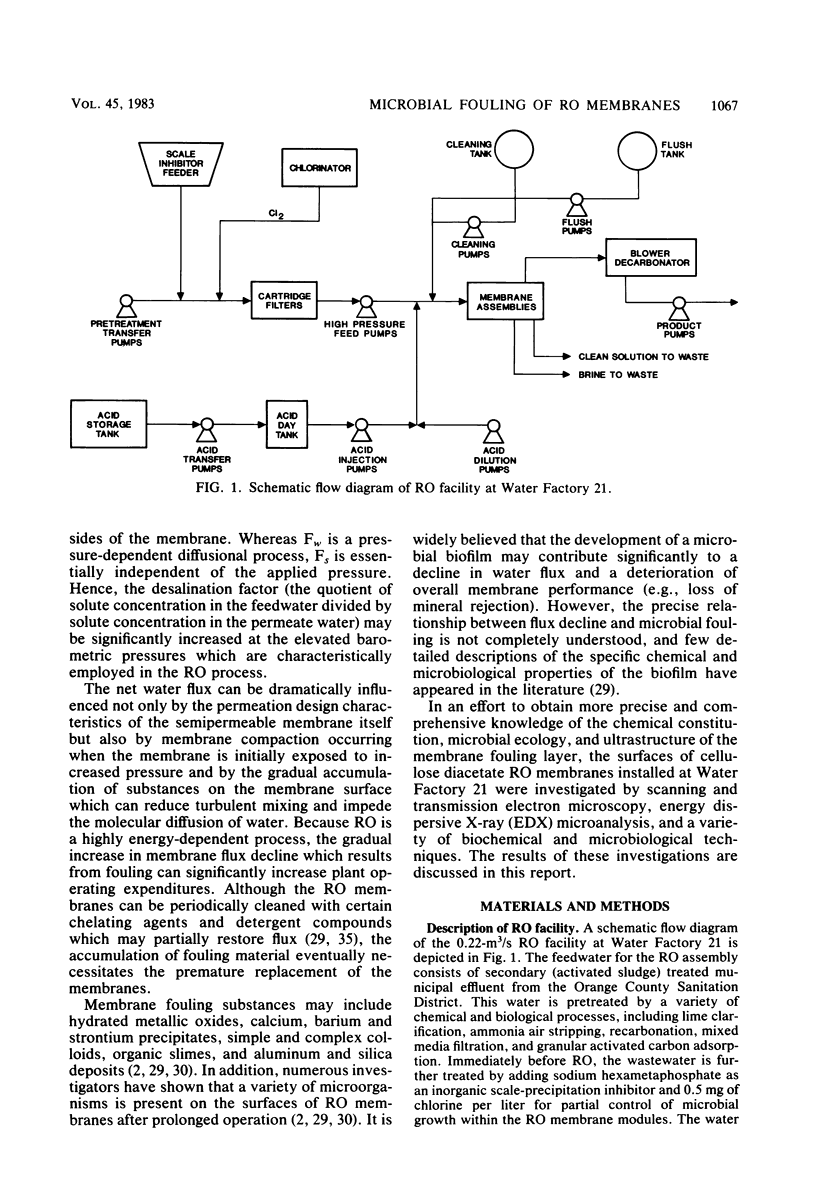
Abstract
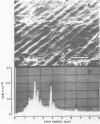
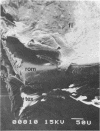
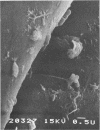
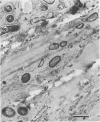
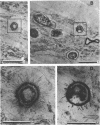
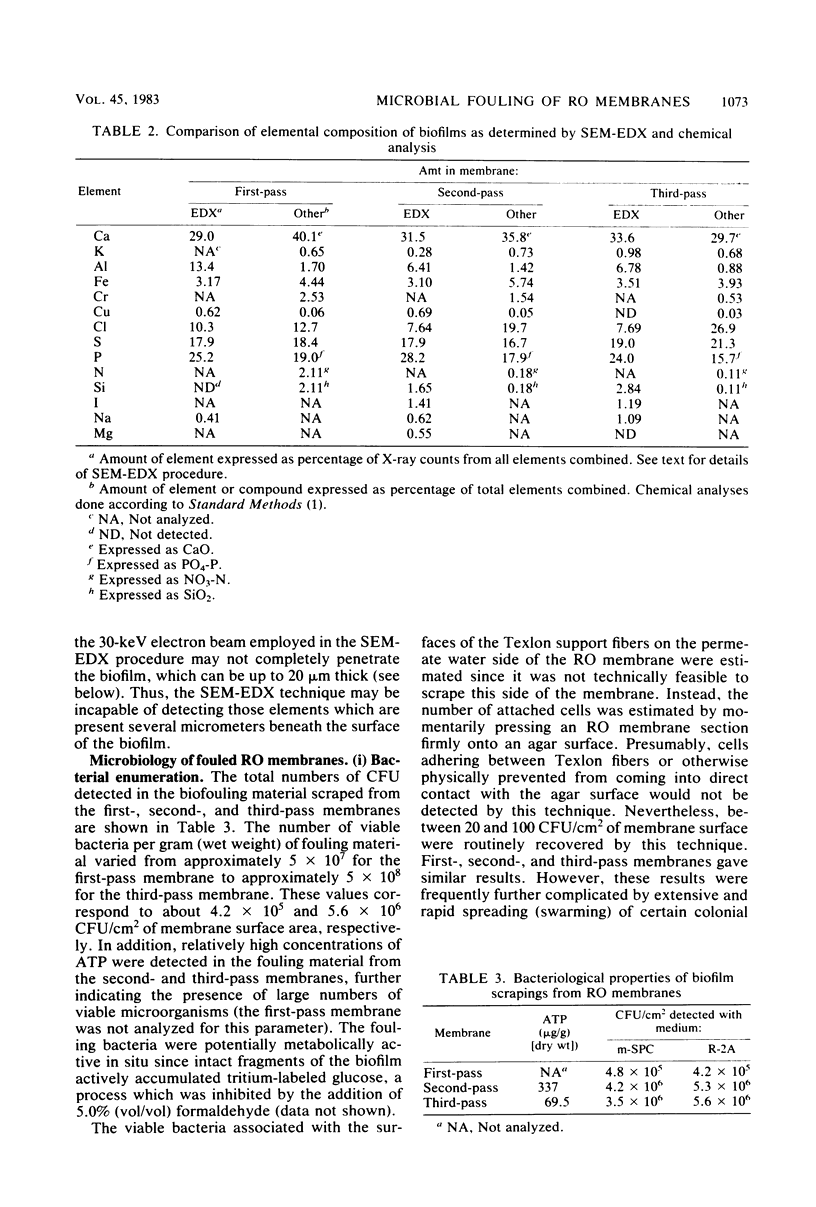
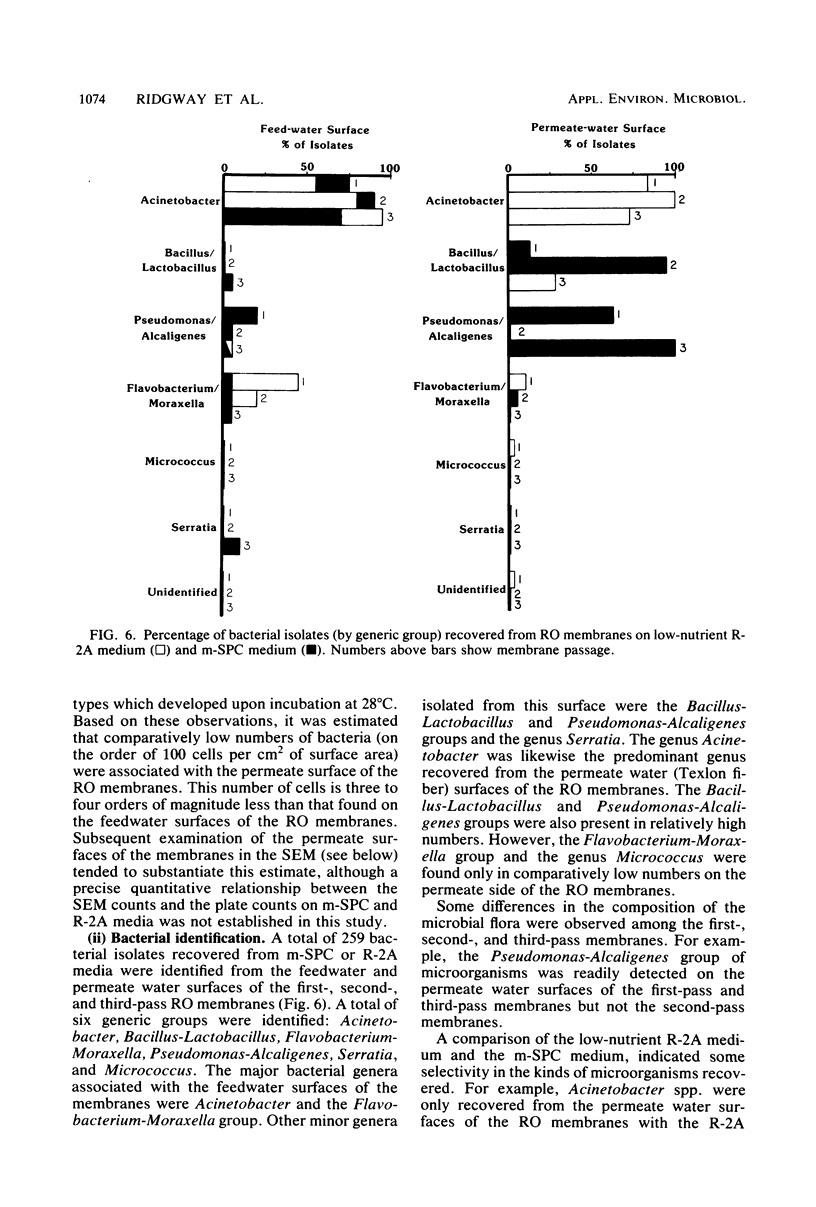
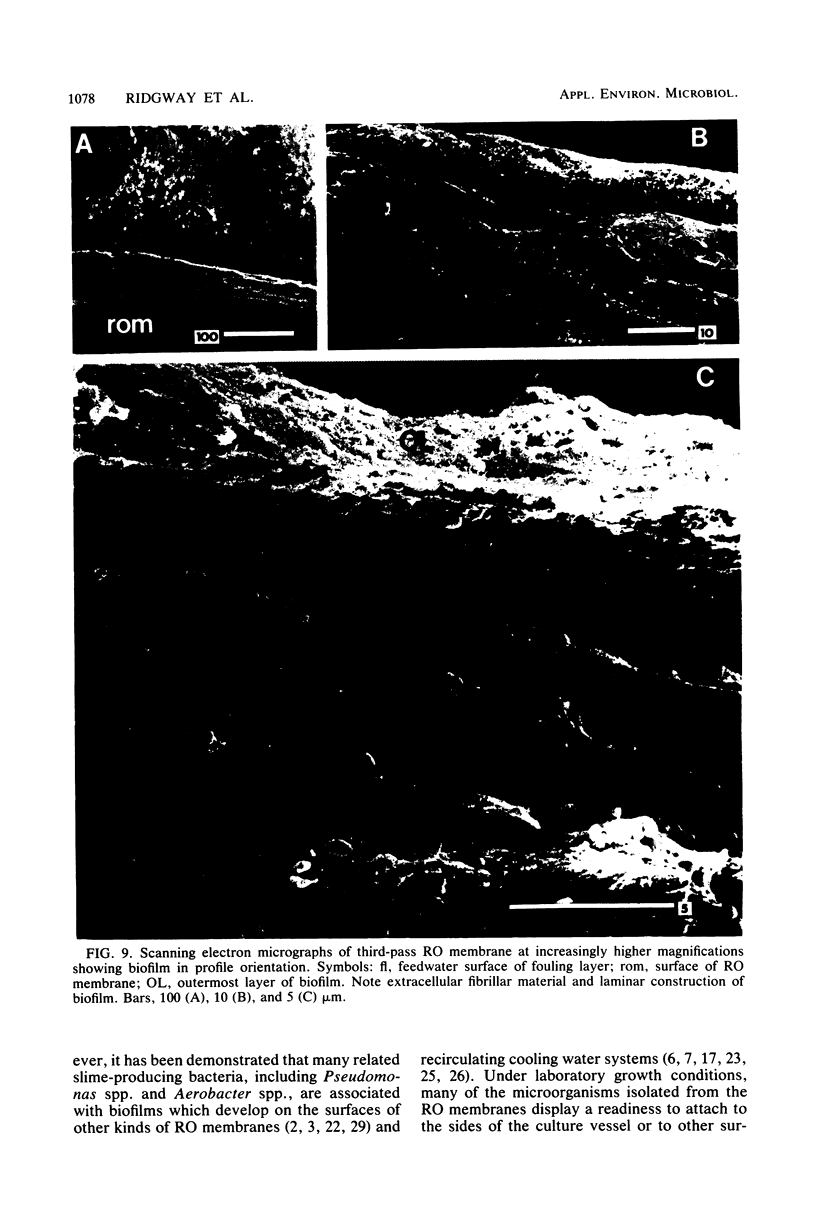
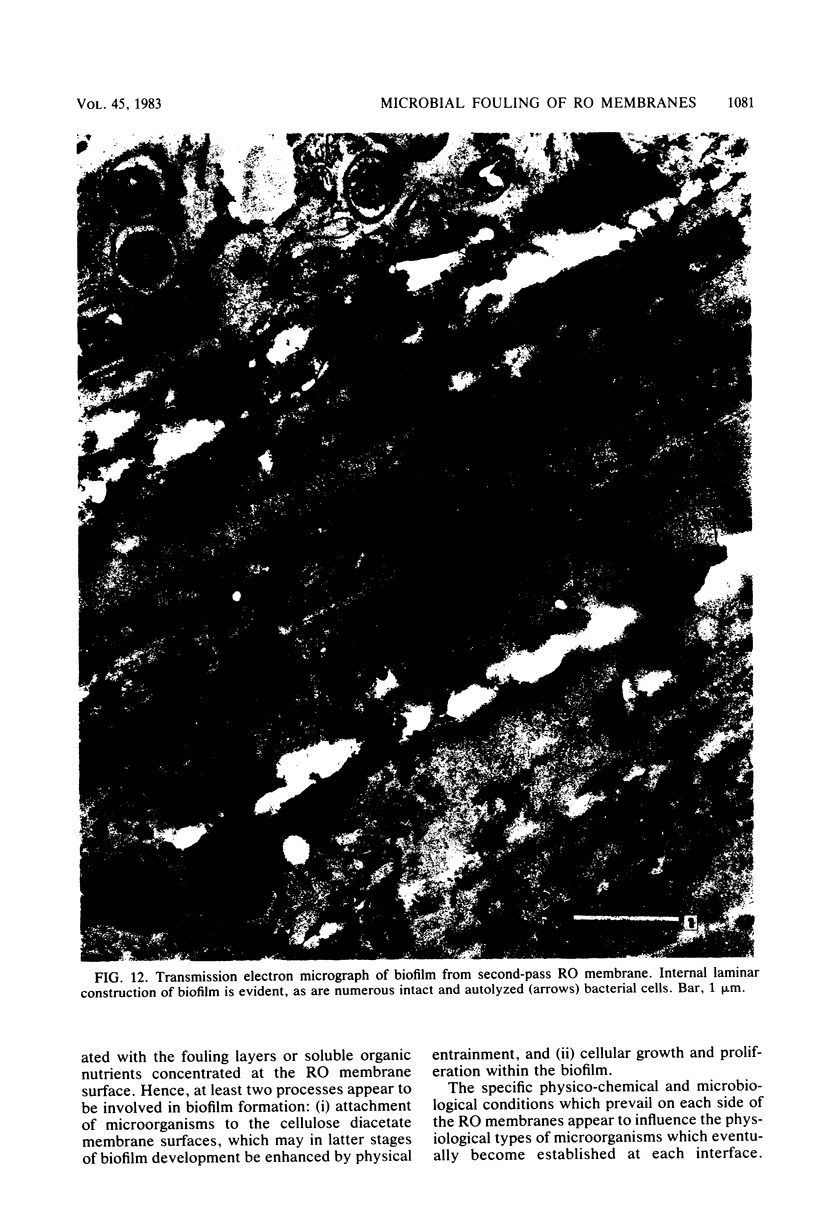
Biofouling of reverse-osmosis membranes was investigated at an advanced wastewater treatment facility. Cellulose diacetate membranes operated for approximately 4,000 h became uniformly coated with a mucilaginous fouling layer. The fouling material was approximately 93% water by weight, and nearly 90% of the dehydrated residue was organic in composition. Calcium, phosphorous, sulfur, and chlorine were the major inorganic constituents detected. Protein and carbohydrate represented as much as 30 and 17%, respectively, of the dry weight of the biofilm. Bacteriological plate counts indicated up to 5.6 X 10(6) CFU/cm2 of membrane surface. Accumulation of [3H]glucose in the biofilm and measurement of ATP indicated that the fouling bacteria were metabolically active in situ. The genus Acinetobacter and the Flavobacterium-Moraxella group were the major generic groups associated with the feedwater surface of the membrane, whereas species of the generic groups Acinetobacter, Pseudomonas-Alcaligenes, and Bacillus-Lactobacillus predominated on the permeate water surface. Electron microscopy revealed that the biofilm on the feedwater surface of the membrane was 10 to 20 microns thick and was composed of several layers of compacted bacterial cells, many of which were partially or completely autolyzed. The bacteria were firmly attached to the membrane surface by an extensive network of extracellular polymeric fibrils. Polyester (Texlon) support fibers located on the permeate surface of the reverse osmosis membranes were sparsely colonized, suggesting bacterial regrowth in the product water collection system.
Full text
PDF
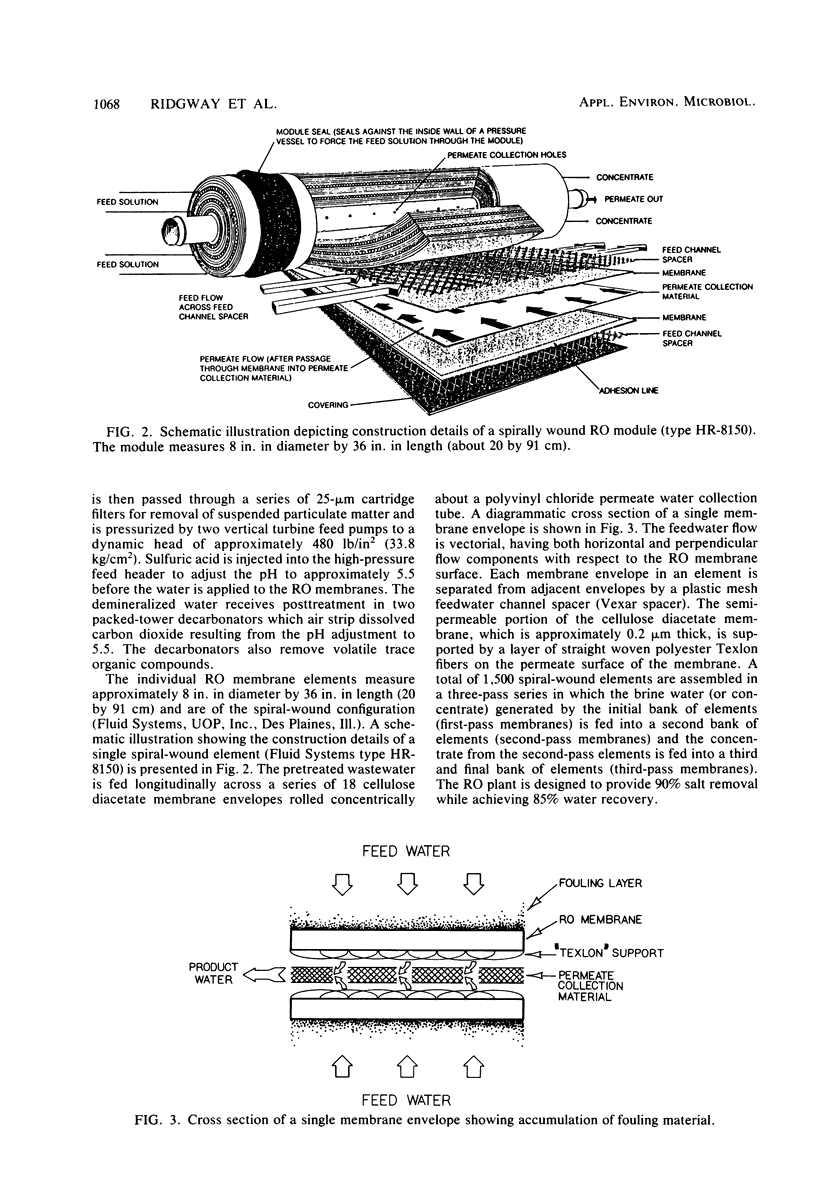



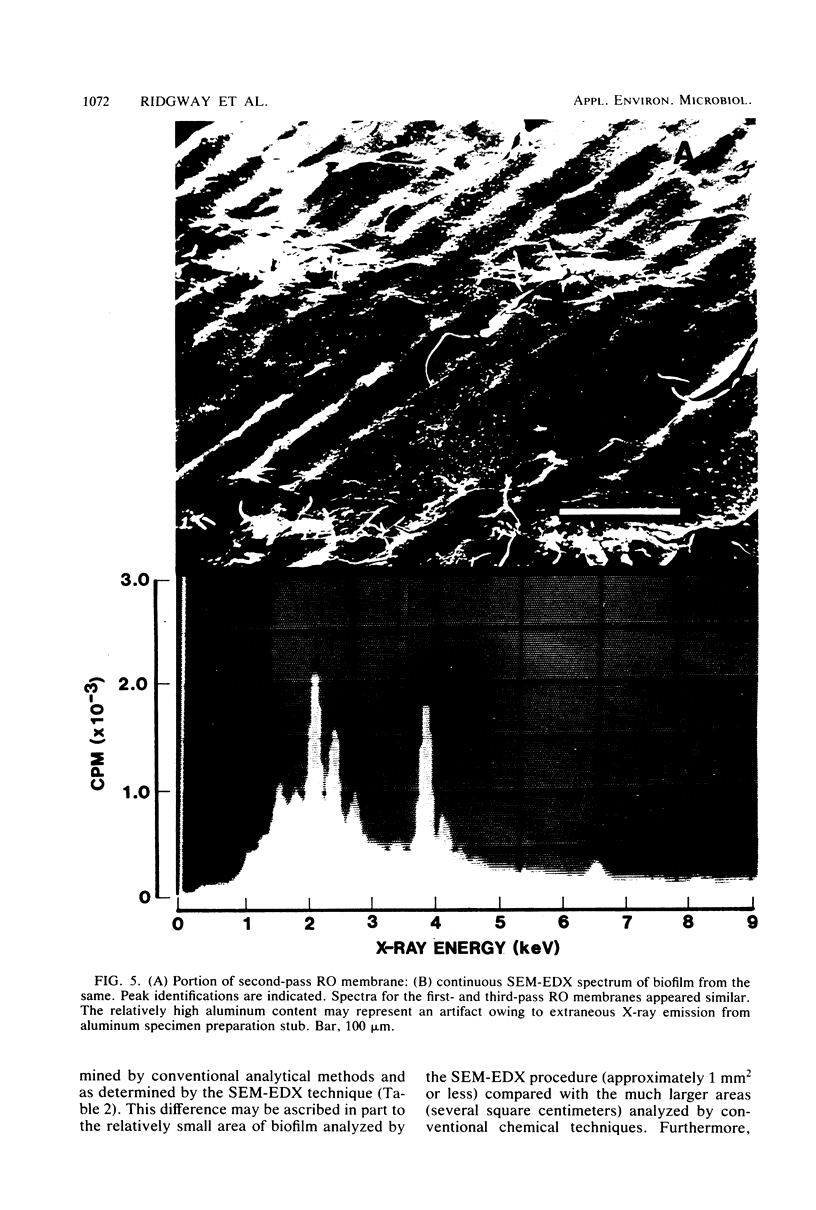







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cagle G. D. Fine structure and distribution of extracellular polymer surrounding selected aerobic bacteria. Can J Microbiol. 1975 Mar;21(3):395–408. doi: 10.1139/m75-055. [DOI] [PubMed] [Google Scholar]
- Favero M. S., Carson L. A., Bond W. W., Petersen N. J. Pseudomonas aeruginosa: growth in distilled water from hospitals. Science. 1971 Aug 27;173(3999):836–838. doi: 10.1126/science.173.3999.836. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lassen J. Rapid identification of gram-negative rods using a three-tube method combined with a dichotomic key. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):525–533. doi: 10.1111/j.1699-0463.1975.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Nickels J. S., Bobbie R. J., Lott D. F., Martz R. F., Benson P. H., White D. C. Effect of manual brush cleaning on biomass and community structure of microfouling film formed on aluminum and titanium surfaces exposed to rapidly flowing seawater. Appl Environ Microbiol. 1981 Jun;41(6):1442–1453. doi: 10.1128/aem.41.6.1442-1453.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meulen H. J., Harder W. Production and characterization of the agarase of Cytoplaga flevensis. Antonie Van Leeuwenhoek. 1975;41(4):431–447. doi: 10.1007/BF02565087. [DOI] [PubMed] [Google Scholar]