Abstract
Objectives. We assessed the impact of parity on tooth loss among American women and examined mediators of this relationship.
Methods. The study sample comprised 2635 White and Black non-Hispanic women who had taken part in the third National Health and Nutrition Examination Survey. We examined the relationship between parity and tooth loss, by age and by socioeconomic position, and tested a theoretical model focusing on direct and indirect influences of parity on dental disease. Robust regression techniques were used to generate path coefficients.
Results. Although parity was associated with tooth loss, the relationship was not moderated through dental care, psychosocial factors, or dental health–damaging behaviors.
Conclusions. Parity is related to tooth loss among American women, but the mechanisms of the association remain undefined. Further investigation is warranted to determine whether disparities in dental health among women who have been pregnant are caused by differences in parity or to physiological and societal changes (e.g., factors related to pregnant women’s access to care) paralleling reproductive choices.
“Jedes kind kostet die mutter einen zahn,” an old German saying that literally means “every child costs the mother one tooth,” embodies a pervasive belief in many cultures, including that of the United States, that tooth loss is a natural consequence of pregnancy.1,2 This belief stems, in part, from the popular view that pregnancy weakens teeth as a result of calcium depletion; such a notion, although wholly unsupported,3–5 is given credibility by findings indicating that pregnancy actually does have an adverse impact on oral tissues.6–13
Despite this widespread conviction, few investigations have explored the association between parity (i.e., the number of live-born children a woman has delivered) and tooth loss. Whereas some have found no association between parity and tooth loss,14–16 others have found that increased parity is related to increased levels of edentulism and fewer teeth.17–19 To our knowledge, however, no studies have examined the mechanisms by which parity may affect tooth loss. Accordingly, our primary goal was to identify mediators of the relationship between parity and tooth loss in a large sample of White and Black American women.
THEORETICAL MODEL
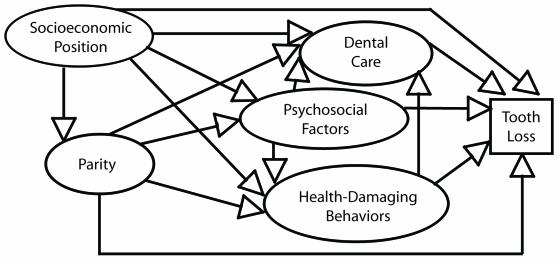
Our theoretical model of the parity–tooth loss relationship (Figure 1 ▶) was based on that of Adler et al.,20 who suggested that one’s socioeconomic position may affect one’s general health via 3 distinct pathways: (1) health care, (2) psychosocial factors, and (3) health behaviors. In our theoretical model, we hypothesized that parity (which has been shown to be closely related to socioeconomic position) would affect tooth loss both directly (i.e., physiologically), via hormonal changes that accompany pregnancy, and indirectly, via 3 pathways similar to those of Adler’s model: dental health care, psychosocial factors (specifically marital status, social support, and financial stress), and dental health–damaging behaviors (specifically smoking and a cariogenic diet).
FIGURE 1—
Theoretical model of relationships between parity and tooth loss.
It is likely that all of these potential mediating variables are affected by parity and, in some cases, by one another. For example, psychosocial factors may influence tooth loss either directly, through their effects on inflammatory periodontal disease levels, or indirectly, through dental care or health-damaging behaviors. Likewise, behaviors that damage dental health may influence tooth loss directly (through physiological changes associated with pregnancy) or indirectly (through dental care).
Pregnancy affects a woman’s hormonal exposure throughout her lifetime, and hormonal exposure is related to periodontal health21,22 and tooth loss.23,24 There are several examples of evidence supporting indirect relationships through the 3 groups of potential mediators included in our theoretical model. First, dental care utilization has been shown to be low among pregnant women,25,26 even though they are often eligible for higher levels of dental insurance coverage.27 Second, increased parity may be related to decreased dental insurance coverage.28
Third, psychosocial status is related to both pregnancy29 and parity,28,30 as well as to dental health (via dental care).31,32 Finally, although smoking typically declines during pregnancy, increased parity is related to relapses in smoking after delivery,33 and smoking is directly related to psychological stress.34 Indeed, smokers are less likely to visit the dentist than nonsmokers despite requiring more regular dental care (because of smoking-related dental disease).35–37
Path analyses of our proposed theoretical model, which included these dental care, psychosocial, and behavioral variables, allowed us to explore the complex relationships between and among variables. We hypothesized that increased parity would be associated with increased tooth loss and that the relationship would be mediated in part by dental care, psychosocial, and behavioral factors.
METHODS
Study Population
We used data from the Third National Health and Nutrition Examination Survey (NHANES III), a nationally representative study of the civilian, noninstitutionalized US population conducted from 1988 to 1994. Dental health data from NHANES III have been used extensively.38–40 White and Black non-Hispanic women between the ages of 18 and 64 years who reported at least 1 pregnancy, had undergone a dental examination, and had retained at least 1 natural tooth were eligible for inclusion. As a result, the sample included nulliparous women who reported a history of pregnancy but had not delivered a live-born infant (e.g., whose pregnancy had ended in miscarriage, abortion, or stillbirth).
Women who were missing data on socioeconomic position measures (education, income, and occupation) and on parity were excluded. Completely edentulous women were not included because of the likelihood that they were different from other women in ways not assessed in our analysis; for example, they may have had a genetic predisposition to medical conditions with a profound effect on dentition (e.g., severe periodontitis), which in turn may have necessitated extraction of all teeth (and these conditions may have been related to parity as well).
Measures
Our outcome variable was tooth loss, measured as the sum of all permanent teeth (excluding third molars) identified as “missing” or “missing and replaced” because of disease. Explanatory variables included measures of socioeconomic position, parity, dental care, psychosocial factors, and health-damaging behaviors.
Socioeconomic measures included family income (calculated as the poverty–income ratio [PIR]), education (number of years of school completed), and occupation (calculated using the Duncan socioeconomic index [SEI]).41 (The PIR is the ratio of the midpoint of a family’s income category to the inflation-adjusted poverty threshold; a ratio below 1 indicates that the family is below the poverty threshold. The SEI, an occupational prestige measure used by the US Census Bureau, is a mixture of occupational prestige scores and census occupation scores.) Parity was based on self-reported information.
Dental care measures included dental insurance coverage and frequency of dental care visits. In NHANES III, participants were asked whether they were covered by any health insurance that paid for any dental care. As a measure of frequency of dental visits, participants were asked “How often do you go to the dentist or dental hygienist?”
Three measures were used to assess psychosocial status: financial stress, marital status, and social support. Women who reported that they had no food or no money for food on 1 or more days during the previous month were considered to be under financial stress. Marital status was dichotomized as married (including women who were married but were not living with their husband, and living together with someone as married) versus unmarried. Six questions exploring frequency of social contact, church or meeting attendance, and club or group membership were used to assess social support.
The dental health–damaging behaviors examined were smoking and consumption of a cariogenic diet. Smoking measures—self-reported smoking, number of cigarettes smoked per day, and serum cotinine concentration—captured both active and passive smoking. Women were categorized as current smokers (those who reported having smoked at least 100 cigarettes in their lifetime and were currently smoking), ex-smokers (those who reported having smoked 100 cigarettes in their lifetime but were not currently smoking), or nonsmokers (those who had not smoked 100 cigarettes in their lifetime).
As part of a food frequency questionnaire, participants were asked about their monthly consumption of 4 groups of cariogenic foods (those high in refined carbohydrates): cakes, cookies, and brownies; chocolate candy and fudge; artificially flavored beverages and fruit drinks; and nondiet sodas. These items were combined as a single measure of number of servings of cariogenic foods consumed per month.
Age at most recent live birth and time elapsed since most recent live birth were based on self-reported data. Women were considered to have diabetes if they reported a history of nonpregnancy-related diabetes.
Statistical Analyses
We first conducted univariate and bivariate analyses, by socioeconomic tertile, focusing on parity, tooth loss, and other variables of interest that would later be used in the modeling procedures. For the most part, we used repeated measures analysis of variance (ANOVA) to examine between-group differences in numbers of missing teeth by age and socioeconomic status; when ANOVA assumptions (e.g., homoskedasticity) were violated, we used the Kruskall–Wallis test. We used the Pearson χ2 test to examine differences between categorical variables.
To maximize use of the available data and avoid collinearity problems in our modeling procedures, we created summed standardized scales for socioeconomic position, smoking, age at most recent live birth, and length of time since most recent live birth. In doing so, we initially assessed correlations between items, then standardized the items, and finally examined Cronbach α reliabilities. When α reliabilities were 0.60 or more, we created scales by summing the standardized items. Because we chose a model-based approach over a sampling-based approach,42 we did not compute population estimates. Monte Carlo simulation was used to impute missing values for variables other than socioeconomic position, parity, and tooth loss in PRELIS43; Stata44 was used in all other analyses. We used Dunn–Sidak corrections to adjust for multiple comparisons.
The scale for socioeconomic position included years of education, PIR score, and SEI score; these variables were positively and significantly correlated at levels of 0.42 (education and PIR score), 0.36 (PIR score and SEI score), and 0.49 (education and SEI score). Smoking included self-reported smoking, number of cigarettes smoked per day, and serum cotinine concentrations; these variables were significantly correlated at levels of 0.67 (self-reported smoking and number of cigarettes smoked per day), 0.70 (numbers of cigarettes smoked per day and serum cotinine concentration), and 0.64 (self-reported smoking and serum cotinine concentration). The correlation between age at most recent live birth and time since most recent live birth was 0.84. Cronbach α reliabilities for the socioeconomic position, smoking, and age–time summary variables were 0.69, 0.87, and 0.89, respectively.
Preliminary analyses demonstrated nonnormal and extremely skewed distributions for several variables, especially tooth loss, and postestimation analyses revealed notable heteroskedasticity and influential outliers in the data. Thus, we used 2 algorithms in our robust path analysis, one that reduced the effects of influential outliers45 and one that asymptotically attenuated heteroskedasticity with a White sandwich variance estimator.46
In the path analysis for each robust regression algorithm, we conducted specific-to-general hierarchical regressions47 for those variables that loaded significantly on the outcome variable to create the initial part of the full model. Then we used a stepwise backward elimination approach to trim the robust regression models of nonsignificant paths and created a path diagram based on this final model. We assessed zero-order relationships between the independent variables that loaded on each endogenous variable and performed intermediate regressions for each of the endogenous variables. All indirect paths were identified, and the sums of each of these indirect effects were calculated as the products of direct paths.
Next, we computed the spurious (unexplained) effects of each independent variable as the difference between zero-order effects and overall effects (direct effects added to the sum of indirect effects),48 and we calculated an error term for each endogenous variable (square root of 1− R2). Finally, we used ANOVA procedures to examine the interaction between parity and socioeconomic position.
RESULTS
Sample Characteristics
Of the 7078 White and Black non-Hispanic women aged 18 to 64 years examined in the NHANES III mobile examination center, 91.8% completed all or part of the dental examination, and of these women 95.2% had at least 1 tooth. Approximately 19% of the women reported no pregnancies, and 9.6% were missing data on socioeconomic position or parity. Of the remaining sample members, 40.4% were missing data on multiple variables; because our primary aim was to identify pathways through which parity affects tooth loss, we excluded these women. Our analysis included 60% of initially eligible women (n = 2635).
Descriptive Analyses
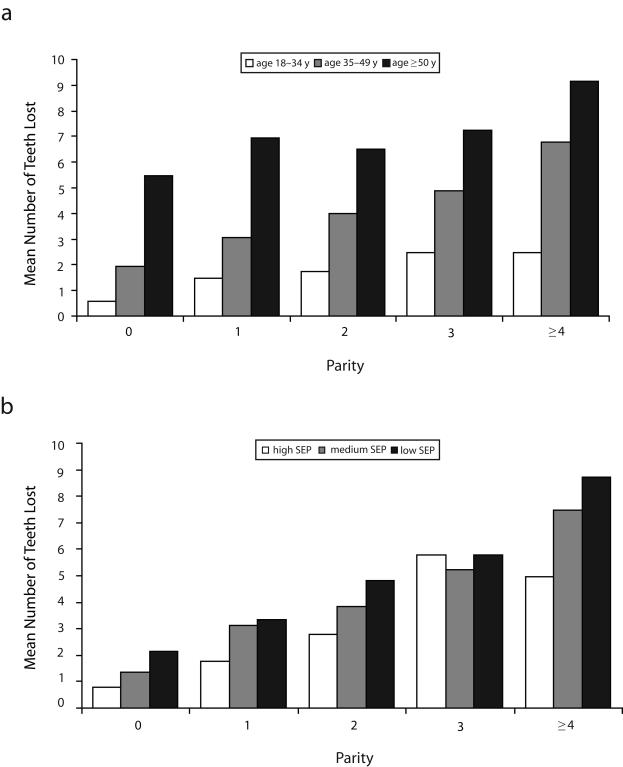
Characteristics of the study participants by socioeconomic position tertile are presented in Table 1 ▶. Socioeconomic position was inversely related to parity and tooth loss. Women in the lowest socioeconomic tertile were far more likely than women in the middle and high tertiles to have borne more children; they also had more missing teeth. Figure 2 ▶ illustrates mean numbers of missing teeth according to parity level, stratified by age group and socioeconomic tertile. Within each age group and each socioeconomic tertile, women who reported more births had more missing teeth than did women who reported fewer births.
TABLE 1—
Demographic Characteristics of Women Aged 18 to 64 Years, by Socioeconomic Position (SEP): Third National Health and Nutrition Examination Survey, 1988–1994
| Low SEP | Middle SEP | High SEP | |
| Total, no. | 878 | 878 | 879 |
| Black, % | 61.3 | 38.8 | 26.5 |
| Age, y, mean (SD) | 36.8 (13.0) | 38.7 (12.7) | 41.2 (11.3) |
| Education, y, % | |||
| ≤ 11 | 36.3 | 6.5 | 0.9 |
| 12 | 53.8 | 51.0 | 19.1 |
| ≥ 13 | 9.9 | 42.4 | 80.0 |
| PIR score,a mean (SD) | 1.5 (0.8) | 3.0 (1.0) | 4.6 (1.8) |
| SEI score,b mean (SD) | 23.6 (6.4) | 31.4 (9.1) | 58.0 (21.1) |
| Parity, no., % | |||
| 0 | 14.7 | 24.6 | 27.9 |
| 1 | 17.1 | 19.3 | 18.8 |
| 2 | 27.1 | 27.9 | 30.8 |
| 3 | 19.8 | 15.8 | 12.5 |
| 4–6 | 18.5 | 11.3 | 9.7 |
| ≥ 7 | 2.9 | 0.9 | 0.3 |
| Parity, mean (SD) | 2.4 (1.9) | 1.8 (1.6) | 1.6 (1.4) |
| No. of years since most recent birth, mean (SD) | 11.7 (10.6) | 13.6 (10.9) | 14.1 (10.3) |
| Dental insurance coverage, % | 53.4 | 67.2 | 68.5 |
| One or more dental visits per year, % | 37.6 | 60.8 | 77.6 |
| Married, % | 49.1 | 61.9 | 73.6 |
| Median no. of contacts per week | 50.7 | 52.0 | 46.3 |
| 1 or more days with no food/money for food in previous month, % | 7.3 | 1.8 | 0.5 |
| Current smoking, % | 33.5 | 24.8 | 16.3 |
| Median serum cotinine level, μg/mL | 1.1 | 0.4 | 0.2 |
| No. of servings of cariogenic foods per month, mean (SD) | 64.4 (55.7) | 50.1 (46.8) | 41.8 (33.3) |
| Diabetes (self-reported), % | 6.5 | 2.3 | 3.2 |
| No. of missing teeth,c mean (SD) | 5.2 (6.37) | 3.8 (5.50) | 2.3 (4.27) |
Note. PIR = poverty–income ratio; SEI = Duncan socioeconomic index. With the exception of social support (P = .60), all demographic factors differed significantly between the 3 socioeconomic tertiles (P ≤ .001).
aPIR is the ratio of the midpoint of a family’s income category to the inflation-adjusted poverty threshold; a ratio below 1 indicates that the family is below the poverty threshold.
bThe SEI is an occupational prestige measure used by the US Census Bureau, which is a mixture of occupational prestige scores and census occupation scores.
cMedian numbers of missing teeth were 3, 1, and 0 in the low, middle, and high tertiles, respectively.
FIGURE 2—
Tooth loss by parity level among 2635 women aged 18 to 64 years, stratified by age (a) and socioeconomic position (b): Third National Health and Nutrition Examination Survey, 1988–1994.
Note. SEP = socioeconomic position.
Robust Regression Models and Path Analyses
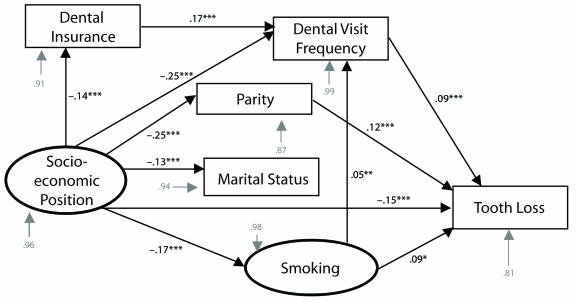
Socioeconomic position, race, parity, frequency of dental care visits, smoking, diabetes, age at most recent live birth, and length of time since most recent live birth were significant predictors of tooth loss in both of our robust regression algorithms (Table 2 ▶). The interaction between parity and socioeconomic position was not significant. The path model based on our final trimmed model is shown in Figure 3 ▶. Values along the paths are robust standardized regression coefficients; these coefficients represent the relative contributions of each term to the endogenous variable at the end of the arrow. Error terms for each variable (shown in gray) are standard errors. All relationships shown were statistically significant in both robust regression algorithms (P ≤ .001). Although the path model controlled for age, length of time since most recent live birth, and diabetes, we did not include these variables in Figure 3 ▶ for the sake of clarity.
TABLE 2—
Results of Robust Regressions for Predictors of Tooth Loss Among Women Aged 18 to 64 Years: Third National Health and Nutrition Examination Survey, 1988–1994
| First Regression Algorithm,a Robust B (SE) | Second Regression Algorithm,b (95% CI) | |
| Socioeconomic position | −0.15 (0.13) | −0.49 (−0.63, −0.36) |
| Race | 0.17 (0.20) | 1.41 (1.22, 1.61) |
| Parity | 0.12 (0.07) | 0.13 (0.07, 0.18) |
| Dental care frequency | 0.09 (0.20) | 0.37 (0.18, 0.57) |
| Smoking | 0.09 (0.11) | 0.25 (0.16, 0.35) |
| Age–timec | 0.45 (0.12) | 1.34 (1.23, 1.44) |
| Diabetes | 0.05 (0.17) | 0.32 (0.19, 0.47) |
Note. See “Methods” section for descriptions of algorithms. With the exception of diabetes in the first algorithm (P = .012), all values were significant at P ≤ .001.
aR2 = 0.35; F7,2635 = 145.7; P ≤ .001.
bF8,2626 = 176.0; P ≤ .001.
cAge at and length of time since most recent live birth.
FIGURE 3—
Path diagram of socioeconomic position, parity, and tooth loss among 2635 women aged 18 to 64 years: Third National Health and Nutrition Examination Survey, 1988–1994.
Note. The model controlled for race, age, length of time since most recent live birth, and diabetes status. Gray arrows denote standard errors. Black arrows indicate a significant relationship was found between the 2 variables. Values along the paths (black numbers) are standardized regression coefficients, which represent the relative contribution of each variable to the variable at the end of the arrow. Error terms for each endogenous variable are provided in grey typeface.
** P ≤ .01; ***P ≤ .001.
In general, higher variable scores indicated less favorable characteristics. The exceptions were socioeconomic position, social support, and dental care, which were reverse scored. We identified direct paths between socioeconomic position and tooth loss and between parity and tooth loss (robust standardized regression coefficients of −0.15 and 0.12, respectively; P ≤ .001 for both), which indicated that women of lower socioeconomic position and women of higher parity were more likely to have increased levels of tooth loss. The direct relationship between frequency of dental care visits and tooth loss indicated that, paradoxically, women who received dental care more frequently had more missing teeth. Smoking was also directly related to tooth loss.
We identified several indirect paths: (1) from socioeconomic position to tooth loss through marital status and parity; (2) from socioeconomic position through parity alone; (3) from socioeconomic position to tooth loss through dental insurance coverage and frequency of dental care visits; (4) from socioeconomic position through frequency of dental care visits alone; (5) from socioeconomic position to tooth loss through smoking and frequency of dental care visits; and (6) from socioeconomic position through smoking alone. The intermediate variables in these paths can be viewed as mediators of the relationship between socioeconomic position and dental disease. Socioeconomic position was related to parity and marital status, and marital status was related to parity: women at higher socioeconomic levels were more likely to be married and to have a low number of children than women at lower socioeconomic levels, and married women had more children than did unmarried women.
DISCUSSION
As hypothesized, we found that increased parity was associated with tooth loss even after adjustment for relevant covariates (age, time since most recent birth, diabetes) and regardless of socioeconomic position. In fact, we found that parity’s impact on tooth loss was stronger than the effects of either smoking or frequency of dental care visits, each of which is a well-established risk factor for tooth loss.49–54
To our knowledge, our results represent the first evidence in a large, heterogeneous sample of US women that parity is so profoundly related to tooth loss, and ours is the first study to attempt to identify possible reasons for this relationship. Although we are not the first to identify a positive relationship between parity and tooth loss,17–19 researchers in previous large-scale investigations examining this connection have used data on elderly Scandinavian women. Thus, the results of these studies may not be generalizable to the racially and socioeconomically diverse US population.
Advantages of our investigation include the large, racially and socioeconomically diverse population included in the NHANES III database; the availability of detailed information on a large number of potential mediators and confounders; the high quality of the dental health data; and the use of a theoretical model examined via path analysis and robust regression techniques. We created our path coefficients through robust regression procedures that allowed us to minimize bias caused by outliers, and ensured that heteroskedasticity of model residuals did not compromise the results of the regression analyses. Because we confirmed the results of each robust regression with the robust algorithms, we are confident that these results are reliable.
Although parity is often defined as number of children born, it may also be viewed in a broader sense: as a composite construct that encompasses pregnancy, parity, and motherhood (raising children). These separate but interconnected facets of a woman’s life may have profound biological, sociological, and behavioral effects. In particular, the pathological oral changes that occur as a result of the physiological alterations accompanying pregnancy are well documented.3,6,7,11–13
Studies have shown that gingivitis, inflammation of tooth-supporting soft tissues without accompanying breakdown of the alveolar bone, occurs in 30% to as many as 100% of pregnant women12,13 and is associated with pathological changes in the oral microflora.55,56 In general, such oral changes are considered transient, because in the vast majority of women, any gingivitis experienced during pregnancy subsides after childbirth. However, periodontal destruction (loss of bony support of the teeth) has been shown to be common among pregnant women,57–59 and investigators have found that periodontitis may worsen during pregnancy60,61 or persist after parturition.62 Gingivitis is a necessary prelude to irreversible periodontal breakdown,63 and it has been postulated that repeated episodes of gingivitis during pregnancy, when combined with underlying periodontitis, could exacerbate the progression of existing periodontitis.64
Alternatively, empirical evidence demonstrates that pregnancy and parity have psychological sequelae, including anxiety and postpartum depression.28–30 Because these psychological states have been shown to affect oral health by modulating behaviors (e.g., cigarette smoking35,36 or compliance with recommended dental treatment8,31,32,35,65) or leading to direct alterations to the immune system,66–68 repeated episodes of depression or psychological stress brought about by life changes such as pregnancy and parity can be hypothesized to ultimately have detrimental effects on oral health.
Because we did not find evidence of moderating effects of parity on dental disease outcomes via dental care, psychosocial factors, or health-damaging behaviors, our results could be viewed as supporting a biological association between parity and tooth loss. However, a perhaps more plausible reason that we did not find such evidence is that we were limited by the data gathered as part of NHANES III, in which the questions that explored key factors may not have captured the essence of these constructs. For example, regarding dental care, participants were asked “How often do you go to the dentist or dental hygienist?” and “Are you covered by any health insurance that paid for any dental care?” Although we assumed that frequency of dental care was positively correlated with regular preventive care, it is possible that, for some women, frequent dental care represented frequent emergency or palliative care.
In addition, in some states, the Medicaid program pays for dental care, and thus women of lower socioeconomic status may have reported having dental insurance coverage. Indeed, we found that 53% of women in the lowest socioeconomic tertile reported having dental insurance. Whereas a large proportion of these women probably had at least some of their dental care covered through Medicaid, it is likely that the majority of women in the highest socioeconomic tertile had private dental insurance. Medicaid reimbursement rates for dental care are generally low,69 and preventive services are often not covered.
One would expect that effects of parity on frequency of dental care visits, financial stress, and social support in particular are strongest among women with young children living in the home. To increase the generalizability of our results, however, we included women up to the age of 64 years in our analyses. Post hoc bivariate correlation (Pearson r ) tests of the effects of parity on tooth loss by age group indeed showed stronger correlations among women younger than 49 years (women 18–34 years, r = 0.23, P ≤ .001; women 35–49 years, r = 0.24, P ≤ .001) than among women 50 years or older (r = 0.19; P ≤ .001).
Our finding that increased parity was associated with increased tooth loss may suggest that women of higher parity are more likely to have teeth extracted, whereas women of lower parity are more likely to have teeth restored or treated. Indeed, it has been shown that both pregnancy and maternity alter patterns of dental treatment among women.25,26,70 In the United States, in fact, it is generally recommended that pregnant women undergo dental care only during the second trimester,71,72 and therefore dentists may be reluctant to offer women treatment either early or later in their pregnancy. Women seeking care during their first or third trimester may be offered alternative treatments, or treatment may be deferred.70,72
Dentists are also likely to alter treatment plans on the basis of a woman’s ability to pay for a particular treatment, comply with scheduled appointments, and engage in adequate oral self-care to prevent disease progression; all of these factors are probably affected by pregnancy or motherhood. In addition, pregnant women may believe that they need to postpone dental therapy until after they have given birth73; however, access to dental care among women with multiple children is likely to be restricted as a result of financial, time, or child-care constraints.74,75
Although recent studies have identified demographic, biological, and behavioral risk factors for dental disease,9,37,49–52 social risk factors remain largely undefined and under-explored, despite the fact that (as is the case with systemic disease) disparities in dental disease are most likely caused by such factors.77 The public health implications of identifying additional predictors of poor dental health are considerable. Women who have experienced changes in dental care, psychological status, or health behaviors because of pregnancy or motherhood are likely to represent a significant proportion of the US population, and interventions aimed at these women could have a positive impact on their dental health status.
Given the strong relationship between parity and tooth loss observed in this study, there is a clear need for future investigations designed to enhance understanding of the ways in which parity influences dental health. Treating depression and anxiety during and after pregnancy, improving pregnant women’s access to and use of preventive dental care services, and enhancing family dental health programs could attenuate parity-related dental health disparities in the future.
Acknowledgments
This work was supported by the National Institute of Dental and Craniofacial Research, New York University College of Dentistry (postdoctoral training grant T32DE14257).
We thank Ralph Katz, Douglas Morse, and Daniel Zelterman for providing input when they served on Stefanie L. Russell’s dissertation committee. In addition, we thank dissertation reviewers Trace Kershaw, Beth Jones, and Susan Reisine. Finally, we acknowledge the individuals who participated in the third National Health and Nutrition Examination Survey; without their contribution, this work would have been impossible.
Human Participant Protection This study was approved by the institutional review boards of New York University and Yale University.
Peer Reviewed
Contributors S. L. Russell and J. R. Ickovics originated the idea for the analysis. S. L. Russell and R. A. Yaffee performed the analyses. All of the authors helped to conceptualize ideas, interpret findings, and review drafts of the article. S. L. Russell led the writing with contributions from J. R. Ickovics and R. A. Yaffee.
References
- 1.O’Connor A. The claim: gain a child, lose a tooth. Available at: http://www.nytimes.com/2007/04/24/health/24real.html. Accessed March 3, 2008.
- 2.Al Habashneh R, Guthmiller JM, Levy S, et al. Factors related to utilization of dental services during pregnancy. J Clin Periodontol. 2005;32:815–821. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg BJ. Women’s oral health issues. J Dent Educ. 1999;63:271–275. [PubMed] [Google Scholar]
- 4.Walker AR, Dison E, Walker BF. Dental caries in South African rural Black women who had large families and long lactations. J Trop Med Hyg. 1983;86:201–205. [PubMed] [Google Scholar]
- 5.Cruikshak DP, Hays PM. Maternal physiology in pregnancy. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies. New York, NY: Churchill Livingstone; 1991:125–146.
- 6.Laine MA. Effect of pregnancy on periodontal and dental health. Acta Odontol Scand. 2002;60:257–264. [DOI] [PubMed] [Google Scholar]
- 7.Casamiassimo P. Maternal oral health. Dent Clin North Am. 2001;45:469–478. [PubMed] [Google Scholar]
- 8.Machuca G, Khoshfeiz O, Lacalle JR, Machuca C, Bullon P. The influence of general health and socio-cultural variables on the periodontal condition of pregnant women. J Periodontol. 1999;70:779–785. [DOI] [PubMed] [Google Scholar]
- 9.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–1049. [DOI] [PubMed] [Google Scholar]
- 10.Redford M. Beyond pregnancy gingivitis: bringing a new focus to women’s oral health. J Dent Educ. 1993; 57:742–748. [PubMed] [Google Scholar]
- 11.Sooriyamoorthy M, Gower DB. Hormonal influences on gingival tissue: relationship to periodontal disease. J Clin Periodontol. 1989;16:201–208. [DOI] [PubMed] [Google Scholar]
- 12.Löe H. Periodontal changes in pregnancy. J Periodontol. 1965;36:209–217. [PubMed] [Google Scholar]
- 13.Löe H, Silness J. Periodontal disease in pregnancy: I. Prevalence and severity. Acta Odontol Scand. 1963; 21:533–551. [DOI] [PubMed] [Google Scholar]
- 14.Scheutz F, Baelum V, Matee MI, Mwangosi I. Motherhood and dental disease. Community Dent Health. 2002;19:67–72. [PubMed] [Google Scholar]
- 15.Wysokiska-Miszczuk J. Effect of the number of pregnancies on the condition of the teeth in older women. Wiad Lek. 1987;40:964–967. [PubMed] [Google Scholar]
- 16.Stalp S, Zuhrt R. Dental caries and pregnancy. Stomatol DDR. 1979;29:481–484. [PubMed] [Google Scholar]
- 17.Christensen K, Gaist D, Jeune B, Vaupel JW. A tooth per child? Lancet. 1998;352:204. [DOI] [PubMed] [Google Scholar]
- 18.Halling A, Bengtsson C. The number of children, use of oral contraceptives and menopausal status in relation to the number of remaining teeth and the periodontal bone height: a population study of women in Gothenburg, Sweden. Community Dent Health. 1989;6:39–45. [PubMed] [Google Scholar]
- 19.Rundgren A, Osterberg T. Dental health and parity in three 70-year-old cohorts. Community Dent Oral Epidemiol. 1987;15:134–136. [DOI] [PubMed] [Google Scholar]
- 20.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: no easy solution. JAMA. 1993;269:3140–3145. [PubMed] [Google Scholar]
- 21.Civitelli R, Pilgram T, Dotson M, et al. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement therapy. Arch Intern Med. 2002;162:1409–1415. [DOI] [PubMed] [Google Scholar]
- 22.Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of US adults from NHANES III. J Clin Periodontol. 2000;27:778–786. [DOI] [PubMed] [Google Scholar]
- 23.Krall EA. Osteoporosis and the risk of tooth loss. Clin Calcium. 2006;16:63–66. [PubMed] [Google Scholar]
- 24.Paganini-Hill A. The benefits of estrogen replacement therapy on oral health: the Leisure World cohort. Arch Intern Med. 1995;155:2325–2329. [PubMed] [Google Scholar]
- 25.Timothe P, Eke PI, Presson SM, Malvitz DM. Dental care use among pregnant women in the United States reported in 1999 and 2002. Prev Chronic Dis. 2005;2:A10. [PMC free article] [PubMed] [Google Scholar]
- 26.Gaffield ML, Gilbert BJ, Malvitz DM, Romaguera R. Oral health during pregnancy: an analysis of information collected by the Pregnancy Risk Assessment Monitoring System. J Am Dent Assoc. 2001;132:1009–1016. [DOI] [PubMed] [Google Scholar]
- 27.Cruz GD, Roldos I, Puerta DI, Salazar CR. Community-based, culturally appropriate oral health promotion program for immigrant pregnant women in New York City. N Y State Dent J. 2005;71:34–38. [PubMed] [Google Scholar]
- 28.D’Elio MA, Ness RB, Matthews KA, Kuller LH. Are life stress and social support related to parity in women? Behav Med. 1997;23:87–94. [DOI] [PubMed] [Google Scholar]
- 29.Moses-Kolko EL, Roth EK. Antepartum and postpartum depression: healthy mom, healthy baby. J Am Med Womens Assoc. 2004;59:181–191. [PubMed] [Google Scholar]
- 30.Glazebrook C, Sheard C, Cox S, Oates M, Ndukwe G. Parenting stress in first-time mothers of twins and triplets conceived after in vitro fertilization. Fertil Steril. 2004;81:505–511. [DOI] [PubMed] [Google Scholar]
- 31.McGrath C, Bedi R. Influences of social support on the oral health of older people in Britain. J Oral Rehabil. 2002;29:918–922. [DOI] [PubMed] [Google Scholar]
- 32.Broder HL, Russell SL, Catapano P, Reisine S. Perceived barriers and facilitators to dental treatment among female caregivers of children with and without HIV and their health care providers. Pediatr Dent. 2002;24:301–308. [PubMed] [Google Scholar]
- 33.Kahn RS, Certain L, Whitaker RC. A reexamination of smoking before, during, and after pregnancy. Am J Public Health. 2002;92:1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bancroft A, Wiltshire S, Parry O, Amos A. “It’s like an addiction first thing . . . afterwards it’s like a habit”: daily smoking behaviour among people living in areas of deprivation. Soc Sci Med. 2003;56:1261–1267. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro da Silva AM, Newman HN, Oakley DA, O’Leary R. Psychosocial factors, dental plaque levels and smoking in periodontitis patients. J Clin Periodontol. 1998;25:517–523. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert GH, Stoller EP, Duncan RP, Earls JL, Campbell AM. Dental self-care among dentate adults: contrasting problem-oriented dental attenders and regular dental attenders. Spec Care Dentist. 2000;20:155–163. [DOI] [PubMed] [Google Scholar]
- 37.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. J Periodontol. 2000;71:743–751. [DOI] [PubMed] [Google Scholar]
- 38.Brown LJ, Winn DM, White BA. Dental caries, restoration and tooth conditions in US adults, 1988–1991: selected findings from the Third National Health and Nutrition Examination Survey. J Am Dent Assoc. 1996;127:1315–1325. [DOI] [PubMed] [Google Scholar]
- 39.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70:13–29. [DOI] [PubMed] [Google Scholar]
- 40.Winn DM, Johnson CL, Kingman A. Periodontal disease estimates in NHANES III: clinical measurement and complex sample design issues. J Public Health Dent. 1999;59:73–78. [DOI] [PubMed] [Google Scholar]
- 41.Stevens G, Cho JH. Socioeconomic indexes and the new 1980 census occupational classification scheme. Soc Sci Res. 1985;14:142–168. [Google Scholar]
- 42.Lohr SL. Sampling Design and Analysis. Pacific Grove, CA: Duxbury Press; 1999.
- 43.PRELIS, Version 2.2. Chicago, IL: Scientific Software International; 1993.
- 44.Stata, Version 8.0 [computer software]. College Station, TX: StataCorp LP; 2004.
- 45.Huber PJ. Robust Statistics. New York, NY: John Wiley & Sons Inc; 1981.
- 46.Davidson R, McKinnon JG. Estimation and Inference in Econometrics. New York, NY: Oxford University Press Inc; 1993.
- 47.Hendry DF, Richard JF. On the formulation of empirical models in dynamic economics. J Econometrics. 1982;20:3–33. [Google Scholar]
- 48.Cohen J, Aiken LS, West SG, Cohen P. Applied Multiple Regression-Correlation Analysis for the Behavioral Sciences. Mahwah, NJ: Lawrence Erlbaum Associates; 2002.
- 49.Copeland LB, Krall EA, Brown LJ, Garcia RI, Streckfus CF. Predictors of tooth loss in two US adult populations. J Public Health Dent. 2004;64:31–37. [DOI] [PubMed] [Google Scholar]
- 50.Ylostalo P, Sakki T, Laitinen J, Jarvelin MR, Knuuttila M. The relation of tobacco smoking to tooth loss among young adults. Eur J Oral Sci. 2004;112:121–126. [DOI] [PubMed] [Google Scholar]
- 51.Kressin NR, Boehmer U, Nunn ME, Spiro A III. Increased preventive practices lead to greater tooth retention. J Dent Res. 2003;82:223–227. [DOI] [PubMed] [Google Scholar]
- 52.Gilbert GH, Duncan RP, Shelton BJ. Social determinants of tooth loss. Health Serv Res. 2003;38:1843–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tonetti MS, Muller-Campanile V, Lang NP. Changes in the prevalence of residual pockets and tooth loss in treated periodontal patients during a supportive maintenance care program. J Clin Periodontol. 1998;25:1008–1016. [DOI] [PubMed] [Google Scholar]
- 54.Murray JJ. Attendance patterns and oral health. Br Dent J. 1996;181:339–342. [DOI] [PubMed] [Google Scholar]
- 55.Bogess KA. Pathogenicity of periodontal pathogens during pregnancy. Am J Obstet Gynecol. 2005;193:311–312. [DOI] [PubMed] [Google Scholar]
- 56.Kornman KS, Loesche WJ. Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infect Immun. 1982;35:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jago JD, Chapman PJ, Aitken JF, McEniery TM. Dental status of pregnant women attending a Brisbane maternity hospital. Community Dent Oral Epidemiol. 1984;12:398–401. [DOI] [PubMed] [Google Scholar]
- 58.Lopez NJ, Smith PC, Gutierrez J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Periodontol. 2002;73:911–924. [DOI] [PubMed] [Google Scholar]
- 59.Moore S, Ide M, Wilson RF, et al. Periodontal health of London women during early pregnancy. Br Dent J. 2001;191:570–573. [DOI] [PubMed] [Google Scholar]
- 60.Lieff S, Boggess KA, Murtha AP, et al. The Oral Conditions and Pregnancy Study: periodontal status of a cohort of pregnant women. J Periodontol. 2004;75:116–126. [DOI] [PubMed] [Google Scholar]
- 61.Albrecht M, Banoczy J, Baranyi E, et al. Studies of dental and oral changes of pregnant diabetic women. Acta Diabetol Lat. 1987;24:1–7. [DOI] [PubMed] [Google Scholar]
- 62.Steinberg BJ. Sex hormonal alterations. In: Rose LF, Kaye D, eds. Internal Medicine for Dentistry. St. Louis, MO: CV Mosby; 1990:1073–1077.
- 63.Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000. 2002; 29:177–206. [DOI] [PubMed] [Google Scholar]
- 64.Hildebolt CF, Pilgram TK, Yokoyama-Crothers N, et al. Alveolar bone height and postcranial bone mineral density: negative effects of cigarette smoking and parity. J Periodontol. 2000;71:683–689. [DOI] [PubMed] [Google Scholar]
- 65.Mendoza AR, Newcomb GM, Nixon KC. Compliance with supportive periodontal therapy. J Periodontol. 1991;62:731–736. [DOI] [PubMed] [Google Scholar]
- 66.LeResche L, Dworkin SF. The role of stress in inflammatory disease, including periodontal disease: review of concepts and current findings. Periodontol 2000. 2002;30:91–103. [DOI] [PubMed] [Google Scholar]
- 67.Saletu A, Pirker-Frühauf H, Saletu F, Linzmayer L, Anderer P, Matejka M. Controlled clinical and psychometric studies on the relation between periodontitis and depressive mood. J Clin Periodontol. 2005;32:1219–1225. [DOI] [PubMed] [Google Scholar]
- 68.Persson GR, Persson RE, MacEntee CI, Wyatt CC, Hollender LG, Kiyak HA. Periodontitis and perceived risk for periodontitis in elders with evidence of depression. J Clin Periodontol. 2003;30:691–696. [DOI] [PubMed] [Google Scholar]
- 69.Oral Health of United States Adults. The National Survey of Oral Health in U.S. Employed Adults and Seniors: 1985–1986, National Findings. Bethesda, MD: National Institutes of Health; 1987.
- 70.Pistorius J, Kraft J, Willershausen B. Dental treatment concepts for pregnant patients—results of a survey. Eur J Med Res. 2003;8:241–246. [PubMed] [Google Scholar]
- 71.American Academy of Periodontology. Statement regarding periodontal management of the pregnant patient. J Periodontol. 2004;75:495. [DOI] [PubMed] [Google Scholar]
- 72.Livingston HM, Dellinger TM, Holder R. Considerations in the management of the pregnant patient. Spec Care Dentist. 1998;18:183–188. [DOI] [PubMed] [Google Scholar]
- 73.Durdyniiazov MK, Alimskii AV. The social hygiene aspects of oral morbidity in multiparous women. Stomatologiia (Mosk). 1993;72:60–65. [PubMed] [Google Scholar]
- 74.Redford M. Beyond pregnancy gingivitis: bringing a new focus to women’s oral health. J Dent Educ. 1993; 57:742–748. [PubMed] [Google Scholar]
- 75.Smith R, Jasinevicius T. Comparison of oral health and psychological indices of women of childbearing age. J Dent Res. 1996;75. Abstract 1341.
- 76.Healthy People 2010: Understanding and Improving Health. Washington, DC: US Dept of Health and Human Services; 2000.