Abstract
Cancer is associated with immune deficiency, but the biologic basis of this is poorly defined. Here we demonstrate that impaired actin polymerization results in CD4+ and CD8+ T cells from patients with chronic lymphocytic leukemia (CLL) exhibiting defective immunological synapse formation with APCs. Although this synapse dysfunction was in part a result of the CLL cells having poor APC function, defective actin polymerization was also identified in T cells from patients with CLL. We further demonstrate that, following contact with CLL cells, defects in immune synapse formation were induced in healthy allogeneic T cells. This required direct contact and was inhibited by blocking adhesion molecules on CLL B cells. In T cells from patients with CLL and in T cells from healthy individuals that had been in contact with CLL cells, recruitment of key regulatory proteins to the immune synapse was inhibited. Treatment of autologous T cells and CLL cells with the immunomodulating drug lenalidomide resulted in improved synapse formation. These results define what we believe to be a novel immune dysfunction in T cells from patients with CLL that has implications for both autologous and allogeneic immunotherapy approaches and identifies repair of immune synapse defects as an essential step in improving cancer immunotherapy approaches.
Introduction
As tumors progress they develop ways to escape immune cell recognition, and this realization has lead to the concept of tumor immunoediting (1–3). Immune dysfunction in the cancer-bearing host can promote tumor cell variants that are able to resist or suppress antitumor immune responses, leading to tumor progression. The complex strategies utilized by tumor cells to escape immune surveillance are not fully characterized but include the production of proinflammatory cytokines, expression of indoleamine 2,3-dioxygenase, differentiation of regulatory T cells, and recruitment of tumor-associated macrophages (4). The continued identification of immunomodulating mechanisms utilized by tumor cells and their repair will help contribute to the development of effective immunotherapy treatments in the clinic.
B cell chronic lymphocytic leukemia (CLL) is characterized by progressive accumulation of long-lived mature monoclonal B lymphocytes and represents an attractive model to study immune cells that have been exposed to circulating tumor cells. Although CLL cells are known to express tumor antigens that can be presented by MHC class I and class II molecules, there is no effective autologous immune response against the tumor cells, and a progressively growing tumor population results over time (5, 6). This can be explained in part by CLL expressing high levels of immune-suppressing factors including TGF-β and IL-10 (7, 8), low levels of expression of adhesion and costimulatory molecules essential for induction of effective immune responses (9–11), and increased numbers of regulatory T cells (12). We hypothesized that T cells from CLL cancer patients become dysfunctional with tumor development and previously characterized the T cell defects in tumor-bearing patients by analyzing the global gene expression of highly purified CD4+ and CD8+ T cells from peripheral blood from individuals with CLL compared with age-matched healthy donors (13). Analysis revealed differentially expressed genes mainly involved in cell differentiation and cytoskeletal formation pathways in CD4+ T cells and in cytoskeletal formation, vesicle trafficking, and cytotoxicity pathways in CD8+ T cells. As complex cytoskeleton-dependent cellular processes are known to regulate T cell activation (14), we speculated that T cells from CLL patients would be defective in immunological function.
T cell antigen receptor (TCR) engagement and recognition of antigen induces dramatic morphological changes in T cells, characterized by polarization of the actin cytoskeleton and accumulation of F-actin at the site of contact with the APC, termed the immunological synapse or immune synapse (15). This cellular signaling structure orchestrates the complex communication between the T cell and the APC in a way that ensures detailed antigen recognition and effective T cell responses. As part of this process, key receptors and signaling molecules are recruited to supramolecular activation clusters (SMACs), major components of the immune synapse. The central SMAC (c-SMAC) contains proteins, including TCR, CD3, and Lck, that cocluster in the center of the mature synapse site. A second zone, the peripheral SMAC (p-SMAC), surrounds the c-SMAC and on T cells is characterized by high concentrations of integrin leukocyte function–associated antigen-1 (LFA-1, also known as CD18/CD11a or αLβ2). The LFA-1 ligand, intracellular adhesion molecule 1 (ICAM-1, or CD54), is expressed in the p-SMAC of APCs. The p-SMAC is thought to provide adhesive anchoring of the T cell to the APC, while the c-SMAC forms a protected zone for TCR signaling (16, 17).
We hypothesized that T cells from cancer patients may inappropriately respond to APCs due to an inability to regulate actin remodeling effectively. In this study we show, by using both primary cells from CLL patients and the transgenic mouse model of CLL (Eμ-TCL1) (18), that CD4+ and CD8+ T cells from tumor-bearing patients have an impaired ability to form immunological synapses. Our results show that critical immunological synapse formation steps are inhibited, including conjugation of T cells with APCs, the subsequent polarization of F-actin, and the recruitment of TCRs, adhesion molecules, and actin cytoskeleton proteins to the synapse contact site. Moreover, we provide evidence that this immunological defect is induced in healthy allogeneic T lymphocytes by direct contact with tumor B cells, identifying a cellular mechanism whereby CLL tumor cells may inhibit immunological recognition, facilitating disease progression. We also identify the potential of improving this immune dysfunction in CLL using an immunomodulatory drug lenalidomide, which appears capable of repairing F-actin polymerization and signaling at the immunological synapse.
Results
Autologous CD4+ and CD8+ T cells from CLL patients have impaired T cell conjugate formation and actin polymerization at the immunological synapse.
Our previous work demonstrated that both the CD4+ and CD8+ T cell populations from untreated patients with CLL have altered global gene expression profiles compared with age-matched healthy donors (13) and identified cytoskeleton organization as a common differentially expressed gene expression pathway. Since cytoskeletal remodeling and F-actin polymerization are essential for the formation of the immunological synapse, we hypothesized that T cells from CLL patients may have an abnormal ability to form a synapse. To test this, conjugates formed with previously untreated patient CD8+ or CD4+ T cells and superantigen-pulsed (sAg-pulsed) autologous CLL cells were examined for the distribution of F-actin after labeling with rhodamine phalloidin. The extent of actin polymerization in T cell conjugates from CLL patients was compared with age-matched healthy donor cells (primary CD4+ or CD8+ T cells conjugated with sAg-pulsed autologous B cells) by quantitative analysis of fixed conjugates by immunofluorescence and confocal microscopy.
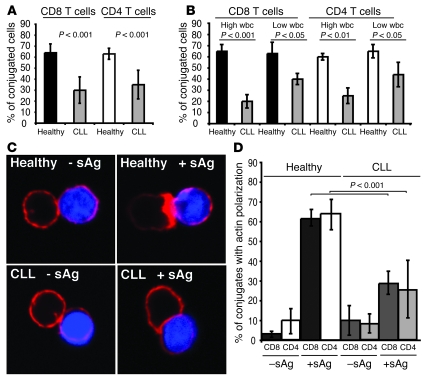
Although TCR and LFA-1 surface levels were similar or slightly higher in T cells from CLL patients than in T cells from controls (data not shown and ref. 19), the percentage of T cells forming conjugates with sAg-pulsed CLL B cells was significantly reduced in CLL patients compared with healthy donor cells (25% versus 65% in the CD8+ population and 35% versus 60% in the CD4+ population, respectively; P < 0.001), suggesting that T lymphocytes from cancer-bearing patients poorly conjugate or interact with CLL B cells (Figure 1A). This conjugation formation defect was present in all CLL patient samples tested but significantly more pronounced with increasing elevation of circulating tumor lymphocyte counts (Figure 1B). As expected, quantification of F-actin polymerization at the T cell–B cell contact site showed that the majority of conjugates formed with healthy donor T cells exhibited a sharp band of F-actin at the immune synapse in the presence of antigen (Figure 1, C and D). The background levels of F-actin polymerization at the synapse were defined by examining autologous conjugates in the absence of antigen, and no difference was identified between CLL patients and healthy donors (Figure 1, C and D). However, analysis identified significantly less antigen-dependent F-actin accumulation at the immune synapse in both autologous CD8+ and CD4+ T cells from CLL patients compared with healthy donor cells (greater than 50% reduction in both T cell populations; P < 0.001) (Figure 1, C and D). This defect was identified in all untreated CLL patient samples tested (n = 18), but again was more pronounced in patients with high circulating tumor lymphocyte counts (data not shown). Similar results were obtained for early and late CD4+ and CD8+ T cell conjugation time points (5 and 30 min; data not shown). Of note, the percentage of conjugates with actin polymerization was not increased following either CpG or CD154 stimulation of CLL B cells (data not shown). Taken together, these data demonstrate that T cells from cancer-bearing patients have a reduced capacity to form immunological synapses.
Figure 1. CLL patients have impaired T cell immune synapse formation.
(A) T cell conjugates formed between T cells from CLL patients or age-matched healthy donors and sAg-pulsed autologous CLL B cells or healthy B cells, respectively, were scored by visual counting using a confocal microscope. Each data set shows the mean ± SD from 6 independent experiments, with 50 random T cells analyzed per experiment. (B) Autologous conjugate experiments were performed as in A, comparing CLL patients with low (less than 20 mm3) absolute wbc versus high wbc (20 mm3 or more) with age-matched healthy donor cell conjugates. Each data set shows the mean ± SD from 3 independent experiments, with 50 random T cells analyzed per experiment. (C) T cells from CLL patients or age-matched healthy donors were allowed to conjugate with autologous CLL cells or healthy B cells, respectively, with or without sAg (blue, CMAC dyed). Conjugates were then fixed and stained with rhodamine phalloidin to detect F-actin (red). Note the lack of F-actin enrichment at the synapse site in T cells from CLL even in the presence of sAg-pulsed CLL cells. Original magnification, ×63. (D) Conjugates were selected at random for imaging and were scored for accumulation of F-actin at the immune synapse. Each data set shows the mean ± SD from 9 independent patient experiments, with 50 conjugates analyzed per experiment.
Defects in CLL B cells and T cells contribute to decreased immunological synapse formation.
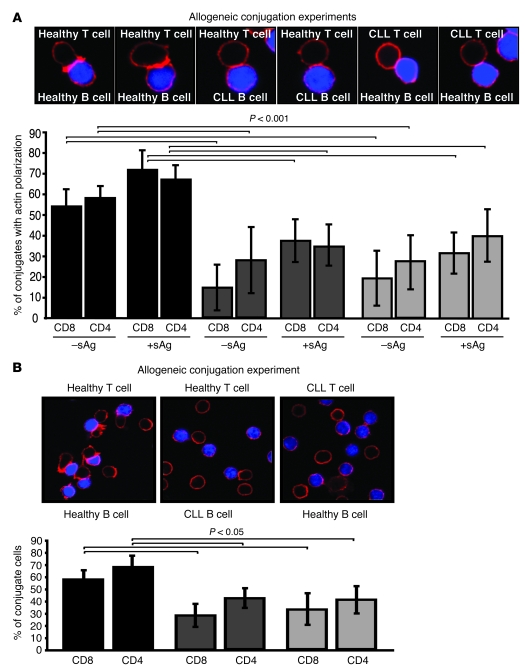
The low APC function of CLL cells has been recognized as a major factor likely to prevent autologous T cell responses against unstimulated CLL cells in patients (11). However, the impaired immune synapse defect identified in CLL could result from not only the poor APC function of the CLL cells but also defects in the T cells or a combination of both factors. To investigate the relative contribution of each factor, we examined synapse formation in mixing experiments using CLL T cells or CLL B cells with healthy allogeneic cells. As expected, conjugates formed with healthy CD8+ or CD4+ T cells and allogeneic healthy B cells exhibited a strong band of F-actin at the immune synapse (Figure 2A). In contrast, significantly less actin polymerization at the synapse was observed in either healthy allogeneic T cells conjugated with CLL B cells or CLL T cells conjugated with healthy allogeneic B cells with or without the presence of antigen (P < 0.001; Figure 2A). These immunological synapse defects were accompanied by defects in the formation of stable conjugates (Figure 2B; P < 0.05). These data indicate that both tumor B cell and T cell defects contribute significantly to impaired immunological synapse formation in CLL patients.
Figure 2. Defects in CLL B cells and T cells contribute to decreased immunological synapse formation.
(A) Conjugates from mixed allogeneic experiments using T cells or CLL B cells from leukemic patients with healthy allogeneic cells were selected at random for imaging and scored for accumulation of F-actin (red) at the immune synapse (with or without sAg-pulsed APCs, blue). As controls, healthy T cells were conjugated with allogeneic healthy B cells. Data are the mean ± SD from 6 independent experiments, with 50 conjugates analyzed per experiment. (B) T cell conjugates from mixed allogeneic experiments (sAg-pulsed CLL B or healthy B cells, blue) were scored by visual counting using a confocal microscope. Each data set shows the mean ± SD from 6 independent experiments, with 50 random T cells analyzed per experiment. Original magnification, ×63.
CLL cells induce defective immunological synapse formation in healthy allogeneic T cells.
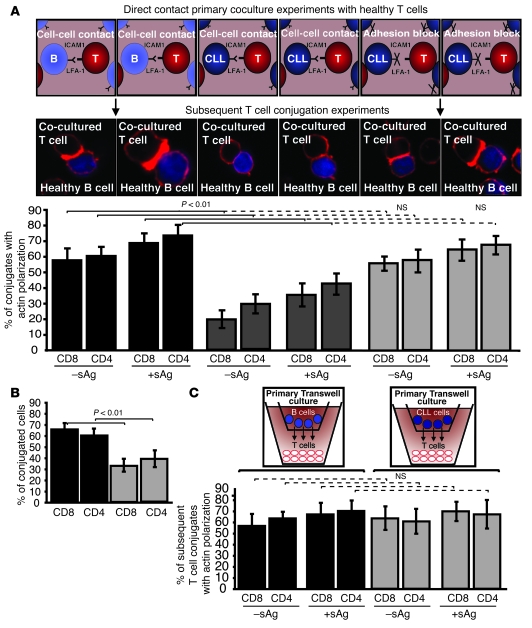
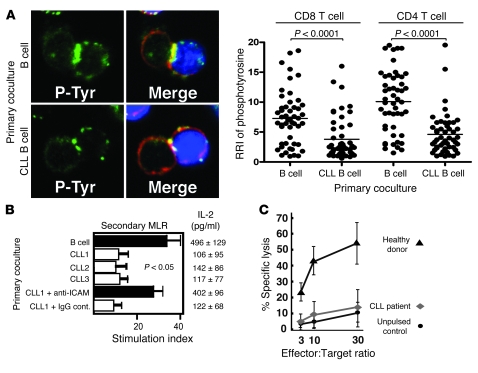
Our previous data demonstrated that direct-contact coculture experiments with CLL B cells and healthy allogeneic T cells induced altered cytoskeletal protein expression profiles, consistent with the comparative gene expression profiling results (13). Thus, we postulated that direct interaction of CLL cells with healthy allogeneic T cells would induce similar immunological synapse defects, as identified in patient-derived T lymphocytes. We first performed experiments to assess the time and CLL B cell number required to induce these T cell defects. T cell defects were induced within 24 h using high numbers of CLL B cells, but maximal induction was seen by 48 h and was then independent of the CLL B cell number added (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI35017DS1). Therefore, in all subsequent experiments, we cocultured healthy CD8+ or CD4+ T cells for 48 h with either allogeneic CLL B cells or allogeneic healthy B cells (primary coculture) and subsequently used these T cells in conjugation assays with third-party healthy allogeneic B cells pulsed with or without antigen. Coculture with CLL B cells resulted in subsequent significant impairment in immune synapse formation compared with coculture with healthy B cells (Figure 3A; P < 0.01). In addition, the percentage of T cell conjugates was significantly reduced (Figure 3B; P < 0.01). This impairment of F-actin polymerization at the immune synapse in the subsequent conjugation assays was not seen when cell adhesion was blocked by pretreatment of CLL B cells with anti–ICAM-1 monoclonal antibody (but not isotype control IgG) prior to primary coculture and subsequent conjugation experiments (Figure 3A). These data indicate that direct contact exposure of T lymphocytes to cancer cells, mediated by cell adhesion, interferes with the subsequent ability of T cells to polymerize F-actin at the immunological synapse site. Further evidence that direct cell contact, and not secreted soluble factors alone, is required to induce this T cell immune defect from the CLL cells was provided by the findings that there was no subsequent immunological synapse impairment when healthy CD8+ or CD4+ T cells were cocultured for 48 h with CLL cells in primary transwell culture assays compared with coculture with allogeneic healthy donor B cells (Figure 3C). The finding that direct contact of CLL cells with allogeneic healthy T cells induces subsequent impairment of immune synapse formation has important clinical relevance for the use of donor lymphocyte infusions in the setting of bulk disease.
Figure 3. CLL B cells induce defective immunological synapse formation in healthy allogeneic T cells by direct cell contact.
(A) Healthy T cells (T) were cocultured for 48 h with either healthy allogeneic B cells (B) or allogeneic CLL B cells and subsequently used in conjugation assays with or without sAg-pulsed third-party allogeneic healthy donor B cells (APCs, stained blue). Conjugates were selected at random for imaging and were scored for accumulation of F-actin (stained red) at the immune synapse. Note the prevention of the synapse defect when cell adhesion was blocked by pretreatment of CLL B cells with anti–ICAM-1 monoclonal antibody prior to primary coculture with healthy T cells. Data are the mean ± SD from 3 independent experiments, with 50 conjugates analyzed per experiment. The confocal images shown are CD8+ T cells. Original magnification, ×63. (B) T cell conjugates from A were scored by visual counting using a confocal microscope. Each data set shows the mean ± SD from 3 independent experiments, with 50 random T cells analyzed per experiment. (C) Healthy T cells cocultured for 48 h with either healthy allogeneic B cells or allogeneic CLL B cells in transwell culture plates and subsequently used in conjugation assays with or without sAg-pulsed third-party allogeneic healthy donor B cells (APCs). Conjugates were selected at random for imaging and were scored for accumulation of F-actin at the immune synapse. Data are the mean ± SD from 3 independent experiments, with 50 conjugates analyzed per experiment.
CLL tumor cells induce defective recruitment of immunological synapse signaling molecules.
To investigate the molecular nature of the CLL-induced T cell immune synapse defect, we examined the targeting of a panel of proteins to the synapse. As before, primary cocultures were set up with healthy CD8+ or CD4+ T cells in direct contact with allogeneic CLL B cells or allogeneic healthy B cells for 48 h. These T cells were subsequently used in conjugation assays with third-party sAg-pulsed allogeneic healthy donor B cells for immunofluorescence and quantitative analysis.
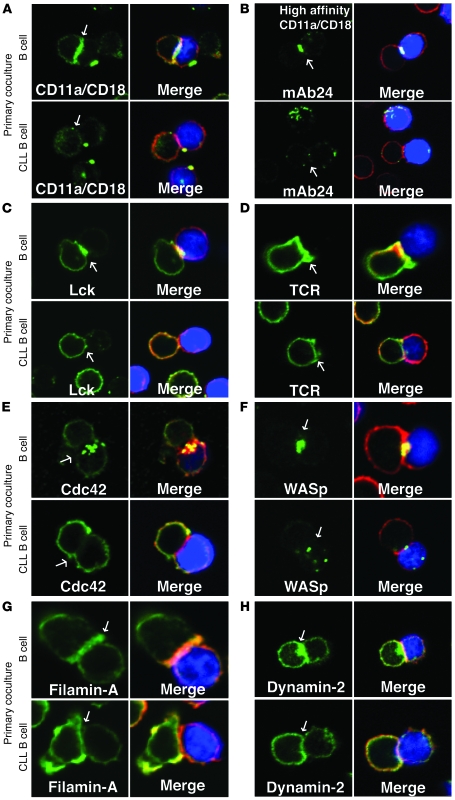
Integrin clustering and activation, in particular LFA-1, is thought to stabilize T cell–APC conjugates when the TCR comes into contact with an appropriate peptide-MHC complex (16). Significantly less LFA-1 clustering at the immunological synapse was observed in CD8+ (P < 0.05) or CD4+ (P < 0.01) T cell conjugates following coculture with CLL B cells compared with allogeneic healthy B cells (Figure 4A and Supplemental Figure 2A). In addition, these conjugate experiments demonstrated significant reduction of high-affinity LFA-1 (20, 21) localized at the immune synapse (P < 0.01 for CD8+ and P < 0.05 for CD4+ T cells) (Figure 4B and Supplemental Figure 2B). This reduced amount of integrin clustering and activity at the cell-cell synapse site is in keeping with the earlier observations that CLL T cells and healthy T cells following cancer cell contact had a reduced ability to conjugate efficiently.
Figure 4. CLL tumor cells induce defective recruitment of signaling molecules to the immune synapse.
Healthy CD8+ and CD4+ T cells were cocultured (primary coculture) for 48 h in direct contact with either healthy allogeneic B cells or allogeneic CLL B cells and subsequently used in conjugation assays with sAg-pulsed third-party allogeneic healthy donor B cells (APCs, blue). T cell conjugates formed were analyzed by immunofluorescence and confocal microscopy (F-actin was stained red using rhodamine phalloidin). Images shown are representative of evaluation of 150 conjugates from 3 independent experiments stained green for (A) LFA-1, (B) high-affinity LFA-1 (mAb24), (C) Lck (5-min conjugation time), (D) TCR (nonpermeabilizing conditions), (E) Cdc42, (F) WASp, (G) filamin-A, and (H) dynamin-2. Quantitative image analysis of protein accumulation (green) at the immunological synapse is shown in Supplemental Figure 2. Arrows denote protein localization at the T cell–APC synapse site. Colocalization of proteins in the merged images is shown in yellow. Original magnification, ×63.
Immunological synapse formation is regulated by TCR signaling, and Lck is the initial tyrosine kinase recruited after TCR clustering and ligation. Conjugate experiments demonstrated impaired recruitment of Lck to the T cell synapse in CD8+ or CD4+ T cells cocultured with CLL B cells compared with those cocultured with healthy B cells (P < 0.0001; Figure 4C and Supplemental Figure 2C), and these findings correlated with the intensity of TCR clustering fluorescence signal (P < 0.0001; Figure 4D and Supplemental Figure 2D).
Further analysis of CD8+ or CD4+ healthy T cells cocultured with CLL cells showed a significant decrease in the recruitment of other actin regulatory proteins to the immunological synapse in T cells, including Cdc42 (P < 0.0001 and P < 0.01, respectively), WASp (P < 0.0001), filamin-A (P < 0.05 and P < 0.01, respectively), and dynamin-2 (P < 0.01) (Figure 4, E–H, and Supplemental Figure 2, E–H). Of note, all of these gene products were noted to be altered on gene expression profiling of T cells from CLL patients compared with healthy donors (13).
Western blot analysis demonstrated alteration in relative total levels of proteins, representative of the cytoskeletal formation pathway in CLL T cells and in healthy T cells following coculture with CLL B cells. In particular, we observed increased expression of Cdc42, Arp3, and filamin-A, all central regulators of actin polymerization and T cell activation (14), consistent with that seen in the verification of the gene expression profiling of T cells from CLL patients (13). However, our present data show that irrespective of the upregulated expression of these proteins, they are not recruited effectively to the immunological synapse. Image quantification confirmed that coculture with CLL cells resulted in subsequently increased amounts of Cdc42 and filamin-A expression in nonconjugated T cells compared with protein expression levels following coculture with healthy B cells (Supplemental Figure 3, A and B). We hypothesize that such elevated expression levels of cytoskeletal proteins in cancer-bearing patient T cells may suggest an intracellular retention of proteins caused by recruitment defects of these key signaling proteins to the immunological synapse. In support of a global T cell recruitment defect in CLL, our previous gene expression profiling identified many altered gene products involved in vesicle and intracellular transport pathways in CLL T cells compared with healthy donor T cells (13). There were also cytoskeletal molecules whose expression was downregulated in T cells from CLL patients, including the large GTPase dynamin-2. Image quantification showed that coculture with CLL cells resulted in decreased dynamin-2 expression in nonconjugated T cells compared with coculture with healthy B cells (Supplemental Figure 3C), confirming our previous gene expression profiling work.
CD8+ and CD4+ T cells from CLL patients had identical molecular recruitment defects at the synapse site as those induced in healthy allogeneic T cells through direct contact with CLL B cells (data not shown). These results suggest that following direct cell contact, CLL cells subsequently have the ability to block T cells recruiting key cytoskeletal signaling molecules to the immune synapse, adding further support for a significant cancer-induced immunological synapse defect in CLL.
CLL T cells have reduced activation and effector function.
T cell activation occurs following TCR signaling and includes rapid recruitment of tyrosine-phosphorylated proteins to the T cell contact site with an APC. Thus, we examined the recruitment of such proteins using a specific phosphotyrosine antibody. Prior coculturing of healthy CD8+ or CD4+ T cells with CLL B cells reduced the subsequent phosphotyrosine fluorescent signal at the immune synapse in comparison with T cells that had been cocultured with healthy B cells (P < 0.0001; Figure 5A). CLL T cells had a similar reduced phosphotyrosine signal compared with age-matched healthy donor T cells (data not shown). Tumor cell–induced synapse defects with diminished early T cell signaling would be expected to lead to impaired T cell proliferation and cytokine production. To investigate this, we cocultured healthy CD3+ T cells for 48 h in direct contact with either allogeneic healthy B cells or CLL B cells. The healthy T cells were then purified and used in secondary mixed lymphocyte reactions (MLRs) with third-party allogeneic PBMCs as stimulators. As shown in Figure 5B, primary coculture with CLL B cells suppressed subsequent proliferation and IL-2 cytokine production in the MLRs compared with coculture with healthy B cells (P < 0.05). This suppression was prevented when the CLL B cells were pretreated with anti–ICAM-1 monoclonal antibody, but not with isotype control IgG. These data demonstrate that direct contact of healthy T cells with CLL B cells induces not only decreased F-actin polymerization at the immune synapse, but also subsequent suppressed T cell proliferation. In addition, IL-2 cytokine production and proliferation upon stimulation with APC or cross-linking with CD3/CD28 were significantly decreased in CLL T cells compared with age-matched healthy donor cells with these stimuli (data not shown). In keeping with our previous observation that cytotoxic function of autologous T cells from CLL patients is rarely detected and proliferation responses are weak against CLL cells (6), we observed impaired ability to induce idiotype-specific CD8+ T cells capable of killing idiotype-pulsed target cells in T cells from CLL patients compared with healthy donors (P < 0.01) (Figure 5C). These results demonstrate that the T cells with immune synapse dysfunction in CLL also have suppressed T cell activation and effector responses.
Figure 5. CLL T cells have reduced activation and effector function.
(A) Healthy CD8+ and CD4+ T cells were cocultured for 48 h as described in Figure 4. T cell conjugates formed during 5 min were fixed, permeabilized, and stained with anti-phosphotyrosine Ab (green) and rhodamine phalloidin (red). Quantitative image analysis (relative recruitment index [RRI]) of phosphotyrosine accumulation at the immunological synapse is shown, representative of evaluation of 150 conjugates from 3 independent experiments (50 conjugates analyzed per experiment), with the mean value shown as a black bar. Original magnification, ×63. (B) Healthy CD3+ T cells were first cocultured in direct contact with allogeneic healthy B cells or 3 different CLL B cells (CLL 1-3) with the addition of anti–ICAM-1 monoclonal antibody or isotype control IgG, and then were used in secondary MLRs with third-party allogeneic PBMCs as stimulators. [3H]-thymidine incorporation was assessed for the last 16 h of a 3-d culture. The stimulation index was calculated as cpm of T cells with stimulator cells/cpm of T cells alone. Results are the mean stimulation index ± SD from 5 independent healthy donor CD3+ T cells. IL-2 was assessed by ELISA, and values represent the mean ± SD from 5 independent healthy donor CD3+ T cells tested. (C) HLA-A*0201–expressing CD8+ cells were stimulated in vitro with DCs pulsed with immunoglobulin heavy chain–derived (IgVH-derived) peptide TLYLQMNSL weekly for 4 wk, and killing of peptide-pulsed H2 cells was assessed at the effector/target ratios shown. The results are the mean ± SD from 6 independent CLL patient and healthy donor experiments.
The immunomodulatory drug lenalidomide enhances autologous immune synapse formation in CLL.
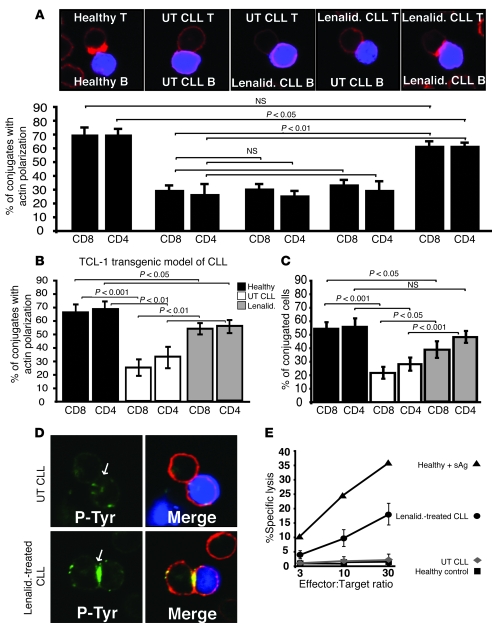
Lenalidomide, a thalidomide analog, is clinically active in patients with relapsed or refractory CLL (22–24), multiple myeloma (25), and myelodysplastic syndrome (26). Lenalidomide has immune-activating properties with T cells including NKT cells (27, 28). Thus, we examined the ability of lenalidomide to modulate formation of the immunological synapse in the autologous CLL patient setting. Pretreatment of ex vivo CD8+ or CD4+ T cells and autologous CLL B cells with lenalidomide 24 h before conjugation assays significantly (P < 0.01) increased the number of conjugates showing F-actin polymerization at the immune synapse compared with untreated CLL cells (P < 0.01) (Figure 6A) as well as the percentage of T cell conjugates formed (Supplemental Figure 4A). Treatment of both CLL B cells and CLL T cells with lenalidomide was required since treatment of either cell alone did not result in increased actin polymerization (Figure 6A) or recruitment of tyrosine-phosphorylated protein to the synapse (Supplemental Figure 5). Culture of the CLL T cells alone for up to 72 h or with addition of exogenous IL-2 did not reverse the T cell defect (Supplemental Figures 6 and 7, respectively).
Figure 6. Treatment of patient and Eμ-TCL1 mouse cells with lenalidomide significantly enhances immunological synapse formation.
Autologous T cell–sAg–pulsed CLL B cell conjugates from untreated (UT) or lenalidomide-treated (Lenalid.) CLL patient (A) or Eμ-TCL1 mouse cells (B) were selected at random for imaging and scored for accumulation of F-actin (red) at the immune synapse. As controls (healthy), autologous age-matched healthy donor or wild-type mice cells were used. Data are the mean ± SD from 3 independent experiments, with 50 conjugates analyzed per experiment. The confocal images shown are CD4+ human T cells. (C) Autologous T cell–sAg–pulsed CLL B cell conjugates from untreated CLL or lenalidomide-treated Eμ-TCL1 mouse cells were scored by visual counting using a confocal microscope. As controls, age-matched wild-type mice cells were used. Data are the mean ± SD from 3 independent experiments, with 50 random T cells analyzed per experiment. (D) Autologous T cell–sAg–pulsed CLL B cell (blue) conjugates from untreated or lenalidomide-treated patient CLL B and CLL T cells were stained for phosphotyrosine (green) and F-actin (red) using immunofluorescence and confocal microscopy. Images shown are representative of evaluation of 150 conjugates from 3 independent experiments. Arrows indicate protein localization at the T cell–APC synapse site. (E) Autologous cytotoxicity of CLL B cells was compared between untreated and lenalidomide-treated patient CLL B and CLL CD8+ T cells. Killing was assessed at the effector/target ratios shown. Data are mean ± SD from 6 independent CLL patient experiments. Autologous healthy donor cells were used as controls (with or without sAg-pulsed B cells). Original magnification, ×63.
Recent work from our group has demonstrated that development of CLL in the Eμ-TCL1 transgenic mouse model of CLL is associated with changes in gene expression profiles and immune function in CD8+ and CD4+ T cells similar to those identified in patients with this disease (Gorgün et al., unpublished observations). Thus, we wished to establish whether the T cells from mice with CLL also have an impaired ability to form an immunological synapse. CD8+ or CD4+ T cells and autologous sAg-pulsed CLL B cells exhibited significantly less actin polymerization at the immune synapse (Figure 6B) and less conjugate formation (Figure 6C) compared with healthy age-matched wild-type mice. Lenalidomide also increased synapse formation in this CLL mouse model. Treatment ex vivo of CD8+ or CD4+ T cells and autologous CLL B cells with lenalidomide significantly increased the number of conjugates showing F-actin polymerization at the immunological synapse site compared with untreated CLL mouse cells (Figure 6B) and T cell conjugate formation (Figure 6C).
In experiments using both human patient and mouse CLL cells, the enhanced immunological synapse formation effect of lenalidomide treatment was associated with an elevated tyrosine-phosphorylated signal at the immune synapse site (P < 0.001) (Figure 6D and Supplemental Figure 4, B and C) as well as increased accumulation of Lck (data not shown) compared with untreated control experiments. Thus, enhanced recruitment of cytoskeletal proteins to the T cell synapse may represent a novel immunomodulatory action of this drug in CLL. Of note, treatment with lenalidomide improved not only synapse formation, but also effector function as demonstrated by increased cytotoxicity of CLL B cells by autologous CD8+ T cells (Figure 6E).
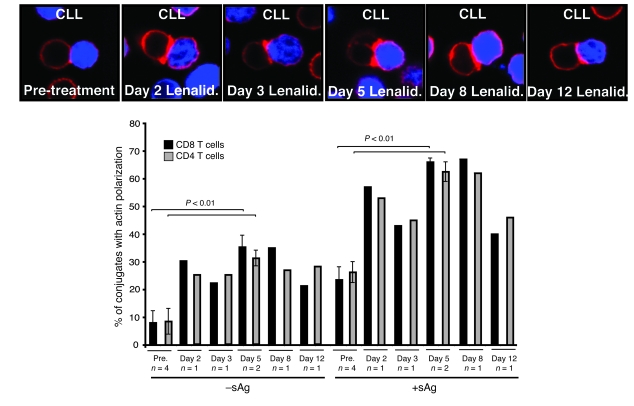
To determine whether in vivo treatment with lenalidomide also enhanced these responses, actin polymerization and recruitment of tyrosine-phosphorylated protein to the synapse were examined in 4 patients from whom samples were available before and after treatment with lenalidomide (24). Comparable with results seen ex vivo, there was enhanced actin polymerization (Figure 7) and recruitment of tyrosine-phosphorylated protein (Supplemental Figure 8) following treatment of patients with this agent.
Figure 7. In vivo treatment with lenalidomide enhances F-actin immune synapse formation.
Autologous T cell–CLL B cell (CLL B cells stained blue) conjugates (with or without sAg) from 4 patients before and after treatment with lenalidomide (Days 2, 3, 5, 8, and 12) were selected at random for imaging and scored for accumulation of F-actin (red) at the immune synapse. Fifty conjugates were analyzed per experiment. Original magnification, ×63.
The findings that lenalidomide can improve the immune synapse defect in CLL has important clinical implications for the treatment of CLL. The in vivo efficacy of lenalidomide is currently under investigation in ongoing clinical trials. An advantage of ongoing studies in the CLL mouse model is that we can examine the effect of this drug in combination with other therapeutic agents. The use of immunofluorescence and confocal microscopy to quantify immunological synapse formation as described here may provide us with a relatively rapid and simple method to assess whether immunotherapy approaches result in improvement in defective immunological synapse formation in CLL.
Discussion
Cancer is associated with immune deficiency, but the biological mechanisms for this are not completely understood. We use CLL as a model cancer to explore this question, as there is widespread interaction of circulating cancer B cells and immune T cells that can both be sampled from peripheral blood. It is well documented that CLL B cells are ineffective APCs, and this in part is due to low levels of expression of costimulatory and adhesion molecules required for effective immune recognition (6, 11). It has been suggested that this may contribute to malignant B cells inducing anergy (6, 29). The defects we have observed in CLL T cells are global and are not restricted to antigen-specific responses, and are not reversed by addition of exogenous IL-2. Taken together, these suggest T cell suppression rather than anergy as a mechanism in this disease. Although the autologous immune response in CLL patients is very weak, we have demonstrated that autologous CTL cell lines can be expanded ex vivo against shared Ig-derived peptides presented by tumor cells that are able to kill patients’ CLL cells (30). It is likely that the low peptide-binding affinity of tumor-associated antigens including Ig-derived peptides to MHC class I molecules contributes to reduced antitumor immune response (30, 31). These data suggest that effective CTLs that have the ability to target CLL tumor cells must be present within the T cell repertoires of cancer patients but are in some way suppressed. We hypothesized that there must be additional mechanisms used by CLL cells to escape immune surveillance and facilitate tumor progression. Our previous studies of gene expression profiling on the nonmalignant T cells from cancer-bearing patients identified profound changes in gene expression pathways in CLL T cells compared with healthy donors. This work demonstrated that these changes could be induced at the protein level in healthy T cells following short-term direct contact culture with CLL B cells.
Here we demonstrate that CD8+ and CD4+ T cells from CLL patients have an impaired ability to form an immunological synapse. Importantly, we demonstrate that short-term direct cell contact between CLL B cells and healthy allogeneic T cells induces the same immunological synapse defects as those identified in CLL T cells. Our results demonstrate one mechanism whereby tumor cells can actively modify T cell immunological recognition and function. These findings have important implications for both autologous and allogeneic immunotherapy treatment approaches including donor lymphocyte infusions in the setting of bulk disease (32). These findings in human patients with CLL are in agreement with those in a murine model of colon carcinoma, where it was demonstrated that tumor-infiltrating lymphocytes had impaired F-actin recruitment and blockade of proximal TCR signaling (33, 34).
Coculture of healthy T cells with CLL B cells results in suppressed F-actin polymerization and recruitment of key molecules including LFA-1, TCR, and Lck to the T cell synapse site, as well as additional signaling molecules associated with the immune synapse, including Cdc42, WASp, dynamin-2, and filamin-A. Dynamin-2 mediates F-actin polymerization at the immunological synapse and subsequent regulation of signals controlling TCR-mediated T cell activation (35). Recruitment of filamin-A to the immune synapse is associated with cytoskeletal rearrangements including lipid raft recruitment that function as TCR-signaling platforms (36). Thus, CLL B cells suppress the recruitment of proximal TCR signaling molecules such as Lck and key cytoskeletal signaling proteins. An intact immune synapse requires sustained TCR engagement with sufficient proximal signaling and an intact T cell cytoskeleton. Treatment of CLL T cells with PMA and ionomycin, which bypass TCR stimulation and immune synapse formation, does not enhance proliferation, IL-2 production, or effector function in human and mouse CLL T cells (data not shown). Taken together with our previous gene expression profiling data demonstrating alteration in expression of multiple components of cytoskeletal regulation suggests that the primary defect in the CLL T cells is a failure of cytoskeletal organization rather than blockade of TCR-mediated signaling.
The function of the immune synapse is to regulate T cell proliferation and activity in accordance with the nature of the T cell and APC involved and the quality and quantity of TCR ligands (17). The formation of the synapse in CD4+ T cells allows the directed secretion of IL-2 and other cytokines toward the immune synapse and eventually T cell proliferation. CTLs form immune synapses that can deliver a lethal hit of cytolytic granules via the microtubule organization center. CLL T cells have reduced levels of tyrosine-phosphorylated proteins at the synapse and a reduced capacity to proliferate and produce IL-2. These defects were induced in healthy T cells by direct-contact coculture with CLL B cells. In addition, we identified a significantly decreased ability to induce antigen-specific CTLs capable of killing target cells from CLL T cells compared with healthy donor cells (P < 0.01). These results demonstrate that CLL impairs the capacity of T cells to form synapses and results in the associated functional responses.
Repair of the defects in T cell function is likely to maximize antitumor immune responses in CLL patients and enhance immunotherapy approaches. Lenalidomide is clinically active as a single agent in patients with relapsed or refractory CLL (22–24). Here we demonstrate that ex vivo and in vivo treatment of both human patient and Eμ-TCL1 transgenic mouse cells with lenalidomide improves the defective immune synapse formation in CLL. The precise mechanisms of action of lenalidomide are not clear, although the drug has multiple immunomodulatory actions (37). Uniquely in CLL, the use of lenalidomide is associated with a tumor flare reaction that may be associated with an immune-mediated antitumor response (22–24, 37). Our ex vivo data suggest that a lower pharmacological concentration of this agent can improve immune synapse formation and effector function, and lower doses of this agent may result in a more acceptable treatment toxicity (23). Ongoing studies are addressing whether the observed tumor flare reactions correlate with altered expression of costimulatory molecules on CLL cells or with improvement in immune synapse formation. Neither lenalidomide treatment of CLL cells alone nor CD154 treatment of CLL cells, both of which result in upregulation of costimulatory molecules (10, 24), resulted in improved synapse formation. Treatment of both CLL cells and T cells with lenalidomide was necessary to improve immune synapse formation, further demonstrating that this drug acts on multiple cell targets. The mechanism(s) whereby lenalidomide mediates this activity in CLL T cells and whether this is unique in CLL or occurs in other B cell malignancies is also under investigation, but we have observed similar induction of defects in healthy T cells on culture with follicular lymphoma or diffuse large B cell lymphoma cells (data not shown).
Immunological synapse impairment is clearly used as an immunomodulating mechanism by tumor cells, and it is tempting to speculate that this can facilitate disease progression in the hostile immune environment of the host, similar to HIV-1 infection (38). Our data identifying immunological synapse formation and the molecules regulating its organization provide functional biomarkers and targets for the reversal of immune deficiency in cancer.
Methods
Cell isolation.
Heparinized venous blood samples from CLL patients and age-matched healthy donors were taken after obtaining written informed consent and approval by the North East London Research Ethics Committee. All CLL patients were previously untreated (median time from diagnosis, 26 [range 7–93] months) at the time that blood was obtained for these studies. CLL patients were selected to represent the heterogeneity of the disease including different Rai stages (I to III) and IgVH mutation status (mutated and unmutated) (data not shown). Of note, none of these factors were shown to be associated with extent of immune synapse defect or to be associated with change in gene expression profile (13). In vivo–derived samples came from an institutional review board–approved trial examining the efficacy of lenalidomide in symptomatic, previously treated CLL patients (24). We used healthy allogeneic B cells rather than CD5+ B cells as controls, based on previous data demonstrating that the gene expression profile of CLL cells was more similar to B cells than CD5+ B cells (39). Additional methodology is described in Supplemental Methods.
Mice.
Ethical approval and a project license from the Animal Procedures Committee of the Home Office were obtained for all animal experiments. Homozygous TCL1 transgenic mice (background strain B6C3) have been previously described (18, 40). Breeding pairs were provided as a generous gift from Carlo M. Croce. B6C3 mice (wild-type controls) were purchased from The Jackson Laboratory. Additional methodology is described in Supplemental Methods.
Antibodies and reagents.
These are listed in Supplemental Methods.
Cell conjugation assays.
Cell conjugates formed were a modification of those formed for previous studies (35, 41–43). Healthy or malignant B cells (1 × 106 healthy B cells or CLL cells) were stained with CellTracker Blue CMAC (7-amino-4-chloromethylcoumarin) following the manufacturer’s instructions and pulsed with or without 2 μg/ml of a cocktail of staphylococcal sAgs (SEA and SEB; Sigma-Aldrich) for 30 min at 37°C. B cells were centrifuged (200 g for 5 min) with an equal number of T cells and incubated at 37°C for 10 min (CD8+ T cells) or 15 min (CD4+ T cells) unless otherwise stated, then plated (centrifuged) onto poly-l-lysine–coated coverslips and fixed for 15 min at room temperature with 3% methanol-free formaldehyde in PBS.
Immunofluorescence and confocal microscopy image acquisition.
Immunofluorescent labeling was done as previously described (41) and are summarized in Supplemental Methods.
Quantitative image analysis of conjugate formation and F-actin polymerization.
Conjugation was scored by visual counting under a confocal microscope as described previously (44) and is summarized in Supplemental Methods. Quantification of F-actin, mAb24, filamin-A, dynamin-2, and phosphotyrosine polarization at the immune synapse was based on a previously described method (43) by random selection of 50 conjugate images containing a CellTracker Blue–stained healthy or CLL B cell in contact with a T cell. Polarization of proteins at the T cell contact site was scored as described in Supplemental Methods.
Quantitative image analysis of fluorescence.
To quantitate recruitment of F-actin, LFA-1, Lck, TCR, Cdc42, WASp, and phosphotyrosine to the immunological synapse, the relative recruitment index was calculated as previously described using the NIH ImageJ program (36). Additional methodology is described in Supplemental Methods.
CLL cell–T cell coculture assays.
Coculture assays were carried out as previously described (13) and are summarized in Supplemental Methods.
Cytotoxicity.
Chromium (51Cr) release assay was performed as previously described (6) and is summarized in Supplemental Methods.
Allogeneic MLR.
MLRs were carried out as previously described (45) and are summarized in Supplemental Methods. IL-2 was assessed by ELISA as previously described (45).
Lenalidomide treatment.
CLL cells and autologous T cells were treated separately with 0.5 μM lenalidomide in acidic PBS (24) for 24 h in full culture medium (10% human serum) at 37°C before cell conjugation assays. Lenalidomide was prepared as previously described (24). Untreated autologous cells were also cultured separately using the acidic PBS alone without drug for 24 h in full culture medium at 37°C before conjugation assays.
Statistics.
Statistical differences between experimental groups were evaluated by 2-tailed Student’s t test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We would like to thank Nancy Hogg and Mark A. McNiven for supplying mAb24 and dynamin-2 antibodies, respectively. Additionally, we would like to acknowledge Ching-Shih Chen for extraction of lenalidomide, Leslie Andritsos and Samir G. Agrawal for providing CLL samples, and Mitch Phelps for LC/MS confirmation of lenalidomide purity. We thank Peter Varney for editorial assistance. This work was supported by grant P01 CA81538 from the National Cancer Institute to the CLL Research Consortium (to J.G. Gribben and J.C. Byrd), grant P01 CA95426 (to J.C. Byrd), and by grants from the Leukemia and Lymphoma Society and the CLL Global Research Foundation.
Footnotes
Nonstandard abbreviations used: CLL, chronic lymphocytic leukemia; LFA-1, leukocyte function–associated antigen-1; MLR, mixed lymphocyte reaction; sAg, superantigen; TCR, T cell antigen receptor.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:2427–2437 (2008). doi:10.1172/JCI35017
References
- 1.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Dunn G.P., Old L.J., Schreiber R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 3.Swann J.B., Smyth M.J. Immune surveillance of tumors. J. Clin. Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhardwaj N. Harnessing the immune system to treat cancer. J. Clin. Invest. 2007;117:1130–1136. doi: 10.1172/JCI32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krackhardt A.M., et al. Identification of tumor-associated antigens in chronic lymphocytic leukemia by SEREX. Blood. 2002;100:2123–2131. doi: 10.1182/blood-2002-02-0513. [DOI] [PubMed] [Google Scholar]
- 6.Krackhardt A.M., et al. T-cell responses against chronic lymphocytic leukemia cells: implications for immunotherapy. Blood. 2002;100:167–173. doi: 10.1182/blood.V100.1.167. [DOI] [PubMed] [Google Scholar]
- 7.Lotz M., Ranheim E., Kipps T.J. Transforming growth factor beta as endogenous growth inhibitor of chronic lymphocytic leukemia B cells. J. Exp. Med. 1994;179:999–1004. doi: 10.1084/jem.179.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayad L., et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97:256–263. doi: 10.1182/blood.V97.1.256. [DOI] [PubMed] [Google Scholar]
- 9.Ranheim E.A., Kipps T.J. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J. Exp. Med. 1993;177:925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantwell M., Hua T., Pappas J., Kipps T.J. Acquired CD40-ligand deficiency in chronic lymphocytic leukemia. Nat. Med. 1997;3:984–989. doi: 10.1038/nm0997-984. [DOI] [PubMed] [Google Scholar]
- 11.Buhmann R., Nolte A., Westhaus D., Emmerich B., Hallek M. CD40-activated B-cell chronic lymphocytic leukemia cells for tumor immunotherapy: stimulation of allogeneic versus autologous T cells generates different types of effector cells. Blood. 1999;93:1992–2002. [PubMed] [Google Scholar]
- 12.Beyer M., et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 13.Gorgun G., Holderried T.A., Zahrieh D., Neuberg D., Gribben J.G. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J. Clin. Invest. 2005;115:1797–1805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billadeau D.D., Nolz J.C., Gomez T.S. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 15.Grakoui A., et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 16.Sims T.N., Dustin M.L. The immunological synapse: integrins take the stage. Immunol. Rev. 2002;186:100–117. doi: 10.1034/j.1600-065X.2002.18610.x. [DOI] [PubMed] [Google Scholar]
- 17.Huppa J.B., Davis M.M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 18.Bichi R., et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6955–6960. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scrivener S., Goddard R.V., Kaminski E.R., Prentice A.G. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk. Lymphoma. 2003;44:383–389. doi: 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- 20.Dransfield I., Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin alpha subunits. EMBO J. 1989;8:3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith A., et al. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol. Rev. 2007;218:135–146. doi: 10.1111/j.1600-065X.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 22.Chanan-Khan A., et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J. Clin. Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 23.Ferrajoli A. , et al. 2008Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 1115291–5297. 10.1182/blood-2007-12-130120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andritsos L., et al. Higher doses of lenalidomide are associated with unacceptable toxicity including life threatening tumor flare in patients with chronic lymphocytic leukemia. J. Clin. Oncol. 2008;26:2519–2525. doi: 10.1200/JCO.2007.13.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson P.G., et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.List A., et al. Efficacy of lenalidomide in myelodysplastic syndromes. N. Engl. J. Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 27.Haslett P.A., Corral L.G., Albert M., Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J. Exp. Med. 1998;187:1885–1892. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang D.H., et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108:618–621. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appleman L.J., Boussiotis V.A. T cell anergy and costimulation. Immunol. Rev. 2003;192:161–180. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 30.Trojan A., et al. Immunoglobulin framework-derived peptides function as cytotoxic T-cell epitopes commonly expressed in B-cell malignancies. Nat. Med. 2000;6:667–672. doi: 10.1038/76243. [DOI] [PubMed] [Google Scholar]
- 31.Harig S., et al. Induction of cytotoxic T-cell responses against immunoglobulin V region-derived peptides modified at human leukocyte antigen-A2 binding residues. Blood. 2001;98:2999–3005. doi: 10.1182/blood.V98.10.2999. [DOI] [PubMed] [Google Scholar]
- 32.Gribben J.G., et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood. 2005;106:4389–4396. doi: 10.1182/blood-2005-05-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koneru M., Schaer D., Monu N., Ayala A., Frey A.B. Defective proximal TCR signaling inhibits CD8+ tumor-infiltrating lymphocyte lytic function. . J. Immunol. . 2005;174:1830–1840. doi: 10.4049/jimmunol.174.4.1830. [DOI] [PubMed] [Google Scholar]
- 34.Monu N., Frey A.B. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. . Cancer Res. . 2007;67:11447–11454. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez T.S., et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat. Immunol. 2005;6:261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- 36.Tavano R., et al. CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunological synapse. Nat. Cell Biol. 2006;8:1270–1276. doi: 10.1038/ncb1492. [DOI] [PubMed] [Google Scholar]
- 37.Chanan-Khan A.A., Cheson B.D. Lenalidomide for the treatment of B-cell malignancies. J. Clin. Oncol. . 2008;26:1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- 38.Fackler O.T., Alcover A., Schwartz O. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat. Rev. Immunol. 2007;7:310–317. doi: 10.1038/nri2041. [DOI] [PubMed] [Google Scholar]
- 39.Klein U., et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. . J. Exp. Med. 2001;194:1625–1638. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson A.J., et al. Characterization of the TCL-1 transgenic mouse as a preclinical drug development tool for human chronic lymphocytic leukemia. Blood. 2006;108:1334–1338. doi: 10.1182/blood-2005-12-011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedwick C.E., et al. TCR, LFA-1, and CD28 play unique and complementary roles in signaling T cell cytoskeletal reorganization. J. Immunol. 1999;162:1367–1375. [PubMed] [Google Scholar]
- 42.Cannon J.L., et al. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity. 2001;15:249–259. doi: 10.1016/S1074-7613(01)00178-9. [DOI] [PubMed] [Google Scholar]
- 43.Labno C.M., et al. Itk functions to control actin polymerization at the immune synapse through localized activation of Cdc42 and WASP. Curr. Biol. 2003;13:1619–1624. doi: 10.1016/j.cub.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thoulouze M.I., et al. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity. 2006;24:547–561. doi: 10.1016/j.immuni.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Schultze J.L., et al. Follicular lymphomas can be induced to present alloantigen efficiently: a conceptual model to improve their tumor immunogenicity. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8200–8204. doi: 10.1073/pnas.92.18.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.