Abstract
In the present study, a tandem-repeat type galectin was characterized from an embryonic cell line (Bge) and circulating hemocytes of the snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni. The predicted B. glabrata galectin (BgGal) protein of 32 kDa possessed 2 carbohydrate recognition domains, each displaying 6 of 8 conserved amino acids involved in galactoside-binding activity. A recombinant BgGal (rBgGal) demonstrated hemagglutinating activity against rabbit erythrocytes, which was specifically inhibited by galactose-containing sugars (lacNAc/lac > galNAc/gal). Although native galectin was immunolocalized in the cytoplasm of Bge cells and the plasma membrane of a subset of snail hemocytes (60%), it was not detected in cell-free plasma by Western blot analysis. The findings that rBgGal selectively recognizes the schistosome-related sugar, lacNAc, and strongly binds to hemocytes and the tegument of S. mansoni sporocysts in a sugar-inhibitable fashion suggest that hemocyte-bound galectin may be serving as pattern recognition receptor for this, or other pathogens possessing appropriate sugar ligands. Based on molecular and functional features, BgGal represents an authentic galectin, the first to be fully characterized in the medically-important molluscan Class Gastropoda.
Keywords: lectin, gastropod mollusk, hemocyte, Bge cell line, innate immunity
1. Introduction
Galectins represent a large family of structurally-related, phylogenetically diverse lectins with a carbohydrate binding specificity primarily to β-galactoside residues. In mammalian species this family is currently represented by 15 members (Gal-1 through -15) that are differentiated on the basis of the number of carbohydrate recognition domains (CRDs), presence/length of a CRD linker peptide or N-/C-terminal tails, amino acid sequence homology, especially of highly-conserved residues within the CRDs, and its metal ion-independent functionality (Barondes et al., 1994; Cooper, 2002; Leffler et al., 2004). Despite their narrow ligand binding affinity for β-galactosides, galectins have been implicated in a diversity of cellular functions including cell adhesion/proliferation, development/morphogenesis, tumor cell metastasis and immune regulation/innate immunity (Hughes, 2002; Vasta et al., 2004a; Zick et al., 2004; Camby et al., 2006). The role of galectins as effectors or modulators of the immune response has been most extensively studied in vertebrates where they have been implicated in apoptotic regulation of B/T-cell populations, cytokine signaling, monocyte/macrophage-mediate inflammation and microbe phagocytosis (Sano et al., 2003; Young and Meeusen, 2004; Acosta-Rodriguez et al., 2004; Liu, 2005; Rubinovitch and Gruppi, 2005; Rubinstein et al., 2006; Barrionuevo et al., 2007).
Metazoan invertebrates representing a diversity of major phyla also possess multiple members of the galectin superfamily as evidenced by both molecular and functional criteria (Vasta et al., 2004b). These are most highly represented in such organisms as nematodes (Hirabayshi et al., 1992; Greenhalgh et al. 1999; Newlands et al., 1999), arthropods (Pace et al., 2002; Pace and Baum, 2004, Barat-Houari et al., 2006; Huang et al., 2007; Kamhawi et al. 2004), tunicates (Parrinello et al., 2007) and sponges (Pfeifer et al., 1993; Stalz et al., 2006). In the Phylum Mollusca, the presence of galactose-binding lectins also has been demonstrated (e.g., Suzuki and Mori, 1989; Mitra and Sakar, 1998; Wilson et al., 1992; Ozeki, 1998), and in some species, the molecular mass of isolated lectins were consistent with galectins possessing single (Mitra and Sakar, 1998) or dual (Ozeki, 1998) CRDs. In addition, expressed sequence tags (EST)/partial sequences for galectin homologues have been identified (Rafferty and Powell, 2002; Mitta et al., 2005; GenBank™ accession nos. AJ550634, BG467428, CO635934, CX6376, EE722624, CK989149, CN476116), including a complete coding sequence from the abalone Haliotis (GenBank™ accession no. EF392832). Thus, there is substantial support for the existence of this 4 gene family in molluscs. However, although galactose-binding proteins previously have been reported in the hemolymph of bivalve (Suzuki and Mori, 1989, Baldo et al., 1975), gastropod (Mitra and Sakar, 1998; Mansour, 1996), and cephalopod (Rogener et al., 1985) molluscs, their molecular structures, expression profiles and specific role(s) in the internal defense system of these organisms remain unknown.
Despite evidence for galectin-like proteins within the molluscs, detailed studies characterizing the structure, ligand-binding properties and protein expression of galectins has been very limited in this animal group. To date only one other molluscan galectin, that of the oyster Crassostrea virginica has been characterized both functionally and at the molecular level (Tasumi and Vasta, 2007). In the present study, we report the cloning and functional characterization of a tandem-repeat type galectin from circulating phagocytic hemocytes of the freshwater snail Biomphalaria glabrata, and demonstrate its binding reactivity with the tegumental surface of larval Schistosoma mansoni, a human blood fluke that utilizes B. glabrata as its intermediate host. To our knowledge this study represents the first investigation of a galectin at the molecular level from a mollusc representing the medically-important Class Gastropoda.
2. Materials and Methods
2.1. Cell and tissue sources used in the study
Cultures of the Biomphalaria glabrata embryonic (Bge) cell line were obtained from American Type Culture Collection (ATCC CRL 1494; Rockville, MD) and maintained in 50 cc culture flasks in complete Bge medium (Hansen, 1976) containing heat-inactivated 10% fetal bovine serum (FBS), penicillin and streptomycin, at 26°C under atmospheric conditions (Yoshino and Laursen, 1995). Whole hemolymph, containing circulating hemocytes, was obtained from lab-reared B. glabrata snails (BS-90 strain) as detailed in Section 2.6. Snails were maintained in 10-gal aquaria at 26°C and fed leaf lettuce ad libitum.
2.2. RNA extraction and Rapid 5’ and 3’ amplification of cDNA ends (RACE)
Bge cells and B. glabrata hemocytes were isolated as previously described (Humphries and Yoshino, 2006). Total RNA from both cell sources was extracted with Trizol (Invitrogen Corporation, Carlsbad, CA), followed by precipitation with isopropanol according to the manufacturer’s protocol and stored at −80°C until further use. An EST (GenBank™ accession no. AW740392) from a BS90 B. glabrata hemocyte library was selected for its high similarities to Gal-4. In order to obtain the complete coding sequence several primers, [forward (65: 5’ TCAATCACCGCATTCACCC 3’; 92: 5’ GTGTGTCTCACTTGAACATCC 3’) and reverse (189: 5’ TGGTCTATTGTCCGCTGCTG 3’; 134: 5’ GACTCAAGTTGACATCACCC 3’)], were designed and used in RACE reactions (First Choice™ RLM-RACE Kit, Ambion, Applied Biosysytem Buisness, Austin, TX) to amplify both 5’- and 3’-ends thus permitting the completion and cloning of the full-length cDNA sequence from Bge cells.
2.3. Cloning and sequencing
Cloning of the complete cDNA sequence was performed following previously described protocols (Dinguirard and Yoshino, 2006). Briefly, specific primers (Fw: 5’: ATGGCATATCCTGTACCTTACTC 3’; Rv: 5’ TTGGTCTATTGTCCGCTGC 3’) were used to amplify the desired 900 bp galectin sequence from Bge cell template cDNA. The amplified product was then purified, cloned and sequenced using the plasmid-based primers T7 (5’GGCCGCGGGAATTCGATT 3’) and sp6 (5’GATTTAGGTGACACTATAG 3’). Samples were sequenced at the Biotechnology Center (University of Wisconsin-Madison) using an Applied Biosystems 3730XL automated DNA analyzer (Foster City, CA) incorporating 50 cm capillary arrays on a POP-7 matrix. Data were analyzed using PE-Biosystems Sequencing Analysis software, version 3.7.
2.4. Sequence analysis and bioinformatics procedures
Complementary DNA sequence identity and homology analyses were performed using the Basic Local Alignment Search Tool (BLAST-X) from the National Center for Biotechnology Information (NCBI) database. The predicted amino acid determinations, sequence alignment analyses, presence/absence of hydrophobic domains using the Kite-Doolittle method, and cluster tree analysis were performed using Vector NTI 8 (InforMax, Inc., Invitrogen). Testing for the presence of signal peptide sequences and nonclassical secretion pathways was accomplished using SignalP (version 3.0) and SecretomeP (version 1.0b), respectively (Center for Biological Sequence Analysis, Technical University of Denmark, http://www.cbs.dtu.dk/index.shtml).
2.5. Protein expression and production of recombinant B. glabrata galectin (rBgGal)
The 900 bp sequence was framed with NotI and SalI restriction sites using the primers: (Fw-SalI-Gal: 5’ GTCGACAATGGCATATCCTGTACCT 3’ and Rv-NotI-Gal: 5’ GCGGCCGCTTATTGTATTGGTCT 3’). Briefly, the cDNA sequence coding for galectin was used as template in a standard amplification reaction at an annealing temperature of 60°C. The NotI/SalI-Galectin PCR fragment was then purified (Qiagen, Valencia, CA) and ligated following standard protocol into a pET28c(+) expression vector (Novagen, EMD Chemicals Inc., San Diego, CA), followed by transformation of the plasmid construct in BL21 (DE3) competent E.coli cells (Novagen) and sequencing to verify its open reading frame. Production of recombinant protein was performed following the standard protocol of the Overnight Express Auto Induction Kit (Novagen) involving cultivation of starter cultures in Teriffic Broth™ (TB; Novagen) containing 15µg/ml kanamycin. This was followed by extraction of the recombinant protein in 10 mL phosphate-buffered imidazole (PBI; pH 7.4) containing 0.025% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Cells were then stored at −80°C prior to purification.
2.5. Purification of recombinant protein and antibody production
Frozen pellets of rBgGal-producing cells were thawed, sonicated (Branson Ultrasonics Corp., Danbury, CT) at an output setting of 4 at 60 pulses/min for 10 min, centrifuged at 10000 × g for 10 min, filtered with a 0.22 µm filter (Fisher, Pittsburgh, PA) and stored at −80°C until use. Samples were loaded on a nickel-Sepharose His-Trap HP column (Amersham, Piscataway, NJ) column, then eluted with 10–40 ml of phosphate buffers containing a range of 100–400 mM imidazole concentrations. Eluted proteins were collected in 1-ml fractions and analyzed for purity by sodium dodecyl sulfate polyacrylamide electrophoresis (SDS-PAGE).
To prepare His-tagged rBgGal protein for polyclonal antibody production, 1-ml elution fractions were combined in a regenerated cellulose 5 kDa MW cutoff centrifugal filter (Millipore, Billerica, MA), washed twice with PBS (pH 7.4) at 4°C to remove imidazole and quantified using the BCA Protein Assay Kit (Pierce, Rockford, IL). Recombinant protein aliquots (2 mg/ml) were stored in 10% glycerol at 4°C and shipped directly to ProteinTech, Inc. (Chicago, IL) for polyclonal antibody production. Standard protocols for generating polyclonal anti-rBgGal antibodies utilized 2 rabbits on a 102-day immunization schedule. Anti-BgGal antisera were purified on a rBgGal affinity column, the titres quantified by enzyme-linked immunosorbent assay (ELISA), aliquoted and stored at −80°C.
2.6. Collection and extraction of proteins from snail hemocytes and Bge cells
Snails were cleaned and bled as previously described by Sminia and Barendsen (1978). Hemolymph containing circulating hemocytes was pooled on iced-petri dishes and transferred to sterile 15-ml tubes containing cold Chernin’s balanced salt solution (CBSS; Chernin, 1963). Blood was then centrifuged at 4000 rpm for 20 min at 4°C, the plasma portion removed, and the pellet was washed once with 3 ml CBSS before a final centrifugation for 15 min (4000 rpm, 4°C). After removal of the CBSS, hemocytes were treated with 1% SDS loading buffer and boiled for 5 min. The recovered plasma was filtered (0.45 µm), concentrated by centrifugation using first a 100 kDa cut-off spin-filter to remove the majority of hemoglobin, and then a 10 kDa cut-off filter (Millipore) to concentrate any BgGal. All protein samples were subjected to SDS-PAGE gels (12.5%), and assayed for the presence of galectin by Western blot analysis (Humphries and Yoshino, 2006).
Preparation of Bge cells for SDS-PAGE/Western blot analysis was as follows: Cells cultures grown to confluency were washed in situ 4 times with snail CBSS+ (CBSS supplemented with 1g/L each of glucose and trehalose) at 26°C. The cells were then harvested and washed in sPBS (Yoshino, 1981; sPBS, pH 7.2), extracted using 1% Triton X-100, quantified using the BCA Protein Assay Kit (Pierce), boiled 5 min, and 20 µg of Bge cell total protein were subjected to SDS-PAGE/ Western blotting.
2.7. Western blot analysis
Protocols used for Western immunoblotting were similar to those previously described (Humphries and Yoshino, 2006). Briefly, snail proteins were separated on a 12.5% SDS-PAGE gel, followed by transfer to a nitrocellulose membrane (Hybond ECL; Amersham Pharmacia Biotech) by semi-dry electroblotting, then blocked overnight in Tris-buffered saline (sTBS; 20 mM Tris, 45 mM NaCl, pH 7.4) containing 5% dry milk, and reacted with anti-rBgGal antibodies (diluted 1:5000 for hemocytes; 1:2000 for plasma proteins; 1:2500 for Bge cell extracts) or rabbit pre-immune serum (1:5000) for 16 hr at 4°C. Membranes were washed in sTBS, incubated for 1 hr with alkaline phosphatase-conjugated goat anti-rabbit antiserum (GAR-AP) in 5% dry milk (Promega, Madison, WI; at 1/10000 dilution for hemocytes and plasma, and 1:5000 dilution for Bge cell extract); and developed in the BCIP/NBT chromogen substrate (Pierce). For initial detection of His-tagged rBgGal during protein purification, blotted protein fractions were probed with a horseradish peroxidase anti-His antibody (Qiagen), followed by chromogenic development with 4-chloro-1- naphtol and 3% H2O2.
2.8. Hemagglutination (HA) and HA sugar inhibition assays
The hemagglutinating properties of BgGal were tested using 1% paraformaldehyde-fixed rabbit erythrocytes (RBCs, Innovative Research Inc., Southfield, MI) as indicator cell. A serial dilution series, starting at 130 µg/ml of rBgGal was used to determine the minimum concentration of galectin necessary to produce a positive agglutinaltion reaction with rabbit RBCs. Briefly, the recombinant-galectin protein was added to the wells of V-bottom hemagglutination microplates and twofold serially diluted in mammalian PBS (mPBS; pH 7.2) before adding an equal volume of 1% rabbit RBCs to each protein dilution. Plates were incubated at 22°C for 1 hr, before visual assessment of agglutination reactions.
After determining the minimum concentration of rBgGal protein required to produce a positive reaction, an agglutinating concentration of 8µg/ml of protein was used to assess its sugar-binding specificity. In these assays, various sugars (Sigma-Aldrich) known to bind (gal, lac, galNAc, lacNAc) and not to bind (glc; man, tre; fuc, glcNAc) galectins (Leffler et al., 2004) were twofold serially diluted (starting 200 µg/ml initial concentrations), followed by addition of recombinant BgGal protein to each reaction well. After a 1 hr preincubation period, 1% rabbit RBCs were added to each well, incubated for an additional hr, and agglutination reactions were visually assessed.
2.9. Immunocytochemistry
Whole hemolymph, containing circulating hemocytes, was obtained from B. glabrata snails as described above and 30 µL was placed on glass coverslips for 1 hr to allow cells to adhere and spread. Hemocyte preparations were washed 5 times with CBSS, fixed with 2% buffered PFA for 15 min, washed 3 times with sPBS, and blocked for 1 hr in sPBS containing 5% normal goat serum (NGS) and 0.03% Triton X-100. Cells were treated with the anti-rBgGal antibody (diluted 1:200 in sPBS) for 2 hr, followed by 5 washes in sPBS, and incubation for 1 hr with Alexa 488-conjugated goat anti-rabbit antiserum (GAR-Alexa; 5µg/mL). After 3 washes in sPBS, cells were mounted in glycerol and viewed with a Nikon inverted epifluorescent microscope (Nikon Eclipse TE300, Nikon Instruments Inc., Melville, NY). Negative specificity controls consisted of treating cells with anti-rBgGal previously absorbed with a nitrocellulose strip (NC) containing immobilized rBgGal, or treatment with GAR-Alexa alone. The positive anti-rBgGal antibody control consisted of antiserum absorbed with a NC strip blocked only with 10% dry milk. Images were captured using a CoolSnap ES camera (Nikon Instrument Inc.) and analyzed with Metamorph (version 7.1.2) imaging system (Universal Imaging Corp., Molecular Devices, Sunnyvale, CA). Methods for localizing of endogenous BgGal in the Bge cell line were similar to those described for hemocytes.
2.10. Sporocyst and sepharose beads binding assays
In order to determine the specific binding of rBgGal to the surface of the parasite, live-sporocysts were treated with rBgGal (16µg/ml), or rBgGal pre-incubated with lac, fuc (50 mM) or lacNAc (20 mM) for 1 hr. The sporocysts were then washed 3 times in CBSS, incubated with anti-rBgGal (1:100 dilution in CBSS) for 1 hr, followed by 3 washes in CBSS before final treatment with GAR-Alexa (5µg/mL; 1 hr at 22°C). After 3 subsequent washes in CBSS, sporocysts were fixed with 1% PFA prior to epifluorescence observation. Approximately 150 sporocysts in each treatment group were qualitatively assessed for anti-rBgGal binding (bright fluorescence = +; weak fluorescence = −; see Fig. 6), counted, and the percent positive (i.e., bright) sporocysts determined for each treatment (Table 2). Data were analyzed by one-way ANOVA, followed by means comparisons using Student’s t-test. In addition, to determine more specifically which glycans might be involved in the rBgGal binding to the sporocysts surface, sepharose beads conjugated with 3 different BSA-neoglycoconjugates [BSA-lacNAc (galactose β1–4 N-acetylglucosamine), BSA-slacNAc (neuraminic acid α2–3/6 galactose β1-4 N-acetylglucosamine), or BSA-LeX (galactose β1–4 [fucose α1–3] N-acetylglucosamine)] were used in a solid-phase anti-rBgGal immunoassays. Beads conjugated to BSA alone served as a negative glycan control. Twenty beads conjugated with BSA alone or glycan-conjugated BSA were incubated for 1 hr with rBgGal (16 µg/mL in CBSS), washed 3 times in CBSS, treated with anti-rBgGal antibody (1:100 diluted in CBSS) for 1 hr, washed 3 times in CBSS, incubated for 30 min with GAR-Alexa (5µg/mL CBSS), again washed and assessed for fluoresecent signal using epifluorescence microscopy. Images of both sporocysts and sepharose beads were normalized to background fluorescence of negative controls, captured using a Nikon Cool-snap digital camera and analyzed with Metamorph (v.7.1.2) as described above.
Table 2.
Percentage of live-sporocysts binding rBgGal, inhibition consisted of pre-incubating the rBgGal with lacNAc or lactose or fucose for 1 hr before treatment with sporocysts. The CBSS control consisted of treating sporocysts with anti-rBgGal antibodies, followed by GAR-Alexa.
| TREATMENT | % SPOROCYSTS BINDING rBgGal |
|---|---|
| rBgGal along | 82.0 ± 8.5 |
| rBgGal + lacNAc | 47.2 ± 7.1 * |
| rBgGal + lac | 15.0 ± 7.6 ** |
| rBgGal + fuc | 85.3 ± 5.5 |
| CBSS along | 1.4 ± 1.3 |
significantly different from rBgGal-alone treatment at P =0.0005 and P <0.0001, respectively, using 1-way ANOVA (F =193.1; P <0.0001), followed by Student’s t-test (2-tailed).
3. Results
3.1. Galectin gene cloning from B. glabrata embryonic (Bge) cells and structural analysis
Based on a high sequence similarity to mammalian galectin-4 (Gal-4), the B. glabrata EST GenBank™ accession no. AW740392 (deposited by N. Raghavan et al.) was subjected to 3’- and 5’- RACE reactions using Bge cell cDNA as templates resulting in the cloning and sequencing of the entire putative B. glabrata galectin cDNA (BgGal; GenBank™ accession no. EF534720) (Fig. 1). In addition, using primers based on the Bge cell galectin sequence, a cDNA from circulating snail hemocytes also was cloned and sequenced (GenBank™ accession no. EF687664), revealing the presence and expression of a nearly identical galectin protein in these cells (Fig. 1). Of the 5 nucleic acid substitutions in the hemocyte sequence, only 2 resulted in amino acid (aa) changes at aa28 and aa47 within the first CRD. Overall, the predicted protein encoded by the BgGal cDNA consisted of 284 amino acids and had a calculated molecular mass of 32017.96 Da.
Fig. 1.
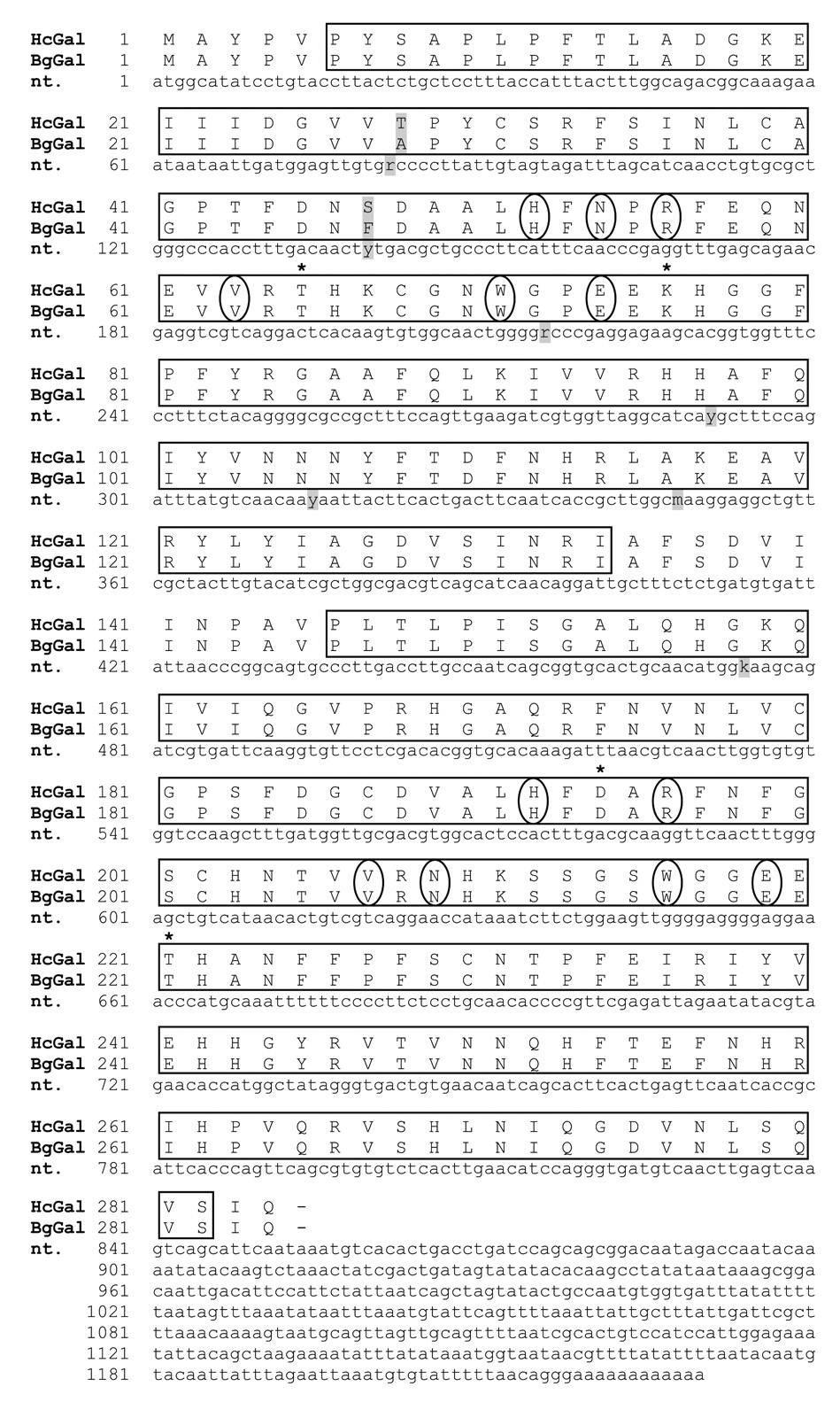
Structural characterization and comparison of the tandem-repeat galectins from the B. glabrata embryonic cell line (BgeGal) and circulating hemocytes (HcGal). Two CRDs were identified (see boxed areas for CRD1: aa6–aa134 and CRD2: aa146–aa282), separated by an 11 aa peptide linker. Comparison of nucleotide (nt) sequences show 5 differences (shaded in gray; r = a or g, y = c or t, m = a or c, k = g or t), that result in 2 aa changes (aa28 and aa47) within CRD1. Circled aa show 6 of 8 highly conserved aa within each CRD, responsible for the galactosyl-binding activity (CRD1: H52, N54, R56, V63, W71, E74; CRD2: H192, R196, V207, N209, W216, E219). Asterisks (*) indicate the 4 substituted aa (CRD1: T65, K76, CRD2: D194, T221).
Further aa sequence analysis of BgGal revealed the presence of two homologous CRDs, characteristic of tandem-repeat type galectins. The dual BgGal CRDs were comprised of a 130 N-and 136 C-terminal amino acids, highly conserved aa motifs within each CRD known to be responsible for β-galactoside sugar binding activity, and a unique 11 aa linking peptide (Fig. 1). Although the two BgGal CRDs share only 43% aa identity between them, each CRD contains 6 of 8 conserved aa known to confer sugar binding activity for invertebrate tandem-repeat type galectins (Pace et al., 2002; Huang et al., 2007). Further comparison of BgGal with two other fully sequenced molluscan multi-CRD galectins reveal that BgGal has highest sequence identity with the abalone Haliotis dual-CRD galectin (41% for CRD1 and 47% for CRD2) compared to any of the 4 CRDs contained in the oyster Crassostrea virginica (~34% for CRDs 1/2 and ~27% for CRDs 3/4) (Fig. 2A). Cluster tree analysis of vertebrate and invertebrate dual-CRD or tandem-repeat type galectins grouped BgGal within the metazoan invertebrate clade, showed closest affinity to other molluscs, including the abalone Haliotis Gal-4-like protein (GenBank™ accession no. EF392832), and partial Gal-4-like oyster Crassostrea spp. sequences (Fig. 2B). Furthermore, BgGal appeared to possess neither signal peptide or transmembrane domains based on SignalP 3.0 and Kyte-Doolittle hydropathy analyses, respectively. However, despite lacking a secretory signal sequence, SecretomeP analysis revealed a neural network output (NN) score of 0.589, which was close to the cutoff of ≥0.6, a value predicting a nonclassical secretory pathway.
Fig. 2.
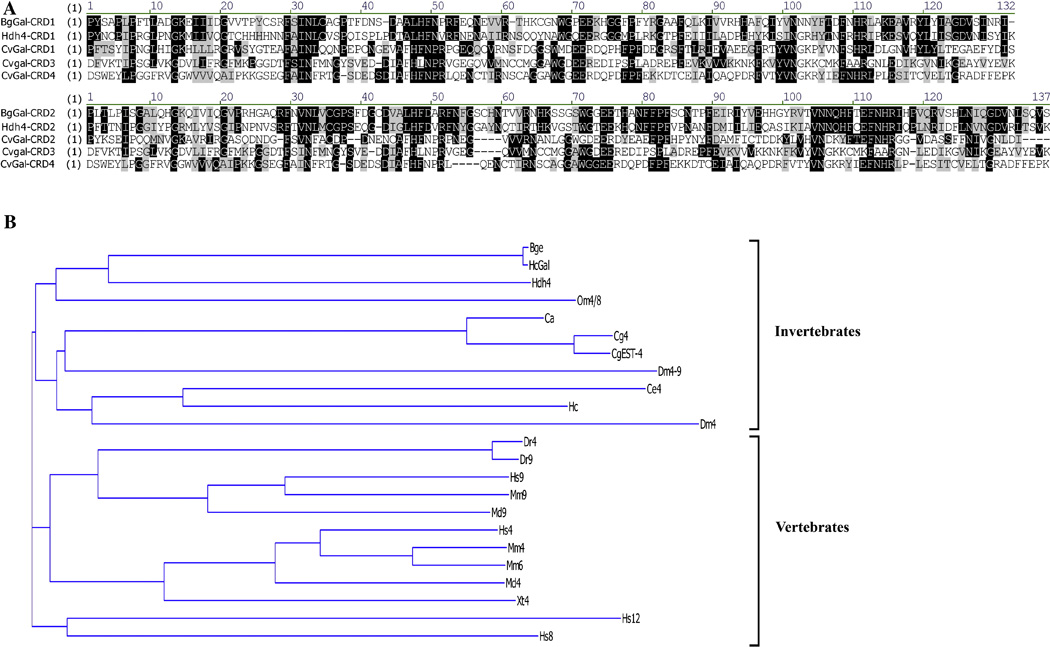
(A) Protein sequence alignment of multi-CRD molluscan galectins: B. glabrata (BgGal), abalone Haliotis (Hdh4) and the oyster C. virginica (CvGal) (GenBank™ accession nos. ABQ09359, ABN54798 and DQ779197, respectively). First alignment series shows the aa identities (shaded in black) and similarities (gray) of the BgGal CRD1 with all other CRD1s, and CRD3 and 4 from the oyster. Second alignment series compares BgGal CRD2 with the other CRD2 sequences and the oyster CRD3 and 4. (B) Cluster tree analysis of selected invertebrate (Bge cells and B. glabrata hemocyte galectins, BgGal, HcGal, respectively; abalone Haliotis discus hannai Gal-4, Hdh4; Ornithodoros moubata Gal-4/8, Om4/8; D. melanogaster Gal-4/9, Dm4/9; C. elegans Gal-4, Ce4; Haemonchus contortus dual-CRD, Hc; Crassostrea spp., CgEST4, Cg4, Ca) and vertebrate (Danio rerio Gal-4 and 9, Dr4 and Dr9; Mus musculus Gal-4, 6 and 9, Mm4, Mm6 and Mm9; Monodelphis domestica Gal-4 and 9, Md4 and Md9; Xenopus tropicalis Gal-4, Xt4; Homo sapien Gal-4, 8, 9 and 12, Hs4, Hs8, Hs9 and Hs12) dual or tandem-repeat type galectins. This cluster analysis demonstrates the higher structural similarities within invertebrate tandem-repeat galectins compared to their vertebrate counterparts. GenBank™ accession nos.: Om4/8: BAF43802; Dm4: AAF57667; Dm4/9: AAL87743; Ca: ABC69709; Cg4: CAD79473; CgEST4: BG467428; Ce4: NP_497763; Hc: AAF63405; Dr4: AAR84192; Dr9: NP_001001817; Mm4: AAH94008; Mm6: O54891; Mm9: AAH03754; Md4: XM_001362670; Md9: XP_001375560; Xt4: NP_001011449; HS4: NP_006140; Hs8: AAF19370; HS9: AAI05945; HS12: NP_149092;
3.2. Expression of recombinant galectin and anti-galectin antibody production
Cloning and expression of the galectin cDNA in BL21 (DE3) E. coli resulted in the production of a histidine-tagged recombinant B. glabrata galectin (rBgGal) of approximately 37 kDa, which was then isolated as a single band with 200 mM imidazole buffer from a nickel-Sepharose His-Trap HP column (Fig. 3A). This band also reacted with an anti-His-tag antibody by Western blot analysis (Fig. 3B) indicating specific immunoreactivity against a protein with the predicted molecular mass of a His-tagged rBgGal. Reactivity of the 37 kDa rBgGal band with affinity-purified rabbit anti-rBgGal antibodies, confirmed the specificity of the anti-rBgGal antiserum to the expressed recombinant galectin (Fig. 4). The anti-rBgGal antisera were subsequently used in Western blot analyses of B. glabrata cells and for immunolocalization studies as described below.
Fig. 3.
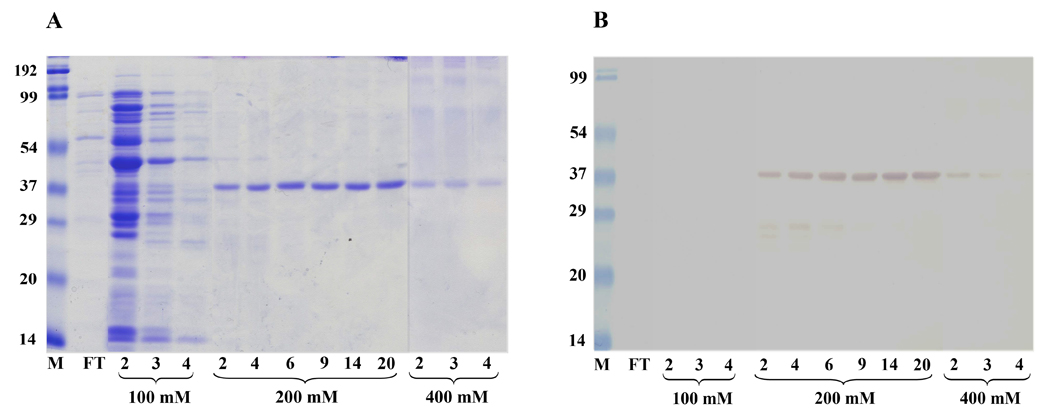
Comassie blue-stained SDS-PAGE gel displaying protein patterns of selected elution fractions taken during the purification of rBgGal on the His-trap column (A). After flow-through (FT), 100 mM imidazole elutions (lanes 2, 3 and 4) show release of many different sized proteins. Elution with 200 mM imidazole released a single 37 kDa protein corresponding to the estimated molecular mass of rBgGal (lanes 2, 4, 6, 9, 14, 20), while final elutions with 400 mM imidazole show a 37 kDa protein and other proteins. (B) Corresponding Western blot of proteins transferred from gel shown in Fig. 3A, probed with anti-His antibody and demonstrating reactivity with the 37 kDa protein eluted with 200 and 400 mM imidazole buffer, confirming that the 37 kDa eluted is the expressed-His-tagged rBgGal protein.
Fig. 4.
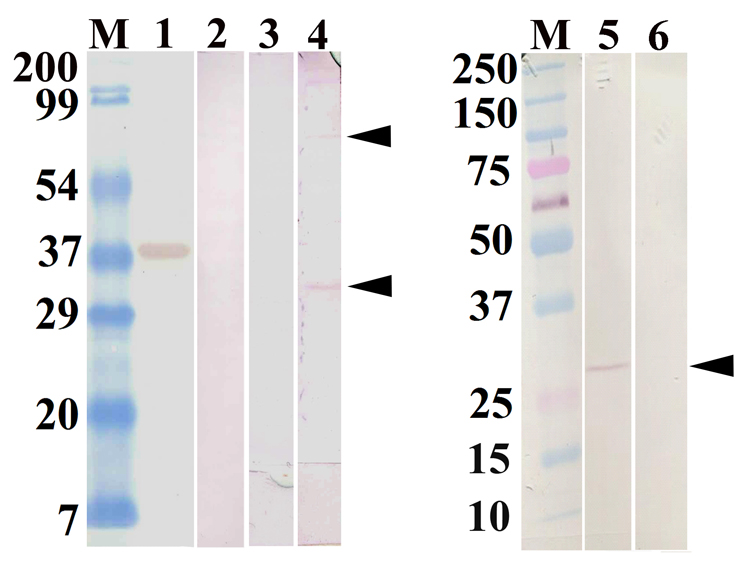
Western blots of SDS-PAGE-separated recombinant and immunoreactive endogenous BgGal probed with an anti-rBgGal polyclonal antiserum. 1: positive control containing the 37 kDa rBgGal only (arrow); 2: negative control containing blotted rBgGal probed with the anti-rBgGal antiserum previously absorbed with rBgGal; 3: Bge cell protein extract showing negative reactivity with the rabbit pre-immune serum; 4: reactivity of anti-rBgGal with 2 Bge cell derived proteins,at 32 kDa and 70 kDa (arrows); 5: immunoreactive of a 32 kDa protein (arrow) from a B. glabrata hemocyte homogenate; 6: B. glabrata cell-free hemolymph (plasma) sample showing negative reactivity with the rBgGal antiserum.
3.3. Western blot analysis and immunolocalization of endogenous BgGal
Western blot analyses of Bge cell extracts revealed two major bands with anti-rBgGal reactivity including one at ~32 kDa and the other at approximately 70 kDa, while in contrast, only a single reactive band of ~32 kDa was seen in B. glabrata hemocyte preparations (Fig. 4). No immunoreactive proteins were detected in cell-free plasma (intact or fractionated) (data not shown). The difference in the molecular mass of rBgGal (37 kDa) and the endogenous snail BgGal (32 kDa) is due to the His-tag sequences flanking both N- and C-termini of the recombinant protein.
In immunocytochemical analyses, Triton-treated Bge cells exhibited a uniform cytoplasmic staining pattern when attached and spread on a glass substrate (Fig. 5A), although cells differed in their staining intensities. By contrast, in circulating hemocytes, immunoreactive BgGal was found asymmetrically distributed in polarized concentrations within the cytoplasm or along the margins of the plasma membrane of filopodia (Fig. 5B). Although filopodial localization appeared to be at the membrane surface, BgGal accumulation at the membrane’s inner edge cannot be excluded. Also, unlike Bge cells, approximately 40% of adherent hemocytes within a given cell population were negative for anti-BgGal-reactivity. Finally, hemocytes, avidly bound exogenous rBgGal to their surface membranes in a lactose-inhibitable fashion, suggesting that, in addition to endogenous galectin expression, hemocytes also possess counter-receptors for this same lectin (Fig. 6).
Fig. 5.
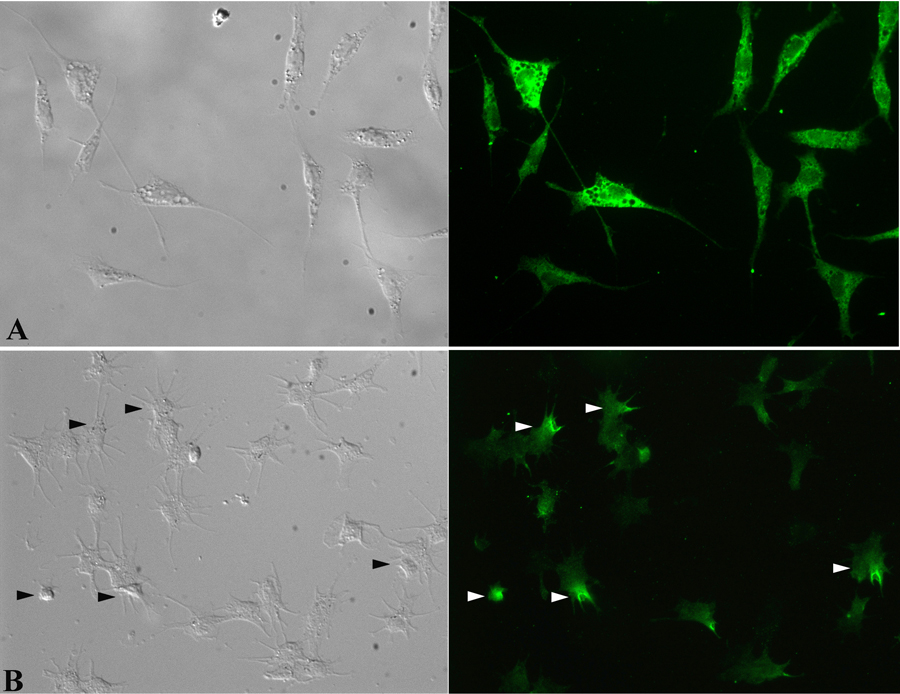
Differential interference contrast (DIC) and immunofluorescence images of Bge cells (A) and hemocytes (B) following treatment with the anti-rBgGal antibody and Alexa 488-conjugated secondary antibody. Bge cells show uniform distribution of the endogenous galectin within the cytoplasm, although the intensity of staining is variable. By contrast, only ~60% of circulating hemocytes expressed native galectin, with compartmentalization within the cytoplasm, and along the margins of filapodial membranes (arrows). (400X).
Fig. 6.
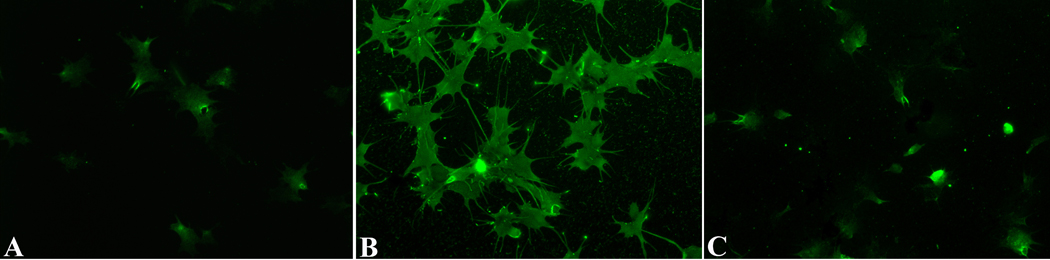
In vitro binding of rBgGal to the surface of Biomphalaria glabrata hemocytes. Live hemocytes, preincubated with rBgGal followed by fixation and treatement with anti-rBgGal antibody demonstrates intense fluorescent surface staining (B) compared to endogenous BgGal staining (A) or staining following exposure of hemocytes to rBgGal in the presence of 40 mM lactose (C). Lactose inhibition of the rBgGal binding to hemocytes demonstrates the presence of specific BgGal counterreceptors (ligands) on the surface of hemocytes.
3.4. rBgGal hemaglutination (HA) and sugar HA-inhibition assays
Using RBCs as indicator cells, at a starting protein concentration of 130 µg/mL, rBgGal exhibited a minimum agglutinating concentration of 2.0 µg/mL. When rBgGal was coincubated with a panel of different sugars, only D-gal, D-galNAc, L-lac, and alpha-L-lacNAc inhibited rBgGal-mediated agglutination reactions at a concentration of 8 µg rBgGal/mL. Calculations of minimum sugar concentrations required to inhibit rBgGal under standard assay conditions further demonstrate that lac and lacNAc had the highest binding affinity for the snail galectin and were approximately equal in their agglutination inhibitiory capacity (Table 1). Gal and galNAc, although still able to inhibit RBC agglutination, did so at minimum concentrations ~5–10-fold higher than lac or lacNAc. Finally, EDTA at a concentration of 10 mM exerted no inhibitory affect on rBgGal hemagglutinating activity (Table 1).
TABLE 1.
Effects of various mono- and disaccharides, and the divalent cation chelating agent, EDTA, on recombinant BgGal hemagglutinating activity. For sugar inhibition tests, values represent the minimum concentration required for complete inhibition of agglutination reactions.
| Glucose | >200 mM* |
| Fucose | >200 mM |
| Mannose | >200 mM |
| Trehelose | >200 mM |
| GlcNAc | >200 mM |
| LacNAc | 5 mM |
| Lactose | 10 mM |
| GalNAc | 25 mM |
| Galactose | 50 mM |
| EDTA | 10 mM** |
highest concentration of sugar tested; no inhibition
only concentration tested; no inhibition
3.5 Binding of rBgGal to the sporocyst tegument and specific neoglycoprotein-conjugated beads
Recombinant BgGal was found to bind in a lac/lacNAc-inhibitable manner to the tegumental surface of S. mansoni sporocysts (Fig. 7). However, enumeration of sporocysts treated with rBgGal in the presence inhibiting sugars revealed that not all larvae within a given population possessed sugar-inhibitable rBgGal determinants. As shown in Table 2, 82% of rBgGal-treated sporocysts were strongly reactive to the recombinant galectin and, although the presence of lac almost completely inhibited the rBgGal binding to sporocysts (15% positive), lacNAc only partially inhibited the rBgGal binding (52% showing positive rBgGal reactivity). Fucose treatment served as negative control and did not inhibit the rBgGal binding to the sporocysts surface.
Fig. 7.
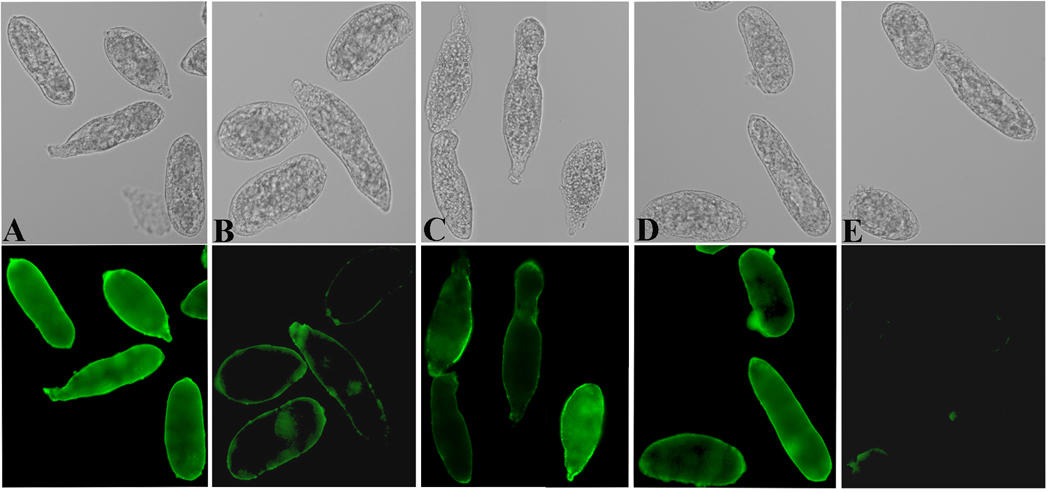
DIC and immunofluorescence image of S. mansoni sporocysts pretreated with rBgGal followed by anti-rBgGal antibodies showing the binding of rBgGal to the sporocyst tegument (A). Partial inhibition of rBgGal binding by lactose (B) or lacNAc (C), but not fucose (D) demonstrates the specificity of rBgGal binding to sporocyst surface. Fig. 7E serves as a control, showing a complete absence of nonspecific or endogenous galectin-like proteins in sporocysts using the antirBgGal antibody (200X).
Direct assessment of rBgGal binding specificity was further investigated using sepharose beads conjugated with specific neoglycoproteins (Fig. 8). Recombinant galectin reacted with BSA-lacNAc and BSA-sialyated lacNAc conjugated beads (Figs. 8B and 8C, respectively), compared to beads conjugated with BSA alone and BSA-LeX, which showed no reactivity to rBgGal (Figs. 8A and 8D). The specificity of the rBgGal to BSA-lacNAc and BSA-sialyated lacNac conjugated beads was confirmed by the inhibition of the binding in presence of lactose (Figs. 8E and 8F).
Fig. 8.
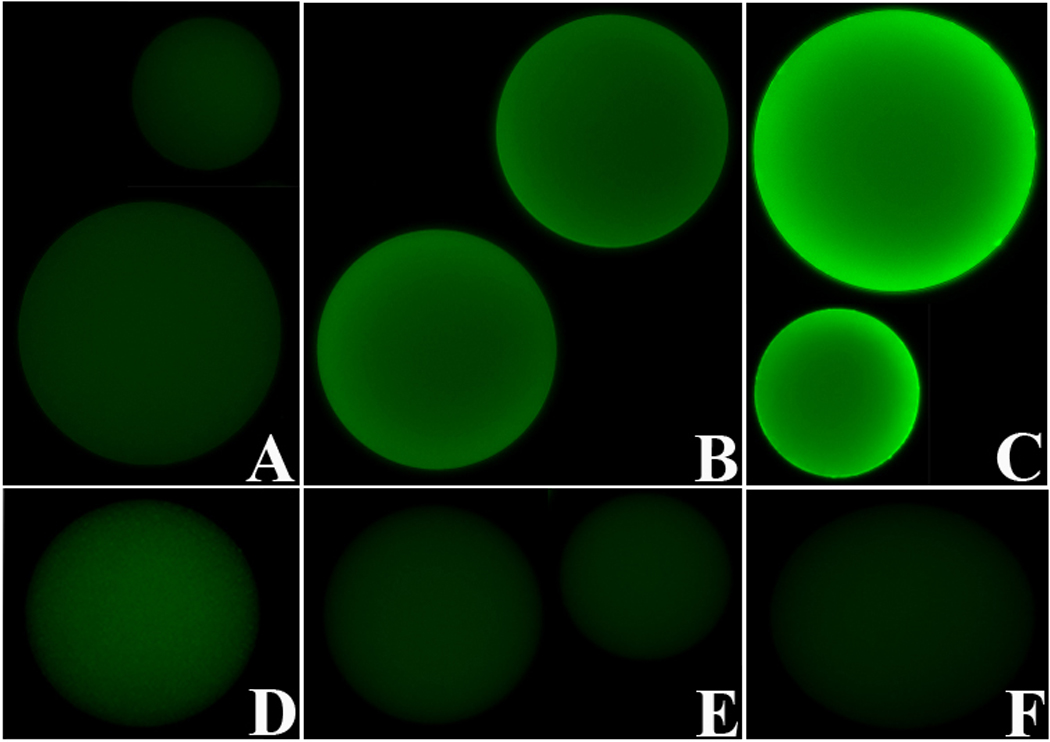
Immunofluorescence images of BSA-conjugated sepharose beads demonstrating the binding of rBgGal to lacNAc (LN) (B, E) and sialyated lacNAc (sLN) (C, F), but not to beads conjugated with BSA-LeX (D) or BSA alone (A).) Inhibition of the binding by lactose confirmed the specificity of rBgGal for LN and sLN (E, F). All immunofluorescent images were normalized to background control beads levels using Metamorph software, v. 7.2. (200X).
4. Discussion
In this study, we have identified and characterized a tandem-repeat type galectin from the freshwater snail Biomphalaria glabrata, which, to our knowledge represents the first molecular characterization of a galectin-related protein from a gastropod mollusc. Evidence that the cloned B. glabrata galectin cDNA is related to the tandem-repeat galectin subfamily is based on both structural and functional characteristics. Structural features include a predicted protein comprised of 284 aa and a calculated molecular mass of ~32 kDa that shares an overall 30–40% sequence identity with other tandem-type galectins, the presence of 2 homologous CRDs each containing 6 of the 8 highly conserved aa reported to be crucial for β-galactoside binding activity (Barondes et al, 1994), and the absence of a signal peptide. Currently, there are 15 recognized galectin member groups of which groups Gal-4, -6, -8, -9 and -12 are characterized as tandem-repeat type galectins (Leffler et al., 2004). Cluster tree analysis shows that BgGal is most closely related to members of the Gal-4 group, exhibiting highest structural affinity with those of other metazoan invertebrates identified as Gal-4 or Gal-9 proteins. However, despite this close species clustering, a few structural inconsistencies with the Gal-4 sequence signatures were noted (Jiang et al., 1999). Prominent among these were aa substitutions for 2 of the 8 conserved aa involved in sugar-reactivity for both CRDs: a Thr and Lys for Asn65 and Arg76, respectively, in CRD1 (N-terminal), and Asp and Thr for Asn194 and Arg221, respectively, in CRD2 (C-terminal). These substitutions do not alter the aa charges in the first CRD, but does add an overall negative charge (via the Asp substitution) in CRD2, thus potentially affecting the lectin’s overall β-galactoside binding affinity or altering its spectrum of sugar-binding specificity (Leffler, 2004; Vasta et al., 2004b). A recently characterized galectin from the eastern oyster Crassostrea virginica was not included in our cluster tree analysis because it possessed a unique quadruple-CRD structure (Tasumi and Vasta, 2007), in which the first 2 CRDs from the N-terminus (CRD1 and 2) shared higher identity (~34%) with the BgGal, than the next 2 CRDs (CRD3 and 4), which exhibited a lower identity (~27%). This unusual CRD structure probably resulted from gene duplication events, although whether the ancestral gene may have been a tandem- or proto-type galectin is still uncertain (Vasta et al., 2004b). It is noted that Bge cells exhibited 2 immunoreactive bands in Western blot analyses with one of the expected size of ~32 kDa and the other weaker band at ~70 kDa. Since the 70 kDa band did not appear in the pre-immune serum control, we speculate that this protein may also represent another galectin homologue similar to the multi-CRD structure reported for the oyster (Tasumi and Vasta, 2007).
The absence of a secretory signal peptide for BgGal also is consistent with other galectins, which these lectins are known to be actively secreted from diverse tissue sources (Cooper, 2002). There is now a general consensus that galectins are secreted from cells via a “nonclassical” secretory pathway, involving protein targeting to the inner plasma membrane followed by membrane blebbing and secretion (Nickel, 2003). Although SecretomeP analysis showed that the BgGal very closely misses the cutoff score for proteins suitable for nonclassical secretion (NN = 0.589), the observed distribution of endogenous immunoreactive BgGal within the cytosol of Bge cells and hemocytes, and not within vesicular compartments typical of proteins trafficking through classical Golgi pathways, is consistent with an unconventional mode of protein secretion.
Given the secretory nature of galectins, one unexpected finding was the lack of detectable BgGal in the cell-free plasma of B. glabrata snails. In earlier studies galactose-reactive lectins have been identified and isolated from the plasma of various molluscs (Suzuki and Mori, 1989; Mitra and Sakar, 1998), including B. glabrata (Mansour, 1996), but their identities as authentic galectins were not determined. Therefore, the presence/absence of plasma galectins within mollusks has not been fully resolved. The absence of anti-BgGal reactivity in plasma certainly may be due to a lack of lectin secretion into hemolymph, or its release at undetectable levels. However, other possibilities include a requirement that cells first be stimulated or induced to release galectins, or that native galectins, when secreted, are rapidly bound by counter-receptors at the extracellular plasma membrane surface or on other matrix proteins, thereby effectively removing these soluble lectins from circulation (Tasumi and Vasta, 2007). Galectin-“self-reactivity” has been proposed as a potential mechanism for modulating or signaling cell adhesive behavior, motility, differentiation and the like in various cell types (Elola et al., 2007). Our finding that rBgGal strongly binds to circulating snail hemocytes in vitro, and that binding is inhibited by lactose, demonstrates the presence of galectin counter-receptors at the surface membrane of these cells, and suggests a potential role of BgGal in regulating cell adhesion/mobility in BgGal-expressing hemocytes. A recently described oyster galectin exhibited a similar pattern of “self-binding”, suggesting that molluscan hemocytes may share a common lectin secretory mechanism (Tasumi and Vasta, 2007).
In addition to structural similarities shared with other tandem-repeat galectins, recombinant BgGal also shares a number of functional characteristics of the galectin superfamily. Chief among these is its ability to specifically bind β-galactoside sugars, as demonstrated by results of the sugar inhibition hemagglutination assays in which agglutination reactions were inhibited exclusively by galactose-containing sugars, with greatest binding affinities associated with the disaccharides lacNAc and lac. This general pattern of sugar binding activity is consistent with other members of the galectin superfamily (Leffler, 2004; Kasai and Hirabayashi, 1996). Also characteristic of the galectins is their lack of dependence on metal ions for binding activity (Kasai and Hirabayashi, 1996), especially divalent cations. BgGal exhibited this feature by retaining full hemagglutinating activity in the presence of the divalent cation chaelator EDTA. Therefore, based both on molecular structure and functional characteristics, the Gal-binding lectin described herein represents an authentic tandem-repeat type member of the galectin family.
One of the important unanswered questions regarding BgGal remains its role or function within the snail. Because of the diverse roles played by galectins in regulating key aspects of the innate immune system of vertebrates (Vasta et al., 2004a; Rabinovitch et al., 2002), such as phagocytosis (Sano et al., 2003), pattern-recognition of parasite antigens (Young and Meeusen, 2004; Van der Berg et al., 2004), and especially regulation of T-cell activation and apoptosis by gal- 4 and gal-9 (Ilarregui et al., 2005; Dai et al., 2005), the finding of BgGal expression in circulating phagocytic cells of B. glabrata was of particular interest. Hemocytes are the primary line of immune defense against invading microbial and parasitic infections in molluscs and, upon pathogen recognition, are capable of eliminating infections through phagocytic or encapsulation responses (Bayne et al., 2001; Labrenche et al., 2006). In resistant strains of B. glabrata, infection by early larval stages of the human blood fluke Schistosoma mansoni elicits hemocytic encapsulation reactions (Loker et al., 1982), although the molecules responsible for parasite recognition (pattern recognition receptors or PRRs) and hemocyte adherence are yet unknown. Because complex carbohydrates associated with glycoproteins and glycolipids represent the predominant antigenic feature of the surface tegument of larval S. mansoni (Nyame et al., 2002), it is likely that lectin-type PRRs may be responsible for mediating cellular immune reactivity with these larvae. However, to date, only 2 such molecules have been identified at the molecular level in Biomphalaria spp., including a C-type lectin with a selectin-like CRD (Duclermortier et al., 1999) and members of the large, highly diverse family of fibrinogen-related proteins (Adema et al., 1994; Zhang et al., 2004). Galectins now represent a third group of putative lectin PRRs to be identified from B. glabrata hemocytes, although we are only beginning to investigate its potential involvement in host-parasite interactions.
The anti-BgGal staining pattern on hemocytes revealed that the granulocytic hemocyte population (immune-reactive cell-type) was heterogeneous in its BgGal expression, with approximately 60% exhibiting immunoreactivity. Moreover, of those BgGal-positive cells, cytosolic localization appeared to be highly compartmentalized, suggesting BgGal synthesis and release may be under strict regulation within hemocytes and/or that cells are in different states of immune activation. Similar heterogeneity in galectin distribution also has been observed within hemocytes of Drosophila (Pace et al., 2002) and the oyster (Tasumi and Vasta, 2007). In addition, our finding that recombinant BgGal binds to hemocytes in a sugar-inhibitable fashion indicates the presence of BgGal counter-receptors (ligands) at the cell surface, and provides a basis for speculating that endogenous BgGal, upon secretion, is activated, and subsequently binds to endogenous ligands exposed at the hemocyte surface. Integrin-like surface proteins expressed by B. glabrata hemocytes (Davids and Yoshino, 1998) may represent one such ligand for BgGal as has been demonstrated in Gal-8/endothelial cell integrin binding studies (Zick et al., 2004). In this way, surface-bound galectin may function to modulate interactions with extracellular matrix proteins or act as PRRs either by cross-bridging reactive ligands at the surface of parasites or binding to pathogens that have been coated (opsonized) by this galectin (Tasumi and Vasta, 2007). In the present study, we demonstrated the sugar-inhibitable binding of rBgGal to the tegumental membrane of S. mansoni sporocysts indicating reactivity with lac- or gal-related determinants at the larval surface. Moreover, rBgGal binding to lacNAc-BSA bound beads, but not control BSA or LeX-BSA beads, further supports the hypothesis that parasite-expressed lac-containing residues may serve as functional ligands mediating BgGal binding. This hypothesis is further reinforced by our finding that rBgGal binding to the sporocyst surface was significantly reduced in the presence of free lacNAc, but not fuc. Taken together, based on results of our rBgGal binding experiments involving sporocysts and CHO-coated beads, larval surface-exposed lacNAc (LN) may represent a putative target ligand for the hemocyte galectin. Recently, macrophage Gal-3 was shown to recognize the lacdiNAc (LDN) epitope in soluble egg secretions of S. mansoni and mediate phagocytosis of LDN-coated particles (Van den Berg et al., 2004). Since lacdiNAc, fucosylated lacdiNAc and possibly other N-acetylated lactosamine derivatives like lacNAc (LN), represent major glycan constituent of the sporocyst tegumental surface (Nyame et al., 2002), future studies will now focus on the potential involvement of BgGal in mediating immune interactions between hemocytes and S. mansoni sporocysts, thereby supporting a putative role for BgGal as a PRR in this host-parasite system.
Acknowledgements
We thank Dr. G. Vasta for permitting the use of the CvGal sequence before its release in GenBank™, and Brandon Wanless for maintaining the B. glabrata snail colony. This study was supported by NIH grant AI015503 to TPY and NIH schistosome supply grant AI30026 to Dr. Fred Lewis (Biomedical Research Institute, Rockville, MD).
Abbreviations
- BgGal
Biomphalaria glabrata galectin
- gal
galactose
- galNAc
N-acetyl-galactosamine
- lac
lactose
- lacNAc
N-acetyl-lactosamine
- glcNAc
N-acetyl-glucosamine
- man
mannose
- glc
glucose
- tre
trehelose
- LDN
lacdiNAc
- Bge
Biomphalaria glabrata embryonic
- CRD
carbohydrate recognition domain
- His
histidine
- RBC
red blood cell
- DIC
differential interference contrast
- PRR
pattern recognition receptor
- aa
amino acid
- EDTA
ethelenediaminetetra acetic acid
- EST
expressed sequence tag
- cDNA
complementary DNA
- RACE
rapid amplification of cDNA ends
- FBS
fetal bovine serum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Timothy P. Yoshino, Department of Pathobiological Sciences, School of Veterinary Medicine University of Wisconsin, Madison, WI 53706, USA
Nathalie Dinguirard, Department of Pathobiological Sciences, School of Veterinary Medicine University of Wisconsin, Madison, WI 53706, USA.
John Kunert, Department of Pathobiological Sciences, School of Veterinary Medicine University of Wisconsin, Madison, WI 53706, USA.
Cornelius H. Hokke, Department of Pathobiological Sciences, School of Veterinary Medicine University of Wisconsin, Madison, WI 53706, USA Department of Parasitology, Leiden University Medical Center, Leiden The Netherlands.
References
- Acosta-Rodriguez EV, Montes CL, Motran CC, Zuniga EI, Liu F-T, Rabinovich GA, Gruppi A. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: functional cross-talk and implications during Trypanosoma cruzi infection. J. Immunol. 2004;172:493–502. doi: 10.4049/jimmunol.172.1.493. [DOI] [PubMed] [Google Scholar]
- Adema CM, Arguello DF, II, Stricker SA, Loker ES. A family of fibrinogen-related proteins that precipitate parasite-derived molecules is produced by an invertebrate after infection. Proc. Natl. Acad. Sci. (USA) 1994;94:8691–8696. doi: 10.1073/pnas.94.16.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Uhlenbruck G. Anti-galactan activity in Tridacna maxima (Roding) haemolymph: Calcium dependence of the haemagglutinins and precipitins. Immunology. 1975;29:1161–1170. [PMC free article] [PubMed] [Google Scholar]
- Barat-Houari M, Hilliou F, Jousset F-X, Sofer L, Deleury E, Rocher J, Ravallec M, Galibert L, Delobel P, Feyereisen R, Fournier P, Volkoff A-N. Gene expression profiling of Spodoptera frugiperda hemocytes and fat body using cDNA microarray reveals polydnavirus-associated variations in lepidoperan host gene transcript levels. BMC Genomics. 2006;7:160–179. doi: 10.1186/1471-2164-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondes SH, Cooper DNW, Gitt MA, Leffler H. Galectins: Structure and function of a large family of animal lectins. J. Biol. Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- Barrionuevo P, Beigier-Bompadre M, Ilarregui JM, Toscano MA, Bianco GA, Isturiz MA, Rabinovich GA. A novel function for galectin-1 at the crossroads of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J. Immunol. 2007;178:436–445. doi: 10.4049/jimmunol.178.1.436. [DOI] [PubMed] [Google Scholar]
- Bayne CJ, Hahn UK, Bender RC. Mechanisms of molluscan host resistance and of parasite strategies for survival. Parasitology. 2001;123:S159–S167. doi: 10.1017/s0031182001008137. [DOI] [PubMed] [Google Scholar]
- Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- Chernin E. Observations on hearts explanted in vitro from the snail Australorbis glabratus. J. Parasitol. 1963;49:353–364. [PubMed] [Google Scholar]
- Cooper DNW. Galectinomics: finding themes in complexity. Biochim. Biophys. Acta. 2002;1572:209–231. doi: 10.1016/s0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- Dai SY, Nakagawa R, Itoh A, Murakami H, Kashio Y, Abe H, Katoh S, Kontani K, Kihara M, Zhang SL, Hata T, Nakamura T, Yamauchi A, Hirashima M. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J. Immunol. 2005;175:2974–2981. doi: 10.4049/jimmunol.175.5.2974. [DOI] [PubMed] [Google Scholar]
- Davids BJ, Yoshino TP. Integrin-like RGD-dependent binding mechanism involved in the spreading response of circulating molluscan phagocytes. Dev. Comp. Immunol. 1998;22:39–53. doi: 10.1016/s0145-305x(97)00039-6. [DOI] [PubMed] [Google Scholar]
- Dinguirard N, Yoshino TP. Potential role of CD36-like class B scavenger receptor in the binding of low-density lipoprotein (acLDL) to the tegumental surface of Schistosoma mansoni sporocysts. Mol. Biochem. Parasitol. 2006;146:219–230. doi: 10.1016/j.molbiopara.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Duclermortier P, Lardans V, Serra E, Trottein F, Dissous C. Biomphalaria glabrata embryonic cells express a protein with a domain homologous to the lectin domain of mammalian selectins. Parasitol. Res. 1999;85:481–486. doi: 10.1007/s004360050581. [DOI] [PubMed] [Google Scholar]
- Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol. Life Sci. 2007;64:1679–1700. doi: 10.1007/s00018-007-7044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh CJ, Loukas A, Newton SE. The organization of a galectin gene from Teladorsagia circumcincta. Mol. Biochem. Parasitol. 1999;101:199–206. doi: 10.1016/s0166-6851(99)00075-4. [DOI] [PubMed] [Google Scholar]
- Hansen EL. A cell line from embryos of Biomphalaria glabrata (Pulmonata): establishment and characteristics. In: Maramorosch K, editor. Invertebrate Tissue Culture: Research Applications. New York: Academic Press; 1976. pp. 75–97. [Google Scholar]
- Hirabayshi J, Satoh M, Kasai K. Evidence that Caenorhabditis elegans 32-kDa beta-galactoside-binding protein is homologous to vertebrate beta-galactoside binding lectins. cDNA cloning and deduced amino acid sequence. J. Biol. Chem. 1992;267:15485–15490. [PubMed] [Google Scholar]
- Huang X, Tsuji N, Miyoshi T, Nakamura-Tsuruta S, Hirabayashi J, Fujisaki K. Molecular characterization and oligosaccharide-binding properties of a galectin from the argasid tick Ornithodoros moubata. Glycobiology. 2007;17:313–323. doi: 10.1093/glycob/cwl070. [DOI] [PubMed] [Google Scholar]
- Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–676. doi: 10.1016/s0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- Humphries JE, Yoshino TP. Schistosoma mansoni excretory-secretory products stimulate a p38 signalling pathway in Biomphalaria glabrata embryonic cells. Int. J. Parasitol. 2006;36:37–46. doi: 10.1016/j.ijpara.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ilarregui JM, Bianco GA, Toscano MA, Rabinovitch GA. The coming of age of galectins as immunomodulatory agents: impact of these carbohydrate binding proteins in T cell physiology and chronic inflammatory disorders. Ann. Rheum. Dis. 2005;64:iv96–iv103. doi: 10.1136/ard.2005.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Puch S, Guo X, Bhavanandan V. Signature sequences for the galectin-4 subfamily. IUBMB Life. 1999:601–605. doi: 10.1080/713803575. [DOI] [PubMed] [Google Scholar]
- Kasai K, Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J. Biochem. 1996;119:1–8. doi: 10.1093/oxfordjournals.jbchem.a021192. [DOI] [PubMed] [Google Scholar]
- Labreuche Y, Lambert C, Soudant P, Boulo V, Huvet A, Nicolas J-L. Cellular and molecular hemocyte responses of the Pacific oyster, Crassostrea gigas, following bacterial infection with Vibrio aestuarianus strain 01/32. Micro. Infect. 2006;8:2715–2724. doi: 10.1016/j.micinf.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconjugate J. 2004;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- Liu F-T. Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol. 2005;136:385–400. doi: 10.1159/000084545. [DOI] [PubMed] [Google Scholar]
- Loker ES, Bayne CJ, Buckley PM, Kruse KT. Ultrastructure of encapsulation of Schistosoma mansoni mother sporocysts by hemocytes of juveniles of the 10-R2 strain of Biomphalaria glabrata. J. Parasitol. 1982;68:84–94. [PubMed] [Google Scholar]
- Mitta G, Galinier R, Tisseyre P, Allienne J-F, Girerd-Chambaz Y, Guillou F, Bouchut A, Coustau C. Gene discovery and expression analysis of immune-relevant genes from Biomphalaria glabrata hemocytes. Dev. Comp. Immunol. 2005;29:393–407. doi: 10.1016/j.dci.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Mitra D, Sarkar MA. Galactose specific agglutinin from the hemolymph of the snail Achatina fulica: purification and characterization. Dev. Comp. Immunol. 1998;12:33–42. doi: 10.1016/0145-305x(88)90022-5. [DOI] [PubMed] [Google Scholar]
- Mansour MH. Evidence for a family of schistosome glycan-binding lectins in Biomphalaria alexandrina. Dev. Comp. Immunol. 1996;19:365–376. doi: 10.1016/0145-305x(95)00022-l. [DOI] [PubMed] [Google Scholar]
- Nickel W. The mystery of nonclassical protein secretion–A current view on cargo proteins and potential export routes. Eur. J. Biochem. 2003;270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- Newlands GG, Skuce PJ, Knox DP, Smith SK, Smith WD. Cloning and characterization of a beta-galactoside-binding protein from the gut of the gastrointestinal nematode parasite Haemonchus contortus. Parasitoly. 1999;119:483–490. doi: 10.1017/s003118209900503x. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Yoshino TP, Cummings RD. Differential expression of LacdiNAc, fucosylated LacdiNAc, and lewis X glycan antigens in intramolluscan stages of Schistosoma mansoni. J. Parasitol. 2002;88:890–897. doi: 10.1645/0022-3395(2002)088[0890:DEOLFL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ozeki Y. Purification and cell attachment activity of a D-galactose-binding lectin from the skin of sea hare, Aplysia kurodai, Biochem. Mol. Biol. Int. 1998;45:989–995. doi: 10.1002/iub.7510450516. [DOI] [PubMed] [Google Scholar]
- Pace KE, Baum LG. Insect galectins: roles in immunity and development. Glycoconj. J. 2004;19:607–614. doi: 10.1023/B:GLYC.0000014092.86763.2f. [DOI] [PubMed] [Google Scholar]
- Pace KE, Lebestky T, Hummel T, Arnoux P, Kwan K, Baum LG. Characterization of a novel Drosophila melanogaster galectin-expression in developing immune, neural, and muscle tissues. J. Biol. Chem. 2002;277:13091–13098. doi: 10.1074/jbc.M112105200. [DOI] [PubMed] [Google Scholar]
- Parrinello N, Arizza V, Cammarata M, Giaramita FT, Pergolizzi M, Vazzana M, Vizzini A, Parrinello D. Inducible lectins with galectin properties and human IL1alpha epitopes opsonize yeast during the inflammatory response of the ascidian Ciona intestinalis. Cell. Tiss. Res. 2007 doi: 10.1007/s00441-007-0415-5. [released online: PMID: 17457616] [DOI] [PubMed] [Google Scholar]
- Pfeifer K, Haasemann M, Gamulin V, Bretting H, Fahrenholz F, Muller WEG. S-type lectins occur also in invertebrates: High conservation of the carbohydrate recognition domain in the lectin genes from the marine sponge Geodia cydonium. Glycobiology. 1993;3:179–184. doi: 10.1093/glycob/3.2.179. [DOI] [PubMed] [Google Scholar]
- Rabinovitch GA, Baum LG, Tinari R, Paganelli R, Natoli C, Liu F-T, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuner of the inflammatory response? Trends Immunol. 2002;23:313–320. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Gruppi A. Galectins as immunoregulators during infectious processes: from microbial invasion to the resolution of disease. Parasite Immunol. 2005;27:103–114. doi: 10.1111/j.1365-3024.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- Rubinstein N, Ilarregui JM, Toscano MA, Rabinovich GA. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tiss. Antig. 2006;64:1–12. doi: 10.1111/j.0001-2815.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Rafferty GP, Powell R. Identification of genes expressed in the gill tissue of the Pacific oyster (Crassostrea gigas) using expressed-sequence tags. J. Mollus. Stud. 2002;68:397–399. [Google Scholar]
- Rogener W, Renwrantz L, Uhlenbruck G. Isolation and characterization of a lectin from the hemolymph of the cephalopod Octopus vulgaris (Lam.) inhibited by alpha-D-lactose and Nacetyl-lactosamine. Dev. Comp. Immunol. 1985;9:605–616. doi: 10.1016/0145-305x(85)90026-6. [DOI] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu F-T. Critical role of galectin-3 in phagocytosis by macrophages. J. Clin. Invest. 2003;112:389–397. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sminia T, Barendsen LA. Comparative morphological and enzyme histochemical study on blood cells of the freshwater snail Lymnaea stagnalis, Biomphalaria glabrata and Bulinus truncates. J. Morphol. 1978;165:31–39. doi: 10.1002/jmor.1051650104. [DOI] [PubMed] [Google Scholar]
- Stalz H, Roth U, Schleuder D, Macht M, Haebel S, Strupat K, Peter-Katalinic J, Hanisch FG. The Geodia cydonium galectin exhibits prototype and chimera-type characteristics and a unique sequence polymorphism within its carbohydrate recognition domain. Glycobiology. 2006;16:402–414. doi: 10.1093/glycob/cwj086. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mori K. A galactose-specific lectin from the hemolymph of the pearl oyster, Pinctata fucata martensii. Comp. Biochem. Physiol. (B) 1989;92:455–462. doi: 10.1016/0305-0491(89)90116-8. [DOI] [PubMed] [Google Scholar]
- Tasumi S, Vasta GR. A galectin of unique domain organization from hemocytes of the eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J. Immunol. 2007 doi: 10.4049/jimmunol.179.5.3086. In press. [DOI] [PubMed] [Google Scholar]
- van den Berg TK, Honing H, Franke N, van Remoortere A, Schiphorst WE, Liu FT, Deelder AM, Cummings RD, Hokke CH, van Die I. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 2004;173:1902–1907. doi: 10.4049/jimmunol.173.3.1902. [DOI] [PubMed] [Google Scholar]
- Vasta GR, Ahmed H, Du S-J, Henrikson D. Galectins in teleost fish: Zebrafish (Danio rerio) as a model species to address their biological roles in development and innate immunity. Glycoconj. J. 2004a;21:503–521. doi: 10.1007/s10719-004-5541-7. [DOI] [PubMed] [Google Scholar]
- Vasta GR, Ahmed H, Odom EW. Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates. Curr. Opin. Struct. Biol. 2004b;14:617–630. doi: 10.1016/j.sbi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Wilson MP, Carrow GM, Levitan IB. Modulation of growth of Aplysia neurons by an endogenous lectin. J. Neurobiol. 1992;23:739–750. doi: 10.1002/neu.480230611. [DOI] [PubMed] [Google Scholar]
- Yoshino TP. Comparison of concanavalin A-reactive determinants on hemocytes of two Biomphalaria glabrata snail stocks: receptor binding and redistribution. Dev. Comp. Immunol. 1981;5:229–240. doi: 10.1016/0145-305x(81)90030-6. [DOI] [PubMed] [Google Scholar]
- Yoshino TP, Laursen JR. Production of Schistosoma mansoni daughter sporocysts from mother sporocysts maintained in synxenic culture with Biomphalaria glabrata embryonic (Bge) cells. J. Parasitol. 1995;81:714–722. [PubMed] [Google Scholar]
- Young AR, Meeusen EN. Galectins in parasite infection and allergic inflammation. Glycoconj. J. 2004;19:601–606. doi: 10.1023/B:GLYC.0000014091.00844.0a. [DOI] [PubMed] [Google Scholar]
- van den Berg TK, Honing H, Franke N, van Remoortere A, Schiphorst WECM, Liu F-T, Deelder AM, Cummings RD, Hokke CH, van Die I. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 2004;173:1902–1907. doi: 10.4049/jimmunol.173.3.1902. [DOI] [PubMed] [Google Scholar]
- Zhang S-M, Adema CM, Kepler TB, Loker ES. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305:251–254. doi: 10.1126/science.1088069. [DOI] [PubMed] [Google Scholar]
- Zick Y, Eisenstein M, Goren RA, Hadari YR, Levy Y, Ronen D. Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj. J. 2004;19:517–526. doi: 10.1023/B:GLYC.0000014081.55445.af. [DOI] [PubMed] [Google Scholar]


