 (Abbruzzese).
Footnotes
Guarantor of the article: Manal M. Hassan, M.D., M.P.H., Ph.D.
Potential competing interests: None.
Specific author contributions: All authors actively participated in the study and read the manuscript. Manal M. Hassan: epidemiologist, study design, statistical analysis, writing; Melissa L. Bondy: epidemiologic and statistical consultation; Robert A. Wolff: patient identification and clinical consultation; James L. Abbruzzese: funding, patient identification, assist in writing; Jean-Nicolas Vauthey: patient identification and clinical consultation (study population); Peter W. Pisters: patient identification and clinical consultation (study population); Douglas B. Evans: patient identification and clinical consultation (study population); Rabia Khan: interviewers for patient and controls (study methods); Ta-Hsu Chou: patient identification and clinical consultation (study population); Renato Lenzi: patient identification and clinical consultation (study population); Li Jiao: data management (study methods); Donghui Li: funding, methodology, assist in writing.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Gudjonsson B. Cancer of the pancreas 50 years of surgery. Cancer. 1987;60:2284–303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Silverman DT, Dunn JA, Hoover RN, et al. Cigarette smoking and pancreas cancer: A case-control study based on direct interviews. J Natl Cancer Inst. 1994;86:1510–6. doi: 10.1093/jnci/86.20.1510. [DOI] [PubMed] [Google Scholar]
- 5.Lee CT, Chang FY, Lee SD. Risk factors for pancreatic cancer in orientals. J Gastroenterol Hepatol. 1996;11:491–5. doi: 10.1111/j.1440-1746.1996.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156:2255–60. [PubMed] [Google Scholar]
- 7.Muscat JE, Stellman SD, Hoffmann D, et al. Smoking and pancreatic cancer in men and women. Cancer Epidemiol Biomarkers Prev. 1997;6:15–9. [PubMed] [Google Scholar]
- 8.Bonelli L, Aste H, Bovo P, et al. Exocrine pancreatic cancer, cigarette smoking, and diabetes mellitus: A case-control study in northern Italy. Pancreas. 2003;27:143–9. doi: 10.1097/00006676-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Silverman DT, Hoover RN, Brown LM, et al. Why do black Americans have a higher risk of pancreatic cancer than white Americans? Epidemiology. 2003;14:45–54. doi: 10.1097/00001648-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Tajima K, Takezaki T, et al. Epidemiology of pancreatic cancer in Japan: A nested case-control study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC) Int J Epidemiol. 2003;32:257–62. doi: 10.1093/ije/dyg062. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Permert J, Hakansson N, et al. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer. 2005;93:1310–5. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowenfels AB, Maisonneuve P. Epidemiology and prevention of pancreatic cancer. Jpn J Clin Oncol. 2004;34:238–44. doi: 10.1093/jjco/hyh045. [DOI] [PubMed] [Google Scholar]
- 13.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis JAMA. 1995;273:1605–9. [PubMed] [Google Scholar]
- 14.Fisher WE. Diabetes: Risk factor for the development of pancreatic cancer or manifestation of the disease? World J Surg. 2001;25:503–8. doi: 10.1007/s002680020344. [DOI] [PubMed] [Google Scholar]
- 15.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology. 1995;109:247–51. doi: 10.1016/0016-5085(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 17.Karlson BM, Ekbom A, Josefsson S, et al. The risk of pancreatic cancer following pancreatitis: An association due to confounding? Gastroenterology. 1997;113:587–92. doi: 10.1053/gast.1997.v113.pm9247480. [DOI] [PubMed] [Google Scholar]
- 18.Talamini G, Bassi C, Falconi M, et al. Early detection of pancreatic cancer following the diagnosis of chronic pancreatitis. Digestion. 1999;60:554–61. doi: 10.1159/000007706. [DOI] [PubMed] [Google Scholar]
- 19.Kamisawa T, Egawa N, Nakajima H, et al. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694–9. doi: 10.1111/j.1572-0241.2003.08775.x. [DOI] [PubMed] [Google Scholar]
- 20.Whitcomb DC. Inflammation and cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G315–9. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 21.Jura N, Archer H, Bar-Sagi D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005;15:72–7. doi: 10.1038/sj.cr.7290269. [DOI] [PubMed] [Google Scholar]
- 22.Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–52. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Partanen TJ, Vainio HU, Ojajarvi IA, et al. Pancreas cancer, tobacco smoking and consumption of alcoholic beverages: A case-control study. Cancer Lett. 1997;116:27–32. doi: 10.1016/s0304-3835(97)04744-7. [DOI] [PubMed] [Google Scholar]
- 24.Silverman DT, Schiffman M, Everhart J, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80:1830–7. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye W, Lagergren J, Weiderpass E, et al. Alcohol abuse and the risk of pancreatic cancer. Gut. 2002;51:236–9. doi: 10.1136/gut.51.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go VL, Gukovskaya A, Pandol SJ. Alcohol and pancreatic cancer. Alcohol. 2005;35:205–11. doi: 10.1016/j.alcohol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Lowenfels AB, Maisonneuve P. Risk factors for pancreatic cancer. J Cell Biochem. 2005;95:649–56. doi: 10.1002/jcb.20461. [DOI] [PubMed] [Google Scholar]
- 28.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Morabia A, Stellman SD, Wynder EL. Smoking prevalence in neighborhood and hospital controls: Implications for hospital-based case-control studies. J Clin Epidemiol. 1996;49:885–9. doi: 10.1016/0895-4356(96)00026-1. [DOI] [PubMed] [Google Scholar]
- 30.Wacholder S, McLaughlin JK, Silverman DT, et al. Selection of controls in case-control studies. I. Principles. Am J Epidemiol. 1992;135:1019–28. doi: 10.1093/oxfordjournals.aje.a116396. [DOI] [PubMed] [Google Scholar]
- 31.Wacholder S, Silverman DT, McLaughlin JK, et al. Selection of controls in case-control studies. II. Types of controls. Am J Epidemiol. 1992;135:1029–41. doi: 10.1093/oxfordjournals.aje.a116397. [DOI] [PubMed] [Google Scholar]
- 32.Wacholder S, Silverman DT, McLaughlin JK, et al. Selection of controls in case-control studies. III Design options. Am J Epidemiol. 1992;135:1042–50. doi: 10.1093/oxfordjournals.aje.a116398. [DOI] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer (IARC) Alcohol Drinking. IARC Monogr Eval Carcinog Risks Hum. 1988;44:xx–xx. [Google Scholar]
- 34.Diaz LE, Montero A, Gonzalez-Gross M, et al. Influence of alcohol consumption on immunological status: A review. Eur J Clin Nutr. 2002;56(Suppl 3):S50–3. doi: 10.1038/sj.ejcn.1601486. [DOI] [PubMed] [Google Scholar]
- 35.Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: Synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–13. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 36.Blot WJ, Day NE. Synergism and interaction: Are they equivalent? Am J Epidemiol. 1979;110:99–100. doi: 10.1093/oxfordjournals.aje.a112793. [DOI] [PubMed] [Google Scholar]
- 37.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112:467–70. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 38.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103:506–11. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 39.Fisher WE, Boros LG, O’Dorisio TM, et al. GI hormonal changes in diabetes influence pancreatic cancer growth. J Surg Res. 1995;58:754–8. doi: 10.1006/jsre.1995.1119. [DOI] [PubMed] [Google Scholar]
- 40.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: Evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res. 1996;63:310–3. doi: 10.1006/jsre.1996.0266. [DOI] [PubMed] [Google Scholar]
- 41.Fisher WE, Boros LG, Schirmer WJ. Reversal of enhanced pancreatic cancer growth in diabetes by insulin. Surgery. 1995;118:453–7. doi: 10.1016/s0039-6060(05)80358-7. [DOI] [PubMed] [Google Scholar]
- 42.Fisher WE, Muscarella P, Boros LG, et al. Variable effect of streptozotocin-diabetes on the growth of hamster pancreatic cancer (H2T) in the Syrian hamster and nude mouse. Surgery. 1998;123:315–20. [PubMed] [Google Scholar]
- 43.Berrington de Gonzalez A, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer. 2003;89:519–23. doi: 10.1038/sj.bjc.6601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saruc M, Pour PM. Diabetes and its relationship to pancreatic carcinoma. Pancreas. 2003;26:381–7. doi: 10.1097/00006676-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Permert J, Adrian TE, Jacobsson P, et al. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am J Surg. 1993;165:61–6. doi: 10.1016/s0002-9610(05)80405-2. [DOI] [PubMed] [Google Scholar]
- 46.Cersosimo E, Pisters PW, Pesola G, et al. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–93. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Nakamori S, Ishikawa O, Ohigashi H, et al. Increased blood proinsulin and decreased C-peptide levels in patients with pancreatic cancer. Hepatogastroenterology. 1999;46:16–24. [PubMed] [Google Scholar]
- 48.Farrow B, Sugiyama Y, Chen A, et al. Inflammatory mechanisms contributing to pancreatic cancer development. Ann Surg. 2004;239:763–9. doi: 10.1097/01.sla.0000128681.76786.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153–69. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 50.Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology. 2005;129:504–11. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalapothaki V, Tzonou A, Hsieh CC, et al. Tobacco, ethanol, coffee, pancreatitis, diabetes mellitus, and cholelithiasis as risk factors for pancreatic carcinoma. Cancer Causes Control. 1993;4:375–82. doi: 10.1007/BF00051341. [DOI] [PubMed] [Google Scholar]
- 52.Feunekes GI, van’t Veer P, van Staveren WA, et al. Alcohol intake assessment: The sober facts. Am J Epidemiol. 1999;150:105–12. doi: 10.1093/oxfordjournals.aje.a009909. [DOI] [PubMed] [Google Scholar]
- 53.Lieber CS, Seitz HK, Garro AJ, et al. Alcohol-related diseases and carcinogenesis. Cancer Res. 1979;39:2863–86. [PubMed] [Google Scholar]
- 54.Stewart S, Jones D, Day CP. Alcoholic liver disease: New insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408–13. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal DP. Molecular genetic aspects of alcohol metabolism and alcoholism. Pharmacopsychiatry. 1997;30:79–84. doi: 10.1055/s-2007-979487. [DOI] [PubMed] [Google Scholar]
- 56.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 57.Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169–70. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 58.Shibata A, Mack TM, Paganini-Hill A, et al. A prospective study of pancreatic cancer in the elderly. Int J Cancer. 1994;58:46–9. doi: 10.1002/ijc.2910580109. [DOI] [PubMed] [Google Scholar]
- 59.Hyvarinen H, Partanen S. Association of cholecystectomy with abdominal cancers. Hepatogastroenterology. 1987;34:280–4. [PubMed] [Google Scholar]
- 60.Hardt PD, Bretz L, Krauss A, et al. Pathological pancreatic exocrine function and duct morphology in patients with cholelithiasis. Dig Dis Sci. 2001;46:536–9. doi: 10.1023/a:1005690930325. [DOI] [PubMed] [Google Scholar]
- 61.Schernhammer ES, Michaud DS, Leitzmann MF, et al. Gallstones, cholecystectomy, and the risk for developing pancreatic cancer. Br J Cancer. 2002;86:1081–4. doi: 10.1038/sj.bjc.6600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talamini G, Falconi M, Bassi C, et al. Previous cholecystectomy, gastrectomy, and diabetes mellitus are not crucial risk factors for pancreatic cancer in patients with chronic pancreatitis. Pancreas. 2001;23:364–7. doi: 10.1097/00006676-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 64.Tersmette AC, Petersen GM, Offerhaus GJ, et al. Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7:738–44. [PubMed] [Google Scholar]
- 65.Rulyak SJ, Lowenfels AB, Maisonneuve P, et al. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124:1292–9. doi: 10.1016/s0016-5085(03)00272-5. [DOI] [PubMed] [Google Scholar]
- 66.James TA, Sheldon DG, Rajput A, et al. Risk factors associated with earlier age of onset in familial pancreatic carcinoma. Cancer. 2004;101:2722–6. doi: 10.1002/cncr.20700. [DOI] [PubMed] [Google Scholar]
- 67.Ghadirian P, Liu G, Gallinger S, et al. Risk of pancreatic cancer among individuals with a family history of cancer of the pancreas. Int J Cancer. 2002;97:807–10. doi: 10.1002/ijc.10123. [DOI] [PubMed] [Google Scholar]
- 68.Schenk M, Schwartz AG, O’Neal E, et al. Familial risk of pancreatic cancer. J Natl Cancer Inst. 2001;93:640–4. doi: 10.1093/jnci/93.8.640. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein AM, Fraser MC, Struewing JP, et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333:970–4. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- 70.Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium. J Natl Cancer Inst. 1999;91:1310–6. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 71.Lynch HT, Voorhees GJ, Lanspa SJ, et al. Pancreatic carcinoma and hereditary nonpolyposis colorectal cancer: A family study. Br J Cancer. 1985;52:271–3. doi: 10.1038/bjc.1985.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lumadue JA, Griffin CA, Osman M, et al. Familial pancreatic cancer and the genetics of pancreatic cancer. Surg Clin North Am. 1995;75:845–55. doi: 10.1016/s0039-6109(16)46731-9. [DOI] [PubMed] [Google Scholar]
- 73.Li D, Jiao L, Li Y, et al. Polymorphisms of cytochrome P4501A2 and N-acetyltransferase genes, smoking, and risk of pancreatic cancer. Carcinogenesis. 2006;27:103–11. doi: 10.1093/carcin/bgi171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duell EJ, Holly EA. Reproductive and menstrual risk factors for pancreatic cancer: A population-based study of San Francisco Bay Area women. Am J Epidemiol. 2005;161:741–7. doi: 10.1093/aje/kwi104. [DOI] [PubMed] [Google Scholar]
- 75.Teras LR, Patel AV, Rodriguez C, et al. Parity, other reproductive factors, and risk of pancreatic cancer mortality in a large cohort of U.S. women (United States) Cancer Causes Control. 2005;16:1035–40. doi: 10.1007/s10552-005-0332-4. [DOI] [PubMed] [Google Scholar]
- 76.Schernhammer ES, Kang JH, Chan AT, et al. A prospective study of aspirin use and the risk of pancreatic cancer in women. J Natl Cancer Inst. 2004;96:22–8. doi: 10.1093/jnci/djh001. [DOI] [PubMed] [Google Scholar]
- 77.Menezes RJ, Huber KR, Mahoney MC, et al. Regular use of aspirin and pancreatic cancer risk. BMC Public Health. 2002;2:18. doi: 10.1186/1471-2458-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sinner PJ, Schmitz KH, Anderson KE, et al. Lack of association of physical activity and obesity with incident pancreatic cancer in elderly women. Cancer Epidemiol Biomarkers Prev. 2005;14:1571–3. doi: 10.1158/1055-9965.EPI-05-0036. [DOI] [PubMed] [Google Scholar]
- 79.Holly EA, Eberle CA, Bracci PM. Prior history of allergies and pancreatic cancer in the San Francisco Bay area. Am J Epidemiol. 2003;158:432–41. doi: 10.1093/aje/kwg174. [DOI] [PubMed] [Google Scholar]
- 80.Kernan GJ, Ji BT, Dosemeci M, et al. Occupational risk factors for pancreatic cancer: A case-control study based on death certificates from 24 U.S. states. Am J Ind Med. 1999;36:260–70. doi: 10.1002/(sici)1097-0274(199908)36:2<260::aid-ajim5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 81.Ji BT, Silverman DT, Stewart PA, et al. Occupational exposure to pesticides and pancreatic cancer. Am J Ind Med. 2001;39:92–9. doi: 10.1002/1097-0274(200101)39:1<92::aid-ajim9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]


