Abstract
Objectives. We assessed the costs, risks, and benefits of possible future major policy decisions on vaccination, surveillance, response plans, and containment following global eradication of wild polioviruses.
Methods. We developed a decision analytic model to estimate the incremental cost-effectiveness ratios and net benefits of risk management options for polio for the 20-year period and stratified the world according to income level to capture important variability between nations.
Results. For low-, lower-middle-, and upper-middle-income groups currently using oral poliovirus vaccine (OPV), we found that after successful eradication of wild polioviruses, OPV cessation would save both costs and lives when compared with continued use of OPV without supplemental immunization activities. We found cost-effectiveness ratios for switching from OPV to inactivated poliovirus vaccine to be higher (i.e., less desirable) than other health investment opportunities, depending on the actual inactivated poliovirus vaccine costs and assumptions about whether supplemental immunization activities with OPV would continue.
Conclusions. Eradication promises billions of dollars of net benefits, although global health policy leaders face difficult choices about future policies. Until successful eradication and coordination of posteradication policies, health authorities should continue routine polio vaccination and supplemental immunization activities.
Before the World Health Assembly committed to eradicating wild polioviruses (types 1, 2, and 3) in 1988,1 these viruses had been paralyzing an estimated 350 000 children per year globally.2 Policymakers anticipated that vaccination would stop following global eradication of wild polioviruses, similar to the cessation of vaccination that occurred after the eradication of smallpox.3 Anticipation of large economic savings and demonstrated successful use of the inexpensive and effective trivalent oral poliovirus vaccine (OPV) to eradicate wild polioviruses in the Americas4,5 supported this international commitment. The Global Polio Eradication Initiative to date has reduced the annual burden of disease by more than 99% to less than 2000 cases of paralysis annually6 and has achieved eradication of wild poliovirus type 2.7 Currently, wild poliovirus types 1 and 3 remain endemic in only 4 countries (Nigeria, India, Pakistan, and Afghanistan). Recent importations of wild poliovirus into previously polio-free areas8,9 demonstrate the importance of finishing the job of eradication, maintaining high levels of vaccination coverage—at least until successful eradication—and carefully considering the risks, costs, and benefits of alternatives.10
The Global Polio Eradication Initiative relies on an eradication strategy that disrupts transmission of wild poliovirus by giving susceptible individuals (mainly children) multiple doses of OPV to ensure high levels of immunity. The Global Polio Eradication Initiative uses the live OPV instead of the inactivated poliovirus vaccine (IPV) as its vaccine of choice because of OPV’s substantially lower cost and ease of administration,11 superior enteric mucosal immunity,12 and ability to enhance population immunity through spread to nonimmunized individuals.13–15 In addition to routine childhood immunization with OPV, the Global Polio Eradication Initiative conducts supplementary immunization activities (SIAs) in the form of mass vaccination campaigns.
Despite its benefits, OPV comes with the relatively rare side effect of vaccine-associated paralytic polio, which does not occur with the more costly IPV.16 Although relatively small in the context of circulating wild poliovirus, the current estimated global burden of 250 to 500 cases of vaccine-associated paralytic polio annually that would result from extended use of OPV appears large in comparison to no naturally occurring polio cases in a world free of wild poliovirus.17 Indeed, with the increasing success of eradication and lower risks of importations, despite its relatively higher cost18 IPV gradually has become the vaccine of choice for routine immunization in many high-income countries that seek to eliminate cases of vaccine-associated paralytic polio while remaining protected from polio.
Within the past decade, polio outbreaks also occurred from circulating vaccine-derived poliovirus—outbreaks in which OPV viruses regained neurovirulence and greater transmissibility as they circulated in susceptible populations.2,19 These events provided an even stronger case for coordinated OPV cessation20–24 while raising the question of whether such outbreaks might persist after OPV cessation.25
Previous studies presented cost-effectiveness analyses for the US domestic polio vaccination program at various points in time18,26 and retrospectively27 for various other countries28–30 and for global31,32 and regional eradication.33 However, no studies provide comprehensive estimates of the cost-effectiveness of the numerous posteradication risk management policy options.34
METHODS
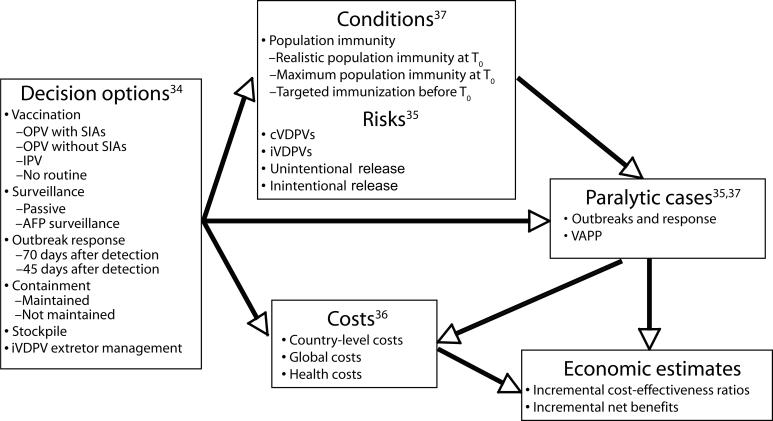
We developed a decision analytic model to estimate the incremental cost-effectiveness ratios and net benefits of risk management options for polio for the 20-year period following the expected end of routine vaccination with OPV (T0). Figure 1 ▶ describes the model components, and Table 1 ▶ summarizes the key inputs. We previously identified 7 major categories of decisions: routine immunization, SIAs, outbreak response, stockpile, surveillance, containment, and management of chronic excretors.34 We then evaluated the risks and costs associated with the options and conditions35,36 and used the results of an outbreak model37 to estimate the potential number of future cases.
FIGURE 1—
Model components leading to health and economic estimates of possible global policies for managing polioviruses.
Note. cVDPVs = circulating vaccine-derived polioviruses; IPV = inactivated poliovirus vaccine; iVDPVs = immunodeficiency-associated vaccine-derived polioviruses; OPV = oral poliovirus vaccine; SIAs = supplemental immunization activities; VAPP = vaccine-associated paralytic polio.
TABLE 1—
Base Case Values or Distributions of Inputs and Alternatives Considered in the Model Exploring Global Policies for Managing Polioviruses
| Model Input | Base Case | Notes |
| Model framework inputs | ||
| Analytic time horizon | 20 years | |
| Population projectionsa38 | Assume 2010–2029 projections reasonably represent this time period 0%, 7% also considered, all costs in 2002 US dollars, adjusted as needed using the Consumer Price Index40 | |
| Discount rate39 | 3% | |
| Policy-related inputs | ||
| Vaccination schedule | ||
| OPV, low- and middle-income groupsb | Three doses | |
| IPV, low- and middle-income groupsc | Two doses, single antigen vaccine | Three doses, combination vaccine also considered |
| IPV, high-income groupd | Three doses, combination vaccine | |
| Number of rounds per year if policy involves SIAs36 (distribution) | Triangular with base 0.67 and range 0.4–2.0 | The distribution implies a mean value of approximately one round per year |
| Population immunity profile at T035,37 | ||
| OPV with SIAs | Maximum population immunity | |
| OPV without SIAs, IPV, or no routine | Realistic population immunity | Maximum population immunity, TIAs also considered |
| Economic inputs | ||
| Costs for establishing a global monovalent OPV stockpile (distribution) | Triangular with base $325 million and range $250–$500 million | Estimate does not include costs to maintain the stockpile; implied mean global costs of $360 million (90% CI $280–$450 million) before T0 are not included in the income group totals |
| Annual costs of the global polio laboratory network36 (distribution) | Triangular with base $22 million and range $15–$30 million | Implied mean global costs of $340 million (90% CI = $270, $420 million) over 20 years are not included in the income group totals |
| Annual costs of maintaining high-level containment36 (distribution) | Triangular with base $300 000 and range $0–$1 million | Implied mean global costs of $6.7 million (90% CI = $1.9, $12 million) over 20 years are not included in the income group totals |
| Costs to achieve population immunity at T0 (if no OPV with SIAs)36 | 0 | $1.1 billion (90% CI of $0.86–$1.3 billion) for maximum population immunity and $81 million (90% CI = $61, $100 million) for TIAs also considered |
| DALYs averted per prevented paralytic polio case41–43 | ||
| Low-income group | 13.3 | Base case estimates assume 3% discount rate with no age-weighing; adjusted estimates for discount rate alternatives also considered |
| Lower-middle-income group | 13.7 | |
| Upper-middle-income group | 13.9 | |
| High-income group | 14.1 | |
| Willingness to pay to prevent a paralytic polio case41,44 | ||
| Low-income group | $5 300 | On the basis of minimal willingness to pay equal to average per capita gross national income per DALY averted45; higher estimates also considered |
| Lower-middle-income group | $17 000 | |
| Upper-middle-income group | $63 000 | |
| High-income group | $340 000 | |
| Treatment cost per paralytic polio case18,31,33,36,46,47 | ||
| Low-income group | $500 | |
| Lower-middle-income group | $5 000 | |
| Upper-middle-income group | $50 000 | |
| High-income group | $500 000 | |
| Outbreak-related inputs | ||
| Probability of circulating VDPV outbreak risk on the basis of observed frequency of35 (distribution) | ||
| Confirmed VDPV outbreaks only | 0.5 | |
| Confirmed and ambiguous VPDVs | 0.5 | |
| Time of outbreak detection37 | Onset of fifth paralytic case (passive surveillance) | Onset of first paralytic case (acute flaccid paralysis surveillance) also considered |
| Outbreak response characteristics37,48 | ||
| Delay from outbreak detection to first response round | 70 days | 45 days also considered |
| Response vaccine | Monovalent OPV | Serotype matched to outbreak virus |
| Number of rounds | 3 | |
| Coverage of each round | 90% | |
| Duration of each round | 3 days | |
| Interval between rounds | 30 days | |
| Target age groups | Cohorts born since cessation of routine vaccination | Rounded to the next multiple of 5 years (e.g., 7 years after cessation, target children younger than 10 years); if continued routine vaccination, target children younger than 5 years |
| Outbreak-specific R037 (distribution) | ||
| Low-income group | 10, 13 | Low, high point estimates shown (base case assumes equal probabilities of each; different probability distributions were also considered) |
| Lower-middle-income group | 8, 11 | |
| Upper-middle-income group | 6, 9 | |
| High-income group | 4, 6 | |
| Outbreak-specific routine immunization coverage since last SIAs49 (distribution) | ||
| Low-income group | 68%, 40%, 25% | Expected, low, and lowest point estimates shown (base case assumes probabilities of, respectively, 0.8, 0.1, and 0.1 for these; different probability distributions were also considered) |
| Lower-middle-income group | 90%, 70%, 50% | |
| Upper-middle-income group | 92%, 70%, 60% | |
| High-income group | 94%, 85%, 80% | |
| Heterogeneity in population immunity if continued SIAs | ||
| Low-income group | Medium | Represents probabilities of 0.75, 0.20, and 0.05 of, respectively, 0%, 10%, and 25% reduction in partially infectibles |
| Middle-income groups | Low | Represents probabilities of 0.9, 0.1, and 0 of, respectively, 0%, 10%, and 25% reduction in partially infectibles |
Note. OPV = oral poliovirus vaccine; IPV = inactivated poliovirus vaccine; SIAs = supplemental immunization activities; TIAs = targeted immunization activities; T0 = period following the expected end of routine vaccination with OPV; CI = confidence interval; DALY = disability-adjusted life year; VDPV = vaccine-derived poliovirus.
aData available from authors upon request.
bWe used best estimates of the OPV price of $0.09, $0.09, and $0.10 per dose in the low-, lower-middle-, and upper-middle-income groups, respectively, and modeled the uncertainty in both antigen price and administration costs using triangular distribution.36
cWe used best estimates of the IPV price of $1.00, $1.75, and $2.50 per dose in the low-, lower-middle-, and upper-middle-income groups, respectively, and modeled the uncertainty in both antigen price and administration costs using triangular distribution.36
dWe used a best estimate of the IPV price of $10.00 per dose and modeled the uncertainty in both antigen price and administration costs using triangular distribution.36
The overall probabilistic model focuses on policy at the global level but stratifies the world into 4 income groups on the basis of the 2002 World Bank classification.41 This captures previously identified important policy, cost, risk, and epidemiological differences between low-, lower-middle-, upper-middle-, and high-income countries.34–37 We estimated the incremental cost-effectiveness ratios and incremental net benefits of different options and characterized uncertainty about these estimates. (Full details are available from the authors on request.)
Policymakers face several important choices (summarized in the left box in Figure 1 ▶), including several vaccination options. The reference case assumes continued IPV routine vaccination in high-income countries and continued OPV use in the rest of the world. We considered continued use of OPV both with SIAs, which would be required to maintain high levels of coverage50 and is currently recommended,51 and without SIAs, which reflects the fact that many countries have reduced or eliminated their use of SIAs following interruption of wild poliovirus transmission. For the IPV option, we assumed that high-income groups would use a 3-dose schedule delivered in a combination vaccine, whereas all other income groups would use 2 doses in a single (trivalent) antigen vaccine formulation.52–54 We modeled the option of continued acute flaccid paralysis surveillance to detect the circulation of polioviruses in the human population (assuming this detects an outbreak at the onset of the first paralytic case)2,55–57 versus passive surveillance (assumed to detect the outbreak at onset of the fifth paralytic case).37
With actual outbreak response plans still under formulation, we modeled multiple response strategies that use monovalent OPV given current recommendations58 and assuming access to sufficient quantities of vaccine (e.g., in the global stockpile).2,48 For the base case, we assumed response with 3 rounds of monovalent OPV starting 70 days after outbreak detection, independent of the outbreak origin (wild or vaccine derived), occurring 30-days apart, lasting 3 days, achieving 90% coverage in each round, and targeting all people born since the past year of IPV or OPV vaccination (rounded to the next multiple of 5).
Given progress toward a global monovalent OPV stockpile with estimated costs of approximately $360 million,59 we modeled only this stockpile option (although some countries may build national ones60). Similarly, given progress toward containment, we assumed for the base case that actively enforcing containment will lead to global discounted costs of approximately $6.7 million (90% confidence interval [CI] = 1.9, 12 million).2,36,61–63 We modeled the risks associated with potential reintroductions of polioviruses from the few individuals with certain primary B-cell–related immunodeficiency syndromes who may continue to excrete vaccine-derived polioviruses,35,64 because the possibility of transmission exists.65 We did not include any related policy options (although future development of antivirals might someday prove helpful in treating immunodeficient individuals who continue to excrete vaccine-derived polioviruses).66
With respect to conditions at T0, policy choices associated with immunization activities just before T0 will influence global population immunity conditions. The base case assumes realistic population immunity,37 which implies no SIAs in low-income countries for 3 years before T0 or in lower-middle- or upper-middle-income countries for 5 years before T0. For high-income countries, IPV routine immunization coverage applies for all years since 1998, the approximate year that most high-income countries switched.54 We explored the implications of spending an estimated cost of $1.1 billion (90% CI = 0.86, 1.3 billion) to conduct one large round of immunization in low- and middle-income groups immediately before T0 that would yield maximum population immunity. Given insufficient current resources despite continued circulation of wild poliovirus, we also considered the impact of conducting targeted immunization activities in high-risk areas (i.e., modeled specifically as targeting the children younger than 5 years within a population of 600 million people in low-income countries and 100 million in lower-middle-income countries), for which we estimated global costs of $81 million (90% CI = 61, 100 million).
To model the risks of outbreaks, we assumed annual Poisson rates per 100 million people and independence of the rates between geographical areas and for different outbreak types (i.e., circulating vaccine-derived polioviruses, immunodeficiency-associated vaccine-derived polioviruses, unintentional releases, or intentional acts).35 We used the distribution of population sizes in the relevant year and income group to determine the outbreak population size38,67 and included uncertainty in our characterization of these risks. For example, we modeled different rates for the risk of circulating vaccine-derived polioviruses assuming an equal chance that the observed frequency of confirmed circulating vaccine-derived poliovirus outbreaks represents the true risk and that the sum of the confirmed circulating vaccine-derived polioviruses and ambiguous vaccine-derived poliovirus events represents the true risk.35
We performed 10 000 iterations to obtain distributions for the uncertain number of total cases (including vaccine-associated paralytic polio) and total costs for each permutation of policies by inputting the risks and conditions into the dynamic disease transmission submodel.37 We then estimated the economic outcomes as a function of disability-adjusted life-years (DALYs) averted assuming no age-weighing,41–43,68 societal willingness to pay on the basis of per capita gross national incomes,44 and treatment cost estimates on the basis of the very limited data that vary significantly across studies,18,31,33,36,46,47 (Table 1 ▶) with the relatively small global-level costs omitted from the income-level incremental cost-effectiveness ratios.
RESULTS
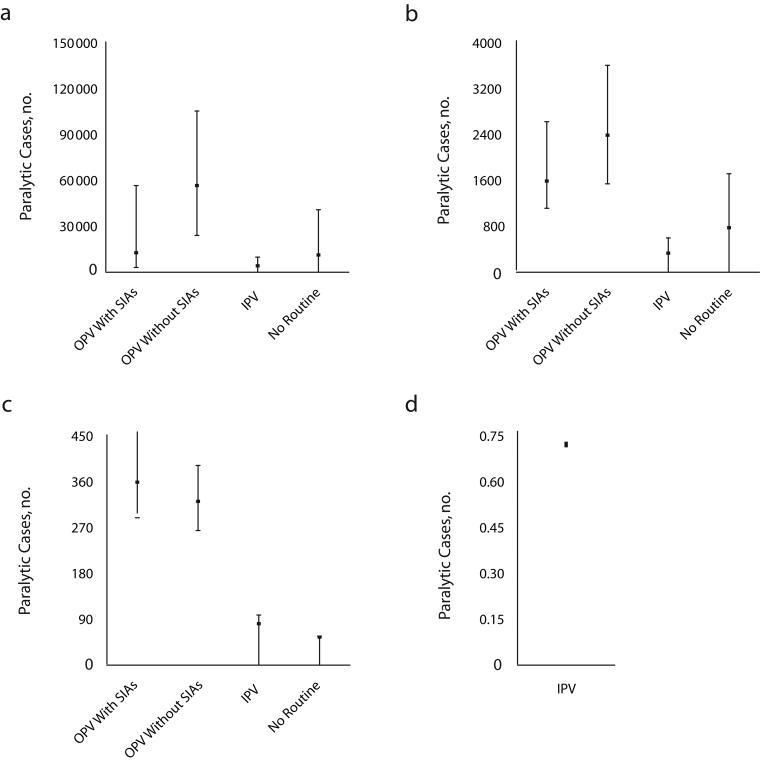
Figure 2 ▶ shows the expected costs (and 5th and 95th percentiles), including the outbreak response costs and treatment costs of paralytic cases, for the different routine vaccination options aggregated by income group over the 20 years. The results suggest costs of billions of dollars for continued vaccination with OPV or IPV in the 20-year period. For countries in the high-income group, we modeled only the option of continued IPV because we did not expect their routine vaccination preference to change. Depending on the income group, either IPV or OPV with SIAs represents the highest-cost option, and no routine consistently offers the lowest cost. Figure 3 ▶ shows the aggregate estimates of paralytic cases.
FIGURE 2—
Expected costs (mean and 95% confidence interval) for the vaccination options considered for managing polioviruses for the 20-year time horizon, by low income (a), lower-middle income (b), upper-middle income (c), and high income (d).
Note. IPV = inactivated poliovirus vaccine; OPV = oral poliovirus vaccine; SIAs = supplemental immunization activities.
FIGURE 3—
Expected paralytic cases (mean and 95% confidence interval) for the vaccination options considered for managing polioviruses for the 20-year time horizon, by low income (a), lower-middle income (b), upper-middle income (c), and high income (d).
Note. IPV = inactivated poliovirus vaccine; OPV = oral poliovirus vaccine; SIAs = supplemental immunization activities.
The numbers of expected future outbreaks differ significantly, with decreasing outbreaks expected with increasing income levels and hence fewer cases expected in higher-income level countries. Most outbreaks associated with OPV cessation result from circulating vaccine-derived polioviruses that occur during the first few years after cessation. However, continued use of OPV for routine vaccination, particularly without SIAs, leads to a relatively large number of expected outbreaks over the 20-year period. IPV and no routine tend to yield the lowest expected number of cases, although in the low-income group OPV with SIAs yields a comparable estimate to these options. The greater expected burden of OPV with SIAs compared with OPV without SIAs in the upper-middle-income group results mainly from a difference in vaccine-associated paralytic polio incidence.
As shown in Table 2 ▶, the no routine vaccination option is both cost and life saving compared with OPV (with or without SIAs), and it yields very high net benefits. Despite the generally lower number of expected paralytic cases with SIAs than without SIAs, the additional costs of the SIAs result in much greater net benefits for no routine compared with OPV with SIAs than without SIAs. The net benefit estimates probably provide policymakers with more useful information than do the cost-effectiveness ratios, and we expect that after global eradication most countries would tend toward OPV without SIAs. Table 2 ▶ also shows that the cost-effectiveness ratios represent very large numbers for the policy of switching from OPV without SIAs to IPV compared with typical values of accepted societal health interventions; and they increase with income group. Comparing IPV to OPV with SIAs, lower-middle- and upper-middle-income countries achieve both cost and life savings with 2 doses of IPV as a single antigen, depending on the actual IPV costs incurred and SIA costs saved. For low-income countries, OPV with SIAs yields fewer costs than IPV, although other IPV formulations could reduce the costs of IPV in these countries.
TABLE 2—
Expected Values of Economic Outcomes of Routine Immunization Policies (in 2002 US Dollars) by Income Group for the 20-Year Time Horizon Described in a Model Exploring Global Policies for Managing Polioviruses
| Incremental Cost Effectiveness Ratio | |||
| Policy Comparison | $/Prevented Paralytic Case | $/DALY Averted | Incremental Net Benefit, $ (Billions) |
| No routine vs OPV (without SIAs) | |||
| Low-income group | Cost and life saving | Cost and life saving | 3.0 |
| Lower-middle-income group | Cost and life saving | Cost and life saving | 1.7 |
| Upper-middle-income group | Cost and life saving | Cost and life saving | 0.8 |
| No routine vs OPV (with SIAs) | |||
| Low-income group | Cost and life saving | Cost and life saving | 4.4 |
| Lower-middle-income group | Cost and life saving | Cost and life saving | 4.3 |
| Upper-middle-income group | Cost and life saving | Cost and life saving | 3.3 |
| IPV vs OPV (without SIAs) | |||
| Low-income group | 51 000 | 3 800 | −2.4 |
| Lower-middle-income group | 1 100 000 | 80 000 | −2.2 |
| Upper-middle-income group | 6 000 000 | 440 000 | −1.4 |
| IPV vs OPV (with SIAs) | |||
| Low-income group | 120 000 | 9 000 | −1.0 |
| Lower-middle-income group | Cost and life saving | Cost and life saving | 0.3 |
| Upper-middle-income group | Cost and life saving | Cost and life saving | 1.1 |
| IPV vs no routine | |||
| Low-income group | 760 000 | 58 000 | −5.4 |
| Lower-middle-income group | 8 900 000 | 650 000 | −4.0 |
| Upper-middle-income group | Dominated | Dominated | −2.2 |
| OPV with SIA vs OPV without SIAs | |||
| Low-income group | 37 000 | 2 800 | −1.4 |
| Lower-middle-income group | 3 200 000 | 230 000 | −2.6 |
| Upper-middle-income group | Dominated | Dominated | −2.5 |
Note. DALY = disability-adjusted life-year; OPV = oral poliovirus vaccine; SIAs = supplemental immunization activities; IPV = inactivated poliovirus vaccine.
Because these results assume routine coverage with IPV at the level currently achieved with routine OPV (and no SIAs with IPV), if policymakers move toward a strategy with greater use of IPV, they will need to consider the impact of different actual levels of coverage. The results in Table 2 ▶ also show that the economic estimates do not favor a policy of initiating IPV given a comparator of no routine (i.e., after OPV cessation), and restarting SIAs if OPV continues may represent an unfavorable option after SIAs stop—except, possibly, for the low-income group, in which SIAs would prevent the most cases.
We found that opportunities to enhance population immunity before T0 may reduce the expected burden of paralytic cases by up to 50% (i.e., by achieving maximum population immunity in the low-income group before OPV cessation). However, given that these relatively small health savings in absolute numbers come at a substantial cost, we did not find favorable economic estimates for these activities. Targeted immunization activities appear somewhat more cost-effective with cost-effectiveness ratios of approximately $19 000 and $42 000 per case of paralytic polio prevented in the low-income group for future vaccination policies of no routine and routine IPV immunization, respectively. Similarly, maintaining acute flaccid paralysis surveillance yields substantial health savings (i.e., up to 75% reduction in expected paralytic cases, depending on the income group and immunization policy), but because of its additional costs, it yielded cost-effectiveness ratios of between $27 000 and over $1 million per case of paralytic polio prevented.
We performed numerous sensitivity analyses. We found that responding to an outbreak at day 45 instead of day 70 after detection provided up to 75% reduction in expected cases. We also found that poor maintenance of containment guidelines led to substantial increases in expected cases, particularly in scenarios with wide use of IPV, given the risk associated with handling large amounts of wild poliovirus for IPV production. In the upper-middle-income group, for which we assumed a much greater likelihood of domestic IPV production than for the 2 lower-income groups, we observed a 375% increase in expected cases associated with a failure to vigilantly maintain long-term containment and, consequently, a higher risk of a virus release from an IPV manufacturing site.35 By contrast, if we assume containment is maintained and the same low risk of IPV production site releases in the upper-middle-income groups as in the 2 lower-income groups, the expected burden with IPV remains lower than with no routine, changing the comparison of IPV versus no routine from “dominated” (Table 2 ▶) to merely cost-ineffective (i.e., a ratio exceeding $85 million per case of paralytic polio prevented).
DISCUSSION
Policymakers will face a number of difficult choices and trade-offs in managing the risks of polio following eradication of wild polioviruses50,69; models like this will provide constructive insights. Given the different risks and conditions, policymakers may rationally prefer and pursue different policies. For example, the no-routine option remains cost and life saving compared with OPV without SIAs in each of the lowest 3 income groups, but countries with relatively higher income and abilities to pay may prefer to switch to (or continue to use) IPV (as we assumed for the high-income group). Successful eradication must be a starting point, and before eradication, clinicians and public health leaders must keep coverage and population immunity high via routine vaccination and campaigns.
Whether the appropriate basis for comparison at T0 is OPV with SIAs or OPV without SIAs is important in the consideration of a switch to IPV for middle-income countries. The tendency of countries to stop or substantially reduce the use of OPV with SIAs following interruption of the transmission of wild polioviruses within their borders suggests that the starting point at T0 is more likely OPV without SIAs. This becomes clearer the more global pressure increases to reduce expenditures on polio and the longer it takes to achieve eradication. Because continued use of OPV with low levels of coverage creates optimal conditions for circulating vaccine-derived polioviruses, modeling the use of OPV with and without SIAs demonstrates that continued use of OPV would necessitate a sustained commitment to SIAs or to very significant improvements in routine coverage for much of the global population. Absent this, these results provide strong economic support for pursuing a policy of global OPV cessation. Along with evidence from recent outbreaks, these results provide a strong warning that countries should not stop vaccination efforts until a global agreement coordinates OPV cessation.
Although important uncertainties exist in the estimates from this study, our results remain remarkably robust.70 Nonetheless, we note a few important limitations. First, our model assumes no undetected circulation of wild polioviruses at T0, because any circulation would not be consistent with eradication and high-quality surveillance remains an essential prerequisite for OPV cessation. Second, we assume that chronic excretors of immunodeficiency-associated vaccine-derived polioviruses will pose a small risk of reintroduction within the time frame, although they represent an important and currently at best partially managed source of potential reintroduction of live polioviruses. Third, our model assumes that the global stockpile contains sufficient vaccine to cover all needs of outbreak response. Failure of the stockpile to do so would result in long response delays and high costs associated with restarting OPV production and much larger expected outbreaks.48
Fourth, the outbreak submodel37 assumes containment of outbreaks within the population of origin and does not incorporate the risk of OPV viruses used during outbreak response, resulting in new outbreaks. Given the likelihood of outbreaks in the post-OPV environment, the development of effective response plans for the post-OPV era that address these factors represents an important challenge.48 Future research using more stochastic and heterogeneous transmission models may help us better understand the risk of circulating vaccine-derived poliovirus emerging because of an outbreak response with OPV and may help us quantify the probability of continued undetected wild poliovirus after apparent eradication.71
Fifth, large uncertainty exists about future prices, and manufacturers’ forecasts of IPV prices for high-income countries proved optimistic upon validation.27 However, the attractiveness of IPV as an option depends significantly on its cost, and it offers the lowest number of expected cases. Given the importance of assumptions about cost, we explored the range of the incremental cost-effectiveness estimates for IPV versus OPV without SIAs as a function of the uncertain IPV price in upper-middle-income countries (see the figure available as a supplement the online version of this article at http://www.ajph.org). Research on IPV fractional doses, alternative administration strategies (intradermal), and attractive combination vaccines may provide a basis for altering the assumptions about IPV in the future, and policymakers can most likely best evaluate the cost-effectiveness of switching to IPV formulations in countries as the actual future IPV vaccine products and prices materialize.
Finally, the model does not address any current or future externalities associated with polio immunization services that were not previously captured in estimating costs (i.e., current cost sharing for epidemiological, laboratory transport, cold chain, surveillance, vaccinators, and other resources that are currently not exclusively maintained for polio eradication). The interaction between vaccine-related services may require that policymakers consider additional potential costs and savings because of externalities and network effects. Although some of these limitations can be addressed through further modeling efforts, they all provide additional motivation for continued research and planning.
We estimate that the highest risks of outbreaks after OPV cessation will occur immediately after cessation, when circulating vaccine-derived poliovirus risk is highest, and policymakers should plan for at least 1 outbreak to occur somewhere in the world and prepare to respond. In addition, although we expect the probability of virus introductions to decline over time, the increasing buildup of susceptible persons means that any outbreaks that occur in the future will lead to much larger numbers of expected cases. Thus, the quality of ongoing surveillance will affect our ability to rapidly detect and respond to any outbreaks and will significantly influence the size of outbreaks that may occur in the future. Efforts to sustain surveillance should address the difficult reality of maintaining recognition for a disease that we hope will no longer harm people following success of the Global Polio Eradication Initiative and implementation of coordinated and well-informed policies. Given the great economic benefits to pursuing a policy of global OPV cessation after successful eradication of wild polioviruses, global policy discussions will need to coordinate and effectively implement posteradication risk management activities. However, countries should not stop vaccination efforts until such a global agreement exists.
Acknowledgments
K.M. Thompson and R.J. Duintjer Tebbens acknowledge support for their work from the Centers for Disease Control and Prevention (grant U50/CCU300860, TS-0675).
We thank Victor Cáceres, Arnold Epstein, Denise Johnson, and Linda Venczel for providing helpful comments.
Peer Reviewed
Note. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the World Health Organization.
Contributors K. M. Thompson and R. J. Duintjer Tebbens developed the model, conducted all the modeling and analysis, wrote the first draft of the article, and edited the article. M.A. Pallansch, R.W. Sutter, R.B. Aylward, and S.L. Cochi contributed sections of writing to the article, participated in discussions related to framing the analysis and conceptualization of this effort from its inception, obtained and synthesized existing data for use in the analysis, and edited the article. O. M. Kew, M. Watkins, H. E. Gary, J. Alexander, and H. Jafari provided comments on the article and contributed to the discussion of the presentation of the results.
Human Participant Protection No protocol approval was needed for this study.
References
- 1.World Health Assembly. Global Eradication of Poliomyelitis by the Year 2000. Geneva, Switzerland: World Health Organization; 1988. Resolution 41.28.
- 2.Aylward RB, Sutter RW, Cochi SL, Thompson KM, Jafari H, Heymann DL. Risk management in a polio-free world. Risk Anal. 2006;26:1441–1448. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Report of the Interim Meeting of the Technical Consultative Group (TCG) on the Global Eradication of Poliomyelitis, Geneva, 13–14 November 2002. Geneva, Switzerland: Vaccines and Biologicals Department, World Health Organization; 2002. Report WHO/V&B/03.04.
- 4.de Quadros CA, Andrus JK, Olive JM, et al. Eradication of poliomyelitis: progress in the Americas. Pediatr Infect Dis J. 1991;10:222–229. [DOI] [PubMed] [Google Scholar]
- 5.de Quadros CA, Andrus JK, Olive JM, Carrasco P. Strategies for poliomyelitis eradication in developing countries. Public Health Rev. 1993;21:65–81. [PubMed] [Google Scholar]
- 6.World Health Organization. Polio case count. Available at: http://www.who.int/vaccines/immunization_monitoring/en/diseases/poliomyelitis/case_count.cfm. Accessed January 30, 2007.
- 7.World Health Organization. Transmission of wild poliovirus type 2—apparent global interruption. Wkly Epidemiol Rec. 2001;76:95–97. [PubMed] [Google Scholar]
- 8.World Health Organization. Outbreak news. Poliomyelitis, Namibia. Wkly Epidemiol Rec. 2006;81:238. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Resurgence of wild poliovirus type 1 transmission and consequences of importation—21 previously polio-free countries, 2002–2005. MMWR Morb Mortality Wkly Rep. 2006;55:145–150. [PubMed] [Google Scholar]
- 10.Thompson KM, Duintjer Tebbens RJ. Eradication versus control for poliomyelitis: an economic analysis. Lancet. 2007;369:1363–1371. [DOI] [PubMed] [Google Scholar]
- 11.Hull HF, Ward NA, Hull BP, Milstien J, de Quadros CA. Paralytic poliomyelitis: seasoned strategies, disappearing disease. Lancet. 1994;343:1331–1337. [DOI] [PubMed] [Google Scholar]
- 12.Onorato IM, Modlin JF, McBean MA, Thoms ML, Losonsky GA, Bernier RH. Mucosal immunity induced by enhanced-potency inactivated and oral polio vaccines. J Infect Dis. 1991;163:1–6. [DOI] [PubMed] [Google Scholar]
- 13.Fine PEM. Herd immunity: history, theory, practice. Epidemiol Rev. 1993;15:265–302. [DOI] [PubMed] [Google Scholar]
- 14.Chen RT, Hausinger S, Dajani AS, et al. Seroprevalence of antibody against poliovirus in inner-city pre-school children. J Am Med Assoc. 1996;275:1639–1645. [PubMed] [Google Scholar]
- 15.Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine—live. In: Plotkin SA, Orenstein WA, eds. Vaccines. Philadelphia, PA: W. B. Saunders; 2004:651–705.
- 16.Andrus JK, Thapa AB, Withana N, Fitzsimmons JW, Abeykoon P, Aylward RB. A new paradigm for international disease control: lessons learned from polio eradication in Southeast Asia. Am J Public Health. 2001;91:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Poliomyelitis prevention in the United States. Updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortality Wkly Rep. 2000;49:1–22. [PubMed] [Google Scholar]
- 18.Miller MA, Sutter RW, Strebel PM, Hadler SC. Cost-effectiveness of incorporating inactivated poliovirus vaccine into the routine childhood immunization schedule. J Am Med Assoc. 1996;276:967–971. [PubMed] [Google Scholar]
- 19.Kew O, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296:356–359. [DOI] [PubMed] [Google Scholar]
- 20.Technical Consultative Group of the World Health Organization on the Global Eradication of Poliomyelitis. “Endgame” issues for the global polio eradication initiative. Clin Infect Dis. 2002;34:72–77. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Conclusions and recommendations of the Ad Hoc Advisory Committee on Poliomyelitis Eradication, Geneva, 21–22 September 2004. Wkly Epidemiol Rec. 2004;79:401–408. [PubMed] [Google Scholar]
- 22.World Health Organization. Conclusions and Recommendations of the Advisory Committee on Poliomyelitis Eradication, Geneva, 11–12 October 2005. Wkly Epidemiol Rec. 2005;80:410–416. [PubMed] [Google Scholar]
- 23.World Health Organization. Polio Eradication Initiative. Cessation of Routine Oral Polio Vaccine (OPV) Use After Global Polio Eradication. Framework for National Policymakers in OPV-Using Countries. Geneva, Switzerland: World Health Organization; 2005. Report WHO/POL/05.02.
- 24.World Health Organization. Report of an Informal Consultation on the Identification and Management of Vaccine-Derived Polioviruses (VDPVs). Geneva, Switzerland: Vaccines and Biologicals Department, World Health Organization; 2004.
- 25.Fine PEM, Carneiro IAM. Transmissibility and persistence of oral poliovirus vaccine viruses: implications for the Global Poliomyelitis Eradication Initiative. Am J Epidemiol. 1999;150:1001–1021. [DOI] [PubMed] [Google Scholar]
- 26.Hinman AR, Koplan JP, Orenstein WA, Brink EW, Nkowane BM. Live or inactivated poliomyelitis vaccine: an analysis of benefits and risks. Am J Public Health. 1988;78:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson KM, Duintjer Tebbens RJ. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 2006;26:1423–1440. [DOI] [PubMed] [Google Scholar]
- 28.Tucker AW, Isaacs D, Burgess M. Cost-effectiveness of changing from live oral poliovirus vaccine to inactivated poliovirus vaccine in Australia. Aust N Z J Public Health. 2001;25:411–416. [DOI] [PubMed] [Google Scholar]
- 29.Baker C, Eberhart-Philips J. Polio vaccine: should New Zealand make the change? N Z Med J. 1997; 110:340–343. [PubMed] [Google Scholar]
- 30.Griffiths U, Botham L, Schoub BD. The cost-effectiveness of alternative polio immunization policies in South Africa. Vaccine. 2006;24:5670–5678. [DOI] [PubMed] [Google Scholar]
- 31.Bart K, Foulds J, Patriarca P. Global eradication of poliomyelitis: benefit-cost analysis. Bull World Health Organ. 1996;74:35–45. [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn MM, Ehreth J. Costs and benefits of polio eradication: a long-run global perspective. Vaccine. 2003; 21:702–705. [DOI] [PubMed] [Google Scholar]
- 33.Musgrove P. Is polio eradication in the Americas economically justified? Bull Pan Am Health Organ. 1988; 22:1–16. [PubMed] [Google Scholar]
- 34.Sangrujee N, Duintjer Tebbens RJ, Cáceres VM, Thompson KM. Policy decision options during the first 5 years following certification of polio eradication. MedGenMed. 2003;5:35. [PubMed] [Google Scholar]
- 35.Duintjer Tebbens RJ, Pallansch MA, Kew OM, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal. 2006; 26:1471–1505. [DOI] [PubMed] [Google Scholar]
- 36.Duintjer Tebbens RJ, Sangrujee N, Thompson KM. The costs of polio risk management policies after eradication. Risk Anal. 2006;26:1507–1531. [DOI] [PubMed] [Google Scholar]
- 37.Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Sutter RW, Thompson KM. A dynamic model of poliomyelitis outbreaks: learning from the past to help inform the future. Am J Epidemiol. 2005; 162:358–372. [DOI] [PubMed] [Google Scholar]
- 38.United Nations Population Division. World Population Prospects Population Database: The 2002 Revision Population Database. Available at: http://esa.un.org/unpp/index.asp?panel=2. Accessed July 31, 2003.
- 39.Gold MR, Siegel JE, Russel LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996.
- 40.Bureau of Labor Statistics, US Department of Labor. Consumer Price Index. Available at: ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt. Accessed July 6, 2006.
- 41.World Bank. World Bank list of economies (July 2002). Available at: http://www.csirwebistad.org/pdf/classi.pdf. Accessed January 23, 2008.
- 42.United Nations Population Division. World Population Prospects Population Database: The 2004 Revision Population Database. Available at: http://esa.un.org/unpp/index.asp?panel=2. Accessed May 10, 2006.
- 43.Murray CJ, Lopez AD. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Vol 1. Cambridge, MA: Harvard University Press; 1996.
- 44.World Bank. World development indicators data query. Available at: http://devdata.worldbank.org/data-query. Accessed May 8, 2006.
- 45.Hutubessy R, Chisholm D, Edejer TT-T, WHO-CHOICE. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatzandrieu EJ, Palmer CS, Halpern MT. A Cost Benefit Analysis of the OPV Vaccine. Atlanta, GA: Centers for Disease Control and Prevention; 1994.
- 47.Ministry of Health, Brazil. Memória sobre estimativa de custos dos casos de poliomielite no Brasil em 1982. Brasilia, Brazil: Ministry of Health; 1984.
- 48.Thompson KM, Duintjer Tebbens RJ, Pallansch MA. Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal. 2006; 26:1541–1556. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. Unpublished Projections: Department of Immunization Vaccines and Biologicals. Geneval, Switzerland: World Health Organization; 2004.
- 50.Henderson DA. Countering the posteradication threat of smallpox and polio. Clin Infect Dis. 2002;34:79–83. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization. Report of the seventh meeting of the Global Technical Consultative Group for Poliomyelitis Eradication. Geneva, Switzerland: Department of Vaccines and Biologicals, World Health Organization; 2002. Report WHO/V&B/02.12.
- 52.World Health Organization. Introduction of inactivated poliovirus vaccine into oral poliovirus vaccine-using countries. WHO position paper. Wkly Epidemiol Rec. 2003;78:241–250. [PubMed] [Google Scholar]
- 53.World Health Organization. Inactivated poliovirus vaccine following oral poliovirus cessation. Wkly Epidemiol Rec. 2006;81:137–144. [PubMed] [Google Scholar]
- 54.Sutter RW, Cáceres VM, Más Lago P. The role of routine immunization in the post-certification era. Bull World Health Organ. 2004;82:31–38. [PMC free article] [PubMed] [Google Scholar]
- 55.Hull BP, Dowdle WR. Poliovirus surveillance: building the global Polio Laboratory Network. J Infect Dis. 1997;175:S113–S116. [DOI] [PubMed] [Google Scholar]
- 56.Pinheiro FP, Kew OM, Hatch MH, da Silveira CM, de Quadros CA. Eradication of wild poliovirus from the Americas: wild poliovirus surveillance—laboratory issues. J Infect Dis. 1997;175:S43–S49. [DOI] [PubMed] [Google Scholar]
- 57.de Gourville EM, Sangrujee N, Duintjer Tebbens RJ, Pallansch MA, Thompson KM. Global surveillance and the value of information: the case of the global polio laboratory network. Risk Anal. 2006;26:1557–1569. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. Advisory committee on polio eradication—standing recommendations for responding to circulating polioviruses in polio-free areas. Wkly Epidemiol Rec. 2005;23:330–331. [PubMed] [Google Scholar]
- 59.Global Alliance for Vaccines and Immunization. Investment Case for the Polio Stockpile. Available at: http://www.vaccinealliance.org/resources/16brd_07._Polio_Stockpile_investment_case.pdf. Accessed January 7, 2006.
- 60.Jenkins PC, Modlin JF. Decision analysis in planning for a polio outbreak in the United States. Pediatrics. 2006;118:611–618. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization. Global Polio Eradication Initiative: Strategic Plan 2004–2008. Geneva, Switzerland: World Health Organization; 2004.
- 62.World Health Organization. WHO Global Action Plan for Laboratory Containment of Wild Polioviruses. 2nd ed. Geneva, Switzerland: World Health Organization; 2004. Report WHO/V&B/03.11.
- 63.World Health Organization. WHO Global Action Plan to Minimize Poliovirus Facility-Associated Risk in the Post Eradication/OPV Era. Geneva, Switzerland: World Health Organization. In press.
- 64.Kew OM, Wright PF, Agol VI, et al. Circulating vaccine-derived polioviruses: current state of knowledge. Bull World Health Organ. 2004;82:16–23. [PMC free article] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention. Poliovirus infections in four unvaccinated children—Minnesota, August–October 2005. MMWR Morb Mortality Wkly Rep. 2005;54:1–3. [PubMed] [Google Scholar]
- 66.National Research Council. Exploring the Role of Antiviral Drugs in the Eradication of Polio: Workshop Report. Washington, DC: National Academies Press; 2006.
- 67.United Nations Children’s Fund. The State of the World’s Children 2003. Available at: http://www.unicef.org/sowc03. Accessed January 23, 2008.
- 68.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Oxford, England: Oxford University Press; 2006.
- 69.Aylward RB, Sutter RW, Heymann DL. OPV cessation—the final step to a “polio-free” world. Science. 2005;310:625–626. [DOI] [PubMed] [Google Scholar]
- 70.Duintjer Tebbens RJ, Pallansch MA, Kew OM, et al. Uncertainty and sensitivity analyses of a decision analytic model for post-eradication polio risk management. Risk Anal. 2008. In press. [DOI] [PubMed]
- 71.Eichner M, Dietz K. Eradication of poliomyelitis: when can one be sure that polio virus transmission has been terminated? Am J Epidemiol. 1996;143:816–822. [DOI] [PubMed] [Google Scholar]