Abstract
To further develop the Leishmania model for porphyria based on their deficiencies in heme biosynthesis, three Old World species were doubly transfected as before for Leishmania amazonensis with cDNAs, encoding the 2nd and 3rd enzymes in the pathway. Expression of the transgenes was verified immunologically at the protein level and functionally by uroporphyrin neogenesis that occurs only after exposure of the double-transfectants to delta-aminolevulinate. All species examined were equally deficient in heme biosynthesis, as indicated by the accumulation of uroporphyrin as the sole porphyrin and the production of coproporphyrin upon further transfection of one representative species with the downstream gene. The results obtained thus demonstrate that at least the first five enzymes for heme biosynthesis are absent in all species examined, rendering their transfectants inducible with aminolevulinate to accumulate porphyrins and thus useful as cellular models for human porphyrias.
Keywords: Uroporphyrin, coproporphyrin, delta-aminolevulinate dehydratase, porphobilinogen deaminase, uroporphyrinogen decarboxylase, heme biosynthesis, transgenes, trypanosomatid protozoa
Introduction
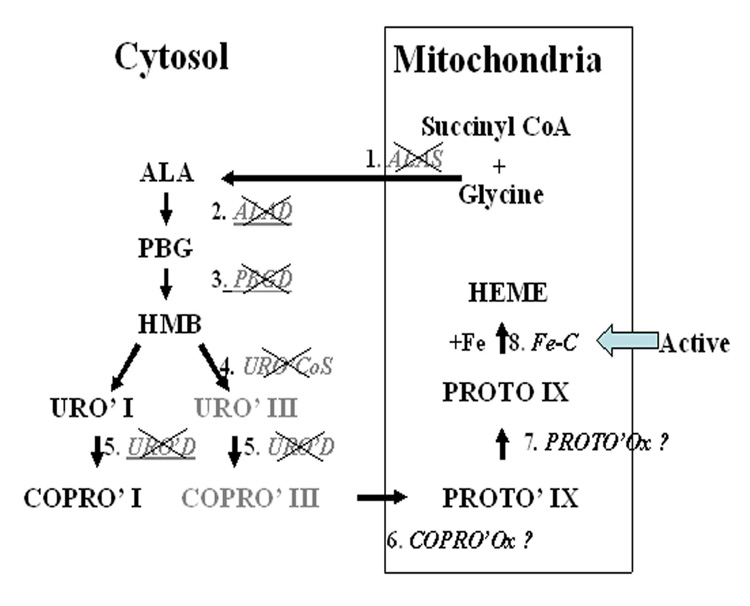
The inability of trypanosomatid protozoa to synthesize heme results in their nutritional requirement for hemin, hematin or hemoglobin as a growth factor (Trager, 1974; Chang et al., 1975; Chang & Chang, 1985; Krishnamurthy et al., 2005). Earlier, this deficiency has been delineated genetically by transfecting Leishmania amazonensis with mammalian cDNAs, encoding the 2nd and 3rd enzymes of the biosynthetic pathway for tetrapyrroles (Sah et al., 2002) (Cf. Fig. 1). Development of uroporphyria in these transfectants in the presence of exogenous delta-aminolevulinate (ALA) led us to suggest that this New World Leishmania is functionally defective in at least the first five and possibly the first seven of the eight enzymes known in heme biosynthetic pathway (Sah et al., 2002) (Fig. 1). Of these eight enzymes, only the last one, i. e. ferrochelatase (Fe-C) has been shown to exist functionally by nutritional studies (Chang et al., 1975) and enzyme assays (Salzman et al, 1982) in different trypanosomatid protozoa. Most recently, we have found the putative Fe-C sequences of high homology in all Leishmania isolates grouped phylogenetically into some 20 genotypes, including most Old World species, i. e. L. infantum/donovani, L. major, L. tropica, L. turanica and L. gerbilli (Waki et al., 2007). Sequence conservation of Fe-C suggests that it may function beyond heme biosynthesis, since protoporphyrin (PROTO) is cytotoxic and thus would not be widely available to serve as a substrate of this enzyme to produce the heme needed by Leishmania. Fe-C and its two preceding enzymes for heme biosynthesis were noted and proposed to have a bacterial origin during in silico construction of Leishmania metabolic pathways from L. major genome sequence database (http://www.genedb.org/genedb/leish/) (Opperdoes & Coombs, 2007).
Fig. 1. Diagramatic depiction of the classic heme biosynthetic pathway and Leishmania enzymic deficiencies in this pathway.
×, Enzymes absent in Leishmania spp.; ?, Putative presence of the last three terminal enzymes based on in silico construction of metabolic pathways according to the L. major genomic database. Abbreviations: ALAS, aminolevulinate synthase ; ALA, delta-aminolevulinate; ALAD, aminolevulinate dehydratase; PBG, porphobilinogen; PBGD, porphobilinogen deaminase; HMB, hydroxymethylbilane; URO CoS, uroporphyrinogen cosynthase, URO’I & III, uroporphyrinogen I & III; URO’D, uroporphyrinogen decarboxylase; COPRO’I & III, coproporphyrinogen I & III; COPRO’Ox, coproporphyrinogen oxidase; PROTO’IX, protoporphyrinogen IX; PROTO’Ox, protoporphyrinogen oxidase; PROTO IX’, protoporphyrin IX; Fe-C, ferrochelatase; Fe, ferrous iron.
Here, we extend our previous findings of this biosynthetic deficiency from L. amazonensis to L. major, L. tropica and L. infantum. As observed previously, only double-transfection of each species with cDNAs encoding ALA dehydratase (ALAD) and porphobilinogen deaminase (PBGD) renders them capable of using exogenous ALA, product of the 1st enzyme, to make uroporphyrin. New experimental conditions were designed to facilitate screening of transfectants for ALA-inducible porphyria and for studying cellular events in this porphyrinogenesis. In addition, genetic complementation of a representative species with an enzyme further downstream resulted in the emergence of coproporphyrin (COPRO) and its intermediates, but not protoporphyrin (PROTO). The results not only provide conclusive evidence for a universal deficiency in heme biosynthesis of all species examined but also make available their mutants suitable for studying porphyric mechanisms at the cellular levels.
Materials and Methods
Leishmania cultivation and transfection
Leishmania spp. used for the present study include L. amazonensis (LV 78 clone 12-1), L. major (RM), L. tropica (K27) and L. infantum (Adana #7) (see http://66.99.255.20/cms/micro/sample%20list.pdf and Waki et al., 2007 for the origin of the strains used).
Promastigotes of all species were grown to stationary phase at 25° C in Medium 199 (Sigma) HEPES-buffered to pH 7.4 and supplemented with 10% heat inactivated fetal bovine serum (HIFBS). Single- and double-transfection of these cells by electroporation with pX-alad and/or p6.5-pbgd, selection for transfectants and their maintenance followed published procedures (Sah et al., 2002). Stable lines of single- and double-transfectants were all maintained in the presence of appropriate selective pressures, e. g. 100 ug/ml of G418 (for pX-alad) and/or 20 ug/ml of tunicamycin (for p6.5-pdgd) (except those of L. infantum, for which the selective pressure was lowered to 5–10 ug/ml of tunicamycin and 50 ug/ml of G418). Double-transfectants of L. major with pX-alad and p6.5-pbgd were further transfected with human cDNA of uroporphyrinogen decarboxylase (~1.1 kb urod Accession # BC001778) placed at the Bam HI expression site of a different plasmid, pXG-hyg. The triple-transfectants were initially selected with hygromycin alone at up to 20 ug/ml and subsequently passaged in media containing a combination of three antibiotics in different concentrations with the highest level of selective pressures as follows: 20 ug/ml of tunicamycin, 100 ug/ml of G418 and 1,000 ug/ml of hygromycin. All transfectants were passaged continuously under above-mentioned selective pressures and grew essentially as well as the wild-type cells in drug-free media.
Western blot analysis
Transfectants were assessed immunologically for their production of ALAD and/or PBGD (Sassa, 1976) using rabbit antisera specific to these mammalian enzymes. Immunoblots were reacted with HRP-conjugated goat anti-rabbit IgG (1:10,000) and developed by using Pierce SuperWest Pico reagent.
Induction of double-transfectants for neogenesis of porphyria
Transfectants were grown under selective conditions to late log phase and then harvested for exposure to 1 mM delta-aminolevulinate (ALA) (Sigma/Porphyrin products, UT) at room temperature in dark at 108 cells/ml in HEPES-buffered Hank’s Balanced Salt Solution (HBSS) (pH 7.4) plus 0.01% bovine serum albumin (BSA) (Sigma). Under these conditions of incubation in the presence of glucose (1 mg/ml), cells did not replicate, but remained fully motile and viable for up to 72 hrs, independent of the presence or absence of ALA. Experiments were terminated at the end of day 3, beyond which time cells began to show signs of degeneration, e. g. sluggish motility and loss of cell integrity.
Spectrofluorimetry of porphyrins
Cells (108) were harvested from each culture by centrifugation for 5 min at 3,500 g at 4 C for porphyrin extraction, as described previously (Sassa et al, 1978). Briefly, sedimented cells were extracted with 3 ml of 1 M perchloric acid + methanol at 1:1 (V/V). Solvent extracts were cleared of debris by centrifugation at 20,000 g for 15 min. Cleared supernatants were scanned for the presence of porphyrins at the excitation wavelength of 400 nm and emission spectra recorded between 550 nm to 670 nm, slit widths being 15 nm and 20 nm, respectively. One nmole of chemically pure uroporphyrin I (Porphyrin Products, UT) in 3 ml of the same solvent (perchloric acid+methanol) was used as the standard. Each sample was scanned thrice to obtain average values for plotting the spectra.
For time course studies, 200 ul aliquots (20 × 106 cells) were withdrawn daily from each sample and centrifuged to separate cells and incubation media. To determine cellular porphyrin levels, each cell pellet was first extracted with 200 ul of the solvent followed by an 8-fold dilution for fluorimetry. To assess released uroporphyrin levels, 50 ul aliquots of incubation media were collected, cleared by centrifugation and mixed each with 350 ul of the solvent for fluorimetry against the uroporphyrin I standard. Fluorescence readings of the experimental samples were the same when blanked against either unused medium or spent medium from the wild-type cells, both being porphyrin-free.
Thin layer chromatography of porphyrins
Porphyrins extracted from samples were subjected to thin layer chromatography (TLC) (Doss et al, 1972). Briefly, a mixture of methanol and sulphuric acid at 12:1 (V/V) was added to sedimented cells at 108 cells/2 ml followed by incubation in dark for 48 hrs. Chloroform was then added at a ratio of 6:1 (V/V), followed by 1 ml of water. The mixture was vigorously shaken in a separating funnel and allowed to stand for phase separation. The lower chloroform phase containing porphyrins was collected, re-washed with 2 ml of water and centrifuged (1,000 g for 20 min); the aqueous phase was then removed by siphoning. This was repeated thrice to clean the chloroform phase. Porphyrins obtained after solvent evaporation were dissolved in 100 ul chloroform for TLC on silica gel plates (Fisher Scientific) using a mixture of 15 ml of ethyl acetate, 80 ml of toluene and 3 ml of ethyl alcohol as the developing solvent. TLC plates were viewed under long wave UV illumination to visualize separated porphyrin species.
Results and Discussion
Fig. 1 presents diagramatically the eight enzymes (#1–8) and their intermediate products of the classic heme biosynthetic pathway (Sassa, 2006). Of the eight enzymes, four are cytosolic (#2–5) and four mitochondrial (#1 and 6–8). Evidence is presented here indicating that all Leishmania spp. examined have the same deficiencies in this pathway and thus can be genetically complemented to become ALA-inducible for the development of porphyria.
All Leishmania species were successfully transfected singly and doubly with pX-alad and/or p6.5-pbgd. The expression of these transgenes was shown by Western blot analyses of stable single- and double-transfectants from all four species, yielding proteins of the expected size (Fig. 2 results for two representative species shown), i. e. ~36 kDa and ~38 kDa for ALAD and PBGD, respectively. Specificity of the reaction products is clearly indicated by the presence of both in the double-transfectants (Lanes 1 and 8), only one or the other in the single-transfectants (Lanes 2, 4, 6 and 7) and neither in the wild-type cells (Lanes 3 and 5). Thus, all Leishmania spp. under study, unless transfected with mammalian alad and pbgd, do not contain ALAD and PBGD recognizable by antisera specific to the respective mammalian enzymes.
Fig. 2. Western blot analyses of Leishmania single- and double-transfectants for delta-aminolevulinate dehydrase and porphobilinogen deaminase.
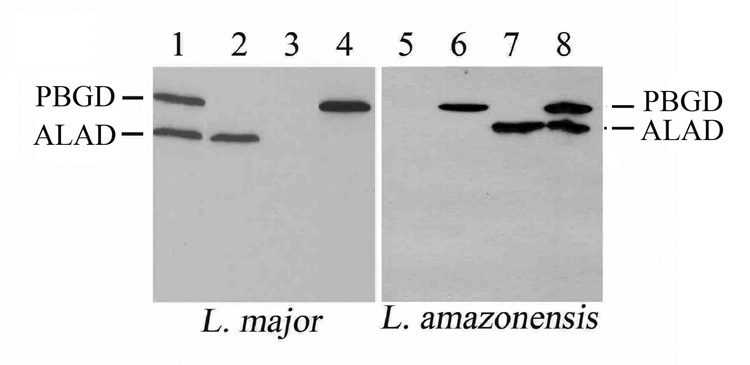
Left and right panels, two representatives of the four Leishmania species examined. All lanes were loaded equally with proteins equivalent to ~107 cells grown to stationary phase and subjected to SDS-PAGE followed by immunoblotting. Lanes 1 and 8, alad/pbgd double transfectants; Lanes 2 and 7, single-transfectants with alad; Lanes 4 and 6, single-transfectants with pbgd; Lanes 3 and 5, wild-type cells. Note: the absence of both enzyme proteins in wild-type cells, and the presence of one or the other in the single transfectants and both only in the double transfectants. pX was used for alad and p6.5 for pbgd expression in all cases.
Functionality of ALAD and PBGD produced by the transgenes was evaluated by examination of the transfectants for their porphyrinogenic ability. For rapid screening of porphyria, cell lysates prepared by freezing-thawing were used. After incubation of the lysates in the presence of ALA for as short as 2–3 hrs at 37 C, porphyrin fluorescence became detectable by long wave UV illumination in the lysates of double-transfectants, but not in those of the controls, i. e. single-transfectants and wild-type cells with or without ALA and double-transfectants without ALA (data not shown). ALAD and PBGD from the transgenes are thus functionally active, but work in tandem, as expected, to convert exogenously supplied ALA for the formation of porphyrins.
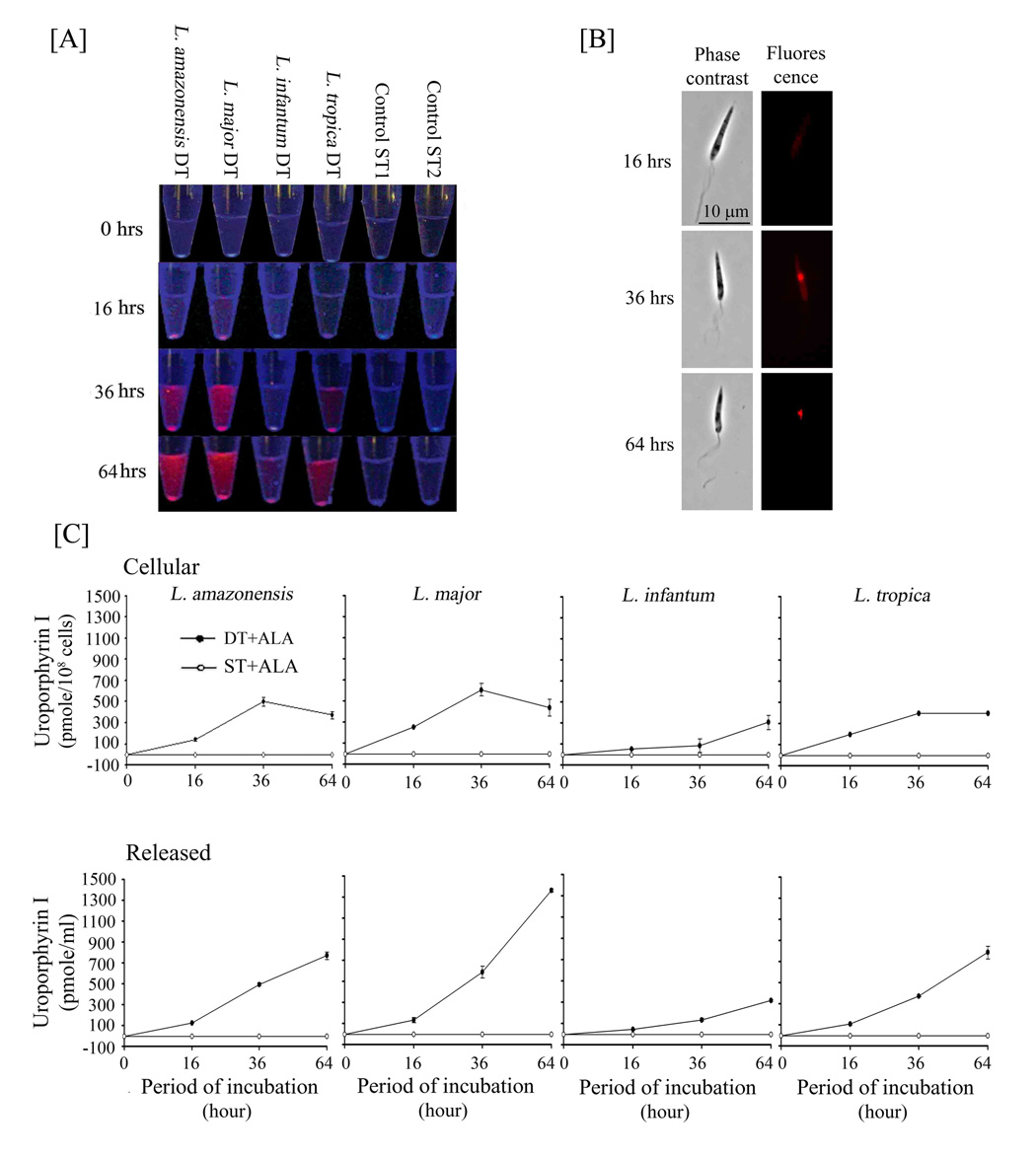
Porphyrinogenesis in the transfectants from all species was subsequently studied in detail under living conditions as described. Namely, live transfectants were incubated in Hank’s Balanced Salt Solution (HBSS) plus 0.01% BSA instead of culture medium, thereby minimizing the potential competition for the uptake of ALA by amino acids therein. Porphyria of double transfectants was indeed found to develop more rapidly and to peak more consistently after exposure to ALA under such conditions within 48 hours. Porphyrins extracted from these samples were analyzed by fluorimetry (Fig. 3 A–B) and TLC (Fig. 3C). No porphyrin was detected by any of these methods in the control samples from all species: [1] ALA-exposed wild-type cells, their transfectants with vector alone and single-transfectants (Fig. 3A ST1 and ST2+ALA; Fig. 3B −alad/−pbgd, +alad/−pbgd or −alad/+pbgd); and [2] double-transfectants without ALA (Fig. 3C DT-ALA). In contrast, all the double-transfectants utilized exogenously supplied ALA to form porphyrin that is identical to uroporphyrin I (URO I) by both fluorescence spectrum (Fig. 3A DT+ALA) and TLC mobility (Fig. 3C DT+ALA, URO). Significantly, TLC revealed neither coproporphyrin (COPRO) nor protoporphyrin (PROTO), products of enzymes subsequent to PBGD in the heme biosynthetic pathway. The presence of these porphyrins would be otherwise expected to emerge as additional fluorescent species, since they are known to migrate farther than URO I. The peak levels of the total URO I from cultures of the double-transfectants varied with different experiments in the range of 0.6–1.2 nmoles in 1 ml at 108 cells/ml among all species, except L. infantum (Fig. 3B +alad/+pbgd). The strain used to represent the latter species was inherently not robust in in vitro culture, accounting apparently for the necessity of using lower selective pressures for its double-transfectants and thus a lower level of their URO I production. The results presented support our previous findings with L. amazonensis as follows: [1] mammalian ALAD and PBGD from the transgenes are functional in the double-transfectants, allowing them to utilize exogenous ALA to produce URO I; [2] these transfectants produced no fluorometrically detectable URO I in the absence of exogenous ALA, indicative of little or no biosynthesis of the latter by Leishmania spp.; and [3] accumulation of only URO I in all double-transfectants suggests the absence of downstream enzymes, i. e. URO co-synthase (UROCoS) and URO-decarboxylase (UROD) (see below).
Fig. 3. Delta-aminolevulinate-induced neogenesis of uroporphyria in Leishmania spp. doubly transfected with mammalian cDNAs encoding delta-aminolevulinate dehydrase and porphobilinogen deaminase.
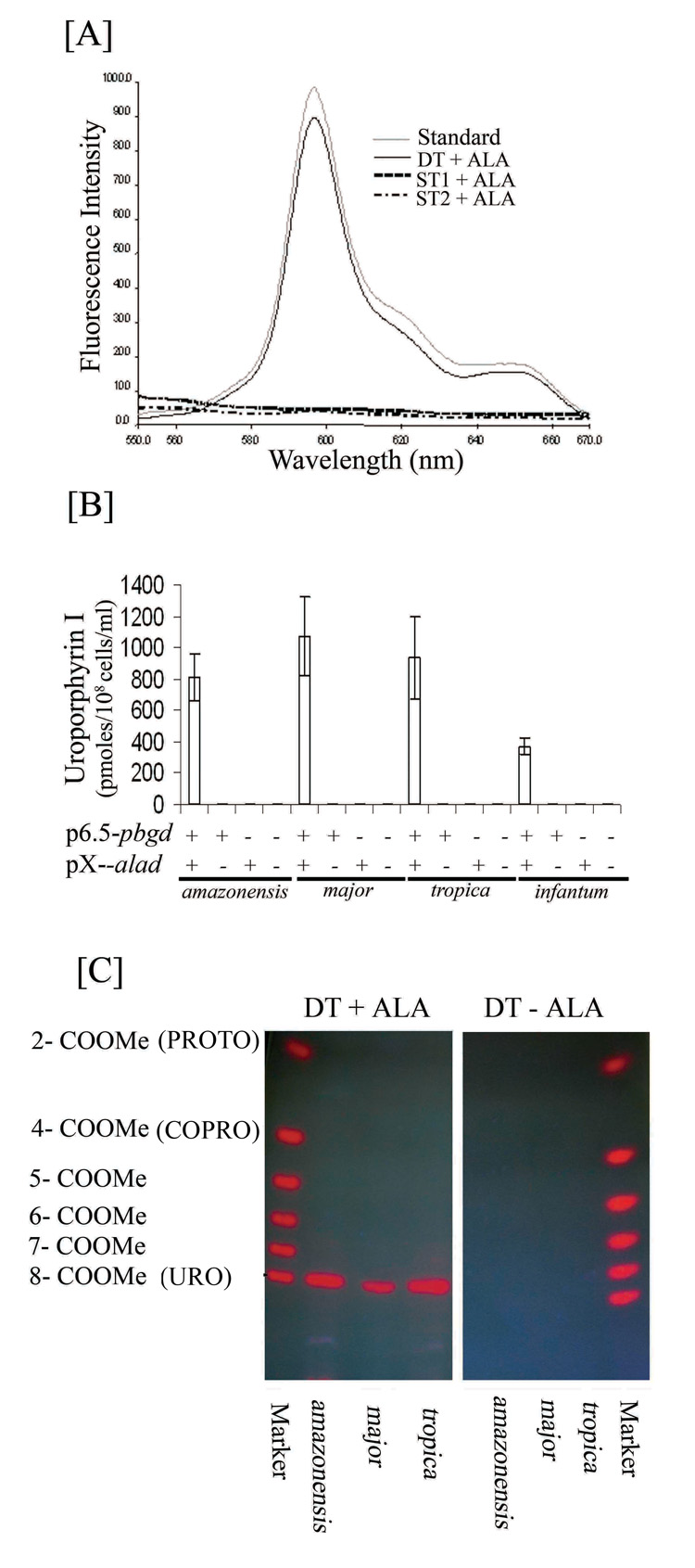
[A] Fluorescence spectrum of uroporphyrin I in extracts of double transfectants (DT), but not those of single transfectants with either alad (ST1) or pbgd (ST2) after exposure to 1 mM delta-aminolevulinate (+ALA) in non-growing conditions. Emission spectra of solvent-extracted porphyrins from these samples were obtained as described in Materials and Methods. Note: the absence of fluorescence in both aporphyric single transfectants (broken and dotted lines) and the virtual superimposition of the spectra between the porphyrin from ALA-exposed DT (Solid line) and uroporphyrin I standard (gray line). DT, L. major doubly transfected with p6.5-pbgd and pX-alad. ST1 and ST2, The same species singly transfected with p6.5-pbgd and pX-alad, respectively. Porphyrins from the ALA-treated double-transfectants of the other three species showed similar emission spectra (Not shown).
[B] Fluorimetric quantitation of uroporphyrin I accumulation in the double transfectants (+p6.5−pbgd and +pX−alad) of four different Leishmania spp. (amazonensis, major, tropica, infantum) and the complete absence of porphyrin in single transfectants with either of the two cDNA (+pbgd/−alad, −pbgd/+alad) or with vectors alone (−pbgd/−alad). Porphyrins were solvent-extracted from 200 ul (20 × 106 cells) of cell suspension for each sample after exposure to ALA under conditions as described in Materials and Methods. Porphyrin concentrations determined fluorometrically against the fluorescence intensities of an uroporphyrin I standard curve (400 nm excitation and 600 nm emission wavelengths). Note: Accumulation of uroporphyrin I to high levels in the DT cells and the absence of any porphyrin in ST and control cells of all species.
[C] The authentication of uroporphyrin I as the sole porphyrin species formed by double-transfectants (DT) of representative species examined (L. amazonensis, L. major and L. tropica) after exposure to ALA (+ALA), but not in its absence (−ALA) under the experimental conditions as described in Materials and Methods. Marker, Porphyrin standards with variable numbers of carboxymethylated side chains and the corresponding porphyrin species in brackets. 2 to 8- COOMe: The number of non-methylated side chains of carboxylated porphyrins.
Leishmanial deficiencies in porphyrin biosynthesis as outlined above were further indicated by evaluating the cellular kinetics of URO I neogenesis, i. e. its cellular emergence, distribution, accumulation and efflux (Fig. 4). Live transfectants under non-growing conditions of incubation were collected at different time points after ALA exposure. URO I began to emerge overnight as weak and diffused fluorescence throughout the cytosol in double-transfectants, as seen by fluorescent microscopy; long wave UV illumination of the cell pellets revealed this fluorescence inconsistently because of the weak signal plus batch-to-batch variations in the proportion of positive cells in any given experiments (Fig. 4A and B 16 hrs). It is clear however that cellular emergence and accumulation of URO I is followed by a switch of its cytosolic to vacuolar fluorescence (Fig. 4B 36–64 hrs) and the coincidence of this event with its increasing release into the extracellular milieu (Fig. 4A and C 36–64 hrs). The kinetics of these cellular events are similar among the double transfectants of all species, except L. infantum, which showed delayed onset of uroporphyrinogenesis and a lower level of uroporphyria for reasons already provided. Porphyrins extracted from the same set of cell samples and their corresponding incubation media were quantitatively assessed by fluorimetry. Clearly, the double-transfectants take up and utilize ALA continuously to produce URO I (Fig. 4C DT+ALA), while the single-transfectants remained totally aporphyric (Fig. 4C ST+ALA) for the entire period of incubation. Kinetics of porphyrinogenesis in the double transfectants of all species, except L. infantum, is marked by a gradual accumulation of URO I up to 0.5 nmoles/108 cells from time 0 to 36 hrs followed by a decline of cell-associated URO I thereafter until 64 hrs (Fig. 4B, Cellular). This decline in cellular URO I is coupled with an increase in the level of this porphyrin found in the extracellular milieu at >0.5 nmoles/ml (Fig. 4C Released), suggestive of extracellular efflux, as seen previously when the L. amazonensis double-transfectants were examined under growing conditions in culture medium (Sah et al., 2002). Under both growing and non-growing conditions, URO I was released by viable cells into the extracellular milieu, independent of inadvertent cytolysis. The kinetics of uroporhyrin neogenesis follows the same pattern under both conditions for all species, although the maximal porphyrin levels reached may vary. We have previously proposed that mobilization of cytosolic URO into vacuoles followed by its extracellular efflux may be related to soluble toxic waste disposal (Sah et al. 2002). Of interest for further investigation is the leishmanial mechanism of URO efflux, for which ABC transporters have been suggested to play a role in mammalian cells (Latunde-Dada et al., 2006). In bacteria, URO is apparently transported by undefined mechanism to the periplasmic space and oxidized subsequently by cytochrome P-450 (Akhtar et al., 2003).
Fig. 4. Kinetics of delta-aminolevulinate-induced cellular neogenesis of uroporphyrin I, its cellular distribution and extracellular release.
[A] Double transfectants (DT) of all four Leishmania species grown to stationary phase were exposed to 1 mM ALA under non-growing conditions for up to 64 hr as described. Samples were withdrawn at the indicated intervals after ALA exposure and centrifuged to separate supernatants and cells. All samples were examined for fluorescence intensity under a UV lamp immediately [A] and for cellular distribution by fluorescent microscopy [B] [using excitation 405 nm (D405/10X), dichroic 485 nm (485DCXR) and emission 610 nm (RG610LP) filter set from Chroma USA] (L. major shown as a representative), and subsequently solvent-extracted for porphyrin quantitation by fluorimetry (400 nm excitation and 600 nm emission wavelengths) (DT+ALA versus ST+ALA) [C]. Concentrations of cellular and released uroporphyrin I were estimated against a standard curve of uroporphyrin I (see Legend to 3B). Note: Cellular emergence (starting from ~16 hrs) and accumulation of uroporphyrin via vacuolar condensation (after ~24 hrs) and its increasing release with time (16–64 hrs) after exposure to ALA (except for a notable delay in L. infantum). See legend to Fig. 3A for ST1 and ST2 single-transfectants included in [A] and [C] as aporphyric controls.
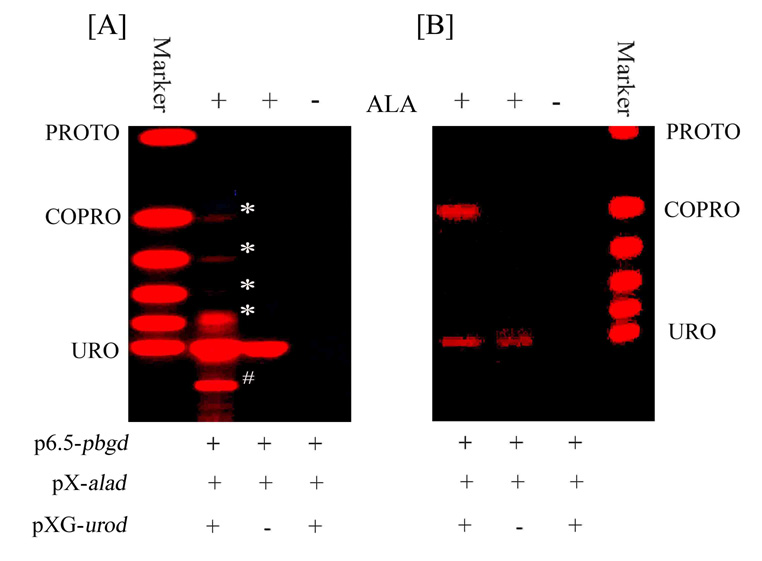
Additional transfection of L. major double-transfectants with UROD provided evidence for the absence of this enzyme. The triple-transfectants with ALAD, PBGD and UROD were produced by transfecting the ALAD/PBGD double-transfectants with human urod using plasmids with a third selectable marker. The stable triple-transfectants obtained were subsequently placed under three selective pressures specific to the plasmids at different concentrations, including the optimal strength of hygromycin at 500–1,000 ug/ml specific for UROD. TLC analyses of the triple transfectants under the optimal strength of selective pressures revealed multiple porphyrin species (Fig. 5A Lane +ALA/+alad+pbgd+urod, white stars), which are absent in ALA-untreated samples (Lane −ALA/+alad+pbgd+urod) or in ALA-treated double-transfectants with the exception of URO I (Lane +ALA/+alad+pdgd−urod). The extra porphyrin species produced by the triple-transfectants included fully decarboxylated URO, i. e. COPRO, but also partially decarboxylated intermediates (de Verneuil et al, 1983: Sassa et al., 1983), judging from their TLC mobilities. There are also fluorescent bands of variable intensities below URO I (Fig. 5A Lane +ALA/+alad+pbgd+urod, pound sign). They may represent COPRO and/or partially decarboxylated intermediates in conjugation with peptides, thereby retarding their migration. The intensities of the extra porphyrin species above URO from the triple transfectants decrease markedly with increasing decarboxylation, suggesting that the URO produced from ALA by the activities of ALAD and PBGD is far too abundant to be completely decarboxylated by the available UROD. Considerable efforts to rectify this by varying the 3 selective pressures for the triple transfectants were initially unsuccessful until they were grown for 4 consecutive passages in 1 month in the presence of hygromycin at 1,000 ug/ml as the only selective pressure for urod. TLC analysis of porphyrins from these cells revealed URO and COPRO as the only porphyrin species, the latter being slightly more abundant; in the absence of incompletely decarboxylated intermediates (Fig. 5B, Lane +ALA/+alad+pbgd+urod). Clearly, the absence of selective pressures for alad and pbgd for the period mentioned substantially reduced the URO level, which was decarboxylated more completely by the UROD available. In spite of more COPRO formation under this condition, no production of PROTO was evident. The absence of COPRO I in ALA-treated double-transfectants (Fig. 5A–B Lane +ALA/+alad+pbgd−urod) signifies the absence of endogenous UROD in Leishmania. It is known that both URO I and COPRO I are spontaneously oxidized to become metabolically dead end products. The scenario presented is consistent with the absence of the first five enzymes as proposed (Sah et al., 2002), and leaves open the possible functionality of the last three in Leishmania for heme biosynthesis. Evaluation of this possibility awaits further investigation to achieve quadruple transfection of wild-type with four functional enzymes, i. e. ALAD, PBGD, UROCoS and UROD.
Fig. 5. delta-aminolevulinate-induced emergence of uroporphyrin, coproporphyrin and their intermediates after triple transfection of L. major with additional cDNA encoding uroporphyrinogen decarboxylase.
Triple-transfectants were generated by transfection of Leishmania major pX-alad and p6.5-pbgd double-transfectants with pXG-urod (see Materials and Methods). [A] Triple-and double-transfectants grown under the full strengths of their respective selective pressures, i. e. 20 ug/ml tunicamycin+100 ug/ml G418+500 ug/ml hygromycin and 20 ug/ml tunicamycin+100 ug/ml G418, respectively; [B] As [A], except the triple-transfectants were grown for 4 passages in 1 month under the single selective pressure of hygromycin at 1 mg/ml for urod. All samples were incubated in the presence and absence of 1 mM ALA (± ALA) for 48 hrs in the dark and processed for porphyrin analyses by TLC as described. Stars and pound sign, Incompletely decarboxylated intermediates in addition to URO and COPRO produced by the triple-transfectants in [A]. URO and COPRO are the only two species produced by the triple-transfectants in [B]. See legend to Fig. 3C for porphyrin markers, and to Fig. 3A and text for ±alad, pbdg and urod.
Notably, none of the four Leishmania spp. studied appears to synthesize ALA in an amount sufficient for utilization by the over-expressed ALAD/PBGD to produce URO, as it is undetectable by the most sensitive fluorometric assay for porphyrins without the provision of exogenous ALA to the double-transfectants. Although biosynthesis of ALA has been reported via either the conventional or alternative enzyme in Leishmania donovani (Sagar et al., 1995; Srivastava et al., 1997) and Trypanosoma Cruzi (Lombardo et al., 2003), there are no gene sequences homologous to ALAS (Fig. 1 enzyme # 1) or the alternative enzyme in the Leishmania genome database (http://www.genedb.org/genedb/leish/) (Opperdoes & Coombs, 2007).
Leishmania defects in heme biosynthesis may be related to the biology of their intracellular parasitism. All Leishmania spp. invariably utilize macrophages as their exclusive host cells by residing in their phagolysosomes wherein senescent erythrocytes are known to end up for disposal, making available hemoglobin and its degradation products (Chang & Chang, 1985). The loss of heme biosynthetic pathway in these unusual parasites may be explained by the intraphagolysosomal availability of this hemoprotein in abundance coupled with the evolution of an effective mechanism for its uptake by receptor-mediated endocytosis (Krishnamurthy et al., 2005). Whatever the evolutionary scenario may be, the transgenic mutants produced here are of value as a cellular model to study monospecific porphyria, not previously available. Photosensitization of these porphyric transfectants for cytolysis further makes its utility possible as inducible suicidal mutants for vaccine/drug delivery to the phagolysosome of macrophages, as discussed previously (see Sah et al. 2002).
Acknowledgements
This work is supported by NIH grant AI-20486, AI-68835 and the School of Graduate and Postgraduate studies, Rosalind Franklin University. We thank John Keller and Bala Kolli for their critical evaluation of this manuscript and to Steve Beverley for providing pX vectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhtar MK, Kaderbhai NN, Hopper DJ, Kelly SL, Kaderbhai MA. Export of a heterologous cytochrome P450 (CYP105D1) in Escherichia coli is associated with periplasmic accumulation of uroporphyrin. Journal of Biological Chemistry. 2003;278:45555–45562. doi: 10.1074/jbc.M212685200. [DOI] [PubMed] [Google Scholar]
- Chang KP, Chang CS, Sassa S. Heme biosynthesis in bacterium-protozoon symbioses: enzymic defects in host hemoflagellates and complemental role of their intracellular symbiotes. Proceedings of the National Academy of Sciences of the United Sates of America. 1975;72:2979–2983. doi: 10.1073/pnas.72.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CS, Chang KP. Heme requirement and acquisition by extracellular and intracellular stages of Leishmania mexicana amazonensis. Molecular and Biochemical Parasitology. 1985;16:267–276. doi: 10.1016/0166-6851(85)90069-6. [DOI] [PubMed] [Google Scholar]
- de Verneuil H, Sassa S, Kappas A. Purification and properties of uroporphyrinogen decarboxylase from human erythrocytes. A single enzyme catalyzing the four sequential decarboxylations of uroporphyrinogens I and III. Journal of Biological Chemistry. 1983;258:2454–2460. [PubMed] [Google Scholar]
- Doss M, Ulshofer B, Philipp-Dormston WK. Quantitative thin-layer chromatography of porphyrins by in situ fluorescence measurements. Journal of Chromatography. 1971;63:113–120. doi: 10.1016/s0021-9673(01)85621-1. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy G, Vikram R, Singh SB, Patel N, Agarwal S, Mukhopadhyay G, Basu SK, Mukhopadhyay A. Hemoglobin receptor in Leishmania is a hexokinase located in the flagellar pocket. Journal of Biological Chemistry. 2005;280:5884–5891. doi: 10.1074/jbc.M411845200. [DOI] [PubMed] [Google Scholar]
- Latunde-Dada GO, Simpson RJ, McKie AT. Recent advances in mammalian haem transport. Trends in Biochemical Sciences. 2006;31:182–188. doi: 10.1016/j.tibs.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Lombardo ME, Araujo LS, Batlle A. 5-Aminolevulinic acid synthesis in epimastigotes of Trypanosoma cruzi. The International Journal of Biochemistry and Cell Biology. 2003;35:1263–1271. doi: 10.1016/s1357-2725(03)00033-5. [DOI] [PubMed] [Google Scholar]
- Opperdoes FR, Coombs GH. Metabolism of Leishmania: proven and predicted. Trends in Parasitology. 2007;23:149–158. doi: 10.1016/j.pt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Sagar R, Salotra P, Bhatnagar R, Datta K. L-alanine: 4,5-dioxovalerate transaminase in Leishmania donovani that differs from mammalian enzyme. Microbiological Research. 1995;150:419–423. doi: 10.1016/S0944-5013(11)80024-8. [DOI] [PubMed] [Google Scholar]
- Sah JF, Ito H, Kolli BK, Peterson DA, Sassa S, Chang KP. Genetic rescue of Leishmania deficiency in porphyrin biosynthesis creates mutants suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. Journal of Biological Chemistry. 2002;277:14902–14909. doi: 10.1074/jbc.M200107200. [DOI] [PubMed] [Google Scholar]
- Salzman TA, Stella AM, Wider de Xifra EA, Batlle AM, Docampo R, Stoppani AO. Porphyrin biosynthesis in parasitic hemoflagellates: functional and defective enzymes in Trypanosoma cruzi. Comparative biochemistry and physiology. B, Comparative biochemistry. 1982;72:663–667. doi: 10.1016/0305-0491(82)90523-5. [DOI] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. Journal of Experimental Medicine. 1976;143:305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. The Hematologic A spects of Porphyria. In: Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, editors. Williams Hematology. 7th. New York: McGraw-Hill, Inc.; 2006. pp. 803–822. [Google Scholar]
- Sassa S, de Verneuil H, Anderson KE, Kappas A. Purification and properties of human erythrocyte uroporphyrinogen decarboxylase: immunological demonstration of the enzyme defect in porphyria cutanea tarda. Transactions of the Association of American Physicians. 1983;96:65–75. [PubMed] [Google Scholar]
- Sassa S, Granick JL, Eisen H, Ostertag W. Regulation of hem e biosynthesis in mouse Friend virus-transform ed cells in culture. In: Murphy MJ Jr, editor. In Vitro A spects of Erythropoiesis. New York: Springer-Verlag; 1978. pp. 135–142. [Google Scholar]
- Srivastava P, Sharma GD, Kamboj KK, Rastogi AK, Pandey VC. Heme metabolism in promastigotes of Leishmania donovani. Molecular and Cellular Biochemistry. 1997;171:65–68. doi: 10.1023/a:1006830113376. [DOI] [PubMed] [Google Scholar]
- Trager W. Trypanosomiasis and Leishmaniasis with special reference to Chagas’ disease, CIBA Foundation Symposium 20 (New series) Amsterdam: Elsevier-Excerpta-North Holland; 1974. Nutrition and biosynthetic capabilities of flagellates: Problems of in vitro cultivation and differentiation; pp. 225–245. [Google Scholar]
- Waki K, Dutta S, Ray D, Kolli BK, Akman L, Kawazu S, Lin CP, Chang KP. Transmembrane molecules for phylogenetic analyses of pathogenic protists: Leishmania-specific informative sites in hydrophilic loops of trans- endoplasmic reticulum N-acetylglucosamine-1-phosphate transferase. Eukaryotic Cell. 2007;6:198–210. doi: 10.1128/EC.00282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]