Abstract
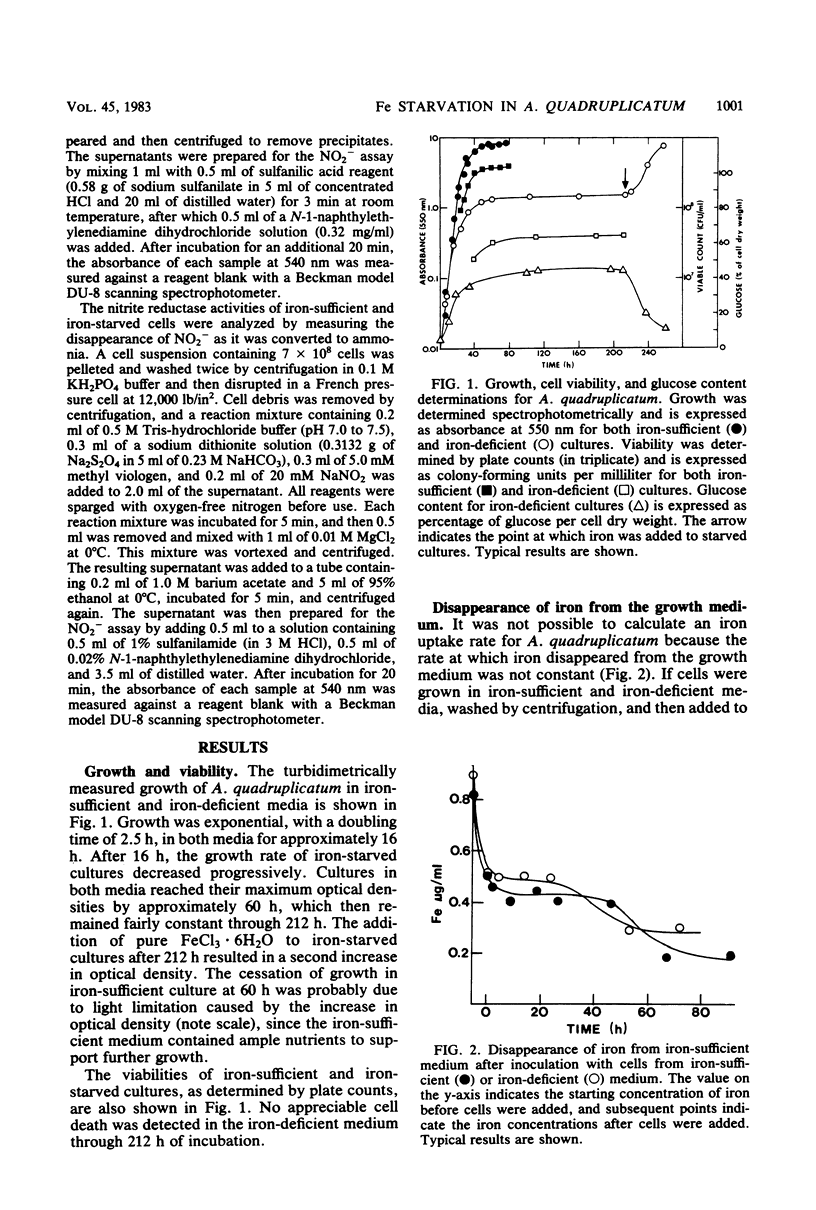
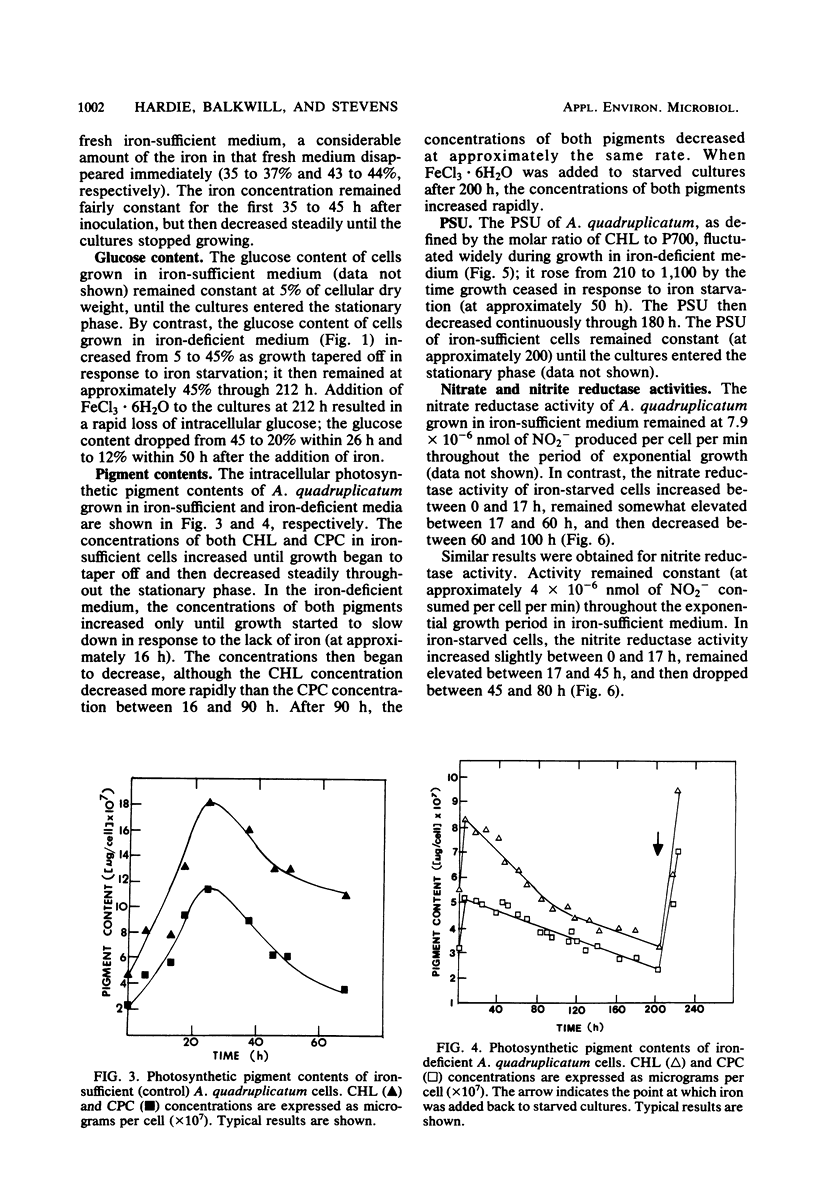
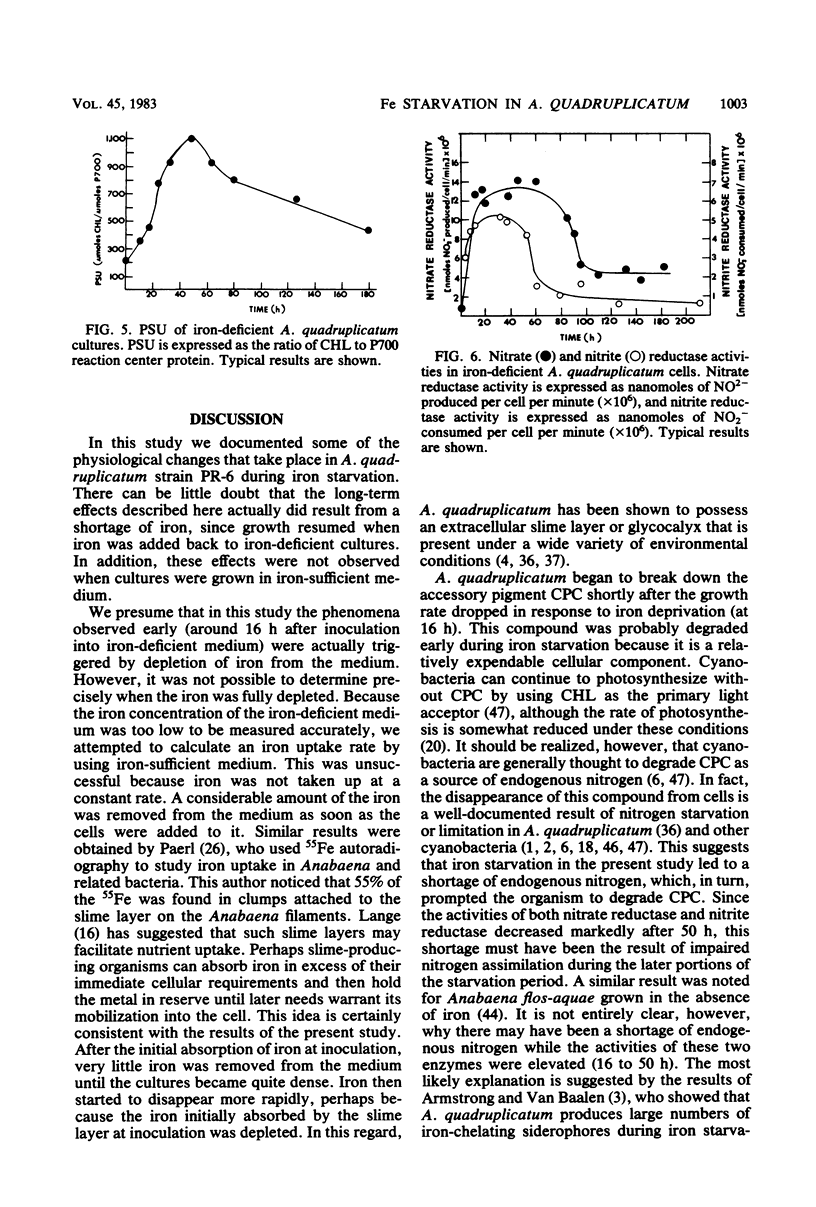
The effects of iron starvation on the growth and physiology of the unicellular cyanobacterium Agmenellum quadruplicatum were studied. Uptake of iron from the medium did not occur at a constant rate. The majority of the iron was removed at two different times, when the cells were initially inoculated into the medium and after the cultures had become quite dense and had stopped growing. Iron became limiting for growth 16 h after transfer to an iron-deficient medium, but cultures retained full viability for at least 212 h. Once iron became limiting, c-phycocyanin and chlorophyll a were degraded concurrently. This was followed by an accumulation of intracellular glucose in place of the c-phycocyanin. Nitrate and nitrite reductase activities were elevated through 50 h, after which they decreased steadily. The photosynthetic unit size also increased through 50 h and then decreased. Once iron was restored to the culture medium, growth resumed. The intracellular pigment levels increased rapidly as the glucose level diminished.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Emery T. Hydroxamic acids of natural origin. Adv Enzymol Relat Areas Mol Biol. 1971;35:135–185. doi: 10.1002/9780470122808.ch4. [DOI] [PubMed] [Google Scholar]
- Eng-Wilmot D. L., Martin D. F. Growth response of the marine blue-green alga, Gomphosphaeria aponina, to inorganic nutrients and significance to management of Florida red tide. Microbios. 1977;19(77-78):167–179. [PubMed] [Google Scholar]
- Kipe-Nolt J. A., Stevens S. E., Jr Effect of levulinic acid on pigment biosynthesis in Agmenellum quadruplicatum. J Bacteriol. 1979 Jan;137(1):146–152. doi: 10.1128/jb.137.1.146-152.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange W. Speculations on a possible essential function of the gelatinous sheath of blue-green algae. Can J Microbiol. 1976 Aug;22(8):1181–1185. doi: 10.1139/m76-171. [DOI] [PubMed] [Google Scholar]
- Lau R. H., MacKenzie M. M., Doolittle W. F. Phycocyanin synthesis and degradation in the blue-green bacterium Anacystis nidulans. J Bacteriol. 1977 Dec;132(3):771–778. doi: 10.1128/jb.132.3.771-778.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M., Wöber G. Accumulation, mobilization and turn-over of glycogen in the blue-green bacterium Anacystis nidulans. Arch Microbiol. 1976 Dec 1;111(1-2):93–97. doi: 10.1007/BF00446554. [DOI] [PubMed] [Google Scholar]
- Lemasson C., Marsac N. T., Cohen-Bazire G. Role of allophycocyanin as light-harvesting pigment in cyanobacteria. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3130–3133. doi: 10.1073/pnas.70.11.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. P., Lean D. R., Nalewajko C. Blue-green algae: their excretion of iron-selective chelators enables them to dominate other algae. Science. 1976 May 28;192(4242):900–902. doi: 10.1126/science.818707. [DOI] [PubMed] [Google Scholar]
- PROVASOLI L., MCLAUGHLIN J. J., DROOP M. R. The development of artificial media for marine algae. Arch Mikrobiol. 1957;25(4):392–428. doi: 10.1007/BF00446694. [DOI] [PubMed] [Google Scholar]
- Paerl H. W. Feasibility of Fe Autoradiography as Performed on N(2)-Fixing Anabaena spp. Populations and Associated Bacteria. Appl Environ Microbiol. 1982 Jan;43(1):210–217. doi: 10.1128/aem.43.1.210-217.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paone D. A., Stevens S. E. Nitrogen Starvation and the Regulation of Glutamine Synthetase in Agmenellum quadruplicatum. Plant Physiol. 1981 Jun;67(6):1097–1100. doi: 10.1104/pp.67.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K., Mathis P., Acker S., van Best J. A. Electron acceptors associated with P-700 in Triton solubilized photosystem I particles from spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):120–134. doi: 10.1016/0005-2728(78)90166-4. [DOI] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Dolan E., Ke B. Spectral and kinetic evidence for two early electron acceptors in photosystem I. Proc Natl Acad Sci U S A. 1979 Feb;76(2):770–773. doi: 10.1073/pnas.76.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. E., Paone D. A. Accumulation of Cyanophycin Granules as a Result of Phosphate Limitation in Agmenellum quadruplicatum. Plant Physiol. 1981 Apr;67(4):716–719. doi: 10.1104/pp.67.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalontai B., Csatorday K. Changes in phycocyanin-carotenoid association during nitrate starvation of Anacystis nidulans. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1294–1200. doi: 10.1016/0006-291x(79)91121-5. [DOI] [PubMed] [Google Scholar]
- Thornber J. P. Comparison of a chlorophyll a- protein complex isolated from a blue-green alga with chlorophyll-protein complexes obtained from green bacteria and higher plants. Biochim Biophys Acta. 1969 Feb 25;172(2):230–241. doi: 10.1016/0005-2728(69)90066-8. [DOI] [PubMed] [Google Scholar]
- Wood N. B., Haselkorn R. Control of phycobiliprotein proteolysis and heterocyst differentiation in Anabaena. J Bacteriol. 1980 Mar;141(3):1375–1385. doi: 10.1128/jb.141.3.1375-1385.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos L., Fay P. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. I. The effect of nitrogen starvation. Arch Mikrobiol. 1974 Mar 28;96(4):271–279. [PubMed] [Google Scholar]


