Abstract
The standard terminal age category in disease reporting in the United States has been 85 years and older since the 1940s, but the dramatically increasing share of the US population reaching this age has rendered the single category inadequate for surveillance, research, and analysis. Important age-specific variations in mortality among the oldest old are masked by the continued use of this category.
Greater specificity in age-specific data for the oldest old would aid in disease surveillance and etiologic research and broaden awareness and understanding of human longevity.
DATA ON DISEASE INCIDENCE and mortality are commonly classified into 5- or 10-year age groups. This practice was established roughly a century ago—when official vital records were uncommon in the United States—because of the tendency for ages to be reported as multiples of 5.1 This issue still applies to immigrants in the United States from countries that do not rigorously maintain vital records,2 but it is no longer a concern overall. Still, 5- and 10-year age groups continue to be widely used for reasons of practicality and convenience. At the same time, researchers are increasingly using single-year-of-age data (with every age in years in its own category) in epidemiological studies, particularly pediatric studies. Regardless of how age data are classified and analyzed, the terminal age category is almost always 85 years and older.
The use of 85 years and older as the terminal age category became common practice around 1940, as reflected in the vital statistics data published in the annual Vital Records of the United States and the Statistical Abstract of the United States, both published by the US Bureau of the Census. Before then, a 75-years-and-older age group was the most common terminal group. Neither series explicitly remarked upon the shift from 75 years and older to 85 years and older even though both had discussed many other fine points of demographic measurement. Given the steady upward trend in longevity in the United States in the early part of the 20th century, perhaps the US Bureau of the Census saw the decision to redefine the oldest age group as too obvious to require explanation. The upward trend in longevity has continued, and an upward revision to the oldest age group is overdue.
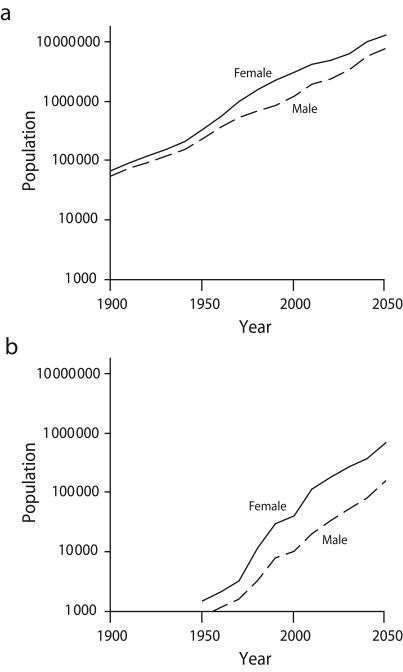
In 1940 there were 365000 people 85 years and older in the United States, representing 0.3% of the total population.3 By 2000, more than 4.2 million (1.5%) Americans were 85 years and older, with 337000 (0.1%) aged 95 years and older,4 nearly the size of the 85-years-and-older population 60 years earlier. By 2030, the population 100 years and older is projected to reach this level (Figure 1 ▶).3–8
FIGURE 1—
Historic and projected populations 85 years and older (a) and 100 years and older (b): United States, 1900–2050.
Source. Data are from the US Census Bureau3–5,7,8 and Siegel and Passel.6
I have found no evidence of federal agencies presenting disease rates by 2 or more age groups for those aged 85 years and older. Mortality counts, by contrast, have been available for 5-year age groups up to the group of 100 years and older ever since Texas became the last state to officially collect mortality data in the mid-1930s.1 Cancer incidence data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program have also long been available by single year of age with an upper limit of 115 years.9 In past decades, these numerator data were not considered especially reliable, but they are now of very high quality, validated with historic census records and Social Security Administration records.10
The limiting factors in the calculation of rates for subgroups of the traditional terminal category of 85 years and older are the insufficient availability and quality of population data. The US Census Bureau publishes data for these age groups stratified by race, ethnicity, state, and county only in decennial census years. Intercensus and postcensus estimates and projections provide only national-level counts. Dicennial census data overstate the numbers in the oldest age groups, particularly centenarians; although the problem has diminished over time, it remains an issue.11
The traditional explanation for the problem of overstatement is age exaggeration—the tendency for those in their nineties to add years to their age to reach the esteemed century mark. However, empirical studies have typically found more of a tendency for people to understate their age.12 The same person might contribute to both patterns, as in the following humorous anecdote, originally published in 1936:
In the matter of prolonging human life, science has played no part whatever. Take the history of one Bessie Singletree. . . . On her twenty-seventh birthday Miss Singletree became twenty-four years of age and was married. At thirty-five she was thirty. At forty she was thirty-nine until she was close to fifty. At fifty Bessie was forty; at sixty, fifty-five. At sixty-five she was sixty-eight and on her seventieth birthday everyone said Grandmother Singletree was pretty chipper for an octogenarian. At seventy-five she had her picture in the paper as the oldest woman in the county, aged ninety-three. Ten years later she passed away at the ripe old age of one hundred and nine.6(p560)
Even when ages are systematically understated overall, the number of centenarians will be exaggerated. To illustrate this, consider 2 age groups, 95 years through 99 years and 100 years and older, and assume that 3% of those aged 95 to 99 years are reported as being 100 years or older (age inflation), 6% of those 100 years or older are reported as being aged 95 to 99 years (age understatement), and that the true ratio of 95- to 99-year-olds to those 100 years or older is 10 to 1. Because there are so many more 95- to 99-year-olds, most of the misclassification will accrue to the 100-years-and-older group even though this group has a lower misclassification rate. This particular example would yield 124 reported centenarians for every 100 actual centenarians.
Beyond the misreporting of age, poor estimates of the extremely aged (90 years and older, but especially centenarians) in past censuses have also resulted from systematic data-collection problems. A prominent example is a problem with the physical design of the 1970 US Census questionnaire that led many respondents to unintentionally indicate they were born in the 1860s.6
MORTALITY AMONG THE OLDEST OLD
Population trends favor the notion of subdividing the 85-years-and-older age group, but doing so will only be useful if the group’s incidence and mortality rates vary meaningfully by age. To determine whether they do, I calculated age-specific mortality for 1999 through 2001 for the most common causes of death in those 85 years and older for the age groups of 85 to 89 years, 90 to 94 years, 95 to 99 years, and 100 years and older. I obtained mortality counts from the National Center for Health Statistics Data Warehouse.13–15
Obtaining population data was somewhat more involved. Numbers for those 100 years and older were from Kestenbaum and Ferguson and were based on the master beneficiary record file of the Social Security Administration.11 Because Medicare enrollment is essentially universal in this age group, the tally is highly accurate and individual ages are verifiable. Kestenbaum and Ferguson tallied 4600 men and 28 000 women as of January 2000, compared with the census-reported 10 000 men and 40 000 women as of April 2000.4
Populations by single year of age from age 85 to 99 years were obtained from Table PCT12 of the 2000 US Census,4 placed into 5-year age groups, and rescaled by assuming that the age misclassification rates implied by the master beneficiary record data also applied to these data. The effect of this rescaling was to reduce the number aged 95 to 99 years moderately while changing only slightly the numbers aged 85 to 89 years and 90 to 94 years. All of the populations were then scaled to sum to the 85 years and older total for 1999 to 2001 as obtained from the Surveillance, Epidemiology, and End Results Program.9
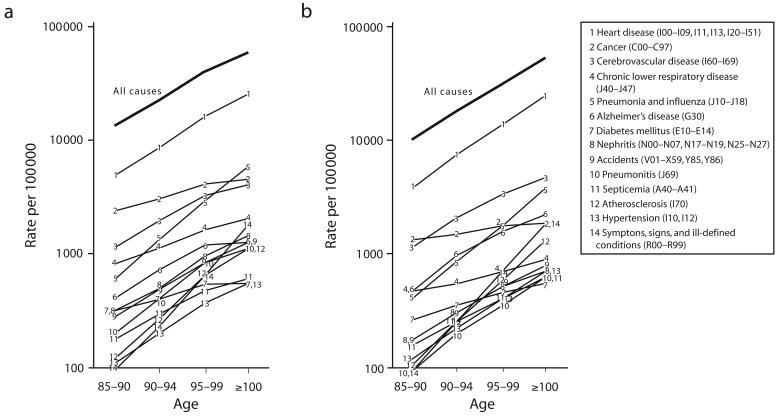
The results indicated substantial variation in mortality at ages above 85 years (Figure 2 ▶). Of the interesting trends and relationships the results revealed, I have briefly discussed 3: the convergence of male–female mortality with increasing age for all-cause mortality and heart disease; the relative rise in “symptoms, signs, and ill-defined conditions” with increasing age; and the relative declines in cancer with increasing age. Each of these phenomena is reasonably well documented, but their discussion elsewhere is confined to specialized literature and does not inform routine disease surveillance.
FIGURE 2—
Mortality rates by age for selected causes of death among men (a) and women (b): United States, 1999–2001.
Note. Data after 2000 are projections.
Current differences in mortality between men and women in their sixties through eighties have been ascribed to behavioral factors, particularly smoking, although ample biological, social, and environmental hypotheses have also been advanced.16 Centenarians are essentially a non-smoking cohort, which eliminates one of the major differences between men and women (men are more likely than are women to smoke), making it sensible that their mortality should be similar. Increasingly disparate rates below the age of 100 years, in turn, reflect higher cigarette consumption rates among men. The steep rise in mortality from “symptoms, signs, and ill-defined conditions” raises the well-documented issue of reduced specificity in assigning a code for the cause of death with increasing age of the deceased. The inevitability of death means that health officials often place a low priority on documenting their oldest patients during workup, screening, or autopsy.17–20
The relative decline in cancer mortality beginning at age 85 years complements an absolute decline in the incidence of prostate, breast, colorectal, and bladder cancers.20–21 There are several hypotheses for this phenomenon, including a lowered genetic susceptibility to cancer in certain individuals; a reduced proliferative ability of all cells, including tumor cells, at advanced age; and a “remodeled” immune system function at advanced age, which, although weakened in most respects, may be better at inhibiting tumor growth.22–25 Evidence from hospital-based autopsy studies and studies of elderly dogs and mice lend plausibility to these hypotheses.17,26,27 However, reduced workup, screening, and autopsy documentation must account for at least some of the pattern, and the presence of age misclassification would tend to exaggerate it. Cancer trends also embed cohort effects, such as the lung cancer peak that aligns with the generation that served in World War II.
CONCLUSION
Age-specific mortality above age 85 years reveals a variety of compelling patterns that have not been well studied. Taken in combination with the rapid growth of this population, the need for greater age specificity is clear. Vital records offices and central disease registries already have the exact ages of all of their cases, and the quality of these data continues to improve. There remain technical hurdles to publishing definitive population data, with ages above 95 years systematically overestimated in the 2000 US Census, but the work of Kestenbaum and Ferguson has shown that this problem is not insurmountable.
The obvious solution is for the US Census Bureau to develop these data and share them through interagency agreements with the National Center for Health Statistics, National Cancer Institute, and other relevant federal health data collection agencies. Such agreements are already in place for intercensus population estimates and projections, bridged race populations, and other specialized data needs.
One cautionary note is that researchers working with more-specific elderly age groups will have to maintain greater vigilance on patient confidentiality. Particularly when data are subdivided by geography or race/ethnicity, they may indicate or appear to indicate a specific individual. Such disclosure risk must be minimized, either through data aggregation or suppression.
When categories are created, there is a tendency to view the world through the prism of those categories, even to the point of forgetting that the categories might have been arbitrary to begin with. Public health researchers have long encountered the category of 85 years and older. Over time, the effect—consciously or not—has been to blur distinctions between those aged 85 years, 95 years, and 105 years. The routine ability to calculate disease rates beyond age 85 would undoubtedly help inspire new hypotheses, direct research questions, and focus surveillance efforts on an ever-aging population. Understanding cancer, diabetes, injuries, and dementia requires studying not only those with these conditions but also those who have evaded them.
The near-exclusive use of 85 years and older as a terminal age category in public health is not unique to the United States. The World Health Organization and most governments of the European Union use the age group of 85 years and older on a routine basis, inspiring some European researchers to advance arguments similar to those presented here.19 The largest exception to this practice is Japan, where more-detailed data for those 85 years and older are readily available and where each September the government publishes a complete list of centenarians and pending centenarians in conjunction with the country’s National Respect for the Aged holiday.28 Coincidentally, Japan boasts the world’s oldest life expectancy at birth and a rich tradition of research on human longevity.29
Acknowledgments
This work is supported in part by the Centers for Disease Control and Prevention’s Cooperative Agreement (U55/ CCU22012-05) awarded to the New York State Department of Health.
I would like to thank research staff of the New York State Cancer Registry for their comments as this article was being prepared.
Note. The contents of this commentary are solely the responsibility of the author and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Human Participant Protection No human participants were involved in this analysis.
Peer Reviewed
References
- 1.Linder FE, Grove RD. Vital Statistics Rates in the United States, 1900–1940. Washington, DC: US Government Printing Office; 1947.
- 2.Denic S, Khatib F, Saadi H. Quality of age data in patients from developing countries. J Public Health (Oxf ). 2004; 26:168–171. [DOI] [PubMed] [Google Scholar]
- 3.Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: US Census Bureau; 2002. Census publication CENSR-4.
- 4.Census 2000 Summary File 1 (SF1). Available at: http://factfinder.census.gov/servlet/DatasetMainPageServlet?_lang=en. Accessed December 1, 2007.
- 5.Krach CA, Velkoff VA. Centenarians in the United States. Washington, DC: US Census Bureau; 1999. Current Population Reports publication P23-199RV.
- 6.Siegel JS, Passel JS. New estimates of the number of centenarians in the United States. J Am Stat Assoc. 1976;71: 559–566. [Google Scholar]
- 7.US Interim Projections by Age, Sex, Race, and Hispanic Origin. Available at: http://www.census.gov/ipc/www/usinterimproj. Accessed December 1, 2007.
- 8.US Population Estimates by Age, Sex, Race, and Hispanic Origin: 1980 to 1999. Available at: http://www.census.gov/popest/archives/1990s. Accessed December 1, 2007.
- 9.SEER*Stat Database: Mortality—All COD, Public-Use With State, Total U.S. (1969–2004). Bethesda, MD: National Cancer Institute, Surveillance, Epidemiology, and End Results Program; 2007.
- 10.Hill ME, Preston SH, Rosenwaike I. Age reporting among White Americans aged 85+: results of a record linkage study. Demography. 2000;37:175–186. [PubMed] [Google Scholar]
- 11.Kestenbaum B, Ferguson BR. The number of centenarians in the United States on January 1, 1990, 2000, and 2010 based on improved Medicare data. N Am Actuar J. 2006;10:1–6. [Google Scholar]
- 12.Preston SH, Elo IT, Stewart Q. Effects of age misreporting on mortality estimates at older ages. Popul Stud. 1999;53:165–177. [Google Scholar]
- 13.Work Table I. Deaths from Each Cause by 5-Year Age Groups, Race, and Sex, United States, 1999. Available at: http://www.cdc.gov/nchs/data/statab/vs00199wktbli.pdf. Accessed December 1, 2007.
- 14.Table 210A. Deaths from 113 Selected Causes, by 5-Year Age Groups, Race, and Sex: United States, 2000. Available at: http://www.cdc.gov/nchs/data/dvs/tabl210a.pdf. Accessed December 1, 2007.
- 15.Work Table 210F. Deaths from 113 Selected Causes, by 5-Year Age Groups, Race, and Sex: United States, 2001. Available at: http://www.cdc.gov/nchs/data/statab/mortfinal2001_work210F.pdf. Accessed December 1, 2007.
- 16.Gjonça A, Tomassini C, Vaupel JW. Male-Female Differences in Mortality in the Developed World. Rostock, Germany: Max Planck Institute for Demographic Research; 1999. Working paper 1999–09.
- 17.Stanta G, Campagner L, Cavallieri F, Giarelli L. Cancer of the oldest old: what we have learned from autopsy studies. Clin Geriatr Med. 1997;13:55–68. [PubMed] [Google Scholar]
- 18.Ries LAG, Devesa SS. Cancer incidence, mortality, and patient survival in the United States. In: Schottenfeld D, Fraumeni JF Jr, eds. Cancer Epidemiology and Prevention. Oxford, England: Oxford University Press; 2007:139–173.
- 19.Meslé F. Causes of death among the oldest-old: validity and comparability. In: Robine J-M, Crimmins EM, Horiuchi S, Yi Z, eds. Human Longevity, Individual Life Duration, and the Growth of the Oldest-Old Population. Dordrecht, Netherlands: Springer; 2006:191–214.
- 20.Saltzstein SL, Behling CA, Baergen RN. Features of cancer in nonagenarians and centenarians. J Am Geriatr Soc. 1998;46:994–998. [DOI] [PubMed] [Google Scholar]
- 21.de Rijke JM, Schouten LJ, Hillen HF, Kiemeney LA, Coebergh JW, van den Brandt PA. Cancer in the very elderly Dutch population. Cancer. 2000;89: 1121–1133. [DOI] [PubMed] [Google Scholar]
- 22.Pompei F, Wilson R. Age distribution of cancer: the incidence turnover at old age. Hum Ecol Risk Assess. 2001;7: 1619–1650. [DOI] [PubMed] [Google Scholar]
- 23.Bonafe M, Barbi C, Storci G, et al. What studies on human longevity tell us about the risk for cancer in the oldest old: data and hypotheses on the genetics and immunology of centenarians. Exp Gerontol. 2002;37(10–11):1263–1271. [DOI] [PubMed] [Google Scholar]
- 24.Lim CS. Cellular senescence, cancer, and organismal aging: a paradigm shift. Biochem Biophys Res Commun. 2006;344:1–2. [DOI] [PubMed] [Google Scholar]
- 25.Macieira-Coelho A. The decline of the clinical incidence of cancers during human senescence. Gerontology. 2003; 49:341–349. [DOI] [PubMed] [Google Scholar]
- 26.Cooley DM, Schlittler DL, Glickman LT, Hayek M, Waters DJ. Exceptional longevity in pet dogs is accompanied by cancer resistance and delayed onset of major diseases. J Gerontol A: Biol Sci Med Sci. 2003; 58(12):B1078–B1084. [DOI] [PubMed] [Google Scholar]
- 27.Pompei F, Polkanov M, Wilson R. Age distribution of cancer in mice: the incidence turnover at old age. Toxicol Ind Health. 2001;17:7–16. [DOI] [PubMed] [Google Scholar]
- 28.Robine J-M, Saito Y. Survival beyond age 100: the case of Japan. In: Carey JR, Tuljapurkar S, eds. Life Span: Evolutionary, Ecological and Demographic Perspectives. New York, NY: Population Council; 2003:208–229.
- 29.The World Health Report 2006: Working Together for Health. Geneva, Switzerland: World Health Organization; 2006.