Abstract
Phentolamine mesylate accelerates recovery from oral soft tissue anesthesia in patients who have received local anesthetic injections containing a vasoconstrictor. The proposed mechanism is that phentolamine, an alpha-adrenergic antagonist, blocks the vasoconstriction associated with the epinephrine used in dental anesthetic formulations, thus enhancing the systemic absorption of the local anesthetic from the injection site. Assessments of the pharmacokinetics of lidocaine and phentolamine, and the impact of phentolamine on the pharmacokinetics of lidocaine with epinephrine were performed to characterize this potentially valuable strategy. The blood levels of phentolamine were determined following its administration intraorally and intravenously. Additionally, the effects of phentolamine mesylate on the pharmacokinetics of intraoral injections of lidocaine with epinephrine were evaluated. Sixteen subjects were enrolled in this phase 1 trial, each receiving 4 drug treatments: 1 cartridge lidocaine/epinephrine followed after 30 minutes by 1 cartridge phentolamine (1L1P), 1 cartridge phentolamine administered intravenously (1Piv), 4 cartridges lidocaine/epinephrine followed after 30 minutes by 2 cartridges phentolamine (4L2P), and 4 cartridges lidocaine/epinephrine followed by no phentolamine (4L). Pharmacokinetic parameters estimated for phentolamine, lidocaine, and epinephrine included peak plasma concentration (Cmax), time to peak plasma concentration (Tmax), area under the plasma concentration-time curve from 0 to the last time point (AUClast) or from time 0 to infinity (AUCinf), elimination half-life (t1/2), clearance (CL), and volume of distribution (Vd). The phentolamine Tmax occurred earlier following the intravenous administration of 1Piv (7 minutes than following its submucosal administration in treatment 1L1P (15 minutes) or 4L2P (11 minutes). The phentolamine t1/2, CL, and Vd values were similar for 1L1P, 1Piv, and 4L2P. The Tmax for lidocaine occurred later and the Cmax for lidocaine was slightly higher when comparing the 4L2P treatment and the 4L treatment. The phentolamine-induced delay of the lidocaine Tmax likely represents phentolamine's ability to accelerate the systemic absorption of lidocaine from oral tissues into the systemic circulation.
Keywords: Pharmacokinetics, Dental local anesthesia, Lidocaine, Phentolamine, Epinephrine
Soft tissue and pulpal anesthesia induced by the administration of local anesthetic agents containing a vasoconstrictor (epinephrine or levonordefrin) is an essential part of outpatient dentistry.1–3 A shortcoming of most dental local anesthetic agents is that the duration of soft tissue anesthesia (numbness to the lip and tongue) typically lasts for 3–5 hours.4 Persistent anesthesia, which is often associated with difficulty in eating, drinking, and speaking and with inadvertent biting of the lips, tongue, and/or cheek, is considered unpleasant, unnecessary and a temporary detriment to the quality of life for many dental patients.
Phentolamine mesylate (0.4 mg, packaged in a 1.7-mL dental cartridge) has been developed to accelerate the return of intraoral and perioral sensations after the completion of routine nonsurgical dental procedures. Phentolamine, a nonselective alpha-adrenergic blocking agent, has been available in the United States since 1952. Although it was originally developed for the treatment of hypertension, the currently FDA-approved intravenous/intramuscular formulation is indicated for the treatment of dermal necrosis resulting from inadvertent extravasation of norepinephrine and for the diagnosis and treatment of patients with pheochromocytoma, a tumor of the adrenal medulla with which excessive catecholamines may result in severe hypertension.5 Like other alpha-adrenergic blocking agents, phentolamine's primary effect is vasodilation.
Following the administration of a local anesthetic with an adrenergic vasoconstrictor, a subsequent phentolamine injection at the same location has been found to induce a rapid return of normal intraoral and perioral sensations.6–10 A phase 2, double-blind, placebo-controlled clinical trial of 122 patients who had received mandibular or maxillary local anesthesia for routine restorative or periodontal maintenance procedures first reported that phentolamine mesylate 0.4 mg decreased the median duration of lip anesthesia by 85 minutes or 54.8% compared to placebo injections (P < .0001).9 In those patients receiving mandibular injections the median duration of tongue anesthesia was reduced by 31.5 minutes or 30% (P = 0.0001) in the phentolamine group compared to placebo. Both the phentolamine and placebo injections were well tolerated with no significant differences in the incidences of any side effect, except for a transient increase in jaw pain 1 hour following phentolamine injection. The average pain rating in the phentolamine group was rated as “weak” compared to “none to faint” in the placebo group. There were no differences in the incidence or intensity of any cardiovascular event (tachycardia, blood pressure changes, extrasystoles) between the phentolamine and placebo groups. For a small subset of study participants requiring 2 local anesthetic injections to complete their dental procedures, the administration of 0.8 mg of phentolamine was also found to be efficacious and well tolerated.9 The clinical findings of 2 additional clinical trials enrolling 484 adults and 152 pediatric patients reported similar reductions in soft tissue anesthesia following the administration of phentolamine.7,8
As part of the overall research development plan to determine the efficacy, safety, and clinical utility of reversing dental local anesthesia with phentolamine, an evaluation of the pharmacokinetics of lidocaine and phentolamine was performed. This article describes the pharmacokinetics of phentolamine following administration of intraoral and intravenous injections. Additionally, the effects of phentolamine mesylate reversal on the pharmacokinetics of lidocaine with epinephrine when administered for maxillary and mandibular local anesthesia were evaluated.
Methods
This was a single-center, open-label, 4-treatment, phase 1 crossover study designed and statistically powered to evaluate the pharmacokinetics of phentolamine mesylate and lidocaine with epinephrine. Local anesthesia characteristics and safety measurements were recorded and are briefly summarized in this report.
To obtain adequate pharmacokinetic data, 16 healthy adult volunteers (7 male, 9 female) were enrolled. A subject was considered to have completed the study if he/she provided evaluable data for the phentolamine and lidocaine pharmacokinetic analyses. The study was designed to have each subject randomly receive each of the 4 drug treatments as follows:
Treatment 1L1P: Subjects received 1 cartridge (1.8 mL) of 2% lidocaine HCl with 1 : 100,000 epinephrine administered as a supraperiosteal infiltration over the maxillary first molar. Thirty minutes later, subjects received 1 cartridge of phentolamine mesylate (0.4 mg in 1.7 mL) at the same location.
Treatment 1Piv: Subjects received 1 cartridge of phentolamine mesylate (0.4 mg in 1.7 mL) injected intravenously over 1 minute. No local anesthetic was administered in this treatment.
Treatment 4L2P: Subjects received 4 cartridges (7.2 mL) of 2% lidocaine HCl with 1 : 100,000 epinephrine; 3.6 mL was administered as an inferior alveolar nerve block and 3.6 mL as a supraperiosteal infiltration over the maxillary first molar. These injections were administered in the same side of the face. Thirty minutes after the first injection of anesthetic, 1 cartridge of phentolamine mesylate (1.7 mL) was injected at the mandibular site and 1 cartridge at the maxillary site where anesthetic had been previously given, using the same injection technique. The total dose of phentolamine in this treatment was 0.8 mg (3.4 mL).
Treatment 4L: Subjects received 4 cartridges of 2% lidocaine HCl with 1 : 100,000 epinephrine; 3.6 mL was administered as an inferior alveolar nerve block and 3.6 mL as a supraperiosteal infiltration over the maxillary first molar. These injections were administered in the same side of the face. Phentolamine mesylate was not administered to subjects in this treatment. The 4L treatment served as a control to the 4L2P treatment.
Subjects received 2 of the 4 treatments during each of 2 clinic admissions. Each admission lasted for 2 full days (2 overnights). Subjects were admitted to the clinical testing facility on the first morning of each 2-day visit and remained in the clinic until the morning after the second treatment. Dosing started in the morning on days 1 and 2, with an interval of at least 24 hours separating each treatment. Subjects were contacted by telephone 2 days after discharge from each clinic admission and were asked about adverse events. Each subject received all 4 treatments in 1 of 4 random sequences.
Study Population
The study protocol and the informed consent document were approved by an Institutional Review Board (Patient Advocacy Council, Inc IRB, 3233 Executive Park Circle, Suite 200, Mobile, Ala). After providing written informed consent, healthy male and female subjects, ages 18–65 years inclusive, were enrolled in the study. Subjects were required to be in good health as determined by medical history, physical examination, vital signs, electrocardiogram, and clinical laboratory evaluations. All subjects were to weigh between 55 and 120 kg (121 and 264 lb). Participants who completed all phases of the trial were eligible for a reimbursement of $825.00.
Treatments
Dental cartridges containing phentolamine mesylate (0.4 mg/1.7 mL) were prepared by Novocol, Inc (Cambridge, Ontario, Canada; Lot #3067) as a sterile, pyrogen-free, isotonic solution. The concentration of the active ingredient phentolamine mesylate was 0.235 mg/mL. Excipients included water for injection, ethylenediaminetetraacetic acid, d-mannitol, sodium acetate, acetic acid, and sodium hydroxide. Dental cartridges of 2% lidocaine with 1 : 100,000 epinephrine (1.8 mL) were obtained from a commercial supplier.
Pharmacokinetics
Blood samples were drawn for measurements of concentrations of phentolamine, lidocaine, epinephrine, and N1-2[N-(3-hydroxyphenyl)-N-(4-toluyl)aminoacetyl] ethylenediamine (HTAEDA). HTAEDA is formed spontaneously in aqueous solutions of phentolamine and its measurement was included to assess its potential formation in the body. Eleven (treatment 1Piv) or 14 (treatments 1L1P, 4L2P, and 4L) blood samples were drawn for pharmacokinetic analysis, starting immediately prior to first injection of local anesthetic (if given) or intravenous injection of phentolamine mesylate, and ending 8.0–8.5 hours later. Because blood levels were expected to be close to physiologic concentrations and at the lower limits of detection, only selected samples were assayed for epinephrine. When values of epinephrine were below levels of detection, a value of zero was applied.
The following parameters were estimated for phentolamine, lidocaine, and, when possible, epinephrine: peak plasma concentration (Cmax), time to peak plasma concentration (Tmax), area under the plasma concentration–time curve from 0 to the last time point with measurable concentration (AUClast), area under the plasma concentration–time curve from time 0 to infinity (AUCinf), elimination half-life (t1/2), clearance (CL), and volume of distribution (Vd). All pharmacokinetic parameters were estimated using noncompartmental assumptions.
Statistical Methods
At least 16 subjects were to be enrolled to achieve the planned target sample size of 12 subjects (6 men and 6 women) evaluable for pharmacokinetic analysis. The effect of phentolamine on the pharmacokinetic parameters of lidocaine was compared with an analysis of variance model. A linear mixed-effect model was used to analyze the data; in this model, sequence, treatment, and period effects were deemed fixed, and subject effect (within sequence) was considered random. The comparison between 4L2P and 4L was made using the log-transformed variables, and was based on the difference of least-square adjusted means and the standard error associated with this difference. The null hypothesis was that the pharmacokinetic parameters of lidocaine were the same with and without subsequent treatment with phentolamine mesylate (comparing pharmacokinetic parameters between 4L2P and 4L).
Because of the limited sample size and the open design of the study, pharmacodynamic outcomes (duration of soft tissue anesthesia, etc) and adverse events are reported only descriptively in the text and tables.
Results
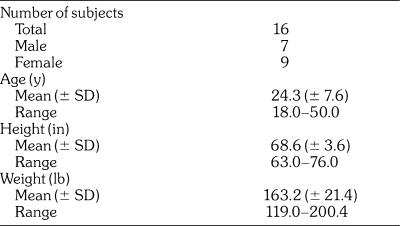
The demographic and baseline characteristics of the subjects are summarized in Table 1. Seven male and 9 female subjects were enrolled and provided evaluable data. The average age of the subjects was 24.3 (± 7.6) years and the average weight was 163.2 (± 21.4) lb.
Table 1.
Subject Demographics/Characteristics
Pharmacokinetics
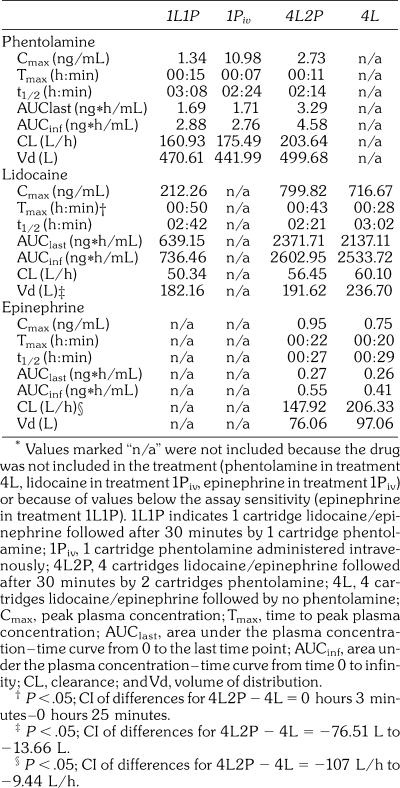
The estimated pharmacokinetic parameters for phentolamine, lidocaine, and epinephrine are summarized in Table 2. Tmax for lidocaine is calculated from time of injection, which occurred 30 minutes prior to the injection time used for phentolamine (see Figures 1 and 2).
Table 2.
Pharmacokinetic Parameters of Phentolamine, Lidocaine, and Epinephrine*
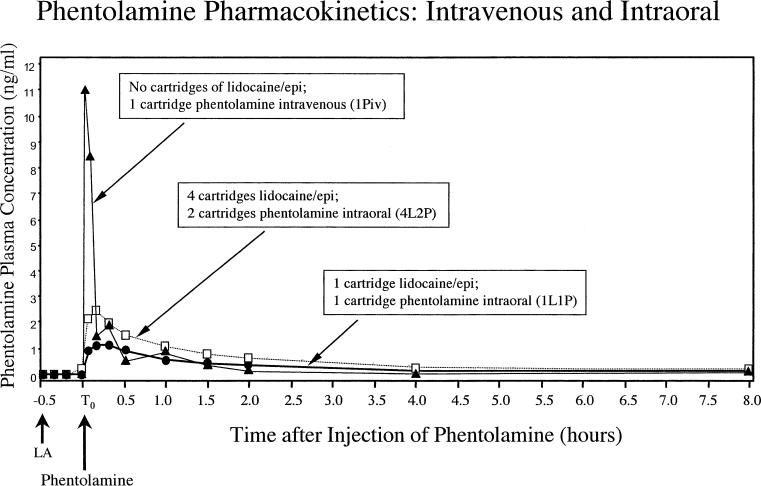
Figure 1.
The plasma concentration–time curves for phentolamine following the administration of phentolamine 0.4 mg intravenously (1Piv; closed triangle), phentolamine 0.4 mg submucosally (1L1P; closed circle), and phentolamine 0.8 mg submucosally (4L2P; open square). Lidocaine (LA) was administered 30 minutes (−0.5 hours) prior to phentolamine administration (T0 hours).
Figure 2.
The plasma concentration–time curves for lidocaine following the administration of 1 cartridge of 2% lidocaine 1 : 100,000 epinephrine followed after 30 minutes with 1 cartridge of phentolamine (1L1P; open circle); 4 cartridges of 2% lidocaine 1 : 100,000 epinephrine followed after 30 minutes with 2 cartridges of phentolamine (4L2P; open square); and 4 cartridges of 2% lidocaine 1 : 100,000 epinephrine followed by no phentolamine (4L; closed square). Lidocaine (LA) was administered 30 minutes (−0.5 hours) prior to phentolamine administration (T0 hours).
Phentolamine
1L1P, 1Piv, and 4L2P were evaluated for phentolamine pharmacokinetic parameters (phentolamine was not administered in 4L). The phentolamine Cmax values for 1L1P and 4L2P were dose-proportional (1.34 vs 2.73 ng/mL). Although the same amount was injected, the phentolamine Cmax value for the intravenous 1Piv was 8 times higher than the phentolamine value for the submucosal 1L1P (10.98 vs 1.34 ng/mL). The phentolamine AUClast and AUCinf values were dose proportional, with 1L1P and 1Piv similar in value and 4L2P approximately twice the value of 1L1P and 1Piv. As illustrated in Figure 1, the estimated Tmax for phentolamine occurred earlier for 1Piv (7 minutes) than for 1L1P (15 minutes) or 4L2P (11 minutes). The phentolamine t1/2, CL, and Vd values were similar for 1L1P, 1Piv, and 4L2P. When comparing the AUC values for 1L1P and 1Piv, phentolamine appeared to be completely bioavailable after intraoral injection (104% or 111%) when compared to intravenous injection.
Lidocaine
1L1P, 4L2P, and 4L were evaluated for lidocaine pharmacokinetic parameters. The lidocaine Cmax values were dose proportional, with similar values for 4L2P and 4L (799.82 vs 716.67 ng/mL), and a value for 1L1P (212.26 ng/mL) that were approximately one fourth the values of 4L2P and 4L. The lidocaine t1/2 and CL values were similar for 1L1P, 4L2P, and 4L. As can be noted in Figure 2, the Cmax of lidocaine was approximately 10% higher for the 4L2P than for the 4L treatments. Subsequently, the Tmax for lidocaine occurred significantly later in 4L2P than in 4L (43 vs 28 minutes; P < .05). The lidocaine Vd value was statistically significantly smaller in 4L2P than in 4L (192 vs 237 L/h; P < .05).
Epinephrine
4L2P and 4L were evaluated for epinephrine pharmacokinetic parameters. No local anesthesia was administered in 1Piv, and it was felt that the epinephrine concentrations resulting from the injections in 1L1P might be so low that they would not be discernable from endogenous epinephrine. The epinephrine Cmax, Tmax, AUClast, AUCinf, t1/2, and Vd values were all similar among the 4L2P and 4L treatment groups. The epinephrine CL for 4L2P was significantly smaller than the epinephrine CL for 4L (147.92 vs 206.33 L/h; P < .05). The decreased CL of exogenous epinephrine in 4L2P relative to 4L, although statistically significant, may not be clinically meaningful because of levels at or below detection and because CL could be estimated for only 8 of the 16 subjects.
HTAEDA
The plasma concentrations of HTAEDA were almost entirely below the limit of quantitation and therefore pharmacokinetic parameters were not estimated.
Pharmacodynamics
The sensation rating for the upper lip was evaluated for 1L1P (maxillary injection), 4L2P (both mandibular and maxillary injections), and 4L (both mandibular and maxillary injections), but not for 1Piv (intravenous phentolamine only). Only subjects who experienced numbness and/or tingling in the upper lip were evaluable for return of normal sensation in the upper lip. For treatments 1L1P, 4L2P, and 4L, the time to return of normal sensation was calculated relative to the time of phentolamine injection. For 4L, phentolamine was not administered and the time to normal sensation was calculated relative to the injection time of the local anesthetic adjusted by a constant equal to the mean time between the first injection of local anesthetic and first injection of phentolamine for 4L2P (30 minutes).
By 60 minutes after injection of phentolamine, the percentage of evaluable subjects with normal sensation in the upper lip was markedly greater with 1L1P and 4L2P than with 4L. By 90 minutes after injection of phentolamine for 4L2P, all evaluable subjects had normal sensation in the upper lip, with normal upper lip sensation maintained through the rest of the 5-hour follow-up period. After 1L1P, all evaluable subjects had normal upper lip sensation by 170 minutes after injection of phentolamine. In contrast, with 4L, not until 230 minutes adjusted time did all evaluable subjects regain normal upper lip sensation. Consistent with these findings, the median time to normal sensation of the upper lip for 4L was approximately twice as long as the median time for 1L1P or 4L2P.
The sensation rating for the tongue was evaluated for 4L2P and 4L (having received both mandibular and maxillary injections), but not for 1L1P (receiving only a maxillary injection) and 1Piv. Only subjects who experienced numbness and/or tingling in the tongue were evaluable for return of normal sensation in the tongue. All evaluable subjects regained normal tongue sensation after 4L2P by 160 minutes following dosing with phentolamine. In contrast, with 4L, at the 160-minute adjusted-time time point only approximately 25% of evaluable subjects had regained normal tongue sensation, and from 260 minutes adjusted time to the end of the 300-minute adjusted-time follow-up period, approximately 95% of evaluable subjects had regained normal tongue sensation. Consistent with these findings, the median time to normal sensation of the tongue for 4L was approximately twice as long as the median time for 4L2P.
Safety
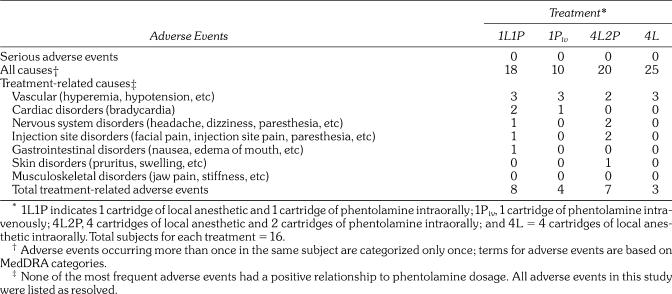
There were no serious adverse events reported and no subjects discontinued because of adverse events. Events were reported as unrelated, possibly related, probably related or related to study medications. As shown in Table 3, similar numbers of subjects reported all-causalities adverse events during each of the treatments. The largest number of all-causalities adverse events was reported after 4L (25), whereas the smallest number was reported after 1Piv (10). The largest total numbers of treatment-related adverse events were after 1L1P and 4L2P (8 and 7, respectively).
Table 3.
Incidence of Adverse Events
The most frequent adverse events are summarized in Table 3. Overall, the most frequent all-causalities adverse event was hypotension, which was defined in the protocol as systolic blood pressure <100 mm Hg. Hypotension was most frequent after 4L, in which no phentolamine was administered. Either all or a majority of the episodes of hypotension were judged to be treatment-related after 1L1P, 1Piv, and 4L2P. All episodes of hypotension were mild and asymptomatic. Other frequent adverse events included bradycardia, headache, and paresthesia (an anticipated side effect of anesthetic), for which either no or no more than half of the episodes were judged to be treatment-related. Episodes of bradycardia, defined in the protocol as a pulse <50 beats per minute, were also asymptomatic.
Discussion
This phase 1 study was designed to evaluate the pharmacokinetics of phentolamine mesylate following its administration by intraoral and intravenous injections. Additionally, the possible impact of phentolamine mesylate upon the pharmacokinetics of lidocaine with epinephrine when administered for oral local anesthesia was assessed. Because the primary outcome responses of interest were pharmacokinetic, a recruitment and enrollment of only 16 subjects was planned. Secondary outcomes relating to the clinical efficacy and safety of phentolamine's use in reversing local anesthesia were not adequately powered to be statistically meaningful. Phase 2 trials using adequately-powered research designs have been completed, and presentations of results demonstrating phentolamine's efficacy and safety have been presented and have been published in abstract format.6–8 Preparation of comprehensive manuscripts of these trials for future publication is ongoing.9–10 The clinical results presented in this report support the findings of these phase 2 studies indicating that the duration of soft tissue anesthesia following dental anesthetic injections was reduced and that there was no apparent increase in the incidence of adverse drug events associated with the use of phentolamine.
It is interesting to note that cardiovascular adverse events were reported for all treatment groups. The slightly higher incidences of adverse events relating to nervous system and injection site may relate to the rapid reversal of anesthesia and subsequent onset of slight postprocedure discomfort in the lidocaine groups receiving phentolamine (1L1P and 4L2P).
Few reports detailing the pharmacokinetics of phentolamine have been published. The manufacturer reports that phentolamine is rapidly redistributed from blood following intravenous administration and that approximately 13% of a single intravenous dose appears in the urine as unchanged drug.11 Following oral administration of phentolamine 80 mg, Goldstein reports a Cmax of 136 ng/mL, a Tmax of 0.67 hours (40 minutes) and a t1/2 of 2.76 hours.12 Our estimates following intravenous and submucosal injection support Goldstein's findings that phentolamine's t1/2 is approximately 2–3 hours.
Our estimated pharmacokinetic profile for lidocaine following dental anesthetic injection is similar to that found in previous reports.13–14 Our most notable finding is seen when comparing 4L2P and 4L, where the administration of phentolamine appears to induce a small but discernable secondary peak in the lidocaine plasma-time curve. This shift is similar to the differences in the plasma-time curves between lidocaine alone and lidocaine with epinephrine reported by Goebel et al.13 They demonstrated that the addition of epinephrine to 2% lidocaine slowed the rate of lidocaine's systemic absorption, delaying the Tmax and lowering the Cmax. Our findings suggest that the administration of phentolamine 30 minutes after anesthesia injection reversed this delay in absorption induced by epinephrine, permitting a more rapid absorption of the lidocaine from the tissue. As illustrated in Figure 2, the phentolamine-induced delay of the lidocaine Tmax in 4L2P, relative to 4L, is best interpreted as a demonstration of phentolamine's ability to accelerate the clearance of lidocaine from oral tissues into the circulatory system. This conclusion is supported when noting a similar “bump” in lidocaine's absorption following the administration of phentolamine that is seen in Figure 2 for 1L1P.
The small difference in the observed Vd for lidocaine (192 L in 4L2P and 237 L in 4L), although statistically significant, is probably not meaningful because neither Cmax nor AUC values differed significantly between these 2 treatments. The decreased CL of epinephrine in 4L2P relative to 4L may not be clinically important because the exogenous epinephrine was often at or below the level of detection and CL could be estimated for only 8 of the 16 subjects, thus possibly biasing the reported value.
Conclusion
After intraoral injection, the pharmacokinetic estimates for phentolamine indicated that its Tmax was short (11–15 minutes after injection); its Cmax, AUClast, and AUCinf were roughly dose proportional; its CL was rapid (approximately 160–200 L/h); its Vd was large (approximately 470–500 L); and its t1/2 was brief (approximately 2–3 hours). After IV injection, the pharmacokinetic estimates for phentolamine indicated that its Tmax occurred earlier (7 minutes after injection) than the values observed after intraoral injection; its Cmax was approximately 8 times that observed after intraoral injection; and its AUClast, AUCinf, CL, Vd, and t1/2 were similar to the values seen with intraoral injection. Phentolamine appears to be completely bioavailable after intraoral injection.
Most interestingly, there appeared to be a second peak in the lidocaine concentration versus time curve immediately following the administration of phentolamine. This secondary peak results in an apparent delay in lidocaine's Tmax, from a mean value of 28 minutes to a mean value of 43 minutes following the administration of phentolamine (4L vs 4L2P). This finding, also seen in the 1L1P treatment group, supports the hypothesis that phentolamine accelerates recovery from oral local anesthesia by reversing the vasoconstrictive properties of epinephrine, subsequently allowing rapid clearance of lidocaine from oral tissues into the systemic circulation.
Acknowledgments
This study was sponsored by Novalar Pharmaceuticals Inc., San Diego, Calif, and carried out through a contract to Jean Brown Research, Salt Lake City, Utah. Participants in the project included Steven E. Christensen, DMD, Principal Investigator; Georgia Blissett, RN, BSN, CCRP; Warren Mueller, RN; and Shannon Holmgren, Study Coordinator. Drs Hersh, Moore, Papas, Goodson, and Yagiela are members of the Clinical Advisory Board for Novalar Pharmaceuticals. Dr Yagiela is a paid consultant.
References
- Yagiela J.A. Local anesthetics. In: Yagiela J.A, Dowd F.J, Neidle E.A, editors. Pharmacology and Therapeutics for Dentistry. 5th ed. St. Louis, Mo: Mosby; 2004. pp. 251–270. [Google Scholar]
- Malamed S.F. Handbook of Local Anesthesia. 5th ed. St. Louis, Mo: Mosby; 2004. [Google Scholar]
- Moore P.A. Manual of Local Anesthesia in Dentistry. 4th ed. Rochester, NY: Eastman-Kodak Co; 1996. [Google Scholar]
- Hersh E.V, Hermann D.G, Lamp C.J, Johnson P.D, Macafee K.A. Assessing the duration of mandibular soft tissue anesthesia. J Am Dent Assoc. 1995;126:1531–1536. doi: 10.14219/jada.archive.1995.0082. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration Center for Drug Evaluation and Research. Approval letter for phentolamine mesylate. 1998. Available at: http://www.fda.gov/cder/foi/anda/98/40235ap.pdf. Accessed May 5, 2007.
- Laviola M, McGavin S.K, Freer G.A, et al. Phentolamine mesylate reverses soft-tissue anesthesia. J Dent Res. 2006;85(B):560. doi: 10.1177/154405910808700717. Available at: http://www.dentalresearch.org/meetings/past/index.html. Accessed September 13, 2007. [DOI] [PubMed] [Google Scholar]
- Yagiela J, Goodson J.M, Hersh E.V, Moore P.A, Papas A.S, Rutherford R.B. Reversal of soft-tissue anesthesia by an a-adrenergic blocker. Proceeding of the 85th General Meeting of the IADR; March 21–24, 2007; New Orleans, La. Available at: http://www.dentalresearch.org/meetings/past/index.html. Accessed September 13, 2007.
- Gordon S.M, Chan J, Chin J, Henegar A, et al. Accelerated reversal of soft-tissue anesthesia in children. Proceeding of the 85th General Meeting of the IADR; March 21–24, 2007; New Orleans, La. Available at: http://www.dentalresearch.org/meetings/past/index.html. Accessed September 13, 2007.
- Laviola M, McGavin S.K, Freer G.A, et al. Randomized study of phentolamine mesylate for reversal of local anesthesia. J Dent Res. Submitted. [DOI] [PubMed]
- Hersh E.V, Moore P.A, Papas A.S, et al. Reversal of soft tissue local anesthesia with phentolamine mesylate. J Am Dent Assoc. In press. [DOI] [PubMed]
- Phentolamine mesylate for injection USP [package insert] Bedford, Oh: Bedford Laboratories; 1999. [Google Scholar]
- Goldstein I. Oral phentolamine: an alpha-1, alpha-2 adrenergic antagonist for the treatment of erectile dysfunction. Int J Impot Res. 2000;12(Suppl 1):S75–S80. [PubMed] [Google Scholar]
- Goebel W.M, Allen G, Randall F. Comparative circulating serum levels of mepivacaine with levonordefrin and lidocaine with epinephrine. Anesth Prog. 1979;26:93–97. [PMC free article] [PubMed] [Google Scholar]
- Cannell H, Walters H, Beckett A.H, Saunders A. Circulating levels of lignocaine after peri-oral injections. Brist Dent J. 1975;138:87–93. doi: 10.1038/sj.bdj.4803383. [DOI] [PubMed] [Google Scholar]