Abstract
The basal forebrain (BF) is known to play important roles in cortical activation and sleep, which are likely mediated by chemically differentiated cell groups including cholinergic, γ-aminobutyric acid (GABA)ergic and other unidentified neurons. One important target of these cells is the lateral hypothalamus (LH), which is critical for arousal and the maintenance of wakefulness. To determine whether chemically specific BF neurons provide an innervation to the LH, we employed anterograde transport of 10,000 MW biotinylated dextran amine (BDA) together with immunohistochemical staining of the vesicular transporter proteins (VTPs) for glutamate (VGluT1, -2, and -3), GABA (VGAT), or acetylcholine (ACh, VAChT). In addition, we applied triple staining for the postsynaptic proteins (PSPs), PSD-95 with VGluT or Gephyrin (Geph) with VGAT, to examine whether the BDA-labeled varicosities may form excitatory or inhibitory synapses in the LH. Axons originating from BDA-labeled neurons in the magnocellular preoptic nucleus (MCPO) and substantia innominata (SI) descended within the medial forebrain bundle and extended collateral varicose fibers to contact LH neurons. In the LH, the BDA-labeled varicosities were immunopositive (+) for VAChT (~10%), VGluT2 (~25%), or VGAT (~50%), revealing an important influence of newly identified glutamatergic together with GABAergic BF inputs. Moreover, in confocal microscopy, VGluT2+ and VGAT+ terminals were apposed to PSD-95+ and Geph+ profiles respectively, indicating that they formed synaptic contacts with LH neurons. The important inputs from glutamatergic and GABAergic BF cells could thus regulate LH neurons in an opposing manner to stimulate vs. suppress cortical activation and behavioral arousal reciprocally.
Indexing terms: glutamatergic, GABAergic, cholinergic, BDA, PSD-95, Gephyrin, rat, sleep-wake states
The basal forebrain (BF) plays an important role in the modulation of cortical activity and regulation of sleep-wake states. As known from early studies, it serves as the ventral extrathalamic relay to the cerebral cortex from the brainstem reticular activating and arousal systems (Starzl et al., 1951; Jones, 2005a). Yet, it is also importantly involved in sleep, because lesions of the BF are associated with insomnia (von Economo, 1931; Nauta, 1946; McGinty and Sterman, 1968; Jones, 2005a). These opposing roles could be mediated by chemically differentiated cell groups in the BF that include cholinergic, γ-aminobutyric acid (GABA)ergic, and non-cholinergic/non-GABAergic, presumed glutamatergic, neurons (Gritti et al., 1993, 1994, 1997; Manns et al., 2001). According to multiple lines of evidence, cholinergic neurons actively stimulate cortical activation during waking and paradoxical sleep (PS, also called rapid eye movement [REM] sleep; Buzsaki et al., 1988; Metherate et al., 1992; Duque et al., 2000; Manns et al., 2000b; Jones, 2004; Lee et al., 2005b). Some putative glutamatergic neurons can act in parallel with the cholinergic neurons in this process (Manns et al., 2003). In contrast, most GABAergic BF neurons are minimally active during cortical activation (Manns et al., 2000a), and, as reflected by c-Fos expression, many are maximally active during sleep (Modirrousta et al., 2004), indicating that they can promote sleep, including slow wave sleep (SWS).
In addition to projections to the cerebral cortex (Gritti et al., 1997), BF neurons give rise to projections to the posterior lateral hypothalamus (LH; Gritti et al., 1994), a region long known to be crucial for waking (von Economo, 1931; Nauta, 1946; Hess, 1957; Swett and Hobson, 1968; Jones, 2005c). Recently, neurons have been localized in the LH that contain the peptide orexin (Orx, also called hypocretin), which is critical for sustaining waking, because absence of the peptide, its receptor, or the Orx neurons results in narcolepsy (Chemelli et al., 1999; Lin et al., 1999; Peyron et al., 2000; Thannickal et al., 2000).
Although previous studies combining retrograde transport with immunohistochemical staining for neurotransmitter enzymes indicated that cholinergic, GABAergic, and other BF neurons project to the LH (Gritti et al., 1994), they did not reveal the efferent BF fibers projecting into the LH nor did they prove the use of acetylcholine (ACh), GABA, or, as proposed, glutamate as neurotransmitters by the projection neurons. Recently, proof of the uptake, storage, and release of specific neurotransmitters has become possible by immunohistochemical staining for specific vesicular transporter proteins (VTPs), including those for ACh (VAChT; Gilmor et al., 1996), GABA (VGAT; Chaudhry et al., 1998) and glutamate (VGluT1, -2, and -3; Fremeau et al., 2001, 2002; Fujiyama et al., 2001). We thus combined anterograde transport of 10,000 MW biotinylated dextran amine (BDA) with immunohistochemical staining for VAChT, VGluT (1, 2, and 3), and VGAT to determine whether cholinergic, glutamatergic, or GABAergic neurons located in the magnocellular preoptic nucleus (MCPO) and substantia innominata (SI) project to the LH. To assess whether the VGluT+ and VGAT+ varicosities form synapses in the LH, we employed triple immunostaining for the postsynaptic proteins (PSPs) PSD-95, a marker for excitatory synapses (Sheng and Pak, 2000), or Gephryin (Geph), a marker for inhibitory synapses (Sassoe-Pognetto and Fritschy, 2000; Sassoe-Pognetto et al., 2000).
MATERIALS AND METHODS
Animals and Surgery
All procedures conformed to the guidelines of the Canadian Council on Animal Care and the U.S. National Institutes of Health and were approved by the McGill University Animal Care Committee.
Long-Evans rats (200–250 g, Charles River Canada, St. Constant, Quebec, Canada) were anesthetized with ketamine/xylazine/acepromazine (65/5/1 mg/kg, i.p.) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) for surgery. Anesthesia level was monitored during the experiment and augmented by boosters if necessary. Because previous studies based on retrograde as well as anterograde tracing showed no evidence of contralateral projections from the BF to the LH (Swanson, 1976; Gritti et al., 1994), injections of BDA were done on left and right sides of each brain. Holes were drilled in the skull, and the dura mater was removed on each side over the BF. Glass micropipettes (tip diameter 15–25 μm) were back-filled with a 0.5 M NaCl solution containing 2% 10,000 MW BDA (BDA-10,000, Molecular Probes, Eugene, OR). With the aid of a micropositioner (model 660, David Kopf Instruments), a BDA-filled pipette was lowered into the BF on each side aimed at the magnocellular preoptic nucleus (MCPO; from Bregma: anterior-posterior [AP], −0.5 lateral [L], ±2.5 mm; vertical [V], 8.5 mm). A holding current of −300 nA was maintained (using a Microionto-phoresis Dual Current Generator 260, World Precision Instruments [WPI], Sarasota, FL) during the descent to avoid leakage of the solution. Once the pipette was in the targeted site, microinjection of BDA was performed by iontophoresis applying positive current pulses (5–10 μA) in a duty cycle of 1 second (0.5 seconds on, 0.5 seconds off) for a period of 25–30 minutes through a stimulator (Pulse-master A300, WPI) and stimulus isolation unit (Iso-Flex, A.M.P.I., Jerusalem, Israel). After the injection, the micropipette was held in place for 10 minutes and removed during renewed application of the holding current.
Rats were maintained for 5 or 6 days with food and water ad libitum to allow anterograde transport of the tracer. They were subsequently perfused transcardially under deep sodium pentobarbital anesthesia (100 mg/kg, i.p.) with ~500 ml 4% paraformaldehyde fixative solution. The brains were removed and put in a 30% sucrose solution for 2–3 days or until they sank, after which they were frozen at −50°C and stored at −80°C for subsequent processing.
Immunohistochemistry
Sections were cut by using a freezing microtome in 25-μm-thick coronal sections and collected in eight adjacent series at 200-μm intervals through the forebrain, including the magnocellular BF area and the tuberal-posterior hypothalamus. Series were processed for evaluation of the BDA injection site in the BF and BDA-labeled fibers in the LH by light microscopy. For this purpose, the avidin-biotin complex (ABC) procedure was performed by using the Vectastain ABC Elite kit (Vector, Burlingame, CA) with nickel-intensified diaminobenzidine (DAB-Ni) and combined with a Nissl counterstain using neutral red (NR).
Adjacent series containing the tuberal-posterior LH region were processed for double or triple fluorescent staining (Table 1). Prior pilot studies were performed to determine the conditions necessary for antibody as well as streptavidin penetration through the full depth of the sections. As viewed through the z-axis under epifluorescent and confocal microscopy, we established that 0.1 or 0.3% Triton X-100 (TX) allowed full penetration of antibodies and streptavidin through 25-μm-thick sections in double- or triple-stained series, respectively. Free-floating sections from each series were rinsed for 30 minutes in Trizma saline buffer (TS; 0.1 M, pH 7.4) followed by incubation for 30 minutes with a blocking solution of normal donkey serum (NDS; 6% in TS) containing TX (0.1 or 0.3%). Subsequent incubations and rinses (30 minutes between incubations) were done by using TS containing NDS (1%) and TX (0.1 or 0.3%). Incubations were performed at room temperature overnight with primary antibodies and for 3 hours with secondary antibodies or streptavidin.
TABLE 1.
List of Primary and Secondary Antibodies Used for Fluorescence Staining of Biotinylated Dextran Amine (BDA), Vesicular Transporter Proteins (VTPs) and Postsynaptic Proteins (PSPs)
| 1ry AB (overnight)
|
2ry AB (3 hours)
|
SA (3 hours)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Series | Antigen | Host | Dilution | Source | Cat. # | Immunogen | Specificity | IgG (Dky)17,18 | Dilution | SA17 | Dilution | n19 |
| Double BDA VTP | VAChT | Gt | 1:5000 | Chemicon1 | AB1578 | Synthetic peptide corresponding to C-terminus of cloned rat VAChT6 (CSPP GPFDGCEDDYNYYSRS)7 | By WB the AB recognizes a ≈65–70 kD band corresponding to VAChT protein4 | Anti-Gt-Cy3 | 1:800 | SA-Cy2 | 1:800 | 8 |
| VAChT | Rb | 1:1000 | Sigma2 | V5387 | Synthetic peptide corresponding to AA 512–530 of C-terminus of cloned rat VAChT (K- SPPGPFDGCEDDYNYYSRS)8 | By WB the AB recognizes a ≈67–70 kD band, corresponding to VAChT protein7 | Anti-Rb-Cy3 | 1:800 | SA-Cy2 | 1:800 | 9 | |
| VGluT1 | Rb | 1:1000 | Gift RHE3 | – | Synthetic peptide corresponding to 68 last AA of C-terminus of rat BNPi (VGluT1)9 | By WB the AB recognizes a ≈62 kD band from rat brain9 | Anti-Rb-Cy3 | 1:800 | SA-Cy2 | 1:800 | 4 | |
| VGluT2 | Rb | 1:5000 | Gift RHE | – | Synthetic peptide corresponding to 64 last AA of C-terminus of rat DNPi (VGluT2)10 | By WB the AB recognizes a ≈50–62 kD band from rat brain10 | Anti-Rb-Cy3 | 1:800 | SA-Cy2 | 1:800 | 14 | |
| VGluT3 | GP | 1:1000 | Chemicon | AB5421 | Synthetic peptide from cloned rat VGluT3 protein11 (AFEGEEPLSYQNEEDFSETS)7 | The AB labels VGluT3+ cells and fibers, in agreement with other VGluT3 antisera11 | Anti-GP-Cy3 | 1:800 | SA-Cy2 | 1:800 | 7 | |
| VGAT | Rb | 1:250 | Chemicon | AB5062P | Synthetic peptide corresponding to a 17 AA peptide near C- terminus region of rat VGAT12 (VHSLEGLIEAYRTNAED)7 | By WB the AB recognizes a band at ≈55–60 kD12 | Anti-Rb-Cy3 | 1:800 | SA-Cy2 | 1:800 | 14 | |
| Double VTP VTP | VAChT/VGluT2 | Gt
Rb |
1:5000
1:5000 |
Chemicon
Gift RHE |
AB1578
- |
(above)
(above) |
(above)
(above) |
Anti-Gt-Cy3
Anti-Rb-Cy2 |
1:800
1:200 |
4 | ||
| VAChT/VGAT | Gt
Rb |
1:5000
1:250 |
Chemicon
Chemicon |
AB1578
AB5062P |
(above)
(above) |
(above)
(above) |
Anti-Gt-Cy3
Anti-Rb-Cy2 |
1:800
1:200 |
4 | |||
| VGAT/VGluT2 | Rb
GP |
1:250
1:5000 |
Chemicon
Chemicon |
AB5062P
AB5907 |
(above)
Synthetic peptide from cloned rat VGluT2 protein13 (VQESAQDAYSYKDRDDYS)7 |
(above)
The AB gives labeling in agreement with other antisera to VGluT213 |
Anti-Rb-Cy2
Anti-GP-Cy3 |
1:200
1:800 |
4 | |||
| Triple BDA VTP PSP | VGluT2/PSD-95 | Rb
Ms |
1:5000
1:100 |
Gift RHE
ABR4 |
-
MA1-045 |
(above)
Purified recombinant of rat PSD- 9514 |
(above)
The AB detects post synaptic density 95kD in rat brain. By WB it recognizes a ≈95 kDa band14 |
Anti-Rb-Cy5
Anti-Ms-Cy3 |
1:800
1:800 |
SA-Cy2
SA-Cy2 |
1:800
1:800 |
3 |
| VGAT/Geph | Rb
Ms |
1:250
1:100 |
Chemicon
SY-SY5 |
AB5062P
147 011 |
(above)
Purified rat gephyrin15 |
(above)
By WB the AB recognizes a ≈93 kD band. It detects a N-terminus epitope16 |
Anti-Rb-Cy5
Anti-Ms-Cy3 |
1:800
1:800 |
SA-Cy2
SA-Cy2 |
1:800
1:800 |
3 | |
Chemicon: Chemicon International, Temecula, CA
Sigma: Sigma, St. Louis, MO
Gift from Edwards, R.H. and Fremeau, R.T. Jr.
ABR: Affinity BioReagents, Golden, CO.
SY-SY: Synaptic Systems, Göttingen, Germany.
Supplied by Chemicon on request
Jackson Immuno Research Laboratories, West Grove, PA.
For multiple labeling (ML) with minimal cross-reactivity (min X) to other species.
n, number of cases (each case referring to an injection site and series from the same side of the brain, thus 1 or 2 per brain).
AA, amino acid; AB, antibody; BNPi, brain specific Na+-dependent phosphate transporter; Cat., catalog; Cy2, cyanine; Cy3, indocarbocyanine; Cy5, indodicarbocyanine; Dky, donkey; DNPi, differentiation-associated Na+-dependent phosphate transporter; Geph, gephyrin; GP, guinea pig; Gt, goat; Ms, mouse (monoclonal); Rb, rabbit; SA, streptavidin; WB, western blot.
For double labeling of BDA and one of the VTPs, sections were incubated (by using 0.1% TX) first with primary antibodies against VAChT, VGluT1, -2, or -3, or VGAT (from various species) and then with appropriate Cy3-conjugated secondary antibodies (from donkey [Dky]) followed by Cy2-conjugated streptavidin for revelation of BDA (see Table 1, Double BDA/VTP).
For double labeling of two VTPs, sections were incubated (by using 0.1% TX) with two primary antibodies against VAChT, VGluT2, and/or VGAT (from different species) and then with appropriate Cy2- and Cy3-conjugated secondary antibodies (from Dky) (see Table 1, Double VTP/VTP).
For triple labeling of BDA, the VTPs, and the PSPs, sections were incubated (by using 0.3% TX) with two primary antibodies against VGluT2 or VGAT and PSD-95 or Geph, respectively (from different species) and then with appropriate Cy3 or Cy5 secondary antibodies (from Dky). They were subsequently incubated with Cy2-conjugated streptavidin for revelation of BDA (see Table 1, Triple BDA/VTP:PSP).
All sections were mounted out of Trizma water, and the mounted sections were dehydrated through alcohols, cleared in xylene, and coverslipped with Permount.
Conventional microscopy, tracing, and stereological analysis
Sections were examined under light and epifluorescent microscopy with a Leica DMLB microscope or Nikon Eclipse E800 equipped with x-y-z motorized stages, a video or digital camera, and filters appropriate for fluorescein isothiocyanate (FITC) (or Cy2), Rhodamine (or Cy3), and (on the Nikon) Cy5 fluorescence. Single as well as composite images were acquired, and drawings were made by using Neurolucida software (MicroBrightField [MBF], Colchester, VT). Cells and varicosities were counted by using the Optical Fractionator probe of Stereo Investigator software (MBF). For tracing or counting, a computer resident atlas of the rat brain was employed that was developed and applied in our laboratory by using standardized procedures for tissue processing (Gritti et al., 1993, 1994). For each application, series of histology sections are matched to appropriate levels of the atlas (at 400-μm intervals) under low magnification (5 or 10× objective). At each level, the atlas image is then rotated if necessary, and the contours are adjusted to fit the relevant nuclei of the histology section optimally.
Injection sites from eight rats (BDA 14, 15, 16, 18, 19, 20, 21, and 22) were examined under brightfield illumination in DAB-Ni-stained material. In 14 cases, the labeled cells were centered in the MCPO-SI (on the left and/or right sides). From these, five injection sites (“cases” on the left and/or right sides) from three rats (BDA 16, 18, and 19) were selected for quantitative estimate of the cells in the injection sites. From the 14 cases, innervation of the LH was studied qualitatively and quantitatively in DAB-Ni and fluorescent stained material (see text and Table 1).
Under brightfield illumination, unbiased estimates of the total number of DAB-Ni-stained, BDA-labeled nerve cell bodies were performed by using the Optical Fractionator probe of Stereo Investigator. Cells were counted within contours of the BF and surrounding nuclei, including the MCPO, SI, nucleus of the diagonal band of Broca (DBB), olfactory tubercle (OTu), lateral preoptic area (LPO), fundus of the striatum (FS), piriform cortex (Pir), nucleus of the lateral olfactory tract (LOT), and anterior amygdaloid area (AA). Counts were made under a 60× oil objective (with 1.40 numerical aperture [NA]) through six or seven BF levels (at 200-μm intervals) by using an x-y sampling grid size (of 120 × 120 μm) that was equal to the counting frame size, so as to sample 100% of each area. Counting was performed through 10 μm in the z-axis (starting 1 μm from the surface in mounted sections having an average of ~12-μm thickness following dehydration). Following the stereological procedures imposed in Stereo Investigator, cells were counted if their tops were contained within the defined counting block.
Projections of the DAB-Ni-stained, BDA-labeled axons were examined through the tuberal-posterior LH and in relation to NR stained neurons viewed under brightfield illumination. The axons and neurons were drawn by using a 100× oil objective within a contour of the LH using Neurolucida software (MBF).
Given evidence for contacts of BF fibers on LH neurons, the total number of NR stained neurons that were or were not contacted by one or more DAB-Ni-stained, BDA+ varicosities (NR+:BDA+ or NR+:BDA−) was estimated by using the Optical Fractionator probe through the LH (n = 3 cases). Counts were performed under a 100× oil objective (1.4 NA) on the Nikon microscope. The cells were sampled in the LH, as defined in the computer resident atlas, through three levels separated by 400-μm intervals (5,800, 6,200, and 6,600 μm anterior [A] to interaural zero [IA0]). The sampling grid size (210 × 210 μm) was set to be larger than the counting frame (70 × 70 μm) so as to sample ~11% of the LH area. Counting was performed through 16 μm of the section thickness (starting 1 μm from the surface of the mounted sections, which had an average thickness of ~19 μm following their minimal dehydration and differentiation for NR). As above, cells were counted if their tops were contained within the defined counting block.
Under epifluorescent illumination, fluorescent stained BDA-positive (+) varicosities were examined for double labeling with the VTPs in the LH. After double labeling with VAChT, VGluT2, and VGAT was found, unbiased estimates of the total numbers of single BDA+ and double-labeled BDA+/VAChT+, BDA+/VGluT2+, or BDA+/VGAT+ varicosities were estimated in the LH for each series by using the Optical Fractionator probe of Stereo Investigator. In five cases analyzed per series in stereology, all VTPs were stained by using antibodies raised in rabbit (Rb; VAChT, VGluT2, and VGAT; see Table 1). Counts were performed under a 100× oil objective (with 1.40 NA) on the Leica microscope. The varicosities were sampled in the LH, as defined in the computer resident atlas, through three levels separated by 400-μm intervals (A 5,800, A 6,200, and A 6,600) of the tuberal-posterior LH (in four cases per series; Table 2). The sampling grid size (180 × 180 μm) was set to be larger than the counting frame (90 × 90 μm) so as to sample 25% of the LH area. Counting was performed through 8 μm of the section thickness (starting 1 μm from the surface of the mounted sections having an average thickness of ~12 μm following dehydration). In each counting block and frame, all BDA+ varicosities (in green, Cy2) were counted, including those that were and were not double-labeled for the VTP (in red, Cy3) to obtain an estimate of the proportion of double-labeled varicosities for each VTP.
TABLE 2.
Proportion of VAChT+, VGluT2+ and VGAT+ BDA-labeled BF Axonal Varicosities in the LH Region Based on Stereological Estimates1
| VAChT series
|
VGluT2 series
|
VGAT series
|
VTP series
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | BDA +var. | BDA+/VAChT+ var. | % VAChT+ var. | BDA +var. | BDA+/VGluT2+ var. | % VGluT2+ var. | BDA +var. | BDA+/VGAT+ var. | % VGAT +var. | ANOVA F-ratio |
| BDA15-L | 38,080 | 4,640 | 12.2 | - | - | - | - | - | - | |
| BDA15-R | 17,920 | 1,040 | 5.8 | - | - | - | - | - | - | |
| BDA16-L | 18,800 | 2,640 | 14.0 | 27,040 | 5,600 | 20.7 | 23,440 | 11,280 | 48.1 | |
| BDA18-L | 17,840 | 2,000 | 11.2 | 10,800 | 2,080 | 19.3 | 17,680 | 8,240 | 46.6 | |
| BDA18-R | 15,120 | 960 | 6.3 | 11,680 | 2,640 | 22.6 | 17,120 | 8,160 | 47.7 | |
| BDA19-L | - | - | - | 18,824 | 6,136 | 32.6 | 20,176 | 10,504 | 52.1 | |
| BDA19-R | - | - | - | 19,032 | 3,432 | 18.0 | 16,536 | 6,864 | 41.5 | |
| Mean ± SEM | 21,552 ± 4,178 | 2,256 ± 673 | 9.9 ± 1.6 | 17,475 ± 2,946 | 3,978 ± 806 | 22.6 ± 2.6 | 18,990 ± 1,274 | 9,010 ± 816 | 47.2 ± 1.7 | 87.3*** |
Data for individual cases (1 or 2 sides from 4 rat brains) is presented together with the mean ± SEM. Estimated numbers of varicosities were obtained by sampling from 3 sections (at levels, A 5800, A 6200 and A 6600), per series per case. The proportions of the three VTP+ varicosities differed significantly (***, P < 0.001 according to one-way ANOVA) across and between VTP+ varicosities (with P < 0.05 according to post-hoc Bonferroni-corrected multiple-comparisons). var., varicosities; VTP, vesicular transporter protein.
Double labeling for VAChT, VGluT2, and/or VGAT was assessed under epifluorescent illumination on the Leica microscope (in four cases per series; Table 1). In the absence of any double labeling, no quantification was undertaken.
Confocal microscopy and image processing
To examine the presence of PSPs in association with BDA+/VTP+ varicosities, triple-stained sections were analyzed by confocal microscopy with a Zeiss LSM 510 laser scanning microscope equipped with Argon 488 nm, helium-neon 543 nm, and helium-neon 633 nm lasers for Cy2, Cy3, and Cy5 excitation as well as with appropriate filters for detection of Cy2 (bandpass 500–530 nm, green), Cy3 (bandpass 565–615 nm, red), and Cy5 (bandpass 697–719 nm, infrared). Scanning was performed through a Plan-Apochromat 100× (with 1.40 NA) objective and pinhole size of 1 (Airy Units) for each of the three channels. Images were acquired for the three chromogens by using the resident LSM 510 software and consisted of stacks taken through the z-axis in optical slices of ~0.33 μm. Rendered 3D views of the image stacks were obtained by using the image software Volocity 3.5.1 (Improvision, Lexington, MA, www.improvision.com), which allowed interactive visualization, magnification, and rotation of the 3D images in order to determine the relative location of each of the elements from the three channels.
In some images, a deconvolution procedure or iterative restoration in Volocity was applied by using a 95% confidence level in order to maximize the signal-to-noise ratio and assess relationships among elements better in the triple-stained material. As assessed in three cases per series (Table 1), contacts between VTP+/BDA+ varicosities and PSP+ profiles were evaluated in the rotated images and validated by lack of separation between the pre- and postsynaptic elements.
Adjustments for brightness and contrast in brightfield images and tonal range for each individual RGB channel (“Adjust/levels” command in Photoshop) in fluorescent images were performed with Adobe Photoshop Creative Suite edition (Adobe Systems, San Jose, CA).
RESULTS
BDA injection site and cellular labeling
Iontophoretic application of BDA-10,000 into the region of the magnocellular preoptic area and substantia innominata (MCPO-SI, Fig. 1A) produced a small and well-restricted, spherical injection site (Fig. 1B) containing labeled cell bodies and dendrites (Fig. 1C). The injection sites ranged in size from 300 to 500 μm in diameter and were consistently located primarily within the MCPO and secondarily in the overlying SI (n = 11 injection sites).
Fig. 1.

BF site of BDA injection. A: Atlas section through the cholinergic cell area (MCPO-SI) where iontophoretic applications of BDA were placed. B: Composite image of typical BDA injection site (case BDA18-R, processed by using ABC with DAB-Ni and counter-stained with neutral red). Note the small size and restricted location of BDA-labeled cells in the MCPO. C: High-magnification image of two BDA-labeled neurons (from B, arrowheads). Brightness and contrast were adjusted in B and C. For abbreviations, see list. Scale bar = 1 mm in B; 20 μm in C.
To appraise the number of cells labeled and their precise location in BF nuclei, stereological estimates were obtained through the BF. The average number of BDA-labeled neurons per injection was ~1,400 (mean ± SEM, 1,430.6 ± 315, n = 5 injection sites). The labeled cells were almost exclusively (96.2 ± 1.7%; range, 90–100%) located within the MCPO (90.2%) and SI (6.0%). A few scattered cells were variably found in immediately adjacent regions including the olfactory tubercle, lateral olfactory tract nucleus, or anterior amygdaloid area (3.5 ± 1.8%). Isolated cells were found in the nearby fundus of the striatum or piriform cortex in some cases. No labeled cells were seen in the more rostral DBB. No labeled cells were seen in distant regions known to project to the MCPO-SI, including the prefrontal cortex or, importantly, the LH, indicating a lack of retrograde transport of the BDA-10,000 in these afferent systems.
BDA was found within axons in the diencephalon (n = 14 cases) and in cortical and subcortical telencephalic regions where different MCPO-SI neurons are known to project from retrograde (Gritti et al., 1994, 1997) and other anterograde tracing studies (Luiten et al., 1987; Grove, 1988).
BDA-labeled fibers and varicosities in the LH
In the diencephalon, thick fascicles of fibers were evident in the ventrolateral posterior LH (Fig. 2A). From these coarse fibers, collateral fine fibers extended out through the LH. Some fibers continued sparsely into the perifornical area, but most remained within the LH. The fine fibers bore varicosities along their axons (boutons en passant, Fig. 2B′) or occasionally at the end of their axons (boutons terminaux, Fig. 2B″). Although many varicose axons did not appear to contact nerve cell bodies in the region (Fig. 2B′), a significant number did appear to do so, innervating either small (Fig. 2B″) or large (Fig. 2B″′) neurons by varicose processes that could entirely envelop the soma. To visualize the fiber distribution and innervation of nerve cell bodies better within the LH, high-magnification tracing of the DAB-Ni-stained axons and neutral red (NR+) stained cells was performed. As seen in Figure 2C, the major axon fascicles were seen to course within the ventrolateral part of the medial forebrain bundle (MFB) from which they extended fine varicose fibers to contact and sometimes entirely surround cells in ventral, central, and dorsal portions of the LH (Fig. 2D′,D″,D″′).
Fig. 2.
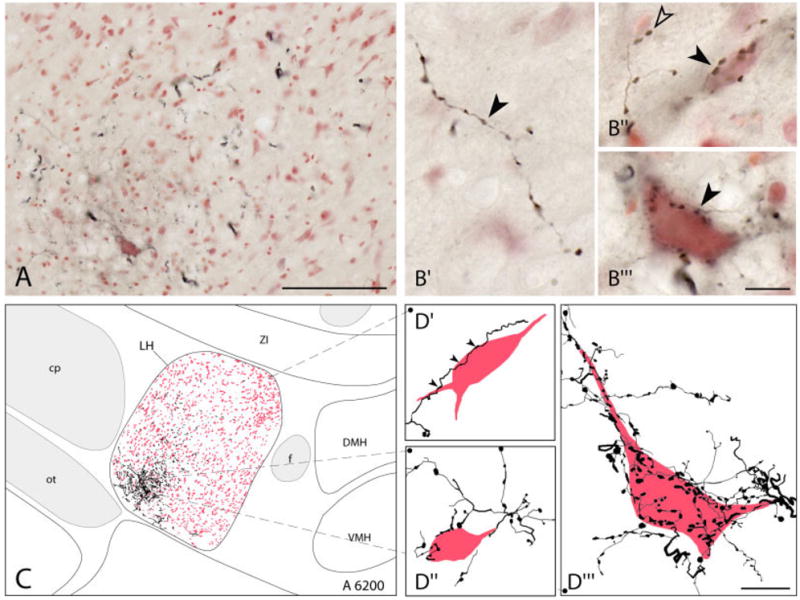
BDA-labeled fibers in the LH. A: Low-magnification composite image of BDA+ axons (in black, DAB-Ni) and neutral red stained (NR+) cells in the LH (case BDA19-L). B: High-magnification images showing BDA+ axons and terminals within the LH area. Varicosities appeared most commonly along axons as boutons en passant (solid arrowhead in B′) but also at the end of axons as boutons terminaux (open arrowhead in B″). Many axonal varicosities (stained with DAB-Ni) were seen in the neuropil (B′) or in close proximity to small (solid arrowhead in B″) or large (solid arrowhead in B″′) nerve cell bodies (stained with NR, B′ and B″, case BDA15-L; B″′, case BDA19-L). C: Tracing of BDA+ axons and NR+ neurons in the LH at ~6,200 μm (from IA0, case BDA19-L). Although concentrated more ventrally, fibers extended through the LH area and formed appositions with neurons therein (D). D: High-magnification tracing of elements in C showing the relationship of BDA+ axons with dorsally (solid arrowheads in D′), as well as more ventrally (D″and D″′) located NR+ neurons. (The neuron in D″′ corresponds to that pictured in B″′.) Tonal ranges for each RGB channel as well as brightness and contrast adjustments were made for pictures in A and B. For abbreviations, see list. Scale bar = 100 μm in A; 10 μm in B,D.
To appraise the extent of the innervation of neurons in the LH, stereological estimates were obtained of the number and proportion of LH cells ostensibly contacted by BDA-labeled varicosities in the light microscope images. In three cases, the NR+ cells that were contacted (NR+: BDA+) together with those that were not contacted (NR+: BDA−) were counted in random sampling through three levels of the LH (~A 5,800, A 6,200, and A 6,600). The number of contacted cells (NR+:BDA+ = 8,027 ± 3,639 neurons) corresponded to ~14% (14.4 ± 5.3%) of the total number of NR+ cells (NR+:BDA+ plus NR+:BDA− = 52,495 ± 5,611 neurons) estimated in the LH.
VAChT, VGluT, and VGAT within BDA-labeled varicosities in the LH
Series that were double stained for BDA and the VTPs were examined to determine whether BF axonal varicosities in the LH were immunopositive (+) for VAChT, VGluT, or VGAT.
VAChT+ varicosities were relatively sparse in the LH. Nonetheless, some BDA-labeled terminals were double-labeled for VAChT (Fig. 3A). The BDA+/VAChT+ varicosities were most often located along axons (Fig. 3A′,A″,A″′).
Fig. 3.

BDA-labeled axons in LH contain VAChT, VGluT2, or VGAT. High-magnification epifluorescent images illustrating antero-gradely labeled BDA+ axons (A′, B′, and C′, green fluorescent Cy2) that are double-labeled for vesicular transporter proteins (VTPs) as shown in single (A″, B″, and C″, red fluorescent Cy3) and merged (right A″′, B″′, and C″′, yellow) images and indicated by white arrowheads for those that are in focus. A: BDA+ varicose fiber (A′) whose varicosities are positive for VAChT (A″ and A″′). B: BDA+ varicose axon (B′) whose varicosities are positive for VGluT2 (B″ and B″′). C: BDA+ axon (C′) whose varicosities are positive for VGAT (C″ and C″′). Tonal range in red and green channels was adjusted individually (see Materials and Methods). For abbreviations, see list. Scale bar = 10 μm in C″′ (applies to A′–C′′′).
Both VGluT1+ (not shown) and VGluT2+ varicosities were densely distributed through the LH, although the VGluT2 most densely so. VGluT3+ varicosities were also present although sparse (not shown). In adjacent series processed for BDA and VGluT1, -2, or -3, only VGluT2 was found to be present in BDA-labeled varicosities (Fig. 3B). The BDA+/VGluT2+ varicosities most frequently appeared to be boutons en passant (Fig. 3B′,B″,B″′), although some appeared to be boutons terminaux.
Varicosities that were VGAT+ were densely distributed within the LH area. Many of the BDA-labeled axonal varicosities were VGAT+ (Fig. 3C). The BDA+/VGAT+ varicosities were most commonly boutons en passant (Fig. 3C′,C″,C″′), although some appeared to be boutons terminaux.
Double staining for different VTPs was subsequently examined (n = 4 cases per series) to determine whether they might be colocalized in the BF terminals. Double labeling for VAChT/VGluT2, VAChT/VGAT, or VGluT2/VGAT was not detected in varicosities of the LH (not shown). These negative results indicated that multiple VTPs are not colocalized in the same BF axonal varicosities innervating the LH.
Proportion and distribution of VAChT+, VGluT2+, and VGAT+ BDA-labeled varicosities
To determine the proportions of cholinergic, glutamatergic, and GABAergic BF axon terminals, stereological analysis was used for estimation of the total numbers of BDA+ and BDA+/VTP+ varicosities in the tuberal-posterior LH (~A 5,800, A 6,200, and A 6,600; Table 2). In the double-stained VAChT series, ~10% of the BDA+ varicosities were BDA+/VAChT+. In the VGluT2 series, ~23% of BDA+ varicosities were BDA+/VGluT2+. In the VGAT series, ~47% of the BDA+ varicosities were BDA+/VGAT+.
As evident in plots of the sampled BDA+/VAChT+, BDA+/VGluT2+, and BDA+/VGAT+ (Fig. 4, representing ~25% of the estimated total number of varicosities), the three types of terminals were codistributed across the LH. The three were commonly most dense within the ventral and central portions of the LH, although they were also scattered through more dorsal portions of the LH.
Fig. 4.

Distribution of VAChT+, VGluT2+, and VGAT+ BDA-labeled varicosities in the LH. Plotted together are the BDA+/VAChT+ (blue circles), BDA+/VGluT2+ (green squares), and BDA+/VGAT+ (red triangles) that were marked and counted by stereological analysis from double-stained fluorescent sections at the three levels through the LH (case BDA16-L). The BDA+/VTP-negative terminals, which were counted in each series, are not included. The stereological analysis was performed by sampling one-fourth of the total area (using a counting frame of 90 × 90 μm within a grid of 180 × 180 μm; see Materials and Methods), and each figure thus reflects about one-fourth of the total number of varicosities in a 25-μm-thick section. Note the concentration of BDA+/VTP+ varicosities within the ventral and central portion of the LH, with scattered varicosities in other portions, especially at the most posterior level (A 5800). For abbreviations, see list.
PSD-95 or Geph in relation to BDA+/VGluT2+ or BDA+/VGAT+ terminals
To assess whether PSPs were present in association with the numerous BDA+/VGluT2+ or BDA+/VGAT+ varicosities, triple fluorescent staining for BDA (Cy2), VGluT2 or VGAT (Cy3), and PSD-95 or Geph (Cy5) was performed in LH sections and analyzed by confocal microscopy by using 3D reconstruction and rotation of the images (in three cases per series).
In the series stained for BDA, VGluT2, and PSD-95, the PSD-95 staining appeared to be punctate and smaller in size than VGluT2+ varicosities. As judged from magnified and rotated 3D images, PSD-95+ puncta were often seen closely associated with VGluT2+ varicosities (Fig. 5A–C, pointers). BDA+/VGluT2+ varicosities were commonly seen in close association with one or more PSD-95+ puncta (BDA+/VGluT2+:PSD-95+, Fig. 5A,B, pointers opposite filled arrowheads). As confirmed in 3D rotations, the BDA+/VGluT2+ varicosities were apposed to the PSD-95+ profiles (Fig. 5A,B, insets). BDA+/VGluT2− varicosities were generally seen unassociated with PSD-95+ puncta (Fig. 5C), although some BDA+/VGluT2−: PSD-95+ profiles were seen (not shown).
Fig. 5.

Relationship of PSD-95+ puncta to VGluT2+ and Geph+ puncta to VGAT+ BDA-labeled varicosities in 3D rendered confocal images. A,B: Large images (8 and 12 serial 0.33-μm-thick optical sections) of BDA-labeled axons and varicosities (solid arrowheads, pseudo-color green, Cy2) that are immunopositive for VGluT2 (pseudo-color blue, Cy3), face PSD-95+ profiles (facing pointers, pseudo-color red, Cy5) and featured in the zoomed images on the right (5 and 12 serial 0.33-μm-thick optical sections). Note also, in the large images, the frequent association of VGluT2+ varicosities with PSD-95+ puncta (pointers). C: Image (eight serial sections) of a BDA+ varicosity (open arrowhead, pseudo-color green, Cy2) that is immunonegative for VGluT2 (pseudo-color blue, Cy3) and does not face a PSD-95+ profile, as is also evident in the zoom images on the right (10 serial sections). Note VGluT2+ varicosities in the vicinity that are associated with PSD-95+ puncta (pointer in large image). D,E: Images (eight and seven serial sections, respectively) of BDA-labeled axons and varicosities (solid arrowheads, pseudo-color green, Cy2) that are positive for VGAT (pseudo-color blue, Cy3) and face Geph+ profiles (facing pointers, pseudo-color red, Cy5), as featured in the zoomed images on the right (nine and seven serial sections). In the large image of D, VGAT+/Geph+ elements appear to surround the unlabeled soma of a neuron with the VGAT+ varicosities outside and the Geph+ puncta (pointers) inside, presumably on the cell membrane. Note in the large panels that many VGAT+ varicosities are associated with Geph+ puncta (pointers). F: Image (seven serial sections) of a BDA+ varicosity (open arrowhead, pseudo-color green, Cy2) that is immunonegative for VGAT (pseudo-color blue, Cy3) and is not associated with any Geph+ puncta, as is also evident in the zoomed images on the right (five serial sections). Note, in contrast, that other VGAT+ varicosities in the vicinity are associated with Geph+ puncta (pointer). Deconvolution was applied to all images with a confidence limit of 95% (see Materials and Methods). For abbreviations, see list. Scale bar = 5 μm in F (applies to A–F, large images; 1 μm in F, lower right corner (applies to A–F, small images).
In series stained for BDA, VGAT, and Geph, Geph staining appeared to be punctate. The Geph+ puncta were generally larger that the PSD-95+ puncta. As judged from magnified and rotated 3D images, Geph+ puncta were frequently seen in close association with VGAT+ varicosities, and conversely VGAT varicosities were frequently seen in close association with Geph+ puncta (Fig. 5D–F, pointers). BDA+/VGAT+ axonal varicosities were apposed to Geph+ puncta (BDA+/VGAT+:Geph+), usually with one Geph+ profile per BDA+/VGAT+ varicosity (Fig. 5D,E). Occasionally, unlabeled LH cells appeared to be surrounded by VGAT+ varicosities, including BDA+/VGAT+ ones that were apposed to Geph+ puncta, located on the inner side of the varicosities and thus presumably in the cell membrane of the innervated cell body (Fig. 5D). BDA+/VGAT− varicosities were not seen in apposition to Geph+ profiles (Fig. 5F).
DISCUSSION
The present study provides evidence that cholinergic, glutamatergic, and GABAergic BF neurons project to the LH. The quantitatively most important, glutamatergic and GABAergic, fibers also appear to form excitatory and inhibitory synapses, respectively, on LH neurons. Through this projection, BF neurons can thus have a dual influence in the LH to excite or inhibit neurons involved in promoting cortical activation and behavioral arousal.
Technical considerations
Confirming its documented utility as an anterograde tracer (Veenman et al., 1992; Wouterlood and Jorritsma-Byham, 1993; Lanciego et al., 2000; Reiner et al., 2000), we found that BDA-10,000 provided discrete labeling of BF cell bodies and reliable anterograde labeling of BF fibers and varicosities. Although we noticed some labeled cells in areas surrounding the injection site in the MCPO-SI, which might have been retrogradely labeled, we did not find cells retrogradely labeled at a distance in the prefrontal cortex or LH, confirming that BDA-10,000, in contrast to BDA-3000 MW, results in neglible retrograde labeling (Wouterlood and Jorritsma-Byham, 1993; Reiner et al., 2000). Here, from the labeled cells located predominantly (>90% on average) in the MCPO of the BF, the BDA-labeled axons were seen to course within the ventro-lateral, a, subdivision of the MFB, which has been known to carry descending fibers from the MCPO (Veening et al., 1982). In the posterior LH, the axons sent collateral branches through the LH region in a manner also previously described from the MCPO by application of other anterograde tracers, including Phaseolus vulgaris leucoagglutinin (PHA-L; Grove, 1988) and proteins synthesized from tritiated amino acids (Swanson, 1976).
This distribution of efferent fibers to the LH from MCPO-SI fits within a lateral to medial topographic organization of projections to the tuberal-posterior hypothalamus from forebrain structures (Veening et al., 1982). Moreover, the BDA-labeled varicose fibers extended through a region in the LH from which neurons in the MCPO and SI had previously been retrogradely labeled with cholera toxin in large numbers in our laboratory (Gritti et al., 1994). Here, we labeled only a small proportion of these afferent neurons, according to their numbers and distribution (Gritti et al., 1993, 1994, 2003), because our injection site was intentionally discrete so as to be maximally restricted to the MCPO and overlying SI. With this relatively small proportion of projection neurons labeled, we found nonetheless that an estimated ~22,000 terminals were labeled with BDA in the LH and ~8,000 cells (~15% of the estimated total LH cell population) were contacted by BDA+ terminals, thus providing a good sample of the basalo-hypothalamic projection to the LH.
Confirming the utility of BDA as an anterograde tracer that can be readily used along with immunostaining for elucidating complex neural circuits (Lanciego et al., 2000), we were able to apply double staining for BDA and the VTPs in order to identify the neurotransmitters utilized by the BF neurons projecting to the LH. We found that with the use of Triton (0.1%), we obtained complete penetration in material double stained for VTP antibodies and streptavidin (through 25-μm-thick sections). Such reliable, homogeneous staining thus permitted the application of stereological analysis to assess the proportions of the different VTP+ terminals in the LH. We were also able to apply triple staining for BDA, the VTPs, and PSPs (by using 0.3% TX).
Double staining with the VTPs allowed unequivocal identification of the neurotransmitter utilized by the projecting neurons (Chaudhry et al., 1998; Bellocchio et al., 2000). Together with other elements necessary for recycling, VTPs form docking and fusion of synaptic vesicles, a critical component of the machinery for neurotransmitter release at presynaptic sites (Liu et al., 1999; Fremeau et al., 2004). Particularly in the case of VGAT and VGluT2, they have been found to be concentrated at symmetrical and asymmetrical synapses, respectively, to co-purify with other synaptic proteins and to colocalize with synaptophysin and/or synaptobrevin in synaptic terminals (Chaudhry et al., 1998; Takamori et al., 2000; Gualix et al., 2003; Fremeau et al., 2004).
Triple staining with PSPs here confirmed that the BDA-labeled VGluT+ and VGAT+ terminals abutted excitatory and inhibitory postsynaptic elements, respectively. The PSPs, PSD-95 and Geph, form part of the postsynaptic scaffolding of excitatory (Kornau et al., 1995; O’Brien et al., 1999; Sheng and Pak, 2000) and inhibitory (Pfeiffer et al., 1984; Sassoe-Pognetto et al., 1995, 2000; Sassoe-Pognetto and Fritschy, 2000) synapses, respectively. Indeed, PSD-95 colocalizes with glutamate receptors by interacting directly with the C-terminus of synaptic NMDA receptor subunits (Kornau et al., 1995) and indirectly through the protein stargazin with AMPA receptors (Chen et al., 2000). Although not binding GABAA receptors directly, Geph colocalizes with the most common and synaptically located GABAA receptor subunits as well as the glycine receptor (Pfeiffer et al., 1984; Sassoe-Pognetto et al., 2000).
We employed laser scanning confocal microscopy and state-of-the-art 3D rendering technology to visualize appositions between BDA+/VTP+ terminals and PSP+ profiles, an approach that has been proposed to be suitable for judging the existence of synaptic contacts by confocal microscopy (Wouterlood et al., 2002, 2003), while recognizing that absolute proof of such contacts necessitates electron microscopy. Thus, we believe that the presynaptic enrichment of VTPs for glutamate and GABA in BDA+ terminals and their apposition with PSPs associated with excitatory and inhibitory synapses respectively, represents strong evidence for the synaptic contacts of glutamatergic and GABAergic BF terminals on LH neurons.
BF cholinergic, glutamatergic, and GABAergic fibers in the LH
The immunohistochemical localization of VAChT, VGluT2, and VGAT in anterogradely labeled BF varicosities demonstrates that BF neurons have the capacity, endowed by the VTPs, to release ACh, glutamate, or GABA, respectively (Gilmor et al., 1996; Chaudhry et al., 1998; Bellocchio et al., 2000; Fremeau et al., 2001). Their neurotransmitter phenotypes would appear to be unambiguous, because, in contrast to previous evidence for the presence of multiple mRNAs or proteins for synthetic enzymes of ACh, glutamate, or GABA in BF cell bodies (Manns et al., 2001; Sotty et al., 2003), no evidence for colocalization of the VTP proteins was found here in terminals within the LH. BF neurons innervating the LH can thus be phenotypically identified as cholinergic, glutamatergic, or GABAergic.
By demonstrating the presence of VGluT2 proteins in BF terminals, the present results provide the first proof for the existence of BF neurons that utilize glutamate as a neurotransmitter and can thus be considered glutamatergic. Another recent report showed by in situ hybridization that cortically projecting BF neurons contain mRNA for VGluT2 and accordingly have the capacity to synthesize the VGluT2 protein (Hur and Zaborszky, 2005). Previous results had also suggested the existence of glutamatergic BF neurons based on the presence of phosphate-activated-glutaminase (PAG; Manns et al., 2001), the enzyme utilized for the synthesis of glutamate from glutamine, yet possibly also used for the synthesis of GABA from the same substrate in some cells (Fujiyama et al., 2001). The presence of glutamatergic BF neurons that utilize VGluT2 follows the principle that in the forebrain, VGluT2 neurons are localized predominantly in subcortical structures, whereas VGluT1 neurons are localized predominantly in the cortex (Fremeau et al., 2001). A third type of vesicular transporter for glutamate (VGluT3) has more recently been visualized in cell bodies within some cortical and subcortical neurons, notably including BF neurons (Fremeau et al., 2002; Schafer et al., 2002; Harkany et al., 2003; Herzog et al., 2004). In the present analysis, we did not find evidence for concentration of VGluT3 in BF varicosities within the LH. It would thus appear that BF neurons might contain VGluT3 in their cell bodies but not transport it to their terminals or, alternatively, that BF neurons that do transport VGluT3 to their terminals do not project caudally to the LH.
Of the total number of BF axonal varicosities in the LH, the smallest proportion was VAChT+ (~10%), a medium proportion was VGluT2+ (~25%), and the largest proportion was VGAT+ (~50%). Previous studies employing retrograde transport with immunostaining for the synthetic enzymes of ACh (choline acetyltransferase [ChAT]) and GABA (glutamic acid decarboxylase [GAD]) found that of the BF cells within the MCPO projecting to the LH, <5% were ChAT+, ~20% were GAD+ and up to ~75% were neither and thus presumed to be glutamatergic (Gritti et al., 1994). The higher proportions of anterogradely labeled VAChT+ and VGAT+ varicosities relative to retrogradely labeled ChAT+ and GAD+ cell bodies might be due to more axonal collateralization per neuron or more varicosities per axon length in GABAergic and cholinergic than non-GABAergic/non-cholinergic neurons, which would include the glutamatergic neurons.
Irrespective of these possible differences, only 25% of the varicosities were found to be glutamatergic, and another ~15% of varicosities could not be accounted for as glutamatergic, cholinergic, or GABAergic. It is possible that this contingent might use another type of excitatory neurotransmitter, such as aspartate, which is not recognized as a substrate by the vesicular glutamate transporters (Bellocchio et al., 2000; Fremeau et al., 2001, 2002). Indeed, aspartate has been found in axon terminals forming asymmetric synapses in the hypothalamus (van den Pol, 1991). Another possibility is that this proportion might simply reflect negative immunohistochemical staining due to insufficient amounts of some or all of the VTPs in terminals.
By showing the presence of PSD-95 and Geph in apposition to BF identified glutamatergic and GABAergic terminals, respectively, in the LH, the results substantiate the synaptic nature of this projection and also corroborate the principle that glutamate and GABA terminals generally form synapses with postsynaptic target neurons (Edwards, 1995). Given the small number and proportion of BDA+/VAChT+ varicosities in the LH and the less well-known association of cholinergic terminals with specific PSPs (Parker et al., 2004) or synaptic specializations (Descarries et al., 1997), we did not examine the relationship of the BDA+/VAChT+ boutons to PSPs. ACh might act predominantly by diffuse transmission through ectopic release and extrasynaptic receptors (Levey et al., 1995; Coggan et al., 2005).
Functional significance of the cholinergic, glutamatergic, and GABAergic BF projections to LH
Neurons in the LH project to brainstem arousal systems and/or spinal cord sympathetic and motor systems as well as forebrain limbic areas and the cerebral cortex (Saper et al., 1979; Saper, 1985; Holstege, 1987). By means of these multiple projections, the LH is well suited to play a central role in arousal. Indeed, the LH has been shown to influence positively several arousal-related processes such as sympathetic tone, locomotion, and exploratory behavior, including food seeking, reward, and cortical activation (Olds and Milner, 1954; Hess, 1957; Berthoud, 2002; DiLeone et al., 2003; Jones, 2005a). Recently, diffusely projecting neurons have been identified in the LH that contain the peptide Orx (de Lecea et al., 1998; Peyron et al., 1998; Sakurai et al., 1998), which appears to be critical for arousal and postural muscle tone because in absence of the peptide or its receptor, narcolepsy with cataplexy occurs (Chemelli et al., 1999; Lin et al., 1999; Peyron et al., 2000; Thannickal et al., 2000; Gerashchenko et al., 2001; Hara et al., 2001). Physiologically as well as anatomically and chemically, the LH is comprised of different cell types, the vast majority of which discharge at high rates during active waking with behavioral arousal (Szymusiak et al., 1989; Sakai et al., 1990; Steininger et al., 1999; Koyama et al., 2003). Some discharge in association with cortical activation during both waking and PS. Identified Orx neurons have recently been found to discharge maximally during active wakefulness and to virtually cease firing during SWS and PS (Lee et al., 2005a; Mileykovskiy et al., 2005).
In the present study, we found that the LH received input from cholinergic BF terminals, which represented ~10% of all BF varicosities. Because cholinergic BF neurons have been found to discharge in association with cortical activation during both waking and PS (Manns et al., 2000b; Lee et al., 2005b), they could possibly influence similar neurons in the LH that project selectively to the cerebral cortex and discharge with cortical activation during both waking and PS (Szymusiak et al., 1989; Sakai et al., 1990; Steininger et al., 1999; Koyama et al., 2003).
Glutamatergic terminals accounted for up to 25% of the total BF innervation of the LH, indicating that glutamatergic BF neurons can exert an important excitatory influence on the LH. This influence could be exerted on LH neurons that discharge in association with cortical activation and presumably project to the cerebral cortex (see above) or on LH neurons that discharge in association with motor activity and presumably project to the brainstem or spinal cord (Szymusiak et al., 1989; Steininger et al., 1999; Alam et al., 2002). Glutamatergic BF neurons could also act on Orx LH neurons, which project diffusely to all targets and could thus simultaneously promote cortical activation along with motor activity and behavioral arousal that occur during waking. Such an excitatory influence could originate from as yet chemically unidentified but possibly glutamatergic BF neurons that are maximally active during waking and minimally active during SWS and PS (Szymusiak and McGinty, 1986; Manns et al., 2003; Lee et al., 2004; Jones, 2005b).
Approximately 50% of BF axon terminals innervating the LH were GABAergic, indicating that a major influence of the BF in this region is inhibitory. Given that the vast majority of neurons in the LH discharge during waking (Steininger et al., 1999; Alam et al., 2002) and that inhibition of these neurons by injections of the GABAA receptor agonist muscimol into the LH suppresses waking (Lin et al., 1989), it can be concluded that the GABAergic inhibitory influence from the BF in the LH would suppress waking and promote sleep. This inhibitory influence could be on multiple LH neurons, including Orx neurons, whose inhibition by GABAergic input would accordingly provide a very powerful impetus for sleep. We propose that this innervation originates from particular GABAergic BF neurons that are sleep-active (Modirrousta et al., 2004) and discharge during SWS or SWS and PS when muscle hypotonia and atonia occur (Szymusiak and McGinty, 1986; Manns et al., 2000a; Lee et al., 2004; Jones, 2005b).
In conclusion, the present study reveals the presence of three phenotypically distinct BF cell groups that, according to their neurotransmitters, differentially modulate the LH. As a minor contingent, cholinergic BF neurons can act to facilitate LH neurons involved in cortical activation. As a larger contingent, newly identified glutamatergic BF neurons can act to promote both cortical and behavioral arousal of waking. As the largest contingent, GABAergic BF neurons can act to suppress arousal and promote sleep.
Acknowledgments
We are grateful to Robert H. Edwards and Robert T. Fremeau (Departments of Neurology and Physiology, University of California San Francisco School of Medicine, San Francisco, CA 94143) for kindly supplying the antibodies for VGluT1 and 2. We thank Lynda Mainville for her excellent technical assistance.
Grant sponsor: Canadian Institutes of Health Research; Grant number: CIHR 13458; Grant sponsor: U.S. National Institutes of Health; Grant number: NIH RO1 MH-60119-01A.
Abbreviations
- ac
anterior commissure
- BDA
biotinylated dextran amine
- BF
basal forebrain
- cp
cerebral peduncle
- DMH
dorsomedial hypothalamus
- f
fornix
- FS
fundus striatum
- Geph
Gephyrin
- LH
lateral hypothalamus
- LPO
lateral preoptic area
- MCPO
magnocellular preoptic nucleus
- MPO
medial preoptic nucleus
- oc
optic chiasm
- ot
optic tract
- Pir
piriform cortex
- PSD-95
< postsynaptic density
- SI
substantia innominata
- SO
supraoptic nucleus
- VAChT
vesicular acetylcholine transporter
- VGAT
vesicular γ-aminobutyric acid transporter
- VGluT2
vesicular glutamate transporter 2
- VMH
ventromedial hypothalamic nucleus
- ZI
zona incerta
LITERATURE CITED
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Coggan JS, Bartol TM, Esquenazi E, Stiles JR, Lamont S, Martone ME, Berg DK, Ellisman MH, Sejnowski TJ. Evidence for ectopic neurotransmission at a neuronal synapse. Science. 2005;309:446–451. doi: 10.1126/science.1108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84:1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Edwards FA. Anatomy and electrophysiology of fast central synapses lead to a structural model for long-term potentiation. Physiol Rev. 1995;75:759–787. doi: 10.1152/physrev.1995.75.4.759. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmor ML, Nash NR, Roghani A, Edwards RH, Yi H, Hersch SM, Levey AI. Expression of the putative vesicular acetylcholine transporter in rat brain and localization in cholinergic synaptic vesicles. J Neurosci. 1996;16:2179–2190. doi: 10.1523/JNEUROSCI.16-07-02179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Jones BE. Codistribution of GABA- with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J Comp Neurol. 1993;329:438–457. doi: 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Jones BE. Projections of GABAergic and cholinergic basal forebrain and GABAergic preoptic-anterior hypothalamic neurons to the posterior lateral hypothalamus of the rat. J Comp Neurol. 1994;339:251–268. doi: 10.1002/cne.903390206. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other non-cholinergic basal forebrain neurons project together with cholinergic neurons to meso- and iso-cortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988;277:347–364. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- Gualix J, Gomez-Villafuertes R, Diaz-Hernandez M, Miras-Portugal MT. Presence of functional ATP and dinucleotide receptors in glutamatergic synaptic terminals from rat midbrain. J Neurochem. 2003;87:160–171. doi: 10.1046/j.1471-4159.2003.01975.x. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Harkany T, Hartig W, Berghuis P, Dobszay MB, Zilberter Y, Edwards RH, Mackie K, Ernfors P. Complementary distribution of type 1 cannabinoid receptors and vesicular glutamate transporter 3 in basal forebrain suggests input-specific retrograde signalling by cholinergic neurons. Eur J Neurosci. 2003;18:1979–1992. doi: 10.1046/j.1460-9568.2003.02898.x. [DOI] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Hess WR. The functional organization of the diencephalon. New York: Grune & Stratton; 1957. [Google Scholar]
- Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: An HRP and autoradiographic tracing study in the cat. J Comp Neurol. 1987;260:98–126. doi: 10.1002/cne.902600109. [DOI] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization. J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Progr Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Jones BE. Basic mechanisms of sleep-wake states. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4. Philadelphia: Elsevier Saunders; 2005a. pp. 136–153. [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005b;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jones BE. Sleep-wake mechanisms: neurophysiology and chemical neuroanatomy. In: Opp MR, editor. SRS basics of sleep guide. Westchester, IL: Sleep Research Society; 2005c. pp. 57–64. [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–1219. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Wouterlood FG, Erro E, Arribas J, Gonzalo N, Urra X, Cervantes S, Gimenez-Amaya JM. Complex brain circuits studied via simultaneous and permanent detection of three transported neuroanatomical tracers in the same histological section. J Neurosci Methods. 2000;103:127–135. doi: 10.1016/s0165-0270(00)00302-2. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani O, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005a;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal fore-brain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005b;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Manns ID, Alonso A, Jones BE. Sleep-wake related discharge properties of basal forebrain neurons recorded with micropipettes in head-fixed rats. J Neurophysiol. 2004;92:1182–1198. doi: 10.1152/jn.01003.2003. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Hersch SM, Wiley RG, Heilman CJ. Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol. 1995;351:339–356. doi: 10.1002/cne.903510303. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Krantz DE, Waites C, Edwards RH. Membrane trafficking of neurotransmitter transporters in the regulation of synaptic transmission. Trends Cell Biol. 1999;9:356–363. doi: 10.1016/s0962-8924(99)01605-0. [DOI] [PubMed] [Google Scholar]
- Luiten PGM, Gaykema RPA, Traber J, Spencer DG. Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Res. 1987;413:229–250. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal fore-brain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000a;20:9252–9263. doi: 10.1523/JNEUROSCI.20-24-09252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000b;20:1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience. 2001;107:249–263. doi: 10.1016/s0306-4522(01)00302-5. [DOI] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003;89:1057–1066. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirrousta M, Mainville L, Jones BE. GABAergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience. 2004;129:803–810. doi: 10.1016/j.neuroscience.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Hypothalamic regulation of sleep in rats. An experimental study. J Neurophysiol. 1946;9:285–316. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Parker MJ, Zhao S, Bredt DS, Sanes JR, Feng G. PSD93 regulates synaptic stability at neuronal cholinergic synapses. J Neurosci. 2004;24:378–388. doi: 10.1523/JNEUROSCI.3865-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F, Simler R, Grenningloh G, Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci U S A. 1984;81:7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Sakai K, El Mansari M, Lin J-S, Zhang G, Vanni-Mercier G. The posterior hypothalamus in the regulation of wakefulness and paradoxical sleep. In: Mancia M, Marini G, editors. The diencephalon and sleep. New York: Raven Press; 1990. pp. 171–198. [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Hypothalamocortical projections. J Comp Neurol. 1985;237:21–46. doi: 10.1002/cne.902370103. [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol. 1979;183:689–706. doi: 10.1002/cne.901830402. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Fritschy JM. Mini-review: gephyrin, a major postsynaptic protein of GABAergic synapses. Eur J Neurosci. 2000;12:2205–2210. doi: 10.1046/j.1460-9568.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Kirsch J, Grunert U, Greferath U, Fritschy JM, Mohler H, Betz H, Wassle H. Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J Comp Neurol. 1995;357:1–14. doi: 10.1002/cne.903570102. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM. Colocalization of multiple GABA(A) receptor subtypes with gephyrin at postsynaptic sites. J Comp Neurol. 2000;420:481–498. doi: 10.1002/(sici)1096-9861(20000515)420:4<481::aid-cne6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Sheng M, Pak DT. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu Rev Physiol. 2000;62:755–778. doi: 10.1146/annurev.physiol.62.1.755. [DOI] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol. 2003;551:927–943. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Taylor CW, Magoun HW. Ascending conduction in reticular activating system, with special reference to the diencephalon. J Neurophysiol. 1951;14:461–477. doi: 10.1152/jn.1951.14.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Res. 1999;840:138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- Swanson LW. An autoradiographic study of the efferent connections of the preoptic region in the rat. J Comp Neurol. 1976;167:227–256. doi: 10.1002/cne.901670207. [DOI] [PubMed] [Google Scholar]
- Swett CP, Hobson JA. The effects of posterior hypothalamic lesions on behavioral and electrographic manifestations of sleep and waking in cats. Arch Ital Biol. 1968;106:283–293. [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370:82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Iriye T, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamic area of cats. Brain Res Bull. 1989;23:111–120. doi: 10.1016/0361-9230(89)90169-x. [DOI] [PubMed] [Google Scholar]
- Takamori S, Riedel D, Jahn R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J Neurosci. 2000;20:4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. J Neurosci. 1991;11:2087–2101. doi: 10.1523/JNEUROSCI.11-07-02087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Cowan WM, Nieuwenhuys R, Geeraedts LMG. The medial forebrain bundle of the rat. II. An autoradiographic study of the topography of the major descending and ascending components. J Comp Neurol. 1982;206:82–108. doi: 10.1002/cne.902060107. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Reiner A, Honig MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 1992;41:239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- von Economo C. Its sequelae and treatment. London: Oxford University Press; 1931. Encephalitis lethargica. [Google Scholar]
- Wouterlood FG, Jorritsma-Byham B. The anterograde neuroanatomical tracer biotinylated dextran-amine: comparison with the tracer Phaseolus vulgaris-leucoagglutinin in preparations for electron microscopy. J Neurosci Methods. 1993;48:75–87. doi: 10.1016/s0165-0270(05)80009-3. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, van Haeften T, Blijleven N, Perez-Templado P, Perez-Templado H. Double-label confocal laser-scanning microscopy, image restoration, and real-time three-dimensional reconstruction to study axons in the central nervous system and their contacts with target neurons. Appl Immunohistochem Mol Morphol. 2002;10:85–95. doi: 10.1097/00129039-200203000-00015. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Bockers T, Witter MP. Synaptic contacts between identified neurons visualized in the confocal laser scanning microscope. Neuroanatomical tracing combined with immunofluorescence detection of post-synaptic density proteins and target neuron-markers. J Neurosci Methods. 2003;128:129–142. doi: 10.1016/s0165-0270(03)00171-7. [DOI] [PubMed] [Google Scholar]


