Abstract
A full-length cDNA for the rat kidney mitochondrial cytochrome P450 mixed function oxidase, 25-hydroxyvitamin D3-1α-hydroxylase (P4501α), was cloned from a vitamin D-deficient rat kidney cDNA library and subcloned into the mammalian expression vector pcDNA 3.1(+). When P4501α cDNA was transfected into COS-7 transformed monkey kidney cells, they expressed 25-hydroxyvitamin D3-1α-hydroxylase activity. The sequence analysis showed that P4501α was of 2,469 bp long and contained an ORF encoding 501 amino acids. The deduced amino acid sequence showed a 53% similarity and 44% identity to the vitamin D3-25-hydroxylase (CYP27), whereas it has 42.6% similarity and 34% identity with the 25-hydroxyvitamin D3-24-hydroxylase (CYP24). Thus, it composes a new subfamily of the CYP27 family. Further, it is more closely related to the CYP27 than to the CYP24. The expression of P4501α mRNA was greatly increased in the kidney of vitamin D-deficient rats. In rats with the enhanced renal production of 1α,25-dihydroxyvitamin D3 (rats fed a low Ca diet), P4501α mRNA was greatly increased in the renal proximal convoluted tubules.
Keywords: vitamin D metabolism, 1α-hydroxylase, 24-hydroxylase, P450
It has been established that vitamin D3 is first metabolized in the liver to 25-hydroxyvitamin D3 [25(OH)D3] and subsequently in the kidney to 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] and 24,25-dihydroxyvitamin D3 [24,25(OH)2D3]. Of these two dihydroxyvitamin D metabolites, 1α,25(OH)2D3 has been demonstrated to be the active form of vitamin D3 (1, 2). The production of 1α,25(OH)2D3 is regulated primarily at the final step in its synthetic pathway; 1α-hydroxylation of 25(OH)D3 by the renal mitochondrial P450 mixed-function oxidase (3, 4). The cytochrome P450 enzymes form a superfamily of heme-containing proteins that are bound to the membranes of the microsomes and mitochondria and serve as an oxidation-reduction component of the mixed-function oxidase system. In in vivo conditions, this system is involved in the oxidative metabolism of a number of steroids. The CYP family is large, with at least 74 families, and each mammalian species is estimated to have between 60 and 200 distinct superfamily members (5, 6). These P450 proteins have more than 40% amino acid homology within a single family and usually more than 55% identity within a subfamily. The cysteine-containing region associated with the heme-binding domain of the P450 protein is highly conserved among all eukaryotic species.
The amino acid sequence similarity between 25(OH)D3-24-hydroxylase (CYP24) and vitamin D3-25-hydroxylase (CYP27) enzymes within the heme-binding domain has been reported to be 60% (7, 8). The homology of the putative ferredoxin-binding domain between CYP27 and CYP24 was 70%. By using a probe from the 3′ region of the rat CYP24 cDNA, which encompasses the heme-binding domain of the molecule, St-Arnaud et al. (9) screened a cDNA library from the kidney of vitamin D-deficient rats under reduced stringency conditions to identify and clone the 25(OH)D3-1α-hydroxylase cDNA. They reported the partial amino acid sequence of P4501α at the 18th Annual Meeting of the American Society for Bone and Mineral Research (September 9–11, 1996, Seattle, WA). The amino acid sequence similarity with CYP24 was calculated to be 78% within the heme-binding domain; the two proteins diverged significantly outside this region for the overall sequence similarity of 26%. However, neither the full-length sequence of 25(OH)D3-1α-hydroxylase nor its regulation in the kidney has been reported.
Recently, Nicoll et al. (10) cloned the third mammalian Na+–Ca+ exchanger, NCX3, by using degenerate oligonucleotide primers derived from two highly conserved regions of NCX1 and NCX2. Shen et al. (11) also cloned a new form of P450 gene by reverse transcription–PCR (RT-PCR) by using degenerate primers containing inosine. To isolate a fragment of 25(OH)D3-1α-hydroxylase, a similar approach was used according to the method of mixed oligonucleotide-primed amplification of cDNA. We hypothesized that 1α-hydroxylase should have a degree of homology with the products of known P450 genes. The P450 genes with homology considered to be likely candidates were CYP27 and CYP24. The primers were targeted at two conserved regions (heme-binding domain and ferredoxin-binding domain) of the products of CYP24 and CYP27. By using the method of mixed oligonucleotide-primed amplification of cDNA, we have cloned a new sequence of the P450 mixed function oxidase from the kidney of vitamin D-deficient rats. This clone designated as P4501α has been sequenced and expressed. Here we report the cloning and sequencing of the P4501α gene and the regulation of its mRNA expression in rat kidney.
MATERIALS AND METHODS
Animals.
Male weanling rats (Sprague–Dawley strain) were maintained for 3 weeks on a synthetic vitamin D-deficient diet containing 0.03% Ca and 0.6% phosphorus (Teklad, Madison, WI). Rats fed the vitamin D-deficient diet were maintained in a room with incandescent lighting, and all potential sources of UV light and vitamin D were excluded. All chemicals were of a highly purified analytical grade. 1α,25(OH)2D3 was purchased from Wako Pure Chemicals (Osaka). 24,25(OH)2D3 was a gift from Kureha Chemical Industries (Tokyo). 26,27-Hexafluoro-1α,25(OH)2D3 [26,27-F6-1α,25(OH)2D3] was kindly donated by Sumitomo Pharmaceuticals (Osaka). 10-Oxo-19-nor-25-hydroxyvitamin D3 was a gift from S. Yamada (Tokyo Medical and Dental University, Tokyo). [26,27-3H]-25(OH)D3 was obtained from Amersham. [α-32P]- and [α-33P]dCTP were purchased from DuPont/New England Nuclear. Taq DNA polymerase, restriction enzymes, vectors, and other molecular biological reagents were obtained from Stratagene, Boehringer Mannheim, Takara Biomedicals (Shiga, Japan), and BRL. COS-7 cells were obtained from the American Type Culture Collection. Trypsin, penicillin G, streptomycin, cell culture media, and fetal bovine serum were obtained from GIBCO. Oligonucleotides described in the text were synthesized by Pharmacia.
Cloning of 1α-Hydroxylase cDNA Fragment.
Total RNA was isolated from a vitamin D-deficient rat kidney by guanidine-hydrochloride method (12) and poly(A)+ RNA was purified through an oligo(dT) cellulose column. Poly(A)+ RNA was reverse-transcribed by a random hexamer primer. PCR with degenerate primers was used to identity a fragment of the 1α-hydroxylase. The primers were derived from the amino acid sequence of conserved regions of CYP24 and CYP27. The forward primer was a 23 mer with 36-fold degeneracy (5′-CTSCTSAARGCHGTSATYAARGA-3′) based on a portion of the proposed ferredoxin-binding site. The reverse primer was a 23 mer with 432-fold degeneracy (5′-CKCTTBCCRAABCCRAARGGVA-3′) based on a portion of the heme-binding region. cDNA from the rat kidney was used as a template for the PCR, which was carried out for 35 cycles (94°C for 30 sec; 42°C for 1 min; 72°C for 1 min). The PCR product was cloned into the pCRII (Invitrogen), and after transformation, four plasmids with appropriately sized inserts were sequenced. All inserts had the same sequence and had a previously undescribed sequence of P450.
PCR Cloning of Rat 25(OH)D3-1α-Hydroxylase cDNA.
Vitamin D-deficient rat kidney poly(A)+ RNA was used to direct synthesis of the first strand cDNA from the T17-tailed rapid amplification of cDNA ends (RACE) oligonucleotide primer. A region of rat mitochondrial 25(OH)D3-1α-hydroxylase cDNA was amplified between the RACE primer and a specific oligonucleotide (5′-AAGGCTGTGATCAAAGAAGTGTTGA-3′). The resulting PCR product, a 1.2-kbp rat kidney 1α-hydroxylase cDNA (confirmed by Northern blot analysis), was cloned into the pCRII (Invitrogen).
Cloning and Sequencing of Full-Length Rat 25(OH)D3-1α-Hydroxylase.
cDNA was prepared from the poly(A)+ RNA of the vitamin D-deficient rat kidney and ligated into Uni-ZAP XR vector arms by a ZAP cDNA Synthesis Kit (Stratagene). The recombinant DNA was packaged with a Gigapack II Gold Packaging Extract (Stratagene) and infected to Escherichia coli, XL1-Blue MRF′. The primary library size was 2.7 × 106 independent recombinants. The primary library was amplified once and suspended to a titer of 3.4 × 109 pfu/ml. The library was plated (1–2 × 104 pfu/150 mm NZY [NZ amine (casein hydrolysate enzymatic) yeast extract] agar plate) and screened by plaque hybridization (13) by using the 1.2-kbp fragment as a probe which was labeled with digoxigenin (DIG) by a PCR DIG Probe Synthesis Kit (Boehringer Mannheim). Hybridization was done at 65°C for 16–20 h with 5× standard saline citrate (SSC) containing 1% blocking reagent, 0.1% N-lauroylsarcosine, 0.02% SDS, and 50–100 ng/ml of probes. The filters were washed twice in 2× SSC containing 0.1% SDS at 60°C for 5 min and once in 0.1× SSC containing 0.1% SDS at 60°C for 15 min. The detection was performed with a DIG Luminescent Detection Kit (Boehringer Mannheim). Positive plaques were isolated and verified by PCR with the specific primers for P4501α. Each clone isolated was excised to a pBluescript by a Rapid Excision Kit (Stratagene) and sequenced by the dideoxy-chain termination method (14).
Expression of cDNA in COS-7 Cells.
A NheI–BamHI fragment of the isolated P4501α was inserted into the same sites of the mammalian expression vector pcDNA 3.1 (+) (Invitrogen) to construct pcDNA-1α. COS-7 cells were subcultured for 20 h before transfection and seeded in 100-mm plates at a density of 1 × 106 cells/dish. Cells were transfected with 10 μg of pcDNA-1α according to the lipofection protocol by using Lipofectin (BRL) as described in the manufacturer’s manual. Twenty-four hours after transfection, the medium was replaced and the cells were cultured for a further 24 h. Then the cells were incubated with [3H]25(OH)D3 (2,000 Bq), and then methanol was added to terminate the reaction. An aliquot of the lipid soluble metabolites was applied to HPLC under the following conditions: column, Finepak SIL 4.6 × 250 mm; mobile phase, n-hexane/2-propanol/methanol (89:5.5:5.5); flow rate, 1.5 ml/min (15). The radioactivity of the eluate was counted. Generation of [3H]1α,25(OH)2D3 was confirmed by comigration of the radioactivity and authentic 1α,25(OH)2D3.
Northern Blot and RT-PCR Analyses.
Total RNA was prepared by the method of Chomczynski and Sacchi (16), and poly(A)+ RNA was isolated by using the Oligotex dT 30 isolation system (Takara Biomedicals) (17). Poly(A)+ RNA was size-fractionated on a 1.4% agarose gel containing 6% formaldehyde. After electrophoresis, RNA was transferred to Hybond-N membrane (Amersham) by capillary diffusion in 20× SSC and fixed by UV cross-linking. 32P-labeled single-stranded DNA probes were hybridized to the membrane overnight at 42°C. The blot was washed for 15 min at 42°C with 2× SSC in 0.1% SDS, followed by washing steps with increasing stringency up to 0.2× SSC at 60°C for 15 min. RT-PCR of microdissected PCT segments were performed by the method as described in the previous papers (18, 19). Two mm of microdissected proximal convoluted tubule (PCT) segments were reverse-transcribed with a random hexamer primer. Synthesized cDNA was used for PCR. The sense (5′-AGTGAGCTGAACAAGTGGTC-3′) and antisense (5′-GTCTGATTGTCAGGCAGCAC-3′) primers used in PCR for CYP24 located in the coding region and corresponded to bases 956–975 and 1,418–1,437, respectively. The sense and antisense primers used in PCR for P4501α were 5′-CTAAAGGCTGTGATCAAAGAAGTGT-3′, which corresponded to bases 1,110–1,134 and 5′-TTCGTTTGCCAAAGCCAAAAGGAAG-3′, which corresponded to bases 1,338–1,362, respectively.
RESULTS
Amplification of a cDNA Fragment with Mixed Primers.
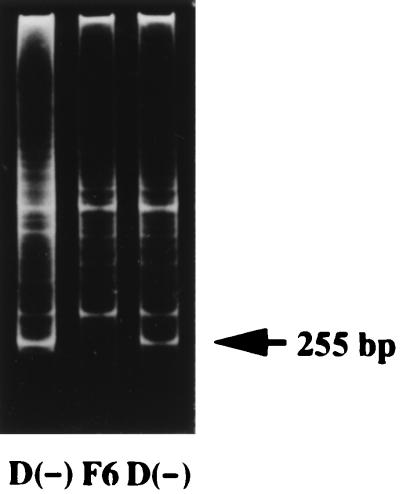
PCR with degenerate primers was used to identify a fragment of 1α-hydroxylase. The primers were targeted at two conserved regions of the products of CYP24 and CYP27. After mRNA was isolated from rat kidney, it was reverse-transcribed into cDNA by using a cDNA synthesis kit (Stratagene), and then the cDNA was amplified with a set of degenerate primers derived from the heme- and ferredoxin-binding domains of the CYP24 and CYP27 genes. By using the method of mixed oligonucleotide-primed amplification of cDNA, we have cloned a novel sequence from the kidney of vitamin D-deficient rats. PCR amplification resulted in three bands on gel electrophoresis (Fig. 1). A PCR product with an approximate size of 255 bp was detected only in cDNA from the vitamin D-deficient rats. This PCR product was cut out, extracted, and cloned into the TA cloning vector pCR II for the sequence analysis. Rapid amplification of 3′ end from P4501α cDNA was carried out according to the manufacturer’s manual (3′-RACE system for rapid amplification of cDNA ends, BRL). A single RACE product about 1.2 kbp was observed (data not shown). The PCR product was subcloned into a plasmid vector, and this insert was used for cloning of the full-length P4501α cDNA.
Figure 1.
Electrophoretic bands (ethidium bromide fluorescence) produced by the method of mixed oligonucleotide-primed amplification of cDNA. Lanes 1 and 3, vitamin D-deficient rats D(−); lane 2, vitamin D-deficient rat treated for 48 h with 1 μg of 26,27-F6-1α,25(OH)2D3 (F6).
DNA Sequences.
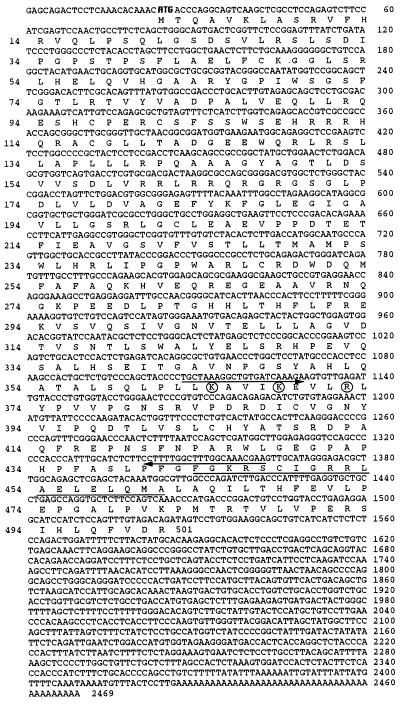
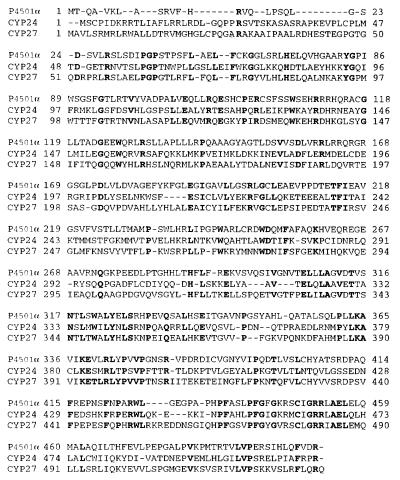
The λZap cDNA library was screened by using the 1.2-kbp fragment as a probe. Eleven positive clones were detected from 8 × 104 plaques at the first screening. The longest insert of these clones was sequenced. A full-length cDNA clone obtained was 2,469 bp long (Fig. 2). The P4501α clone contained an ORF of 1,503 nucleotides, starting at base 24 and ending at base 1,526, which encoded a protein of 501 amino acids. The predicted polypeptides were compared with the protein databases and found to possess the hallmark features of the CYP superfamily (Fig. 3). There was a significant homology to CYP members in the ferredoxin- and heme-binding domains. A heme-binding, highly conserved region could be recognized from residues 441 to 461, in which Cys-448 was considered as the heme-binding site. Amino acid characteristics of the ferredoxin-binding site in P450 (20) were observed at Lys-364, Lys-368, and Arg-378. A sequence search that used the University of Wisconsin Genetics Computer Group (gap program) indicated that the deduced amino acid sequence of P4501α has a 52.9% similarity and 43.6% identity with CYP27. Its similarity is 42.6% and its identity is 33.9% with CYP24. It is thus related more to the liver mitochondrial vitamin D 25-hydroxylase than to the kidney mitochondrial 24-hydroxylase. Based on these findings and the current nomenclature (5, 6), we propose P4501α be known as CYP27B1.
Figure 2.
The nucleotide sequence of rat P4501α cDNA and its deduced amino acid sequence. Arrows indicate primer positions for isolation of P4501α fragment. The region of the heme-binding site is underlined. The conserved amino acids known to be important in ferredoxin-binding are circled. The start codon is shown in bold type.
Figure 3.
Alignment of P4501α with closely related other CYPs. The closest relatives to P4501α were determined by using the blastp program provided by the National Center for Biotechnology Information, and the amino acid sequences were manually aligned to P4501α. CYP27, the predicted protein from the rat mitochondrial vitamin D3-25-hydroxylase (GenBank accession no. M38566); CYP24, the predicted protein from the rat mitochondrial 25(OH)D3-24-hydroxylase (GenBank accession no. X59506). Identical residues are shown in bold type, and dashes represent gaps for alignment purposes.
Metabolism of 25(OH)D3 in the P4501α-Transfected COS-7 Cells.
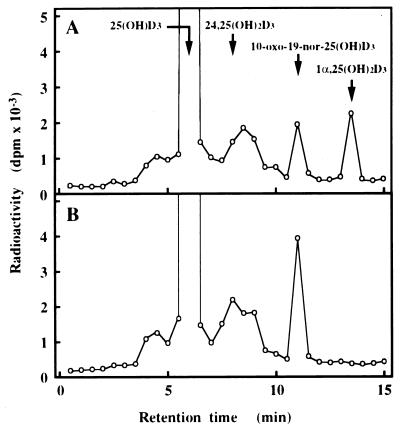
To confirm that P4501α can catalyze the hydroxylation of 25(OH)D3 at 1α-position, we examined the metabolism of 25(OH)D3 in COS-7 cells transfected with the plasmid expressing P4501α. Expression of P4501α mRNA was confirmed by RT-PCR. Control COS-7 cells did not express the P4501α mRNA. A marked expression of P4501α mRNA was observed in the cells transfected with cDNA P4501α (data not shown). Following a 20-h incubation with 25(OH)D3, COS-7 cells transfected with pcDNA-1α generated a metabolite that comigrated with authentic 1α,25(OH)2D3 (Fig. 4). This experiment has been repeated three times with identical results. 1α,25(OH)2D3 was not generated in the cells transfected with a pcDNA empty vector under the same conditions. These results indicate that P4501α cDNA catalyzes the 1α -hydroxylation reaction of 25(OH)D3.
Figure 4.
Metabolism of 25(OH)D3 in COS-7 cells transfected with the expression plasmid for P4501α. Metabolism of 25(OH)D3 by the cells transfected with 10 μg of pcDNA 1α (A) and the same amount of pcDNA empty vector (B). The retention times of 25(OH)D3, 24,25(OH)2D3, 10-oxo-19-nor-25(OH)D3, and 1α,25(OH)2D3 are indicated. 25(OH)D3 was metabolized into 1α,25(OH)2D3 only in the cells transfected with pcDNA 1α. The fraction containing 1α,25(OH)2D3 was further applied to HPLC under the following two conditions: column, Finepak SIL 4.6 × 250 mm; mobile phase, 8% 2-propanol in hexane; flow rate, 1 ml/min; column, J′-sphere ODS-AM 4.6 × 150 mm (YMC Co., Kyoto, Japan); mobile phase, 10% water in methanol; flow rate, 1.0 ml/min (data not shown). In all cases the putative 1α,25(OH)2D3 comigrated with authentic 1α,25(OH)2D3. Similar results were obtained in three independent sets of experiments.
Expression of CYP24 and P4501α in the Kidney.
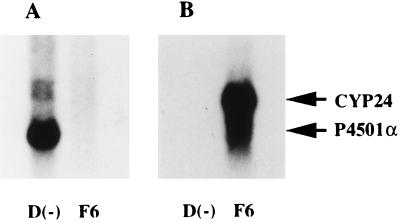
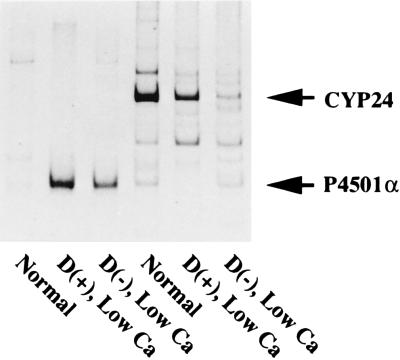
Next, we examined the expression of CYP24 and P4501α mRNAs in the kidney, the endocrine organ of vitamin D. The expression level of the CYP24 gene was increased by the administration of 26,27-F6-1α,25(OH)2D3 (Fig. 5B). No CYP24 mRNA was detected in the kidney of vitamin D-deficient rats. In contrast, the expression level of renal P4501α mRNA was greatly increased in the kidney of vitamin D-deficient rats (Fig. 5A). No transcript was detected in the kidney of 26,27-F6-1α,25(OH)2D3-treated rats. 1α,25(OH)2D3 is synthesized from 25(OH)D3 by P4501α exclusively in the mitochondria of renal PCT (21, 22). Fig. 6 shows the preferential expression of CYP24 and P4501α mRNAs in microdissected PCT of each rat model. Interestingly, P4501α mRNA in PCT was increased significantly in rats with enhanced renal 1α-hydroxylase activity (rats fed a low calcium diet). In contrast, expression of CYP24 mRNA in PCT was down-regulated in rats fed a vitamin D-deficient or -repleted low Ca diet. The expression level of P4501α mRNA in PCT was inversely correlated with the expression of CYP24 mRNA.
Figure 5.
Expression of CYP24 and P4501α mRNAs in rat kidney. Poly(A)+ RNAs (5 μg) were prepared from rats that had been treated with either 26,27-F6-1α,25(OH)2D3 or vehicle. 26,27-F6-1α,25(OH)2D3 (1 μg/rat) was intravenously administered to vitamin D-deficient low Ca rats. Rats were killed 48 h after administration of either 26,27-F6-1α,25(OH)2D3 or vehicle. The blots were probed with [32P]dCTP labeled P4501α (A) and CYP24 (B). Similar results were obtained in three independent sets of experiments.
Figure 6.
Expression of CYP24 and P4501α mRNAs in the kidney of each rat model. Radioautography of RT-PCR products of CYP24 and P4501α mRNAs in microdissected PCT. Three-week-old rats were fed a vitamin D-replete or a vitamin D-deficient low Ca diet for 2 weeks. Microdissection and RT-PCR were performed as described (18, 19) with a slight modification. Final volume of the PCR reaction was 20 μl, and composition of the solution was 10 mM Tris⋅HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, gelatin 0.01%, 200 nM sense and antisense primers, 0.1 mM dNTPs, 74 kBq of [33P]dCTP, and 1 unit of Taq DNA polymerase. The program of PCR was as follows: 25 cycles of 94°C for 40 sec, 50°C for 1 min, and 72°C for 1 min. The expected size of RT-PCR products for P4501α and CYP24 were 253 and 482 bp, respectively.
Effects of Low Calcium and Low Phosphorus Feeding on the Expression of P4501α.
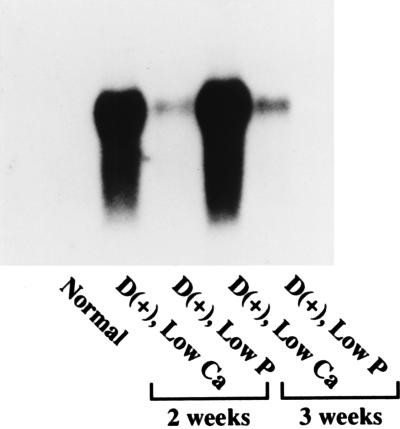
Fig. 7 shows the expression of renal P4501α mRNA after rats had been maintained on a vitamin D-replete diet containing 1.2% calcium and 0.6% phosphorus (Normal), 0.03% calcium and 0.6% phosphorus (Low Ca), or 1.2% calcium and 0.1% phosphorus (Low P) for 2 or 3 weeks. In the low calcium group, expression of P4501α mRNA was greatly and time-dependently increased as compared with that in the normal group. In contrast, the expression level of P4501α mRNA in the low P feeding group was moderately increased as compared with the low Ca group.
Figure 7.
Expression of CYP24 and P4501α mRNAs in 3-week-old rats maintained for 2–3 weeks on a vitamin D-repleted diet containing 1.2% Ca and 0.6% P (Normal), 0.03% Ca and 0.6% P (Low Ca), or 1.2% Ca and 0.1% P (Low P). Poly(A)+ RNA (5 μg) was electrophoresed and probed for P4501α. Similar results were obtained in three independent sets of experiments.
DISCUSSION
The present study describes the cloning and sequencing of 25(OH)D3-1α-hydroxylase (P4501α). The P4501α has been expressed in COS-7 cells and metabolism of 25(OH)D3 into 1α,25(OH)2D3 could be observed, thus verifying that P4501α encodes a 25(OH)D3-1α-hydroxylase. Vitamin D is metabolized by three major cytochrome P450-containing enzymes—the liver 25-hydroxylase (CYP27), the renal 1α-hydroxylase, and the renal and intestinal 24-hydroxylase (CYP24) (3, 4). P4501α and CYP24 are renal P450 enzymes synthesized from nuclear transcripts. P4501α and CYP24 are both embedded in the inner membrane of the mitochondria facing the matrix. The major location of P4501α and CYP24 is in the inner mitochondrial membrane of PCT cells of the kidney (21, 22). In agreement with the previous studies (18, 19, 21, 22), P4501α and CYP24 mRNAs were expressed in the PCT of the renal nephron (Fig. 6). They receive electrons from a common electron donor, ferredoxin, and catalyze the vitamin D hydroxylation reactions. These common features must be related to the conserved region of the molecules. As described previously, the putative heme-binding portion is located in the highly conserved regions (5, 6). The peptide region that interacts with ferredoxin must also be conserved (20). The amino acid sequence of 1α-hydroxylase (P4501α) contained a conserved cysteine residue located at position 448 that was considered to be a ligand for the heme ion (Figs. 1 and 2). Subjecting the deduced amino acid sequence to a computer homology search, it was found that the enzyme is 43.6% identical and 53% similar to a cytochrome P450 isolated from rat liver mitochondria catalyzing CYP27. It is 42.6% similar and 34% identical to the 25-hydroxyvitamin D-24-hydroxylase. Based on current nomenclature (5, 6), we propose P4501α be known as CYP27B1.
Recently, Guo et al. (23) isolated a cDNA for the human liver mitochondrial vitamin D3-25-hydroxylase by using PCR products of a fraction of rat liver 25-hydroxylase cDNA as a hybridization probe. The structure of the isolated human cDNA was virtually identical to that of the human cholestanetriol 27-hydroxylase cDNA. Axén et al. (24) also reported that CYP27 of pigs and humans catalyzes not only 27-hydroxylation of cholestanetriol and 25-hydroxylation of vitamin D3, but also 1α-hydroxylation of 25(OH)D3. Because the 1α-hydroxylase activity toward 25(OH)D3 is much lower than that of the other two enzyme activities and also the liver does not seem to be involved in the 1α-hydroxylation reaction of 25(OH)D3 at least in mammals, the physiological meaning of this activity remains to be elucidated. Andersson et al. (25) reported that CYP27 is localized not only in the liver but also in the kidney, lung, and spleen. However, the degree to which the CYP27 is regulated hormonally is not clear.
P4501α is the only enzyme physiologically responsible for the biosynthesis of 1α,25(OH)2D3 from 25(OH)D3 (1, 2). The regulatory mechanism of 1α-hydroxylase in the kidney remains unclear, because its cDNA has not yet been cloned. Renal 1α-hydroxylase activity is regulated by a number of physiological factors including parathyroid hormone (PTH), 1α,25(OH)2D3, calcitonin, insulin, calcium, phosphorus, growth hormone, and prolactin (1, 2). Among these factors, PTH is a major regulator of 1α,25(OH)2D3 synthesis in the kidney. The mechanism of action of PTH on renal 1α-hydroxylase has not been fully elucidated. Previous studies have demonstrated that cAMP is a major intracellular mediator in the stimulation of 1α-hydroxylase activity by PTH (26). On the other hand, 1α,25(OH)2D3 production is inhibited by high serum concentration of calcium and 1α,25(OH)2D3 per se. In agreement with the previous reports (17, 19), renal expression of P4501α mRNA was increased in the state of the enhanced synthesis of 1α,25(OH)2D3 (Figs. 5 and 7).
In rats fed a low phosphorus diet, the direction of regulation of serum levels of PTH and calcium was opposite to that of intact rats fed a low calcium diet. A possible common event that can occur in both of the rats fed a low calcium diet and a low phosphorus diet may be a decrease of the intracellular phosphorus level in renal proximal tubules (19). Feeding of rats with a low calcium diet or a vitamin D-deficient diet markedly stimulates 1α-hydroxylase activity (17, 19). In contrast, feeding a low phosphorus diet moderately stimulates 1α-hydroxylase activity (19). To clarify whether this increase of the enzyme activity is caused by a pretranslational induction, changes of the mRNA level in the kidney were examined by Northern blot analysis. The results showed that expression level of 1α-hydroxylase was regulated at the mRNA level (Fig. 7).
The cloning of the P4501α gene will now make possible a full understanding how 1,25(OH)2D3 synthesis in the renal PCTs is regulated at the molecular level.
Acknowledgments
We thank Prof. Colin Jefcoate (University of Wisconsin, Madison) and Prof. Yoshihiko Ohyama (Hiroshima University) for their help with P450 nomenclature as well as their helpful reviews.
ABBREVIATIONS
- 25(OH)D3
25-hydroxyvitamin D3
- 1α
25(OH)2D3, 1α,25-dihydroxyvitamin D3
- 26
27-F6-1α,25(OH)2D3, 26,27-hexafluoro-1α,25-dihydroxyvitamin D3
- 24
25(OH)2D3, 24,25-dihydroxyvitamin D3
- P4501α
25-hydroxyvitamin D3-1α-hydroxylase
- CYP24
25-hydroxyvitamin D3-24-hydroxylase
- CYP27
vitamin D3-25-hydroxylase
- PCT
proximal convoluted tubules
- RACE
rapid amplification of cDNA ends
- RT-PCR
reverse transcription–PCR
- PTH
parathyroid hormone
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. 001992).
References
- 1.DeLuca H F. FASEB J. 1988;2:224–236. [PubMed] [Google Scholar]
- 2.Suda T, Shinki T, Kurokawa K. Curr Opin Nephrol Hypertens. 1994;3:59–64. doi: 10.1097/00041552-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Armbrecht H J, Okuda K, Wongsurawat N, Nemani R K, Chen M L, Boltz M A. J Steroid Biochem Mol Biol. 1992;43:1073–1081. doi: 10.1016/0960-0760(92)90334-F. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K, Usui E, Ohyama Y. J Lipid Res. 1995;36:1641–1652. [PubMed] [Google Scholar]
- 5.Nelson D R, Koymans L, Kamataki T, Stegeman J J, Feyereisen R, Waxman D J, Waterman M R, Gotoh O, Coon M J, Estabrook R W, Gunsalus I C, Nebert D W. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Graham-Lorence S E, Peterson J A. Methods Enzymol. 1996;272:315–326. doi: 10.1016/s0076-6879(96)72037-2. [DOI] [PubMed] [Google Scholar]
- 7.Usui E, Noshiro M, Okuda K. FEBS Lett. 1990;262:135–138. doi: 10.1016/0014-5793(90)80172-f. [DOI] [PubMed] [Google Scholar]
- 8.Ohyama Y, Noshiro M, Okuda K. FEBS Lett. 1991;278:195–198. doi: 10.1016/0014-5793(91)80115-j. [DOI] [PubMed] [Google Scholar]
- 9.St-Arnaud, R., Moir, M., Messerlian, S. & Glorieux, F. H. (1996) J. Bone Miner. Res. 11 (Suppl. 1), S-124 (abstr.). [DOI] [PubMed]
- 10.Nicoll D A, Quednau B D, Qui Z, Xia Y-R, Lusis A J, Philipson K D. J Biol Chem. 1996;271:24914–24921. doi: 10.1074/jbc.271.40.24914. [DOI] [PubMed] [Google Scholar]
- 11.Shen Z, Wells R L, Liu J, Elkind M M. Proc Natl Acad Sci USA. 1993;90:11483–11487. doi: 10.1073/pnas.90.24.11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald R J, Swift G H, Przybyla A E, Chirgwin J M. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 14.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto Y, Shinki T, Yamamoto K, Ohyama Y, Iwasaki H, Hosotani R, Kasama T, Takayama H, Yamada S, Suda T. J Biol Chem. 1997;272:14115–14119. doi: 10.1074/jbc.272.22.14115. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Shinki T, Jin C H, Nishimura A, Nagai Y, Ohyama Y, Noshiro M, Okuda K, Suda T. J Biol Chem. 1992;267:13757–13762. [PubMed] [Google Scholar]
- 18.Iida K, Taniguchi S, Kurokawa K. Biochem Biophys Res Commun. 1993;194:659–664. doi: 10.1006/bbrc.1993.1872. [DOI] [PubMed] [Google Scholar]
- 19.Iida K, Shinki T, Yamaguchi A, DeLuca H F, Kurokawa K, Suda T. Proc Natl Acad Sci USA. 1995;92:6112–6116. doi: 10.1073/pnas.92.13.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nebert D W, Gonzalez F J. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- 21.Brunette M G, Chan M, Ferriere C, Roberts K D. Nature (London) 1978;276:287–289. doi: 10.1038/276287a0. [DOI] [PubMed] [Google Scholar]
- 22.Kawashima H, Torikai S, Kurokawa K. Proc Natl Acad Sci USA. 1981;78:1199–1203. doi: 10.1073/pnas.78.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y-D, Strugnell S, Back D W, Jones G. Proc Natl Acad Sci USA. 1993;90:8668–8672. doi: 10.1073/pnas.90.18.8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axén E, Postlind H, Sjöberg H, Wikvall K. Proc Natl Acad Sci USA. 1994;91:10014–10018. doi: 10.1073/pnas.91.21.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson S, Davis D L, Dahlbäck H, Jörnvall H, Russell D W. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- 26.Horiuchi N, Suda T, Takahashi H, Shimazawa E, Ogata E. Endocrinology. 1977;101:969–974. doi: 10.1210/endo-101-3-969. [DOI] [PubMed] [Google Scholar]