Summary
Cytolysis of target cells by natural killer (NK) cells and by some cytotoxic T cells occurs unless prevented by inhibitory receptors that recognize MHC class I on target cells. Human NK cells express a p58 inhibitory receptor specific for HLA-C. We report association of the tyrosine phosphatase HCP with the p58 receptor in NK cells. HCP association was dependent on tyrosine phosphorylation of p58. Phosphotyrosyl peptides corresponding to the p58 tail bound and activated HCP in vitro. Furthermore, introduction of an inactive mutant HCP into an NK cell line prevented the p58-mediated inhibition of target cell lysis. These data imply that the inhibitory function of p58 is dependent on its tyrosine phosphorylation and on recruitment and activation of HCP.
Introduction
Natural killer (NK) cells recognize various target cells, including tumor cells and cells infected by viruses. The ability of most cells to resist assault by NK cells is due to recognition of major histocompatibility complex (MHC) class I molecules expressed on target cells by inhibitory receptors on NK cells (reviewed by Yokoyama, 1995; Raulet and Held, 1995). The first such receptors to be described at the molecular level belong to the mouse Ly-49 family of type II transmembrane molecules with lectin-like domains (reviewed by Yokoyama and Seaman, 1993). Ly-49 molecules recognize H-2 class I molecules and are involved in the delivery of an inhibitory signal (Karlhofer et al., 1992; Correa et al., 1994; Daniels et al., 1994). In contrast, human NK cells express a family of immunoglobulin-related molecules called p58 and p70 that provide specific recognition of HLA-C and HLA-B molecules, respectively (Wagtmann et al., 1995a; Colonna and Samaridis, 1995; D’Andrea et al., 1995). p58 and p70 molecules also function as inhibitory receptors in a subset of T cells (Ferrini et al., 1994; Mingari et al., 1995; Phillips et al., 1995). Functional transfers of individual p58 and p70 killer cell inhibitory receptors (KIRs) into NK clones demonstrated that a single receptor confers both the specificity for HLA molecules on target cells and the ability to receive a signal that prevents target cell lysis (Wagtmann et al., 1995b). The specific distinction between HLA-Cw3 and HLA-Cw4 alleles by NK cells (Colonna et al., 1993; Vitale et al., 1995) is determined at the level of direct binding by different members of the p58 family (Wagtmann et al., 1995b). What is not known is how KIRs prevent activation of the cytotoxic response.
Some members of the p58 receptor family have a long cytoplasmic tail, while others have a short form (Wagtmann et al., 1995a). The long form, containing two tyrosine residues in the context of YxxL motifs, is present in p58 receptors that can deliver the inhibitory signal (Wagtmann et al., 1995b). The configuration of tyrosine residues in p58 is reminiscent of the immune receptor tyrosine-based activation motif (ITAM) associated with the Fc, B, and T cell receptors (Chan et al., 1994). ITAMs (YxxL(x)6–8YxxL) transduce activation signals upon receptor cross-linking by serving as substrates for src family tyrosine kinases such as lck or lyn, and by subsequent association with SH2 domains contained in tyrosine kinases such as ZAP-70 and syk (Chan et al., 1994). The YxxL motifs of p58 may serve to recruit proteins containing SH2 domains. However, the greater spacing between the two tyrosine residues (YxxL(x)26YxxL), as compared with that in ITAMs, suggests a different role for p58.
Understanding how p58 receptors inhibit NK cells is complicated because the receptors on NK cells responsible for inducing lysis of tumor and virus-infected cells are not defined. However, several known surface molecules can activate NK cells, including the low affinity Fc receptor CD16 (Lanier et al., 1983), CD2 (Siliciano et al., 1985), and receptors belonging to the C-type lectin family, such as NKR-P1 (Chambers et al., 1989) and CD69 (Moretta et al., 1991; Lopez-Cabrera et al., 1993). The ability to activate lysis through CD16 has been exploited to develop assays for p58-mediated inhibition, such as the redirected lysis of FcR+ cells coated with antibodies specific for CD16. Simultaneous antibody-mediated engagement of both p58 and CD16 at the site of contact with the target cell overrides the activation signals and prevents target cell lysis (Vitale et al., 1995). Therefore, engagement of p58 molecules somehow blocks the biochemical cascade elicited through an activating receptor. The CD16 activation pathway is dependent on tyrosine phosphorylation events (Einspahr et al., 1991), as is the lytic activity of NK cells directed at tumor cells (Einspahr et al., 1991; Ting et al., 1991; Stahls et al., 1992). Hence, recruitment of proteins that can disrupt tyrosine kinase–mediated activation would be a plausible mechanism by which an inhibitory receptor could interfere with activation signals.
Association of the hematopoietic cell-specific tyrosine phosphatase (HCP) (Yi et al., 1992; Shen et al., 1991; Matthews et al., 1992; Plutzky et al., 1992) with several receptors has suggested a role for this phosphatase in either modulating or blocking activation signals (reviewed by Thomas, 1995). Association of HCP with CD22 results in the down-regulation of activation signals by the B cell receptor (Doody et al., 1995). The lower antigen threshold for B cell activation in mice that are deficient in HCP suggested that signals transmitted by the B cell receptor are modulated by HCP (Cyster and Goodnow, 1995). The only case in which a substrate for HCP has been defined is in the recruitment of HCP by the erythropoietin receptor (EPO-R): the HCP-mediated termination of EPO-R signaling occurs through dephosphorylation of Jak2 kinase (Klingmuller et al., 1995). A function of the FcγRIIB on B cells is to block activation signals completely through the B cell receptor when these two receptors are cocross-linked (Amigorena et al., 1992; Muta et al., 1994). A short sequence in the cytoplasmic tail of FcγRIIB, including a YxxL motif, is critical for this inhibitory effect (Muta et al., 1994) and for association with HCP (D’Ambrosio et al., 1995). Although functional evidence for a role of HCP in blocking activation signals is still lacking, these findings provided a potential mechanism for an inhibitory signal. We therefore tested whether HCP was involved in the inhibition of target cell lysis by KIR. We report here that HCP associates with tyrosine-phosphorylated p58 and we provide functional evidence that HCP plays a role in the delivery of a negative signal that prevents target cell lysis by NK cells.
Results
Tyrosine Phosphorylation Induced by Antibody-Mediated Cross-Linking of p58 Molecules
The presence of an ITAM-related sequence in the p58 cytoplasmic tail (Figure 1) suggested that the p58 receptor may become tyrosine phosphorylated. The status of tyrosine phosphorylation of p58 receptors in NK cells was determined by immunoprecipitation of p58 from NK clones followed by Western blotting with phosphotyrosine-specific antibodies. The receptor was not detectably phosphorylated in untreated NK cells (Figure 2). To explore the possibility that p58 could become phosphorylated when engaged by its ligand on a target cell, aggregation of p58 molecules was induced by antibody-mediated cross-linking. Unlike interactions with target cells, such treatment engages a large fraction of the receptors at the same time, which may facilitate detection of phosphorylation events in the limited number of cells available from individual NK clones. As previously reported (Melero et al., 1994), antibody cross-linking of p58 led to increases in tyrosine phosphorylation of cellular substrates (Figure 2). This was also observed with F(ab′)2-fragments, ruling out a requirement for a contribution from Fc receptors expressed by the NK cells. These results indicate that p58 alone is able to engage a cascade of tyrosine phosphorylation. Antibody cross-linking induced detectable tyrosine phosphorylation of a protein corresponding in size and migration to p58 in the p58 immunoprecipitations (Figure 2). Treatment with intact GL183 antibody led to a marked reduction in the amount of p58 recovered in the immunoprecipitation. Since such a loss of p58 protein is not observed upon F(ab′)2 cross-linking, it is possible that cross-linking with intact antibody leads to cocross-linking with Fc receptors. Fc receptor cross-linking is known to induce attachment with cytoskeletal structures and result in detergent insolubility of the complex (see Beaven and Metzger, 1993). In addition, since Fc receptor cross-linking activates lck (Salcedo et al., 1993; Cone et al., 1993; Pignata et al., 1993), it is possible that a portion of the tyrosine phosphorylation observed after antibody cross-linking is due to Fc receptor cocross-linking. A tyrosine-phosphorylated protein of about 30 kDa was detected in the p58 precipitations independently of p58 cross-linking. Attempts to study proteins that may associate with p58 in NK clones were hindered by the limited number of cells available.
Figure 1. Tyrosine Residues in Cytoplasmic Tails of the p58 NK Receptor Family.
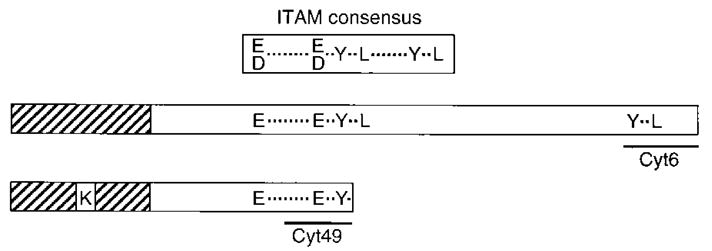
Schematic representation of two types of cytoplasmic tails found in members of the p58 receptor family expressed on NK cells. The hatched and open boxes represent the transmembrane and cytoplasmic domains, respectively. Amino acid residues are shown in single letter code. Spacing between the amino acid residues is shown to scale. The ITAM as found in the CD3 subunits of the TCR is aligned with the first YxxL of the p58 tail. The underlined regions correspond to peptides used for generation of antisera.
Figure 2. Cross-Linking p58 with Antibody in NK Clones Induces Tyrosine Phosphorylation of a 58 kDa Protein.
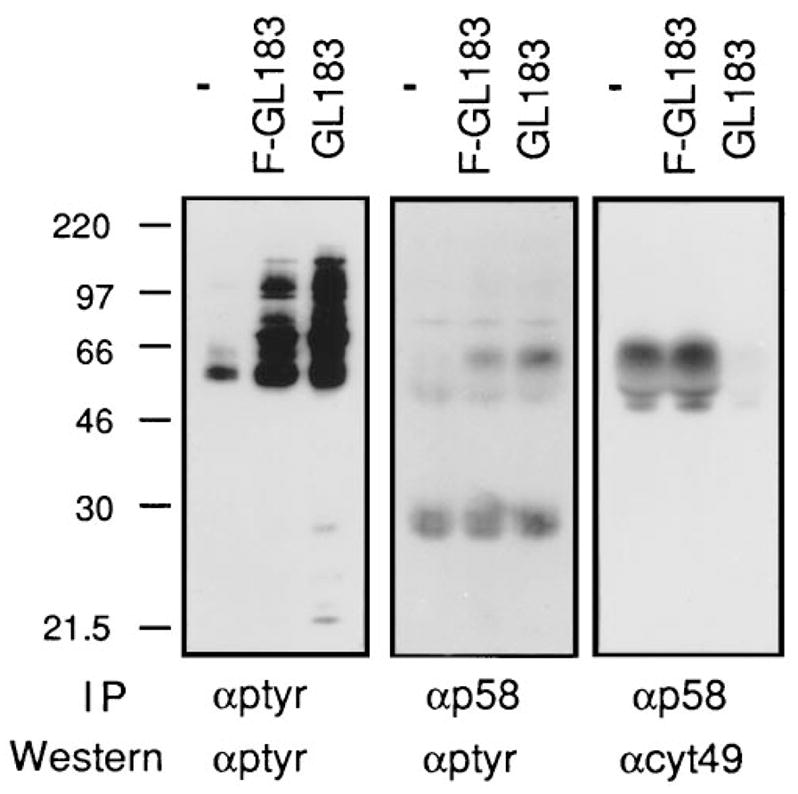
Three NK clones expressing the GL183 determinant (SR70, SR47, and SR64) were pooled. The cells (3 × 106 per condition) were preincubated in the absence of primary antibody (minus), with F(ab′)2–GL183 (F–GL183), or intact GL183 (GL183). Following addition of F(ab′)2–goat anti-mouse IgG, cells were incubated for 1 min at 37°C and lysed. GL183 was added to the control samples (minus) after lysis. The GL183 immune complexes (αp58) were collected with mouse anti-goat IgG and the supernatants were subjected to a second immunoprecipitation with anti-phosphotyrosine MAb 4G10 (αptyr). The immunoprecipitated samples were electrophoresed on the same gel and analyzed by Western blot with anti-phosphotyrosine (αptyr). The right panel represents a sequential probing of the membrane in the middle panel using antisera αcyt49. Molecular mass markers are indicated in kilodaltons on the left.
HCP Association with p58 in NK Cells
To determine whether p58 associates with the phosphatase HCP, populations of NK cells from two donors were lysed under gentle conditions. Western blot analysis with anti-HCP antisera was performed on material immunoprecipitated with anti-p58. HCP association with p58 was observed in the absence of Ab-mediated cross-linking (Figure 3a). The only readily detectable tyrosine-phosphorylated protein in the anti-p58 immunoprecipitations from these NK cells was a 58 kDa protein comigrating with p58 (Figure 3b). The detection of this phosphorylated 58 kDa protein in this experiment (as opposed to the experiment with NK clones) is possibly due to the greater number of cells (3 × 107 cells per lane in Figure 3 and 3 × 106 cells per lane in Figure 2), or the prior activation of p58 receptors in mixed populations of NK cells that may not have occurred in NK clones, or both. The relationship between p58 phosphorylation and HCP association was evaluated by inducing further phosphorylation by monoclonal antibody (MAb)-mediated p58 cross-linking. Despite a drastic decrease in the amount of p58 recovered under these conditions, the strength of the phosphotyrosine signal was maintained or even increased. Correction for p58 loss by densitometric analysis of the data in Figure 3 indicated that tyrosine phosphorylation of p58 after cross-linking was increased 8- and 18-fold in NK cells from each donor, respectively. Similarly, the amount of coprecipitating HCP increased. These results suggested that, provided the phosphorylated 58 kDa protein is p58, the small amount of p58 recovered after cross-linking is heavily phosphorylated and that HCP association correlates with tyrosine phosphorylation of the p58 receptor.
Figure 3. p58 Association with HCP in NK Cells.
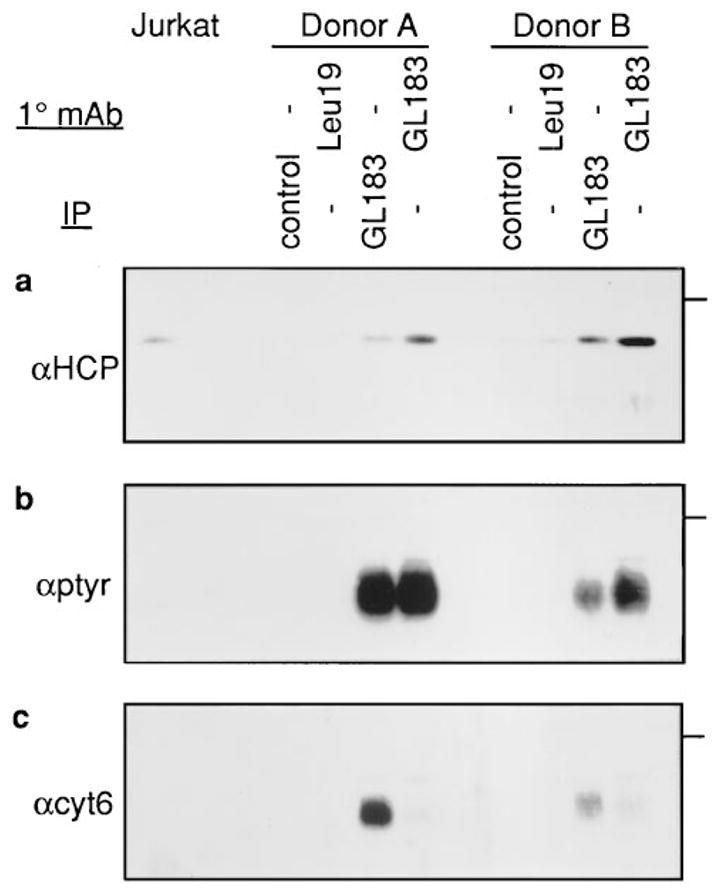
NK cell populations derived from two donors were treated with MAbs specific for CD56 (Leu19) and p58 (GL183) and cross-linked for 1 min at 37°C with goat anti-mouse IgG. The cells were lysed in Lysis-L buffer and the associated proteins immunoprecipitated (the control precipitation is with MOPC-21) and Western blotted for HCP (a). The samples correspond to 4 × 107 cells/lane from donor A and 3 × 107 cells/lane from donor B. The far-left lane contains total cell lysate from Jurkat cells and indicates the position of HCP. The membrane was reprobed with anti-phosphotyrosine (b). The membrane was then stripped and reprobed to determine the amount of p58 reactive with αcyt6 (c). The same region of the gel is presented in each panel, and the position of a 66 kDa molecular mass marker is indicated by a bar on the right.
Tyrosine-Phosphorylated p58 Recruits HCP
To establish directly whether p58 receptors can be tyrosine phosphorylated and whether this phosphorylation leads to HCP association, we reconstituted these interactions by vaccinia virus–driven expression of each component in fibroblast cells. Fibroblasts do not normally express p58, hematopoietic cell-specific src family kinases such as lyn, or the phosphatase HCP. The YxxL motifs of p58, and the reported association of p58 with lck (Bottino et al., 1994), suggested that src family kinases may phosphorylate p58. Indeed, tyrosine phosphorylation of p58 was observed after coexpression of the src family kinase lyn (Figure 4). In addition, a band of lower molecular mass (~48 kDa) phosphorylated in the presence of lyn was also detected by the anti-p58 antisera. It corresponds in size to the core glycosylated p58 (Wagtmann et al., 1995b) and may represent an immature form of p58 that is more prevalent during vaccinia-driven expression than in normal NK cells.
Figure 4. Tyrosine Phosphorylation of p58 and Phosphorylation-Dependent Association with HCP Reconstituted in Nonhematopoietic.
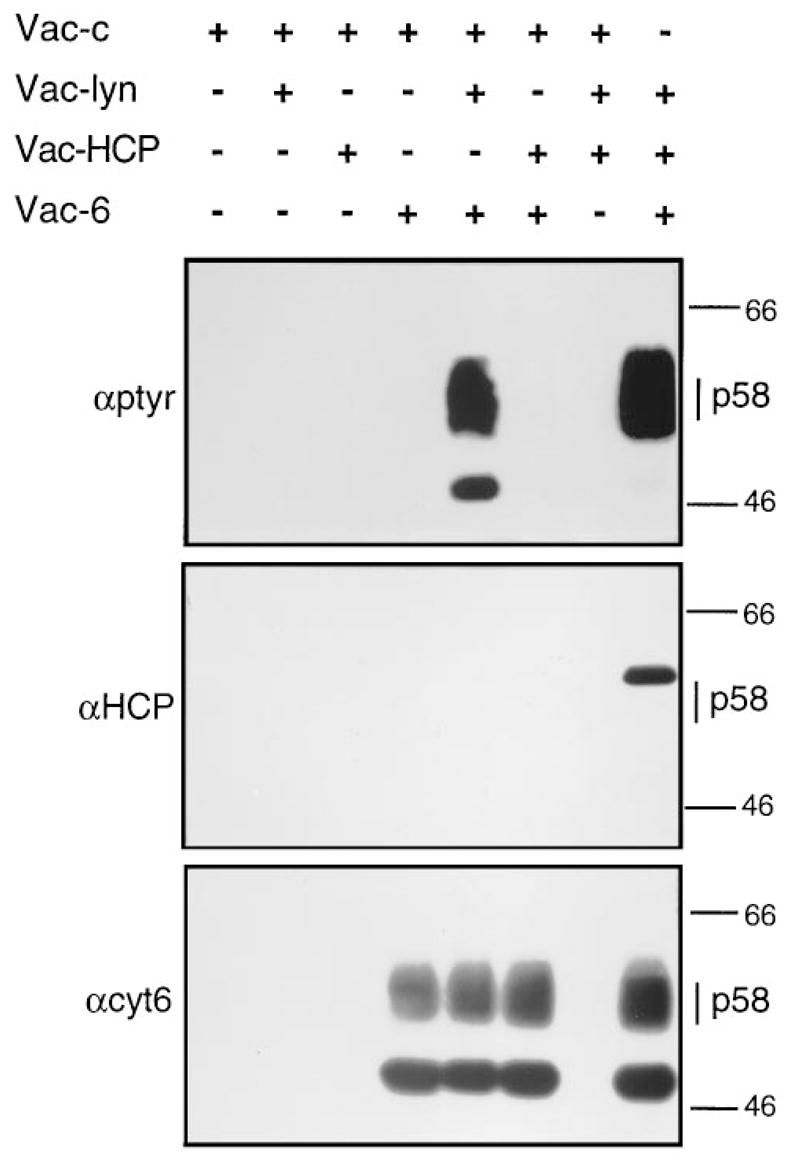
Cells 3T3E cells (5 × 106) were infected for 6 hr with 5 pfu/cell of recombinant vaccinia viruses in combination with the appropriate dose of the control virus (Vac–c) to normalize the infections to 15 pfu/cell. The recombinant viruses express lyn tyrosine kinase (Vac–lyn), HCP (Vac–HCP), p58-cl6 (Vac–6). Following lysis with Lysis-L buffer, all samples were subjected to immunoprecipitation with GL183. The samples were divided into two lanes of the same gel such that, following transfer to a membrane, they were probed in parallel with anti-phosphotyrosine (αptyr) or anti-HCP (αHCP). The amount of p58 in each sample was determined by reprobing with αcyt6 the membrane that had been first probed with αHCP. The position corresponding to the migration of p58 is indicated on the right, along with those of molecular mass markers.
This engineered phosphorylation of p58 was used to test whether phosphorylation was required for HCP association. HCP did not coimmunoprecipitate with lyn (data not shown) and did not coprecipitate with non-phosphorylated p58 (Figure 4). Coimmunoprecipitation of HCP with p58 was observed only when the fibroblast cells were also infected with a vaccinia virus encoding lyn. Interestingly, the presence of HCP associated with p58 did not decrease the tyrosine phosphorylation of the mature p58 (Figure 4). The reduced phosphorylation of the 48 kDa protein in the presence of HCP was not observed in all experiments. The reason for the difference is unclear. The failure of HCP to dephosphorylate mature p58 indicates that p58 is not a substrate for the phosphatase activity of HCP. It is likely that phosphotyrosine residues associated with SH2 domains become protected from phosphatase activity (Scharenberg et al., 1995).
HCP Binds to and Is Activated by p58 Peptides
HCP contains two SH2 domains that regulate HCP activity. Removal of the SH2 domains or occupancy of the SH2 binding sites with peptides increases HCP catalytic activity (Townley et al., 1993; Pei et al., 1994). Therefore, we tested phosphotyrosyl peptide sequences of p58 for direct binding with HCP and assessed the effect on HCP enzymatic activity. Tyrosine-phosphorylated peptides corresponding to each YxxL motif of p58 bound cellular HCP in Jurkat cell lysates (Figure 5A). Using a direct binding assay with GST fusion proteins carrying either one of the two HCP SH2 domains, we determined that the peptides interacted specifically with the second SH2 domain (SH2c) of HCP (Figure 5B). The GST fusion proteins were used in excess and therefore did not provide information about the relative affinity of each peptide for the SH2c domain. The p58 phosphotyrosyl peptides also activated the enzymatic activity of HCP in vitro (Figure 5C). The membrane-proximal peptide sequence (pY1) was a more potent activator of HCP than the membrane-distal peptide (pY2) (Figure 5C).
Figure 5. p58-Derived Phosphotyrosyl Peptides Bind and Activate HCP.
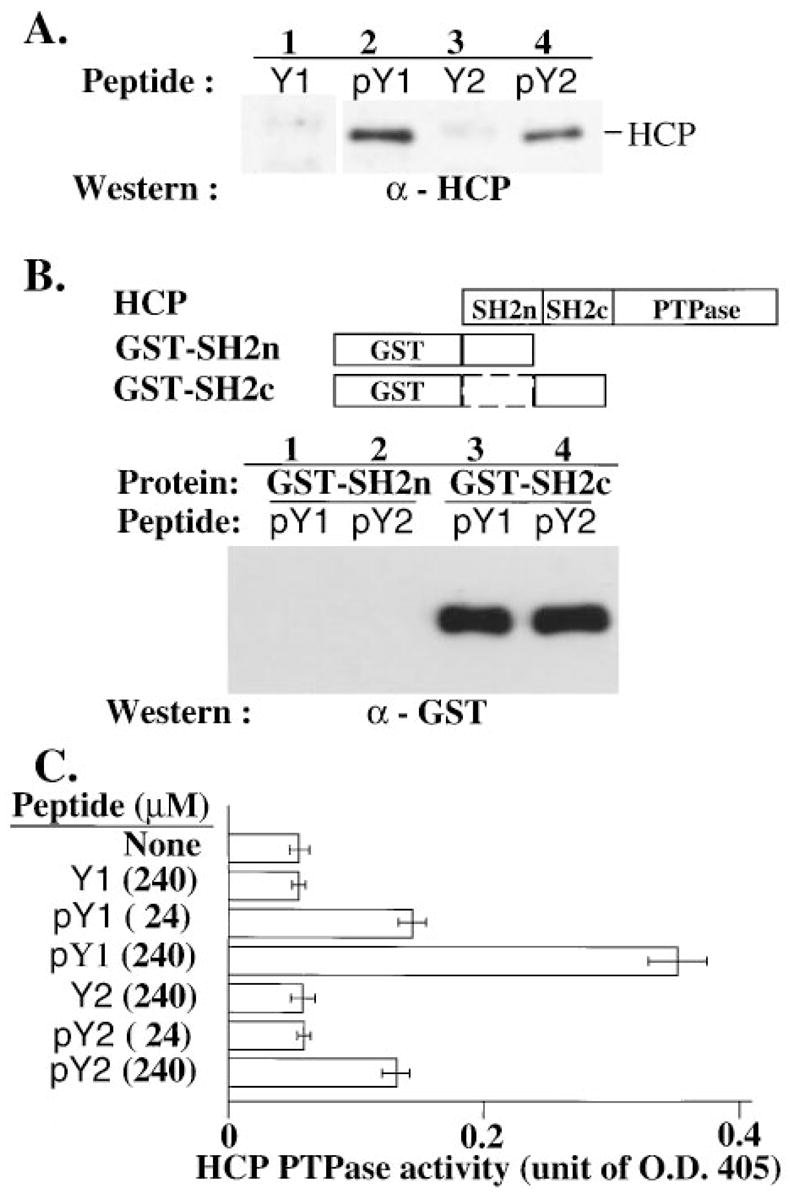
(A) Binding of cellular HCP to p58 phosphotyrosyl peptides. Peptides corresponding to each of the two p58 YxxL sequences were synthesized with a tyrosine (Y1 and Y2) or a phosphotyrosine (pY1 and pY2). Bead-conjugated peptides were incubated with a lysate of Jurkat cells. Cellular proteins associated with the peptides were analyzed by SDS–PAGE and Western blotting with antibody against HCP.
(B) HCP interacts via its SH2c domain with the p58 peptides. Beads bearing the indicated peptides were incubated with GST fusion proteins containing either one of the two SH2 domains of HCP as indicated in the diagram. Proteins associated with the peptides were analyzed by SDS–PAGE and Western blotting with an antibody against GST.
(C) Activation of phosphatase activity by p58 peptides. The PTPase activity of a complete HCP–GST fusion protein was determined in the presence of 0–240 μM of peptide as indicated. The data represent the mean ± SD values of duplicate samples from three independent experiments.
An Inactive Mutant of HCP Blocks p58 Function
The phosphotyrosine-dependent association of HCP with p58 and the association of HCP with p58 in NK cells suggests that this association is important for p58 function. To address this possibility, the functional reconstitution of p58-mediated inhibition of target cell lysis in vaccinia virus–infected NK clones (Wagtmann et al., 1995b) was used to express a catalytically inactive form of HCP (HCP-C453S) in NK cells. The NK cell line NK-92 was used because it is highly active in killing assays, it does not express detectable endogenous p58 (data not shown), and it has a detectable but low level of endogenous HCP as compared with NK clones (Figure 6). Therefore, we reasoned that competition with HCP-C453S would be more effective in NK-92 than in NK clones. HCP-C453S has no phosphatase activity in vitro (K. B. and T. Y., unpublished data).
Figure 6. Endogenous HCP in NK Cells.
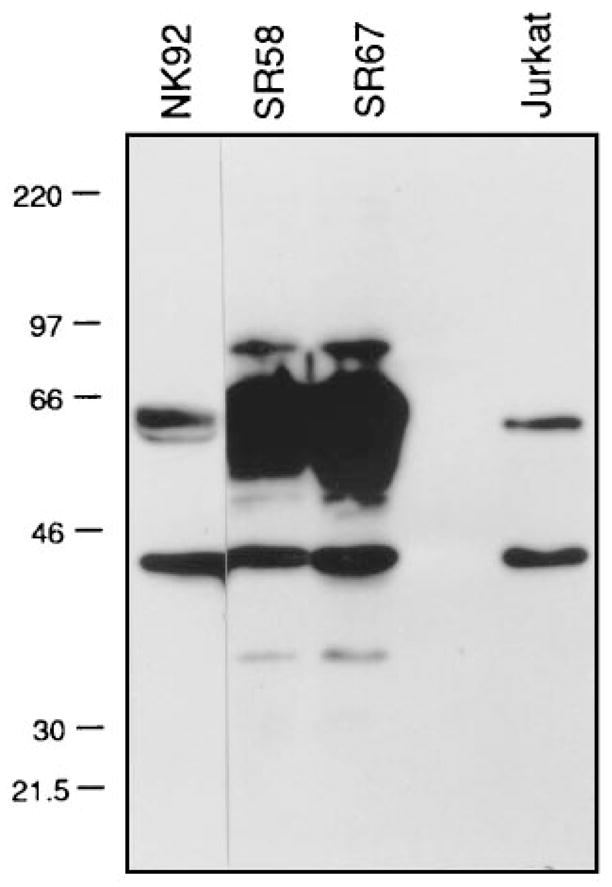
The amount of HCP in 1 × 105 cell equivalents of total cell lysate from the cell line NK-92 is compared with that in two NK clones (SR58 and SR67) by Western blot analysis. Commercially supplied Jurkat lysate is included as a migration marker for HCP but not for quantitative comparison. The intact HCP has an apparent molecular mass of 60 kDa. The 40 kDa band detected with the anti-sera corresponds to a degradation product of HCP.
NK-92 cells were infected with recombinant vaccinia viruses encoding the p58-cl6 receptor or the p58-cl42 receptor, specific for HLA-Cw3 and HLA-Cw4, respectively (Wagtmann et al., 1995b). As shown previously in NK clones (Wagtmann et al., 1995b), these receptors conferred to NK-92 cells the ability to recognize HLA-C alleles specifically and to receive an inhibitory signal (Figure 7). Vaccinia virus infections also caused some nonspecific reduction in the lytic activity of NK-92 cells, which was particularly evident with the .221-Cw3 target cells and with infected NK-92 expressing high levels of p58-cl42. Nevertheless, despite these reductions in lysis caused by vaccinia virus, coexpression of HCP-C453S reversed the specific protection mediated by p58 and restored lysis of target cells. The effect of HCP-C453S was specific because it reversed the specific p58-mediated protection but not the vaccinia virus–induced non-specific inhibition of lysis (i.e., with cl42 plus HCP-C453S on .221-Cw3 targets).
Figure 7. A Catalytically Inactive Mutant HCP Prevents the p58-Mediated Inhibition of Target Cell Lysis.
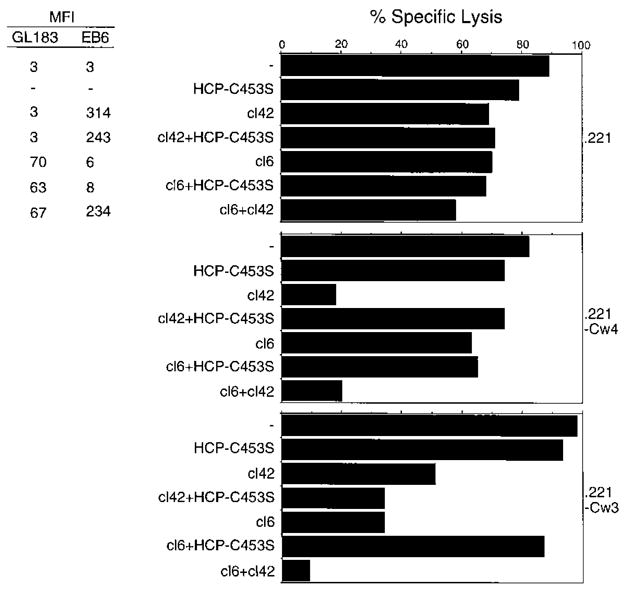
NK-92 cells were infected with recombinant vaccinia viruses encoding p58 molecules cl6 and cl42 and a catalytically inactive form of HCP, HCP-C453S, as indicated. The mean fluorescence intensities (MFI) given on the left correspond to the surface expression of p58-cl6 or p58-cl42 for each infection as determined with MAbs GL183 and EB6. The same infected NK-92 cells were then tested for their ability to lyse the target cells .221, .221-Cw3, and .221-Cw4 cells by infected NK-92 cells was determined in a 3 hr 51Cr release assay. The data presented are for an E:T ratio of 4. Similar results were observed at an E:T of 1 and in three independent experiments.
Discussion
Lysis of target cells by NK cells and by a subset of cytotoxic T cells is inhibited by KIRs that recognize MHC class I molecules on target cells. Here, we have provided evidence that the inhibitory function of the p58 KIR involves tyrosine phosphorylation of p58 and the recruitment of the tyrosine phosphatase HCP.
Functional Interaction of HCP with Tyrosine-Phosphorylated p58 KIR
In contrast with antigen receptors that initiate activation signals through associated molecules carrying ITAMs, the p58 receptor carries potential tyrosine phosphorylation motifs in its own cytoplasmic tail. Aggregation of p58 receptors on NK cells with MAbs caused tyrosine phosphorylation of many proteins, including a molecule that comigrated with p58 in anti-p58 immunoprecipitations. The tyrosine phosphorylation dependence of HCP association with p58 suggests that the interaction of p58 and HCP may be regulated by kinases that are activated upon contact with target cells. Although the kinase responsible for tyrosine phosphorylation of p58 in NK cells has not been identified, it is likely to be a member of the src family of kinases, because tyrosine phosphorylation of p58 by lyn in fibroblasts reproduced the association with HCP. NK cells express several src family kinases (Eiseman and Bolen, 1990). An obvious candidate for p58 phosphorylation is lck, which is found in association with p58 in NK cells (Bottino et al., 1994). The tyrosine phosphorylation of p58 implies that p58 has the potential to transduce signals by interacting directly with proteins containing SH2 domains.
HCP was found associated with p58 in normal NK cells. The amount of HCP associated with p58 was increased by p58 cross-linking with antibodies, suggesting a role for this interaction in the mechanism of p58-mediated signaling. Tyrosine phosphorylation–dependent recruitment of catalytic molecules containing SH2 domains is a common mechanism for signal transduction by lymphocyte receptors. Therefore, it was not surprising that HCP bound only to tyrosine-phosphorylated p58 in fibroblasts and to tyrosine-phosphorylated peptides corresponding to the p58 cytoplasmic tail in vitro. Interestingly, phosphorylated peptides from p58 bound only to the second SH2 domain of HCP and this binding stimulated the phosphatase activity of HCP. These observations indicated that HCP bound to p58 in NK cells may be catalytically active and suggested that the role of the interaction with p58 is to recruit and activate HCP, as opposed to sequester HCP.
Evidence that the interaction between HCP and p58 is necessary for p58 function was obtained through a dominant-negative mutant approach and by use of an expression system in NK cells. Vaccinia virus–driven expression of individual p58 receptors (Wagtmann et al., 1995b) in the highly lytic cell line NK-92 led to specific recognition of HLA-C molecules expressed on target cells and to inhibition of lysis. Coexpression of a dominant-negative mutant of HCP (HCP-C453S), which is defective for catalysis but has normal SH2 substrate binding activity, resulted in reversal of the p58-mediated inhibition of lysis. HCP-C453S did not augment the lytic capacity of the infected NK-92 cells, except with respect to target cells protected by HLA-C-mediated engagement of the p58 receptor. Therefore, HCP-C453S interfered specifically with the p58 inhibitory function, most likely by competing with endogenous HCP for the binding site on p58. The high abundance of HCP and its association with p58 in NK cells suggest that HCP is the main protein forming functional complexes with tyrosine-phosphorylated p58 in its natural environment.
An important conclusion from this study is that tyrosine phosphorylation of p58 is required for the p58-mediated negative signal. As HCP bound to tyrosine-phosphorylated but not unphosphorylated p58, the ability of the dominant-negative mutant HCP to interfere with p58-mediated signaling in NK-92 cells must be through an interaction with tyrosine-phosphorylated p58.
A Motif for Interaction with HCP?
The EPO, interleukin-2 (IL-2), IL-3 and c-kit receptors all bind the amino-terminal SH2 domain (SH2n) of HCP and contain the [pY]Fx(F/P/L/Y) motif, as determined by direct binding of SH2n to a degenerate peptide library in which the three residues following the phosphotyrosine varied (Songyang et al., 1994). At least in the case of the EPO-R, recruitment of HCP is known to be involved in terminating signals that are initiated by the receptor itself (Klingmuller et al., 1995). In contrast, for several other receptors, such as FcγRIIB (D’Ambrosio et al., 1995), CD22 (Doody et al., 1995), and p58, HCP recruitment serves to block or to down-regulate signals initiated by another receptor. FcγRIIB and CD22 both negatively regulate activation through the B cell receptor, whereas p58 regulates activation signals in cytotoxic T and NK cells. Interestingly, we found that, in contrast with the first group of receptors, phosphotyrosyl peptides from p58 bound to the second SH2 domain (SH2c) of HCP.
To test whether the binding motif for the SH2c domain of HCP may be different from that for the SH2n domain, and whether the p58 binding motif may be shared by other receptors, we aligned the sequences flanking the tyrosine residues from KIR (p58 and p70) and from FcγRIIB and CD22. The FcγRIIB has a single tyrosine in the cytoplasmic tail, whereas CD22 has six, three of which can interact with HCP (Doody et al., 1995). The only common feature that was not shared with other SH2 binding proteins was the presence of V or I at position −2, relative to the YxxL (Figure 8). This motif differs from the (T/S)xx[pY]xxL motif proposed earlier for peptides that interact with HCP (Thomas, 1995) and from the motif for binding to the SH2n domain of HCP (Songyang et al., 1994). The significance of the conserved (V/I)xYxxL putative immunoreceptor tyrosine-based inhibitory motif (ITIM, see Cambier, 1995) was strengthened by its absence in over 35 sequences reported to interact with SH2 domains and in many more putative SH2-binding sequences (Songyang et al., 1993). In addition, the conserved V/I at −2 was found in two other NK-specific molecules: the Ly-49 receptor family and NKG2-A. Even though these are type II transmembrane molecules with an amino-terminal cytoplasmic tail, they both carry a QE(V/I)TY sequence identical to that in KIR (Figure 8). Ly-49 serves as an MHC class I–specific NK inhibitory receptor in mouse (Karlhofer et al., 1992), whereas NKG2-A is a human NK-specific molecule of unknown function (Yabe et al., 1993). It will be interesting to test whether these two receptors inhibit NK activation by a mechanism similar to that used by p58 KIR.
Figure 8. NK Receptors and HCP Binding Proteins Share a Unique Immunoreceptor Tyrosine-based Inhibition Motif (ITIM).
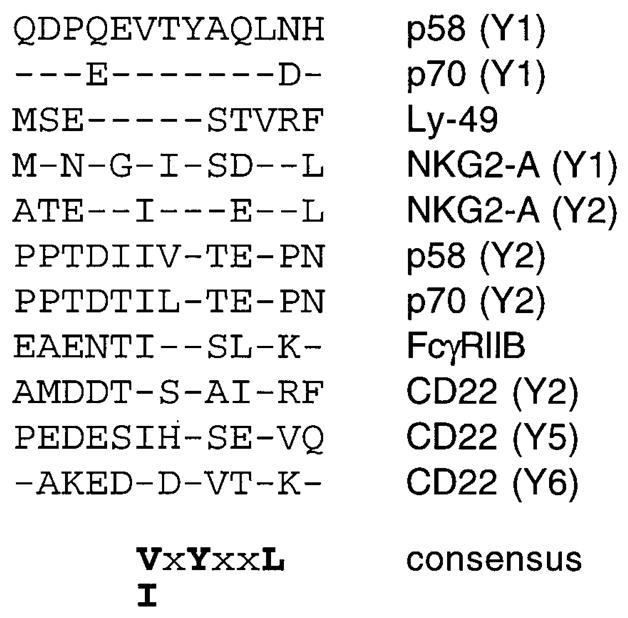
Amino acid sequences (in single letter code) surrounding the YxxL motifs of the indicated proteins are aligned. Dashes indicate identity with the sequence around the membrane-proximal tyrosine of the p58 cytoplasmic tail, p58 (Y1). Sequences were taken from the NK inhibitory receptors p58-cl6 (Wagtmann et al., 1995a), p70 (D’Andrea et al., 1995; Wagtmann et al., 1995b), and Ly-49 (Yokoyama and Seaman, 1993). NKG2-A is a molecule of unknown function expressed in human NK cells (Yabe et al., 1993). The single tyrosine residue in mouse FcγRIIB (D’Ambrosio et al., 1995) and three of the six tyrosines (i.e., Y2, Y5, and Y6) in CD22 (Doody et al., 1995) are known to interact with HCP.
A Potential Mechanism for Inhibition
The KIR–HCP complex could act at various points along the activation pathway of NK cells by directly inactivating a specific protein required for cytolysis, or by activating proteins that interfere with signal transduction. Recent data suggest that inhibition of NK cells by their MHC class I–specific receptors occurs upstream of calcium fluxes and inositol phosphate metabolism (Kaufman et al., 1995). Furthermore, when mouse NK cells are presented with susceptible and MHC class I–protected target cells at the same time, protected targets do not interfere with the killing of susceptible cells (Ljunggren et al., 1988). Assuming the human and mouse KIR are similar in this respect, p58-mediated inhibition is unlikely to use long-lived inhibitory factors that diffuse throughout the NK cell. It is more likely that p58 prevents early and localized events that occur at the site of contact with target cells. Recruitment and activation of the phosphatase HCP by p58 could easily fulfill these criteria.
It has been proposed that the inhibition of B cell activation by FcγRIIB on B cells is mediated by recruitment of HCP to phosphorylated FcγRIIB (D’Ambrosio et al., 1995). The inhibition by FcγRIIB and tyrosine phosphorylation of FcγRIIB are dependent on cocross-linking of FcγRIIB with the B cell receptor via antigen–antibody complexes. In comparison, we have observed that clustering of p58 alone leads to increased tyrosine phosphorylation of p58 in NK cells. However, in the context of an NK–target interaction, kinases activated by other receptors on the NK cell may be involved in phosphorylation of p58. A reasonable prediction is that, as long as MHC class I molecules that bind p58 are expressed by the target cells, p58 receptors and activating receptors cocluster at the interface of the NK and target cells. In this manner, p58 would serve to recruit and activate HCP specifically at the effector–target contact site. This localized activation of HCP could dephosphorylate and inactivate a triggering receptor or a kinase recruited by that receptor and thereby stop transduction of the activation signal. Therefore, in a subsequent interaction with a cell that does not express p58 ligands, the NK cell would be able to receive the activation signal and lyse this susceptible target. Identification of the substrates for the phosphatase activity of HCP in NK cells will shed light on the mechanism by which p58 inhibits NK-mediated lysis of target cells.
Experimental Procedures
MAbs, Antisera, and Peptides
The MAb Leu-19 (immunoglobulin G1 [IgG1]) (Becton Dickinson) is specific for human CD56; MOPC-21 (Sigma), of unknown specificity, served as an IgG1 control. GL183 and EB6 (Moretta et al., 1990) ascites and purified GL183 (IgG1) specific for p58 were provided by A. Moretta and C. Bottino (University of Genova, Italy). GL183 reacts with p58-cl6 and EB6 with p58-cl42. AffiniPure goat anti-mouse IgG and F(ab′)2 goat anti-mouse IgG, mouse anti-goat IgG, peroxidase-conjugated goat anti-rabbit IgG, and fluorescein isothiocyanate–conjugated goat anti-mouse IgG were purchased from Jackson Research Laboratories. The antiphosphotyrosine-specific antibody 4G10 (IgG2b), biotin-conjugated 4G10, and polyclonal anti-SH-PTP1 (αHCP) were purchased from Upstate Biotechnology, Incorporated. αcyt6 was generated against peptides corresponding to the carboxy-terminal residues of p58-cl6, CVYTELPNAES. The antiserum was affinity purified on the immunizing peptide coupled to SulfoLink (Pierce). αcyt6 reacts also with p58 molecules having a very similar but slightly longer tail. αcyt49 was generated against the cl-49 peptide CEQDHQEVSYA and supplied affinity purified (Research Genetics). αcyt49 reacts with the protein corresponding to the cl6 sequence. F(ab′)2 fragments of GL183 were generated by digestion with pepsin (Calbiochem) and purified by depletion on protein G–agarose or by high pressure liquid chromatography on a DEAE column. Purity was determined by SDS–PAGE and Coomassie staining of reduced and nonreduced samples. A microbicinchoninic acid assay (Pierce) was usedto determine the protein concentration.
The peptides Y1 (EQDPQEVTYAQLNH), pY1 (EQDPQEVT[pY]AQL NH), Y2 (KTPPTDIIVYTELPNA), and pY2 (KTPPTDIIV[pY]TELPNA) were purchased from Quality Controlled Biochemicals, Inc. and correspond to the sequences flanking each of the two tyrosines in the cytoplasmic tail of p58-cl6. [pY] denotes synthesis with a phosphotyrosine residue.
Cell Lines and Culture
Human NK populations were enriched from human PBL using the MACS NK isolation kit (Miltenyi Biotec, Incorporated, California) and analyzed for purity by flow cytometry. Populations were between 90%–99% CD3 negative and 90%–99% CD56 positive. The cells were seeded in microtiter plates at 105 cells/well with 105 irradiated autologous feeder cells and 0.5 μg/ml phytohemagglutinin. Fresh feeder cells were added on day 5. The cultures were maintained in Iscove’s media supplemented with 10% O+ serum, L-glutamine, 5% purified IL-2 (Schiaparelli), and 100 U/ml recombinant IL-2 (a gift from Hoffmann–La Roche). NK cells were used for experiments between days 10 and 17 of culture. Human NK clones were generated and maintained as previously described (Malnati et al., 1993). The cell line 3T3E expresses the human FcεR (Scharenberg et al., 1995). The human NK cell line NK-92 (a gift from H.-G. Klingemann) (Gong et al., 1994) was maintained in MyeloCult H5100 (StemCell Technologies) supplemented with 100 U/ml recombinant IL-2. Cell lines .221, .221-Cw3, and .221-Cw4 (a gift from J. Gumperz and P. Parham) were maintained as described (Wagtmann et al., 1995b).
Receptor Cross-Linking, Immunoprecipitations, and Western Blotting
Unless otherwise indicated in the figure legends, the cells were treated by the following procedure. Cells were washed once in cold Iscove’s media, resuspended at 5 × 107–5 × 108 cells/ml, divided into 100 μl aliquots, and maintained on ice. Cells were preincubated for 5 min with primary antibodies (1 μg for intact antibodies and 0.69 μg of GL183 F(ab′)2). Secondary antibodies, F(ab′)2 (5.2 μg), or intact goat anti-mouse IgG (5.4 μg) were added just prior to incubating at 37°C for 1 min. Control samples received only the secondary antibody. Cross-linking was stopped by addition of 0.4 ml cold lysis buffer (1% Triton X-100, 0.15 M NaCl, 20 mM Tris–HCl [pH 8], 5 mM iodoacetamide, and 2 mM NaVO3) or Lysis-L (0.5% Triton-X 100, 75 mM NaCl, 20 mM Tris–HCl [pH 8], 5 mM iodoacetamide, 0.5 mM PMSF, 2 mM NaVO3, and 5 mM NaF). MAbs were added where appropriate for control precipitations prior to centrifugation at 14,000 × g for 10–15 min. Immune complexes were collected by addition of 12–20 μl of protein G–agarose beads (GIBCO BRL). The pellets were washed five times with lysis buffer and eluted by boiling in SDS–PAGE sample buffer with 5% 2-mercaptoethanol. The proteins were separated by SDS–PAGE and transferred to Immobilon-P membranes (Millipore). Membranes were blocked in phosphate-buffered saline containing 5% bovine serum albumin, 0.1% Tween-20, or in SuperBlock (Pierce). Western blotting was performed with the appropriate antisera and peroxidase-conjugated goat anti-rabbit IgG antisera or biotinylated 4G10 and peroxidase-conjugated avidin (Amersham) and detected by enhanced chemiluminescence (Amersham or NEN-Dupont). Membranes were stripped by incubation at 60°C for 1 hr in 2% SDS, 100 mM 2-mercaptoethanol, 62.5 mM Tris–HCl (pH 8). Jurkat lysate (Transduction Laboratories) was used a migration marker for HCP.
Vaccinia Virus Infections
Recombinant vaccinia Vac–c was generated with the plasmid pSC-65 (S. Chakrabarti and B. Moss, personal communication). Vac–lyn containing murine lyn (Scharenberg et al., 1995), and Vac–6 and Vac–42, containing p58-cl6 and p58-cl42, respectively, (Wagtmann et al., 1995b) have been described. Vac–HCP and Vac–HCP-C453S were generated as described (Earl and Moss, 1988), using cDNA of mouse HCP or HCP with the cysteine residue 453 mutated to serine. 3T3E monolayers were infected in DMEM containing 0.1% bovine serum albumin, 25 mM HEPES, and L-glutamine for 6 hr. Cells were collected in 1 mM EDTA, 0.1% bovine serum albumin in phosphate-buffered saline, washed once, and lysed in 0.4 ml of Lysis-L buffer. Immunoprecipitations were performed with 1 μl of GL183 ascites essentially as described above. NK-92 cells (3 × 105) were infected for 1.5 hr with purified viruses essentially as previously described for NK clones (Wagtmann et al., 1995b). The infection with recombinant vaccinia virus Vac–6 was at 5 pfu/cell and for all other viruses at 10 pfu/cell. Under these conditions, approximately 80% of the cells expressed p58-cl6 at the surface of the cell, and greater than 95% expressed p58-cl42.
Peptide Conjugation, Binding Assay, and Phosphatase Assay
The peptide conjugation and binding assay were performed as previously described (Yi et al., 1995). In brief, the peptides were conjugated to Affigel 10 (BioRad Laboratories), following the instructions of the manufacturer. For each binding assay, 20 μl of peptide conjugated beads (approximately 0.2 μg of peptide) were incubated with 200 μl of Jurkat cell lysate at 4°C for 90 min. The samples were washed gently two times with cold lysis buffer, eluted by boiling in SDS sample buffer, analyzed by SDS–PAGE and Western blotting with antibody against HCP. The preparation of GST fusion protein of HCP has been described previously (Yi et al., 1992). The phosphatase (PTPase) activity of the GST–HCP fusion protein was determined using pNPP (Sigma) as a substrate. The PTPase assay was carried out at 30°C for 5 min in 50 μl of reaction mixture (100 mM HEPES [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 10 mM pNPP, 20 mM GST–HCP). The reaction was terminated by adding 950 μl of 1 N NaOH. The reaction product p-nitrophenolate was quantified by measuring absorbance at 405 nm.
Acknowledgments
We thank S. Rojo for assistance in generating the NK populations, A. Moretta and C. Bottino for antibodies, J. Gumperz and P. Parham for transfected cells, H.-G. Klingemann for NK-92, B. Moss for plasmid pSC65, the National Institutes of Health Blood Bank for human blood samples, Hoffmann–La Roche for rIL-2, D. Wiest for assistance with densitometry, and J. O’Shea for helpful discussions. This work was supported in part by American Cancer Society grant DB-74554 and American Heart Association grant NEO-94-074-GIA to T. Y.
References
- Amigorena S, Bonnerot C, Drake JR, Choquet D, Hunziker W, Guillet JG, Webster P, Sautes C, Mellman I, Fridman WH. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- Beaven MA, Metzger H. Signal transduction by Fc receptors: the Fc epsilon RI case. Immunol Today. 1993;14:222–226. doi: 10.1016/0167-5699(93)90167-j. [DOI] [PubMed] [Google Scholar]
- Bottino C, Vitale M, Olcese L, Sivori S, Morelli L, Augugliaro R, Ciccone E, Moretta L, Moretta A. The human natural killer cell receptor for major histocompatibility complex class I molecules: surface modulation of p58 molecules and their linkage to CD3 zeta chain, FcepsilonRI gamma chain and the p56lck kinase. Eur J Immunol. 1994;24:2527–2534. doi: 10.1002/eji.1830241040. [DOI] [PubMed] [Google Scholar]
- Cambier JC. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL) Immunol Today. 1995;16:110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, Hiserodt JC. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989;169:1373–1389. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405–408. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- Colonna M, Borsellino G, Falco M, Ferrara GB, Strominger JL. HLA-C is the inhibitory ligand that determines dominant resistance to lysis by NK1- and NK2-specific natural killer cells. Proc Natl Acad Sci USA. 1993;90:12000–12004. doi: 10.1073/pnas.90.24.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JC, Lu Y, Trevillyan JM, Bjorndahl JM, Phillips CA. Association of the p56lck protein tyrosine kinase with the FcgammaRIIIA/CD16 complex in human natural killer cells. Eur J Immunol. 1993;23:2488–2497. doi: 10.1002/eji.1830231017. [DOI] [PubMed] [Google Scholar]
- Correa I, Corral L, Raulet DH. Multiple natural killer cell–activating signals are inhibited by major histocompatibility complex class I expression in target cells. Eur J Immunol. 1994;24:1323–1331. doi: 10.1002/eji.1830240613. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Goodnow CC. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio D, Hippen KL, Minskoff SA, Mellman I, Pani G, Siminovitch KA, Cambier JC. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by FcgammaRIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- D’Andrea A, Chang C, Franz-Bacon K, McClanahan T, Phillips JH, Lanier LL. Molecular cloning of NKB1: a natural killer cell receptor for HLA-B allotypes. J Immunol. 1995;155:2306–2310. [PubMed] [Google Scholar]
- Daniels BF, Nakamura MC, Rosen SD, Yokoyama WM, Seaman WE. Ly-49A, a receptor for H-2Dd, has a functional carbohydrate recognition domain. Immunity. 1994;1:785–792. doi: 10.1016/s1074-7613(94)80020-0. [DOI] [PubMed] [Google Scholar]
- Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin J, Thomas ML, Fearon DT. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- Earl PL, Moss B. Generation of recombinant vaccinia viruses. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley and Sons; 1988. pp. 16.17.1–16.17.16. [Google Scholar]
- Einspahr KJ, Abraham RT, Binstadt BA, Uehara Y, Leibson PJ. Tyrosine phosphorylation provides an early and requisite signal for the activation of natural killer cell cytotoxic function. Proc Natl Acad Sci USA. 1991;88:6279–6283. doi: 10.1073/pnas.88.14.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseman E, Bolen JB. src-related tyrosine protein kinases as signaling components in hematopoietic cells. Cancer Cells. 1990;2:303–310. [PubMed] [Google Scholar]
- Ferrini S, Cambiaggi A, Meazza R, Sforzini S, Marciano S, Mingari MC, Moretta L. T cell clones expressing the natural killer cell–related p58 receptor molecule display heterogeneity in phenotypic properties and p58 function. Eur J Immunol. 1994;24:2294–2298. doi: 10.1002/eji.1830241005. [DOI] [PubMed] [Google Scholar]
- Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- Kaufman DS, Schoon RA, Robertson MJ, Leibson PJ. Inhibition of selective signaling events in natural killer cells recognizing major histocompatibility complex class I. Proc Natl Acad Sci USA. 1995;92:6484–6488. doi: 10.1073/pnas.92.14.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983;131:1789–1796. [PubMed] [Google Scholar]
- Ljunggren HG, Ohlen C, Hoglund P, Yamasaki T, Klein G, Kärre K. Afferent and efferent cellular interactions in natural resistance directed against MHC class I deficient tumor grafts. J Immunol. 1988;140:671–678. [PubMed] [Google Scholar]
- Lopez-Cabrera M, Santis AG, Fernandez-Ruiz E, Blacher R, Esch F, Sanchez-Mateos P, Sanchez-Madrid F. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J Exp Med. 1993;178:537–547. doi: 10.1084/jem.178.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnati MS, Lusso P, Ciccone E, Moretta A, Moretta L, Long EO. Recognition of virus-infected cells by natural killer cell clones is controlled by polymorphic target cell elements. J Exp Med. 1993;178:961–969. doi: 10.1084/jem.178.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RJ, Bowne DB, Flores E, Thomas ML. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992;12:2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero I, Salmeron A, Balboa MA, Aramburu J, Lopez-Botet M. Tyrosine kinase–dependent activation of human NK cell functions upon stimulation through a 58-kDa surface antigen selectively expressed on discrete subsets of NK cells and T lymphocytes. J Immunol. 1994;152:1662–1673. [PubMed] [Google Scholar]
- Mingari MC, Vitale C, Cambiaggi A, Schiavetti F, Melioli G, Ferrini S, Poggi A. Cytolytic T lymphocytes displaying natural killer (NK)-like activity: expression of NK-related functional receptors for HLA class I molecules (p58 and CD94) and inhibitory effect on the TCR-mediated target cell lysis or lymphokine production. Int Immunol. 1995;7:697–703. doi: 10.1093/intimm/7.4.697. [DOI] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Pende D, Tripodi G, Tambussi G, Viale O, Orengo A, Barbaresi M, Merli A, Ciccone E, Moretta L. Identification of four subsets of human CD3−CD16+ natural killer (NK) cells by the expression of clonally distributed functional surface molecules: correlation between subset assignment of NK clones and ability to mediate specific alloantigen recognition. J Exp Med. 1990;172:1589–1598. doi: 10.1084/jem.172.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A, Poggi A, Pende D, Tripodi G, Orengo AM, Pella N, Augugliaro R, Bottino C, Ciccone E, Moretta L. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J Exp Med. 1991;174:1393–1398. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signaling. Nature. 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- Pei D, Lorenz U, Klingmuller U, Neel BG, Walsh CT. Intramolecular regulation of protein tyrosine phosphatase SH-PTP1: a new function for Src homology 2 domains. Biochemistry. 1994;33:15483–15493. doi: 10.1021/bi00255a030. [DOI] [PubMed] [Google Scholar]
- Phillips JH, Gumperz JE, Parham P, Lanier LL. Superantigen-dependent, cell-mediated cytotoxicity inhibited by MHC class I receptors on T lymphocytes. Science. 1995;268:403–405. doi: 10.1126/science.7716542. [DOI] [PubMed] [Google Scholar]
- Pignata C, Prasad KVS, Robertson MJ, Levine H, Rudd CE, Ritz J. FcgammaRIIIA-mediated signaling involves src-family lck in human natural killer cells. J Immunol. 1993;151:6794–6800. [PubMed] [Google Scholar]
- Plutzky J, Neel BG, Rosenberg RD. Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci USA. 1992;89:1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH, Held W. Natural killer cell receptors: the offs and ons of NK cell recognition. Cell. 1995;82:697–700. doi: 10.1016/0092-8674(95)90466-2. [DOI] [PubMed] [Google Scholar]
- Salcedo TW, Kurosaki T, Kanakaraj P, Ravetch JV, Perussia B. Physical and functional association of p56lck with Fc gamma RIIIA (CD16) in natural killer cells. J Exp Med. 1993;177:1475–1480. doi: 10.1084/jem.177.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg AM, Lin S, Cuenod B, Yamamura H, Kinet JP. Reconstitution of interactions between tyrosine kinases and the high affinity IgE receptor which are controlled by receptor clustering. EMBO J. 1995;14:3385–3394. doi: 10.1002/j.1460-2075.1995.tb07344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen SH, Bastien L, Posner BI, Chretien P. A protein–tyrosine phosphatase with sequence similarity to the SH2 domain of the protein–tyrosine kinases. Nature. 1991;352:736–739. doi: 10.1038/352736a0. [DOI] [PubMed] [Google Scholar]
- Siliciano RF, Pratt JC, Schmidt RE, Ritz J, Reinherz EL. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985;317:428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, McGlade J, Olivier P, Pawson T, Bustelo XR, Barbacid M, Sabe H, Hanafusa H, Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994;14:2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahls A, Heiskala M, Mustelin T, Andersson LC. Activation of natural killer cells via the Fc gamma RIII (CD16) requires initial tyrosine phosphorylation. Eur J Immunol. 1992;22:611–614. doi: 10.1002/eji.1830220249. [DOI] [PubMed] [Google Scholar]
- Thomas ML. Of ITAMs and ITIMs: turning on and off the B cell antigen receptor. J Exp Med. 1995;181:1953–1956. doi: 10.1084/jem.181.6.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AT, Einspahr KJ, Abraham RT, Leibson PJ. Fc gamma receptor signal transduction in natural killer cells: coupling to phospholipase C via a G protein–independent, but tyrosine kinase–dependent pathway. J Immunol. 1991;147:3122–3127. [PubMed] [Google Scholar]
- Townley R, Shen SH, Banville D, Ramachandran C. Inhibition of the activity of protein tyrosine phosphate 1C by its SH2 domains. Biochemistry. 1993;32:13414–13418. doi: 10.1021/bi00212a006. [DOI] [PubMed] [Google Scholar]
- Vitale M, Sivori S, Pende D, Moretta L, Moretta A. Coexpression of two functionally independent p58 inhibitory receptors in human natural killer cell clones results in the inability to kill all normal allogeneic target cells. Proc Natl Acad Sci USA. 1995;92:3536–3540. doi: 10.1073/pnas.92.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagtmann N, Biassoni R, Cantoni C, Verdiani S, Malnati M, Vitale M, Bottino C, Moretta L, Moretta A, Long EO. Molecular clones of the p58 natural killer cell receptor reveal immunoglobulin-related molecules with diversity in both the extra-and intracellular domains. Immunity. 1995a;2:439–449. doi: 10.1016/1074-7613(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Wagtmann N, Rajagopalan S, Winter CC, Peruzzi M, Long EO. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity. 1995b;3:801–809. doi: 10.1016/1074-7613(95)90069-1. [DOI] [PubMed] [Google Scholar]
- Yabe T, McSherry C, Bach FH, Fisch P, Schall RP, Sondel PM, Houchins JP. A multigene family on human chromosome 12 encodes natural killer-cell lectins. Immunogenetics. 1993;37:455–460. doi: 10.1007/BF00222470. [DOI] [PubMed] [Google Scholar]
- Yi T, Zhang J, Miura O, Ihle JN. Hematopoietic cell phosphatase associates with erythropoietin (Epo) receptor after Epo-induced receptor tyrosine phosphorylation: identification of potential binding sites. Blood. 1995;85:87–95. [PubMed] [Google Scholar]
- Yi TL, Cleveland JL, Ihle JN. Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol Cell Biol. 1992;12:836–846. doi: 10.1128/mcb.12.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM. Natural killer cell receptors specific for major histocompatibility complex class I molecules. Proc Natl Acad Sci USA. 1995;92:3081–3085. doi: 10.1073/pnas.92.8.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]


