Abstract
Objectives. We tested whether social integration protects against memory loss and other cognitive disorders in late life in a nationally representative US sample of elderly adults, whether effects were stronger among disadvantaged individuals, and whether earlier cognitive losses explained the association (reverse causation).
Methods. Using data from the Health and Retirement Study (N = 16 638), we examined whether social integration predicted memory change over 6 years. Memory was measured by immediate and delayed recall of a 10-word list. Social integration was assessed by marital status, volunteer activity, and frequency of contact with children, parents, and neighbors. We examined growth-curve models for the whole sample and within subgroups.
Results. The mean memory score declined from 11.0 in 1998 to 10.0 in 2004. Higher baseline social integration predicted slower memory decline in fully adjusted models (P<.01). Memory among the least integrated declined at twice the rate as among the most integrated. This association was largest for respondents with fewer than 12 years of education. There was no evidence of reverse causation.
Conclusions. Our study provides evidence that social integration delays memory loss among elderly Americans. Future research should focus on identifying the specific aspects of social integration most important for preserving memory.
Memory loss is a prominent feature of aging and is associated with substantial declines in quality of life and increased risk of dementia,1,2 institutionalization,3 and mortality.4 Few effective strategies for prevention or treatment have been identified.5 Several studies have suggested that features of the social environment are important predictors of cognitive outcomes among the elderly (those aged 50 and older; for a review, see Fratiglioni et al.6). For example, Fratiglioni et al. reported that limited social networks were associated with increased risk of incident dementia,7 Barnes et al. found that high social networks and high social engagement reduced the rate of cognitive decline,8 Bassuk et al. reported that individuals with many social ties were at lower risk of incident cognitive decline than were individuals with few social ties,9 and results from Zunzunegui et al. indicated that elderly men and women with few social ties, poor social integration, and social disengagement were at greater risk of cognitive decline.10 These strong studies clearly indicate the importance of social relations in shaping cognitive decline.
We examined the association between social integration and memory loss in a large, representative sample of US residents born before 1948. We explored 3 related issues: whether the associations found in previous studies would be generalizable to a nationally representative sample of elderly in the United States, whether the effects would be stronger among individuals in socially and economically disadvantaged conditions, and whether the results could be attributable to reverse causation. Although it has limited clinical data, the Health and Retirement Study (HRS) provided an excellent opportunity to address these 3 issues.
METHODS
Study Population
The HRS is a longitudinal, biennial interview survey of a nationally representative cohort of US adults 50 years and older. Details regarding HRS sampling and interview methods have been published elsewhere.11,12 Briefly, enrollment was based on a multistage area probability sample of households, with supplemental sampling from a Medicare list for those born in 1914 or earlier. Communities with high fractions of Black or Hispanic residents were oversampled. Enrollment occurred in 3 waves (1992, 1993, and 1998), depending on birth year and spouse’s birth year. Most interviews were conducted by telephone. Our analyses were restricted to noninstitutionalized individuals interviewed in 1998, the first year with adequate social integration measurements. Respondents were followed for up to 6 years (4 assessments), through the 2004 interview. All data were based on self-report or direct assessment.
For our study, we used information on social integration, sociodemographic characteristics, and health conditions gathered in 1998 and memory scores assessed in 1998, 2000, 2002, and 2004. Of 20325 eligible respondents, 18733 (92.2%) provided at least 1 memory score during the follow-up period and 11571 (56.9%) provided memory scores at all 4 assessments. We excluded respondents for whom data on covariates were missing (n = 542) or who scored below the 10th percentile of memory scores at baseline (n = 1553), for a final sample of 16638. We excluded respondents with the lowest memory scores to avoid bias caused by floor effects on the memory score. Respondents excluded because of low memory scores were significantly (P < .01) different from the rest of the study sample for all covariates; for all health measures, the excluded had statistically significant worse baseline scores.
Assessment of Memory
The interviewers read a list of 10 common nouns to the respondents. Immediately afterward, the respondents were asked to recall as many words as possible. After a 5-minute delay during which other (unrelated) questions were asked, the interviewers asked the respondents to recall the words again. Details regarding the development, implementation, and validation of the recall test are available elsewhere.12 The sum of words correctly remembered in the immediate and delayed recall tasks made up the memory score used in our analysis. There are several alternative methods of calculating a memory score from the recall test administered in the HRS, including using the immediate recall score alone, using the delayed recall score alone, or creating a savings score (delayed recall score divided by immediate recall score). We examined the use of each of these alternative methods, and the results were consistent with the results obtained by using the sum of immediate and delayed recall. The sum score, however, showed the best construct validity, and results from the sum score were conservative; thus, we present the results from the sum score only.
Although impaired memory score is not a clinical diagnosis, a substantial body of research shows its importance as a health outcome and a potential early warning sign of more severe cognitive impairment.13,14 In the HRS sample, memory score predicted subsequent risk of self-reported memory-related disease, institutionalization, and mortality (results available from the authors). The HRS interviews included additional cognitive measures, notably the Telephone Interview for Cognitive Status (TICS). We focused only on the memory measure because the TICS was not assessed for all age-eligible respondents; additionally, the TICS was unlikely to be sensitive to early cognitive losses because of the restricted range (0–13) of scores, which resulted in a large fraction of respondents at each wave obtaining the highest score.
Assessment of Social Integration
We assessed baseline social integration across 5 domains of social activity: marital status, volunteer activities, and contact with parents, children, and neighbors. We set specific criteria for integration in each domain and assigned 1 point for each domain in which respondents were integrated. If respondents were missing data for a domain, their integration score for that domain was set to missing. The sum of nonmissing values for all domains was the individual’s social integration score (range = 0–5). If respondents were missing all domains, their social integration score was set to missing.
Currently married respondents received 1 point for marital status; all others (currently separated, divorced, widowed, or never married) received 0 points. Respondents were asked if they spent any time volunteering for religious, educational, health-related, or other charitable organizations. Respondents who volunteered at least 1 hour in the past year received 1 point in this domain; respondents who did not volunteer any hours received 0 points. Respondents were considered to have contact with parents if they had weekly or more frequent contact (by phone, mail, or in person) with any parent (including mother, father, mother-in-law, or father-in-law). Respondents with no living parents were coded as missing this domain. Contact with children was dichotomized on the basis of whether the participant or the participant’s spouse had contact (by phone, in person, or by mail) with offspring (including children-in-law and stepchildren) once a week or more frequently. Unfortunately, for married participants, both spouses’ contact with children was assessed with a single combined question. Respondents without living children were coded as missing this domain. Contact with neighbors was based on whether the respondents reported getting together with neighbors just to chat or for social visits weekly or more frequently.
In initial analyses, for simplicity of display, we compared respondents with the highest quartile of integration with all others. In subsequent analyses, we modeled social integration as a continuous variable.
Assessment of Covariates
The following information about health was measured at baseline: prevalent health conditions (self-reported presence of high blood pressure, diabetes, cancer, lung disease, heart disease, stroke, psychiatric problems, and arthritis), mobility, large muscle index (difficulty sitting for 2 hours, getting up from a chair, stooping or kneeling or crouching, and pushing or pulling a large object), limitations on basic activities of daily living (bathing, eating, dressing, walking across a room, and getting in and out of bed), fine motor skills, instrumental activities of daily living (using a telephone, taking medication, handling money, shopping, and preparing meals), and depressive symptoms (measured with a modified 7-item Center for Epidemiological Studies Depression Scale). Presence of vascular disease was defined by the self-reported presence of at least 1 of the following conditions: diabetes, hypertension, and stroke.
Age, gender, race (White, Black, other), years of completed schooling (range = 0–17) household income, and household wealth were assessed at baseline. Household income and wealth were adjusted for household size and natural log transformed to bring in the right tail of the distribution. Coding from the Research and Development Corporation HRS data set15 was used for income, wealth, and physical health variables.
Statistical Analysis
We used linear growth-curve models to test the hypothesis that individuals with higher baseline social integration would experience a slower rate of decline in memory scores during the follow-up period. Growth-curve models allowed us to examine the trajectory of memory scores by level of baseline social integration, average rate of memory change over time, and differences in rate of change by level of social integration (i.e., the interaction of social integration with time). We used linear modeling to aid in the interpretability of results.
Covariates were added to the model in 3 stages, with only those significant at the α < .05 level remaining in the model. The first stage included core sociodemographic covariates. The second stage added adjustment for baseline health characteristics (previous research has indicated that social integration affects physical health, so we assessed the association of social integration and memory change with and without simultaneous adjustment for physical health). The third stage added interaction terms between time and each covariate that predicted baseline memory score. Interaction terms were retained in the final model only if they were significant.
Stratified models were examined for subgroups defined by gender, age (younger than 65 years or 65 years or older), race (Black or White), years of education (less than 12 vs 12 or more), and presence of at least 1 of 3 vascular disorders (diabetes, hypertension, and stroke) that are risk factors for memory-related diseases.16,17 All covariates selected for the final population-wide model were included in stratified models.
To examine the possibility that memory loss before baseline confounded the association between social integration and subsequent memory decline, we performed additional analyses on 2 subsamples. For 1 subsample, we excluded respondents who were most likely to have already experienced memory loss: respondents in the lowest 25th percentile of baseline memory score. For the second subsample, we focused on respondents who participated in memory assessments in 1993 (n = 3762), 5 years before our social integration assessment. In this subsample, we tested whether 1993 memory scores predicted 1998 social integration.
To account for the complex sampling design of the HRS, we used 1998 sample weights and clustering variables. We present 95% confidence intervals (CIs) for hypothesis tests. All analyses were conducted with SAS 9.0 (SAS Institute Inc, Cary, NC). The PROC MIXED procedure was used for growth-curve models.
RESULTS
There was substantial heterogeneity in the extent of social integration in our study sample. Nearly one half of the sample reported contacts in 3 or more domains, whereas more than 20% of the sample reported contacts in 0 or 1 domain. The baseline (1998) characteristics of the respondents and their memory scores at each assessment by level of baseline social integration are shown in Table 1 ▶. Respondents with high social integration were significantly (P<.01) different from respondents with low social integration for every covariate examined: they were younger, were more likely to be male and White, were more highly educated, were healthier, and had better memory scores at each assessment. Respondents in this sample were aged 51 to 99 years at baseline.
TABLE 1—
Sample Characteristics Among Participants, by Social Integration Quartile: Health and Retirement Study, United States, 1998
| All Participants (N = 16 638) | Participants With High Social Integration (Highest Quartile) | Participants With Low Social Integration (Lowest 3 Quartiles) | ||||
| Mean (SE) or % | No. | Mean (SE) or % | No. | Mean (SE) or % | No. | |
| Age, y | 64.5 (0.08) | 16 638 | 59.3 (0.11) | 3496 | 65.9 (0.09) | 13 142 |
| Women | 57.6 | 16 638 | 50.2 | 3496 | 59.7 | 13 142 |
| Education, y | 12.6 (0.02) | 16 638 | 13.4 (0.04) | 3496 | 12.3 (0.03) | 13 142 |
| White | 88.4 | 16 638 | 91.4 | 3496 | 87.5 | 13 142 |
| CES-D score | 1.5 (0.01) | 16 638 | 1.1 (0.03) | 3496 | 1.6 (0.02) | 13 142 |
| Health conditions | 1.5 (0.01) | 16 638 | 1.2 (0.02) | 3496 | 1.6 (0.01) | 13 142 |
| Memory score at each wave | ||||||
| 1998 | 11.0 (0.03) | 16 638 | 11.9 (0.05) | 3496 | 10.7 (0.03) | 13 142 |
| 2000 | 10.4 (0.03) | 14 614 | 11.3 (0.06) | 3226 | 10.1 (0.03) | 11 388 |
| 2002 | 10.2 (0.03) | 13 341 | 11.2 (0.06) | 3053 | 9.9 (0.04) | 10 288 |
| 2004 | 10.0 (0.03) | 12 335 | 10.9 (0.06) | 2914 | 9.7 (0.04) | 9421 |
Note. CES-D = Center for Epidemiological Studies Depression Scale. Means, standard errors, and percentage values were weighted to account for the complex sampling design of the Health and Retirement Study. All differences between participants with high and low social integration were statistically significant (P < .01).
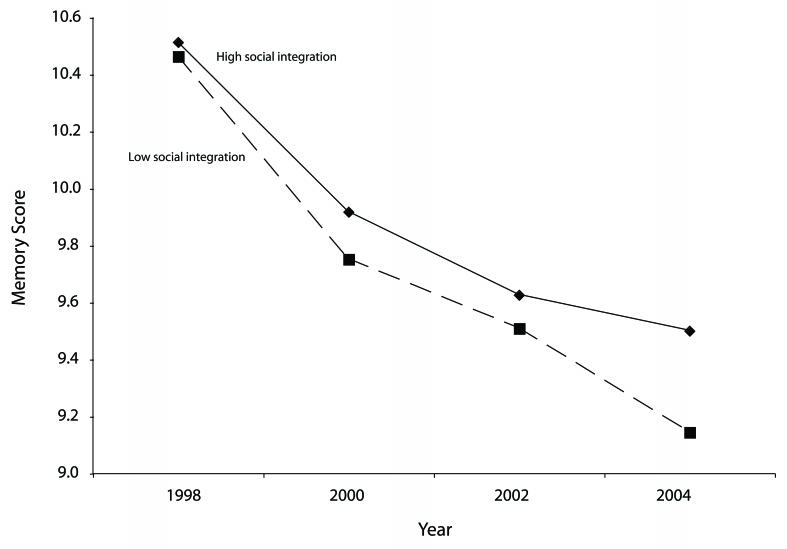
We first examined how memory scores changed over the 6-year follow-up period by comparing people with high versus low social integration and adjusting for baseline sociodemographic and health variables (Figure 1 ▶). Respondents with high social integration and low social integration had similar memory scores at baseline (1998) but diverged over successive assessments. Compared with respondents with low social integration, respondents with high social integration in 1998 had slower rates of memory decline over time. When we allowed a flexible (i.e., nonlinear) characterization of change over time, the decline in memory scores was nearly linear. To aid in interpretation, we treated time as a linear variable in subsequent models.
FIGURE 1—
Flexible growth-curve models showing predicted change in memory scores across 6 years of follow-up, by level of social integration at baseline: Health and Retirement Study, United States, 1998–2004
Note. Models adjusted for age at baseline; age squared; sex; wealth; income; race; education; Center for Epidemiological Studies Depression Scale; health conditions; mobility; large muscle index; activities of daily living; instrumental activities of daily living; time indicator variables for 2000,2002, and 2004; and interaction terms between each time variable and level of social integration.
The growth-curve models of the relation between baseline social integration and memory change over 6 years are shown in Table 2 ▶. In model 1, memory score declined an average of 0.32 (95% CI = −0.34, −0.30) points per year. Respondents with higher baseline social integration had slower rates of decline in memory: the average rate of decline was 0.04 (95% CI = 0.03, 0.05) points faster for each decrease in number of domains of social integration. The results were largely unchanged after adjustment for health status (model 2). In the final model (model 3), which was additionally adjusted for significant predictors of change in memory over time, memory score declined an average of 0.29 (95% CI = −0.32, −0.26) points per year. On the basis of the final model, we predicted that in 1 year, individuals with the lowest social integration (integration = 0) would have, on average, a 0.29-point decline in memory score per year, compared with a 0.14-point annual decline for individuals with the highest social integration (integration = 5). Thus, memory decline among the most integrated was less than half the rate of the least integrated. To assess whether these results primarily reflected the effect of one particular component of integration, we repeated these analyses excluding each item of the social integration measure one by one. With each version of the social integration score, individuals with higher social integration had slower rates of memory decline and the significance of social integration remained largely unchanged.
TABLE 2—
Estimates of Memory Decline For Time (In Years), Baseline Social Integration, and the Interaction Between Time and Social Integration: Health and Retirement Study, United States, 1998–2004
| Estimate (95% CI) | P | |
| Model 1 | ||
| Time | –0.32 (−0.34, −0.30) | <.01 |
| Social integration | 0.01 (–0.03, 0.05) | .74 |
| Time × social integration | 0.04 (0.03, 0.05) | <.01 |
| Model 2 | ||
| Time | –0.33 (−0.35, −0.31) | <.01 |
| Social integration | –0.02 (–0.06, 0.02) | .35 |
| Time × social integration | 0.04 (0.03, 0.05) | <.01 |
| Model 3 | ||
| Time | –0.29 (−0.32, −0.26) | <.01 |
| Social integration | 0.00 (–0.04, 0.04) | .84 |
| Time × social integration | 0.03 (0.02, 0.04) | <.01 |
Note. CI = confidence interval. Model 1 was adjusted for sociodemographic factors (age at baseline, age squared, gender, wealth, income, race, and education). Model 2 was adjusted for sociodemographic factors and baseline health conditions (depressive symptoms, health conditions, mobility, large muscle index, basic activities of daily living [bathing, eating, dressing, walking across a room, and getting in and out of bed], and instrumental activities of daily living [using a telephone, taking medication, handling money, shopping, and preparing meals]). Model 3 was adjusted for sociodemographic factors, baseline health conditions, and the following interaction terms: education × time and health conditions × time.
We then examined growth-curve models in subgroups to explore whether the association between social integration and memory differed by gender, age, race, education, or presence of a vascular disorder (Table 3 ▶). In analyses stratified by gender, education, and vascular disorder, the results were similar to those for the full study sample: social integration was protective against memory decline. The results of models stratified by age and race were slightly different. Similar to the full study sample, in the subgroup of respondents 65 years and older and in the subgroup of White respondents, social integration was protective against memory decline. Among those younger than 65 years, however, social integration was associated with memory score at baseline but not with memory decline over time. Among Blacks, the interaction of time and social integration was not significant, although the wide confidence intervals suggested that this may have been because of reduced statistical power in this relatively small subgroup.
TABLE 3—
Estimates of Memory Decline in Stratified Models For Time (In Years), Baseline Social Integration, and the Interaction Between Time and Social Integration: Health and Retirement Study, United States, 1998–2004
| No. | Unstandardized Parameter Estimate (95% CI) | P | |
| Gender | |||
| Men | 6 774 | ||
| Time | –0.28 (−0.32, −0.24) | <.01 | |
| Social integration | –0.01 (–0.07, 0.05) | .69 | |
| Time × social integration | 0.03 (0.02, 0.04) | <.01 | |
| Women | 9 864 | ||
| Time | –0.29 (−0.33, −0.25) | <.01 | |
| Social integration | 0.00 (–0.05, 0.05) | .96 | |
| Time × social integration | 0.03 (0.02, 0.04) | <.01 | |
| Age | |||
| Younger than 65 y | 8 320 | ||
| Time | –0.16 (−0.20, −0.12) | <.01 | |
| Social integration | 0.07 (0.01, 0.13) | .02 | |
| Time × social integration | 0.00 (–0.01, 0.01) | .89 | |
| 65 y or older | 8 318 | ||
| Time | –0.38 (−0.42, −0.34) | <.01 | |
| Social integration | –0.04 (–0.10, 0.02) | .22 | |
| Time × social integration | 0.04 (0.03, 0.05) | <.01 | |
| Race | |||
| White | 13 967 | ||
| Time | –0.29 (−0.32, −0.26) | <.01 | |
| Social integration | –0.01 (–0.05, 0.03) | .77 | |
| Time × social integration | 0.03 (0.02, 0.04) | <.01 | |
| Black | 2 133 | ||
| Time | –0.24 (−0.32, −0.16) | <.01 | |
| Social integration | 0.03 (–0.08, 0.14) | .55 | |
| Time × social integration | 0.02 (–0.01, 0.05) | .12 | |
| Education | |||
| 12 y or more | 12 347 | ||
| Time | –0.26 (−0.29, −0.23) | <.01 | |
| Social integration | 0.01 (–0.04, 0.06) | .73 | |
| Time × social integration | 0.03 (0.02, 0.04) | .02 | |
| Fewer than 12 y | 4 291 | ||
| Time | –0.36 (−0.41, −0.31) | <.01 | |
| Social integration | –0.05 (–0.13, 0.03) | .23 | |
| Time × social integration | 0.05 (0.03, 0.07) | <.01 | |
| Vascular disordera | |||
| No | 8 039 | ||
| Time | –0.27(−0.31, −0.23) | <.01 | |
| Social integration | 0.00 (–0.06, 0.06) | .98 | |
| Time × social integration | 0.02 (0.01, 0.03) | <.01 | |
| Yes | 8 599 | ||
| Time | –0.31 (−0.35, −0.27) | <.01 | |
| Social integration | 0.02 (–0.04, 0.08) | .52 | |
| Time × social integration | 0.04 (0.03, 0.05) | <.01 | |
Note. CI = confidence interval. Models were adjusted for age at baseline, age squared, gender, wealth, income, race, education, depressive symptoms, health conditions, mobility, large muscle index, basic activities of daily living [bathing, eating, dressing, walking across a room, and getting in and out of bed], instrumental activities of daily living [using a telephone, taking medication, handling money, shopping, and preparing meals], and interactions between time and education and health conditions. Models within gender, age, and education were not controlled for clustering within households.
aThis was defined as the presence of at least 1 of the following: diabetes, hypertension, or stroke.
Education-stratified models suggested that social integration may be particularly important for individuals with fewer than 12 years of education (interaction of time and social integration: unstandardized parameter estimate [B]=0.05; 95% CI=0.03, 0.07). Respondents with low education showed a particularly precipitous decline as time progressed (B= −0.36; 95% CI=−0.41, −0.31), averaging a decrease in memory score of 1 point every 3 years. Finally, results stratified by the presence of a vascular disorder showed that among individuals with strong risk factors for memory-related disease, social integration may provide an important buffer against memory decline (interaction of time and social integration: B=0.04; 95% CI=0.03, 0.05).
These results suggest that low social integration puts people at risk of accelerated memory loss, but it is also possible that our results were attributable to reverse causation (i.e., poor memory or memory decline causing social withdrawal).18 This could bias our results only if prior memory losses predicted both low social integration and accelerated future memory loss. To test this possibility, we repeated our analyses by excluding respondents below the 25th percentile of memory score in 1998, who may have already experienced some memory loss. The results in this restricted sample were similar to the results in the whole sample (interaction of time and social integration: B=0.03; 95% CI = 0.02, 0.03). This suggests that declines in cognitive function before baseline were unlikely to explain the observed longitudinal association between 1998 social integration and memory decline over 6 years of follow-up.
As a further test of reverse causation, we examined the association between memory score in 1993 and social integration in 1998 (because of the staggered enrollment in the HRS, it was possible to conduct this test in only a subsample of oldest respondents). After adjustment for sociodemographic characteristics and measures of health in 1993, memory score in 1993 explained less than 1% of the variability in social integration in 1998 (B=0.01; 95% CI = 0.00, 0.02).
DISCUSSION
We found that high levels of social integration predicted a slower rate of memory decline in a nationally representative sample of US residents 50 years and older who were followed prospectively for 6 years. The association between higher social integration and reduced memory decline was consistent in most subgroups and was largest among respondents with fewer than 12 years of education and with vascular conditions. Being in the highest level of social integration ameliorated more than half of the age-related decline in memory. There is evidence that recency and speed of cognitive decline are more potent predictors of mortality than is stable but low cognitive function,19–21 thus making changes in the rate of decline particularly salient.
The major limitations of this study included the nonrandomized study design and limitations in the measures of social integration and health. Our measure of social integration did not include all possible types of social connections. By using the sum of integration in multiple domains, we believe we obtained an overall indicator of the level of integration. This measure, however, did not contain information about the quality of these contacts; the HRS did not assess quality of relationships during this time frame. At least 1 study has indicated that emotional support, rather than contacts, is important for cognitive outcomes.22 Data on health conditions were self-reported, and therefore it is possible that unmeasured health experiences may have affected levels of memory and integration. We addressed this possibility by excluding respondents at baseline who had poor memory scores, but further studies with more-intensive data collection on health status will have to explore this possibility. Additionally, although we controlled for activities of daily living and instrumental activities of daily living in our analyses, the HRS did not have physical or cognitive activity data that would have allowed us to further control for activities that may be related to cognitive function and decline.
Our findings are consistent with prior studies based in Chicago, Illinois,8 New Haven, Connecticut,9 Honolulu, Hawaii,23 urban Sweden,7 and suburban Spain,10 all of which reported that social engagement or social network structure predicted reduced rate of cognitive decline or lower risk of incident dementia.
Our study overcame some of the important challenges in previous research on social integration and cognitive decline. The HRS is the largest longitudinal, nationally representative study of the US elderly population; the large sample allowed informative assessment of effects of social integration across population subgroups. Additionally, because the HRS conducted memory assessments before 1998, we were able to provide evidence that reverse causation was an unlikely explanation for our results.
Social integration may help to preserve memory through several mechanisms. One possible mechanism is physical health: research strongly implicates vascular conditions such as diabetes, unmanaged hypertension, and stroke in the etiology of dementia.16,17 Social integration may reduce the onset of such conditions and help to ameliorate their consequences through direct neurohormonal pathways and behavioral modifications.24 Social ties may create pressure, either through explicit reminders or implicit behavioral norms, to take care of oneself, for example, by careful management of chronic conditions.25 Another possible mechanism is through cognitive aspects of social interactions: by presenting complex cognitive and memory challenges, social interactions may enhance cognitive reserve,26 improve compensation in response to neurophysiologic decline,18 and increase resilience after neuronal injury.27 Finally, contacts with friends and loved ones may provide a greater sense of purpose and emotional validation that has direct neurohormonal benefits.6 In our study, we had limited capacity to understand which of these pathways was most important.
Memory loss is a strong risk factor for and hallmark of dementia, which is a syndrome estimated to affect up to 10% of the US population aged 65 years and older.28 Memory loss and dementia pose a tremendous public health and clinical burden in elderly populations, and with the aging of the US population, this burden is expected to increase substantially.13 Interventions to prevent or treat these outcomes have been largely unsuccessful.29,30 Our results suggest that increasing social integration may be an important component of efforts to protect older Americans from memory decline. Future research should focus on identifying the specific aspects of social integration most important for preserving memory.
Acknowledgments
The authors gratefully acknowledge funding from the National Institute of Aging (grant AG023399).
Human Participant Protection The Health and Retirement Study was approved by the institutional review board at the University of Michigan.
Peer Reviewed
Contributors K.A. Ertel participated in the conception of this study, took primary responsibility for data management and analysis, and was the primary author of the article. M.M. Glymour participated in the conception of this study, provided expertise regarding the Health and Retirement Study data and analytic techniques, and contributed substantially to the writing of the article. L.F. Berkman provided overall conceptual support for data analysis and contributed substantially to the writing of the article.
References
- 1.Gunten A, Giannakopoulos P, Duc R. Cognitive and demographic determinants of dementia in depressed patients with subjective memory complaints. Eur Neurol. 2005;54:154–158. [DOI] [PubMed] [Google Scholar]
- 2.Hogan D, Ebly E. Predicting who will develop dementia in a cohort of Canadian seniors. Can J Neurol Sci. 2000;27:18–24. [DOI] [PubMed] [Google Scholar]
- 3.Aguero-Torres H, von Strauss E, Viitanen M, Windblad B, Fratiglioni L. Institutionalization in the elderly: the role of chronic diseases and dementia. Cross-sectional and longitudinal data from a population-based study. J Clin Epidemiol. 2001;54:795–801. [DOI] [PubMed] [Google Scholar]
- 4.Smits C, Deeg D, Kriegsman D, Schmand B. Cognitive functioning and health as determinants of mortality in an older population. Am J Epidemiol. 1999; 150:978–986. [DOI] [PubMed] [Google Scholar]
- 5.Lyketsos C, Colenda C, Beck C, et al. Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer’s disease. Am J Geriatr Psychiatry. 2006;14:561–572. [DOI] [PubMed] [Google Scholar]
- 6.Fratiglioni L, Paillard-Borg S, Windblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet. 2004;3:343–353. [DOI] [PubMed] [Google Scholar]
- 7.Fratiglioni L, Wang H, Ericsson K, Maytan M, Windblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355:1315–1319. [DOI] [PubMed] [Google Scholar]
- 8.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63:2322–2326. [DOI] [PubMed] [Google Scholar]
- 9.Bassuk S, Glass T, Berkman L. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Ann Intern Med. 1999;131:165–173. [DOI] [PubMed] [Google Scholar]
- 10.Zunzunegui M, Alvarado B, Del Ser T, Otero A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. J Gerontol. 2003;58B:S93–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juster F, Suzman R. An overview of the Health and Retirement Study. J Hum Resources. 1995; 30(suppl):S7–S56. [Google Scholar]
- 12.Ofstedal M, McAuley GF, Herzog AR. Documentation of Cognitive Functioning Measures in the Health and Retirement Study. 2005. Available at: http://hrsonline.isr.umich.edu/docs/userg/dr-006.pdf. Accessed January 30, 2008.
- 13.Chapman D, Williams S, Strine T, Anda R, Moore M. Dementia and its implications for public health. Prev Chronic Dis. 2006;3(2):A34. [PMC free article] [PubMed] [Google Scholar]
- 14.Chodosh J, Reuben D, Albert M, Seeman T. Predicting cognitive impairment in high-functioning community-dwelling older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2002;50:1051–1060. [DOI] [PubMed] [Google Scholar]
- 15.St Clair P, Bugliari D, Chien S, et al. RAND HRS Data Documentation, Version E. Santa Monica, CA: Labor & Population Program, RAND Center for the Study of Aging; 2005.
- 16.de la Torre J. Vascular basis of Alzheimer’s pathogenesis. Ann NY Acad Sci. 2002;977:196–215. [DOI] [PubMed] [Google Scholar]
- 17.de la Torre J. Is Alzheimer’s disease a neurode-generative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. [DOI] [PubMed] [Google Scholar]
- 18.Hultsch D, Small B, Hertzog C, Dixon R. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14:245–263. [DOI] [PubMed] [Google Scholar]
- 19.Bassuk S, Wypig D, Berkman L. Cognitive impairment and mortality in the community-dwelling elderly. Am J Epidemiol. 2000;151:676–688. [DOI] [PubMed] [Google Scholar]
- 20.Schupf N, Tang M, Albert S, et al. Decline in cognitive and functional skills increases mortality risk in non-demented elderly. Neurology. 2005;65:1218–1226. [DOI] [PubMed] [Google Scholar]
- 21.Wilson R, Li Y, Aggarwal N, et al. Cognitive decline and survival in Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:365–362. [DOI] [PubMed] [Google Scholar]
- 22.Seeman T, Albert M, Lusignolog T, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur Studies of Successful Aging. Health Psychol. 2001;20:243–255. [DOI] [PubMed] [Google Scholar]
- 23.Saczynski J, Pheifer L, Masaki K, et al. The effect of social engagement on incident dementia. Am J Epidemiol. 2006;163:433–440. [DOI] [PubMed] [Google Scholar]
- 24.Uchino B. Social support and health: A review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;4:377–387. [DOI] [PubMed] [Google Scholar]
- 25.Berkman L, Glass T. Social integration, social networks, social support, and health. In: Berkman L, Kawachi I, eds. Social Epidemiology. New York, NY: Oxford University Press; 2000:137–173.
- 26.Bennett D, Schneider J, Tang Y, Arnold S, Wilson R. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–412. [DOI] [PubMed] [Google Scholar]
- 27.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 28.Hendrie H. Epidemiology of dementia and Alzheimer’s disease. Am J Geriatr Psychiatry. 1998;6:S3–S18. [DOI] [PubMed] [Google Scholar]
- 29.Blennow K, de Leon M, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. [DOI] [PubMed] [Google Scholar]
- 30.Bianchetti A, Ranieri P, Margiotta A, Trabucchi M. Pharmacological treatment of Alzheimer’s disease. Aging Clin Exp Res. 2006;18(2):158–162. [DOI] [PubMed] [Google Scholar]