Abstract
Objectives. We sought to determine whether an elevated burden of chronic kidney disease is found among disadvantaged groups living in the United States, Australia, and Thailand.
Methods. We used data on participants 35 years or older for whom a valid serum creatinine measurement was available from studies in the United States, Thailand, and Australia. We used logistic regression to analyze the association of income, education, and employment with the prevalence of chronic kidney disease (estimated glomerular filtration rate<60 mL/min/1.73 m2).
Results. Age- and gender-adjusted odds of having chronic kidney disease were increased 86% for US Whites in the lowest income quartile versus the highest quartile (odds ratio [OR] = 1.86; 95% confidence interval [CI] = 1.27, 2.72). Odds were increased 2 times and 6 times, respectively, among unemployed (not retired) versus employed non-Hispanic Black and Mexican American participants (OR=2.89; 95% CI=1.53, 5.46; OR=6.62; 95% CI=1.94, 22.64. respectively). Similar associations were not evident for the Australian or Thai populations.
Conclusions. Higher kidney disease prevalence among financially disadvantaged groups in the United States should be considered when chronic kidney disease prevention and management strategies are created. This approach is less likely to be of benefit to the Australian and Thai populations.
Chronic kidney disease refers to a chronic, irreversible loss of kidney function, ranging from asymptomatic kidney damage to end-stage kidney disease (ESKD), in which death would occur without renal replacement therapy. Principal risk factors include diabetes and hypertension.1 Loss of kidney function usually takes place gradually over many years. Whether an individual develops ESKD depends on the type of primary kidney disease, how well it is managed, and other risk factors and comorbidities. Most people with chronic kidney disease will die of a comorbid condition, usually cardiovascular disease, before experiencing complete kidney failure requiring dialysis or transplantation.2 However, the onset and progression of chronic kidney disease are highly preventable, and early treatment of complications can significantly improve long-term patient outcomes.3
There is strong evidence that low socioeconomic status (SES) is associated with elevated rates of cardiovascular morbidity and mortality.4–6 Recent reports have observed similar associations between SES and the prevalence and progression of chronic kidney disease,7–10 suggesting the existence of an unrecognized group at risk for ESKD and cardiovascular complications of chronic kidney disease. However, it is difficult to assess whether we can generalize these findings beyond the few countries for which data are available or beyond high-income countries. Environmental and infectious causes of chronic kidney disease11 disproportionately affect the poor of low- and middle-income countries. Combined with a growing prevalence of vascular risk factors accompanying epidemiological transitions12 and inequities in access to medical services, this may result in a similar excess burden of chronic kidney disease among the disadvantaged populations of these countries; but this remains largely unexplored.
Population representative datasets, including data on kidney damage, are now available for Australia, from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), and Thailand, from the International Collaborative Study of Cardiovascular Disease in Asia (InterASIA). We analyzed data from these 2 studies, conducted during 1999–2001 and 2000 respectively, alongside US data from the third National Health and Nutrition Examination Survey (NHANES III).13–15 We hypothesized that the association between disadvantage and elevated rates of chronic kidney disease observed for the US population would also be observed in Australia. We also hypothesized that we would see a similar association for the Thai population, despite differing levels of economic development. Relative advantage versus disadvantage was defined in terms of categories of income, educational attainment, and employment status and explored for its association with chronic kidney disease.
There is currently an international call for primary and secondary prevention of chronic kidney disease, particularly in developing regions in which resources are lacking to sustain expensive dialysis and transplant services.16 Understanding the relation between SES and chronic kidney disease may help to define new population groups at risk and identify important barriers to disease detection and appropriate management; this information would have implications for the design of preventive interventions and for health service planning and delivery. We sought to determine whether associations between socioeconomic disadvantage and chronic kidney disease are consistent across high-income countries and whether such relations exist outside high-income countries.
METHODS
Study Samples
NHANES III, AusDiab I, and InterASIA (Thailand) are nationally representative studies of the health and nutritional status of the US, Australian, and Thai populations, respectively. NHANES III was conducted from 1988 to 1994, AusDiab I from 1999 to 2001, and InterASIA (Thailand) in 2000. Details of sample selection and data collection methods have been described elsewhere.13–15 The minimum age for inclusion in our analysis was 35 years, the inclusion criteria stipulated for the InterASIA study.14
Individual participant data were obtained from the 3 studies (NHANES III: n = 10625; InterASIA (Thailand): n = 5099; AusDiab I: n = 9852) for participants 35 years or older who had a valid serum creatinine measurement. Valid measurements on all required variables were available for 9098 participants from NHANES III, 5063 from InterA-SIA (Thailand), and 9329 from AusDiab I. Because of documented differences in rates and outcomes of chronic kidney disease among US White, non-Hispanic Black, and Mexican American individuals17,18 as well as differences in relative advantage, we also conducted separate analyses of these groups (n = 4482, n = 2214, and n = 2049, respectively). Although indigenous Australians suffer a disproportionately high burden of chronic kidney disease and ESKD, only 88 people in the AusDiab I study had identified as Aboriginal or Torres Strait Islander (0.8%) and were therefore not examined separately. No data on ethnicity were collected as part of the InterASIA study.
Measures
The outcome of interest was prevalence of chronic kidney disease stage 3 or above,19 defined as estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2. We estimated GFR from serum creatinine level using the Modification of Diet in Renal Disease Study prediction formula20,21:
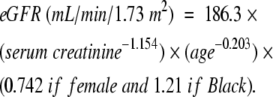 |
(1) |
Correction is required for gender and race because for any given GFR, serum creatinine concentration is significantly higher in men than in women and in Black persons than in White persons because of differences in muscle mass.20
In each study, serum creatinine was measured at a central laboratory using the modified kinetic Jaffe reaction.14,17,22 Serum creatinine measurements from NHANES III and InterASIA (Thailand) were standardized to samples stored at the Cleveland Clinic (Cleveland, Ohio).23 Serum creatinine measurements from AusDiab I have not been standardized. Although this may affect estimated prevalence of chronic kidney disease for the Australian population, it does not necessarily affect the relation between prevalence and socioeconomic factors.
We compared household income, employment status, and level of education across the 3 countries. From data on household income and educational attainment, we constructed categories to reflect relative disadvantage in each country. A square root equivalence scale was applied to total household income to calculate a figure for household income corrected for the number of people dependent on that income.24 Absolute household income (or the upper limit of the income bracket where actual income was not recorded) was divided by the square root of household size and then grouped into quartiles to form income equivalence groups.
For the NHANES population, quartiles from lowest to highest were defined as corrected household income less than US $12000, $12000 to $20499, $20500 to $27999, and greater than $28000. For the AusDiab population, quartiles were defined as less than Aus $16000, $16000 to $29999, $30000 to $46999, and greater than $47000. For the InterASIA population, quartiles were defined as less than 17000, 17000 to 31999, 32 000 to 59 999, and 60000 or more Thai baht. For the US and Australian populations, the majority of adults 35 years or older had completed at least 12 years of schooling, with a large proportion having completed exactly 12 years, corresponding with the completion of high school. Therefore, we grouped educational attainment into categories of fewer than 12 years, 12 years, or more than 12 years. By contrast, approximately two thirds of the Thai population had completed exactly 4 years of education. Because of the very large size of this group, results for the Thai population were compared for fewer than 4 years, 4 years, and more than 4 years of education.
Statistical Analysis
Logistic regression was used to calculate the odds ratio for the association between an eGFR of less than 60 mL/min/1.73 m2 and each of the socioeconomic variables (household income, employment status, and educational attainment) separately. We constructed multivariate models to account for potential explanatory variables and separate models estimated for each socioeconomic variable. The first set of models adjusted for diabetes and hypertension status, which are principal risk factors for chronic kidney disease1,25,26 and have been demonstrated to be more prevalent with lower education and lower income.27 The second set of models, additionally, adjusted for urban or rural area of residence, abdominal obesity, smoking status, and previous cardiovascular events (stroke or heart attack), factors that have also been linked to chronic kidney disease prevalence and incidence.25,28 Analyses were carried out in SAS software version 9.1 (SAS Institute, Cary, North Carolina) using proc survey logistic. In all analyses, we adjusted standard errors for the sampling design of each survey, using the appropriate clustering, stratification, and sampling weights.
RESULTS
Characteristics of the Study Participants
Demographic, socioeconomic, and relevant health characteristics of the US, Australian, and Thai participants appear in Table 1 ▶. The Thai population had a younger distribution than the US and Australian populations, which were alike in their age structure. The Australian population was the most urbanized, and the Thai population the least. A significantly higher proportion of the Thai population was employed than the US and Australian populations (77.1% compared with 59.9% and 57.0%, respectively). The prevalence of stages 3 to 5 chronic kidney disease (eGFR < 60 mL/min/1.73 m2) among adults 35 years and older was 6.6% in the United States (7.2% among non-Hispanic Whites, 5.6% among non-Hispanic Blacks, and 2.1% among Mexican Americans), 13.9% in Thailand, and 10.0% in Australia. The Thai population had notably lower rates of hypertension and cardiovascular problems. The US population had the highest rate of abdominal obesity.
TABLE 1—
Population Weighted Demographic, Socioeconomic, and Health-Related Characteristics Among US, Australian, and Thai Populations 35 Years or Older, by Study: NHANES III, AusDiab I, and InterASIA (Thailand)
| NHANES III (n = 9098), % | AusDiab I (n = 9329), % | InterASIA (Thailand) (n = 5063), % | |
| Age group, y | |||
| 35–44 | 34.4 | 31.4 | 40.8 |
| 45–54 | 21.8 | 26.6 | 26.6 |
| 55–64 | 18.3 | 17.4 | 17.2 |
| 65–74 | 15.7 | 16.2 | 12.7 |
| ≥ 75 | 9.7 | 8.5 | 2.6 |
| Gender | |||
| Men | 46.5 | 48.6 | 48.7 |
| Women | 53.5 | 51.4 | 51.3 |
| Area of residence | |||
| Urban | 48.2 | 58.8 | 32.3 |
| Rural | 51.8 | 41.2 | 67.7 |
| Schooling, y | |||
| < 4 | . . . | . . . | 11.9 |
| 4 | . . . | . . . | 63.1 |
| > 4 | . . . | . . . | 25.1 |
| < 12 | 27.1 | 42.8 | . . . |
| 12 | 32.4 | 18.4 | . . . |
| > 12 | 40.5 | 38.8 | . . . |
| Income equivalence groupsa | |||
| Lowest quartile | 23.5 | 26.5 | 24.5 |
| Second quartile | 25.3 | 25.4 | 24.9 |
| Third quartile | 22.9 | 23.3 | 25.1 |
| Highest quartile | 28.3 | 24.8 | 25.5 |
| Employment status | |||
| Employed | 59.9 | 57.0 | 77.1 |
| Not employed | 19.6 | 16.4 | 16.4 |
| Retired | 20.5 | 26.6 | 6.5 |
| eGFR | |||
| ≥ 60 mL/min/1.73 m2 | 93.4 | 90.0 | 86.1 |
| < 60 mL/min/1.73 m2 | 6.6 | 10.0 | 13.9 |
| Hypertensionb | |||
| Yes | 41.2 | 43.2 | 24.3 |
| No | 58.8 | 56.8 | 75.7 |
| Stroke or heart attack | |||
| Yes | 7.3 | 6.4 | .8 |
| No | 92.7 | 93.6 | 99.2 |
| Diabetesc | |||
| Yes | 10.6 | 7.4 | 9.8 |
| No | 89.4 | 92.6 | 90.4 |
| Abdominal obesityd | |||
| Yes | 69.4 | 32.0 | 37.8 |
| No | 30.6 | 68.0 | 62.2 |
| Smoking status | |||
| Current smoker | 24.3 | 14.6 | 24.8 |
| Ex-smoker | 32.7 | 29.3 | 13.9 |
| Never smoked | 42.9 | 56.1 | 61.3 |
Note. NHANES III = Third National Health and Nutrition Examination Survey; AusDiab I = Australian Diabetes, Obesity, and Lifestyle Study, and InterASIA (Thailand) = International Collaborative Study of Cardiovascular Disease in Asia; eGFR = estimated glomerular filtration rate.
aIncome equivalence was calculated by dividing total household income by square root of the number of people dependent on that income. The 25th, 50th, and 75th percentiles for construction of income equivalence groups are US $12 000, US $20 500, and US $28 000 (United States); Aus $16 000, Aus $30 000, and Aus $47 000 (Australia); and 17 000, 32 000, and 60 000 Thai baht (Thailand).
bHypertension was defined as systolic blood pressure at more than 140 mm Hg and diastolic blood pressure at more than 90 mm Hg, taking antihypertensive medication or if a doctor ever told the participant that he or she has high blood pressure or hypertension.
cDiabetes was defined as random plasma glucose at more than 7 mmol/L, taking insulin or oral agents, or if a doctor ever told the participant that he or she has diabetes.
dAbdominal obesity defined according to waist circumference: more than 80 cm for women, more than 94 cm for non-Hispanic White or Black men, and more than 90 cm for Asian or Mexican American men.29
Approximately 40% of adults 35 years and older in the United States and Australia had received more than 12 years of education. The great majority (63.1%) of the Thai population 35 years or older had received exactly 4 years of education. The distribution of educational attainment above 4 years was 6.8% with 5 to 6 years, 6.8% with 7 to 11 years, 4.2% with 12 years, and 7.0% with more than 12 years of education. These figures correspond with World Bank statistics on education, which estimate that, for the population older than 25 years in 2000, an average duration of schooling of 12.25 years in the United States, 10.57 years in Australia, and 6.10 years in Thailand.30
Results of Crude Analysis
The crude associations between risk factors for chronic kidney disease and prevalence of eGFR less than 60 mL/min/1.73 m2 across all groups are shown in Table 2 ▶. All associations were in the expected direction except for smoking—people who had never smoked or were ex-smokers were more likely than were current smokers to have an eGFR at less than 60 mL/min/1.73 m2. However, this association became nonsignificant after adjusting for age. Income in the lowest quartile, shorter duration of education, and being unemployed were associated (P< .01) with significantly increased odds of eGFR at less than 60 mL/min/1.73 m2 on crude analysis among US non-Hispanic Whites, US non-Hispanic Blacks, the Thai population, and the Australian population (Table 3 ▶). Education level was not significantly associated with chronic kidney disease prevalence among Mexican Americans, although point estimates were similar to those for other ethnic groups, and the wide confidence intervals probably result from the smaller size of this group.
TABLE 2—
Crude Odds Ratios (95% Confidence Interval) for the Association Between Prevalence of Chronic Kidney Disease and Extraneous Variables for US, Australian, and Thai Populations 35 Years or Older: NHANES III, AusDiab I, and InterASIA (Thailand)
| United States | |||||
| Non-Hispanic White (n = 4482) | Non-Hispanic Black (n = 2214) | Mexican American (n = 2049) | Thailand (n = 5063) | Australia (n = 9329) | |
| Age,a y | |||||
| 45–54 | 1.50 (0.56, 4.02) | 1.96 (0.79, 4.82) | . . . | 4.14 (2.47, 6.94) | 6.85 (4.86, 12.15) |
| 55–64 | 4.02 (1.77, 9.14) | 8.07 (3.81, 17.09) | 8.10 (2.81, 23.38) | 14.11 (8.35, 23.83) | 13.26 (7.49, 23.46) |
| 65–74 | 12.05 (5.75, 25.22) | 18.70 (9.64, 36.27) | 17.41 (6.71, 45.20) | 34.08 (19.48, 59.62) | 59.66 (34.27, 103.86) |
| ≥ 75 | 46.01 (22.16, 95.51) | 38.51 (19.38, 76.52) | 41.93 (16.79, 104.73) | 48.95 (24.84, 96.46) | 121.15 (69.83, 210.20) |
| Women (vs men) | 1.58 (1.20, 2.05) | 1.31 (0.90, 1.92) | .99 (0.57, 1.71) | 1.34 (.87, 2.06) | 2.32 (1.75, 3.07) |
| Smokingb | |||||
| Ex-smoker | 2.97 (2.11, 4.18) | 2.34 (1.48, 3.72) | 3.39 (1.31, 8.75) | 1.85 (1.29, 2.66) | 2.65 (1.71, 4.11) |
| Never smoked | 2.57 (1.83, 3.61) | 1.83 (1.16, 2.88) | 1.98 (0.96, 4.08) | 1.68 (1.05, 2.70) | 2.51 (1.62, 3.90) |
| Rural area of residence (vs urban) | 1.12 (0.86, 1.46) | 1.36 (0.96, 1.93) | 1.37 (0.64, 2.91) | 1.31 (0.76, 2.25) | 1.43 (0.86, 2.38) |
| Hypertensivec (vs not) | 4.85 (3.79, 6.20) | 7.94 (5.35, 11.79) | 6.09 (3.21, 11.58) | 2.74 (2.05, 3.66) | 3.62 (3.01,4.35) |
| History of stroke or heart attack (vs none) | 5.65 (4.42, 7.22) | 5.38 (3.62, 7.99) | 5.38 (2.90, 9.99) | 3.16 (1.47, 6.80) | 4.71 (3.43, 6.47) |
| Diabeticd (vs not) | 3.26 (2.30, 4.62) | 4.71 (2.87, 7.73) | 2.99 (2.33, 3.85) | 1.97 (1.13, 3.41) | 1.49 (1.16, 1.90) |
| Has abdominal obesitye (vs does not) | 2.06 (1.53, 2.79) | 1.43 (0.91, 2.25) | 1.48 (0.72, 3.06) | 1.49 (0.72, 3.06) | 2.27 (1.68, 3.07) |
Note. NHANES III = Third National Health and Nutrition Examination Survey; AusDiab I = Australian Diabetes, Obesity, and Lifestyle Study, and InterASIA (Thailand) = International Collaborative Study of Cardiovascular Disease in Asia; eGFR = estimated glomerular filtration rate. Chronic kidney disease was defined as a GFR of less than 60 mL/min/1.73 m2 (chronic kidney disease severity of Stage 3 or greater).31 Ellipses indicate that there were insufficient numbers for the calculation of an estimate.
aAge 35–44 was the reference category.
bCurrent smoker was the reference category.
cHypertension defined as systolic blood pressure at more than 140 mm Hg and diastolic blood pressure at more than 90 mm Hg, taking antihypertensive medication, or if a doctor ever told participant they have high blood pressure or hypertension.
dDiabetes was defined as random plasma glucose at more than 7 mmol/L, taking insulin or oral agents, or if a doctor ever told the participant he or she has diabetes.
eAbdominal obesity defined according to waist circumference: more than 80 cm for women, more than 94 cm for non-Hispanic White or Black men, and more than 90 cm for Asian or Mexican American men.29
TABLE 3—
Crude Age-, Gender-, and Multivariate-Adjusted Odds Ratios (95% Confidence Interval) for the Association Between Socioeconomimc Variables and Chronic Kidney Disease Among US, Australian, and Thai Populations 35 Years or Older: NHANES III, AusDiab I, and InterASIA (Thailand)
| Model 1 (Crude) | Model 2 | Model 3 | Model 4 | |
| United States, non-Hispanic White (n = 4482) | ||||
| Education,a y | ||||
| < 12 | 3.18 (2.41, 4.20) | 1.33 (1.01, 1.76) | 1.28 (.97, 1.70) | 1.34 (1.01, 1.77) |
| 12 | 1.29 (.99, 1.68) | .99 (.75, 1.29) | .94 (.72, 1.24) | 1.00 (.76, 1.30) |
| Incomeb | ||||
| Lowest quartile | 3.92 (2.69, 5.72) | 1.86 ( 1.27, 2.72) | 1.77 (1.22, 2.56) | 1.80 (1.17, 2.77) |
| Second quartile | 2.00 (1.32, 3.03) | 1.44 (.93, 2.22) | 1.38 (.89, 2.12) | 1.34 (.84, 2.15) |
| Third quartile | .99 (.67, 1.47) | 1.23 (.84, 1.80) | 1.24 (.84, 1.82) | 1.18 (.77, 1.79) |
| Not employed, not retired (vs employed) | 3.54 (2.52, 4.98) | 1.00 (.73, 1.38) | .95 (.69, 1.30) | .81 (.57, 1.16) |
| United States, non-Hispanic Black (n = 2214) | ||||
| Education,a y | ||||
| < 12 | 4.97 (2.94, 8.38) | 2.07 (1.17, 3.68) | 1.82 (1.00, 3.34) | 1.79 (.92, 3.47) |
| 12 | 1.79 (1.04, 3.08) | 1.63 (.95, 2.81) | 1.48 (.87, 2.54) | 1.31 (.75, 2.30) |
| Incomeb | ||||
| Lowest quartile | 3.73 (1.58, 8.79) | 1.81 (.75, 4.38) | 1.49 (.58, 3.82) | 1.47 (.57, 3.81) |
| Second quartile | 2.05 (.81, 5.17) | 1.44 (.55, 3.77) | 1.25 (.46, 3.39) | 1.25 (.46, 3.40) |
| Third quartile | 1.17 (.38, 3.61) | 1.06 (.35, 3.28) | 1.00 (.32, 3.16) | .84 (.25, 2.81) |
| Not employed, not retired (vs employed) | 4.72 (2.73, 8.18) | 2.89 (1.53, 5.46) | 2.47 (1.30, 4.71) | 2.38 (1.24, 4.58) |
| United States, Mexican American (n = 2049) | ||||
| Education,a y | ||||
| < 12 | 2.37 (.73, 7.68) | .86 (.25, 2.96) | .79 (.24, 2.68) | 1.40 (.40, 4.81) |
| 12 | 2.43 (.63, 9.29) | 1.86 (.47, 7.31) | 1.97 (.48, 8.05) | 3.52 (.86, 14.44) |
| Incomeb | ||||
| Lowest quartile | 1.84 (.66, 5.13) | 1.32 (.47, 3.70) | 1.07 (.37, 3.07) | .98 (.32, 2.99) |
| Second quartile | 1.02 (.32, 3.29) | .97 (.32, 2.99) | .87 (.28, 2.75) | .81 (.25, 2.59) |
| Third quartile | .42 (.07, 2.47) | .51 (.09, 2.99) | .38 (.06, 2.57) | .21 (.04, 1.20) |
| Not employed, not retired (vs employed) | 10.12 (3.66, 27.99) | 6.62 (1.94, 22.64) | 5.52 (1.51, 20.15) | 4.49 (1.15, 17.55) |
| Australia (n = 9329) | ||||
| Education,a y | ||||
| < 12 | 2.69 (2.20, 3.28) | 1.07 (.88, 1.30) | 1.07 (.88, 1.29) | 1.01 (.80, 1.28) |
| 12 | .97 (.76, 1.23) | .70 (.52, .95) | .70 (.52, .94) | .72 (.53, .99) |
| Incomeb | ||||
| Lowest quartile | 5.81 (3.86, 8.73) | 1.37 (.87, 2.14) | 1.34 (.86, 2.09) | 1.31 (.81, 2.13) |
| Second quartile | 3.99 (2.86, 5.57) | 1.23 (.83, 1.82) | 1.21 (.83, 1.76) | 1.18 (.79, 1.78) |
| Third quartile | 1.27 (.85, 1.91) | 1.34 (.82, 2.19) | 1.33 (.82, 2.16) | 1.32 (.78, 2.25) |
| Not employed, not retired (vs employed) | 4.51 (3.02, 6.72) | 1.38 (.94, 2.02) | 1.35 (.91, 1.99) | 1.26 (.88, 1.83) |
| Thailand (n = 5063) | ||||
| Education,c y | ||||
| < 4 | 5.90 (3.79, 9.19) | 1.17 (.73, 1.88) | 1.22 (.77, 1.93) | 1.11 (.70, 1.76) |
| 4 | 2.55 (1.75, 3.72) | 1.35 (.96, 1.90) | 1.43 (1.01, 2.04) | 1.31 (.92, 1.85) |
| Incomeb | ||||
| Lowest quartile | 1.61 (1.01, 2.58) | .96 (.67, 1.40) | 1.04 (.71, 1.53) | .93 (.63, 1.36) |
| Second quartile | 1.03 (.72, 1.47) | .94 (.66, 1.34) | 1.00 (.70, 1.43) | .92 (.65, 1.32) |
| Third quartile | .91 (.67, 1.25) | .83 (.57, 1.23) | .89 (.60, 1.30) | .82 (.56, 1.20) |
| Not employed, not retired (vs employed) | 3.38 (2.46, 4.64) | 1.25 (.92, 1.70) | 1.20 (.88, 1.63) | 1.27 (.92, 1.77) |
Note. NHANES III = Third National Health and Nutrition Examination Survey; AusDiab I = Australian Diabetes, Obesity, and Lifestyle Study, and InterASIA (Thailand) = International Collaborative Study of Cardiovascular Disease in Asia; eGFR = estimated glomerular filtration rate. Chronic kidney disease was defined as a GFR at less than 60 mL/min/1.73 m2(chronic kidney disease severity of Stage 3 or greater).31 Model 2 was adjusted for age and gender. Model 3 was adjusted for age (continuous), gender, diabetes status (plasma glucose ≥ 7 mmol/L, taking insulin or oral agents, or doctor ever told participant he or she has diabetes), and hypertension status (systolic blood pressure > 140 mm Hg and diastolic blood pressure > 90 mm Hg, taking antihypertensive medication, or doctor ever told participant he or she has high blood pressure or hypertension). Model 4 was adjusted for the same set of covariates as model 3, with additional adjustment for area of residence (urban vs rural), obesity (as defined by waist circumference), smoking status, and previous cardiovascular events (stroke or heart attack).
aMore than 12 years was the reference category.
bHighest quartile was the reference category. Income equivalence was calculated by dividing total household income by the square root of the number of people dependent on that income. The 25th, 50th, and 75th percentiles for construction of income equivalence groups are US $12 000, US $20 500, and US $28 000 (United States); Aus $16 000, Aus $30 000, and Aus $47 000 (Australia); and 17 000, 32 000, and 60 000 Thai baht (Thailand).
aMore than 4 years was the reference category.
Results of Multivariate Analysis
Age- and gender- and multivariate-adjusted ORs for the associations between socioeconomic variables and prevalence of eGFR less than 60 mL/min/1.73 m2 are given in Table 3 ▶. The crude effects of SES reduced substantially with adjustment for age and gender. The odds ratios for prevalence of eGFR at less than 60 mL/min/1.73 m2 for participants with fewer than 12 years of education, compared with those with more than 12 years of education, remained significantly high for US non-Hispanic White and non-Hispanic Black participants. Odds ratios also remained significantly high for unemployed non-Hispanic Blacks and Mexican Americans compared with employed groups, and for US non-Hispanic Whites in the lowest quartile for income compared with the highest quartile.
Testing for trend across income quartile groups indicated a significant negative gradient in the age- and gender-adjusted association between income group and prevalence of eGFR at less than 60 mL/min/1.73 m2 among the US White (P = .002) and non-Hispanic Black (P = .04) populations. That is, the odds of prevalent chronic kidney disease increased with descent in income group for these populations. A gradient effect of education is also suggested for non-Hispanic Blacks, whereby the odds of prevalent chronic kidney disease increased with lower categories of educational attainment. None of the results for the Australian or Thai populations was significant after adjustment for age and gender, and there was no evidence of a gradient in the effect of income for either population.
Adjusting for diabetes and hypertension status (Table 3 ▶, model 3) and additional potential explanatory variables (Table 3 ▶, model 4) did not substantially alter these findings. In the fully adjusted model, the remaining statistically significant associations were unemployment for non-Hispanic Black and Mexican Americans and income in the lowest quartile (vs highest) or fewer than 12 years education (vs greater than 12 years) for US non-Hispanic Whites. The association between chronic kidney disease prevalence and fewer than 12 years education (vs greater than 12 years) was of similar magnitude in the non-Hispanic Black population compared with the US non-Hispanic White population; however, this was borderline significant only, possibly because of the smaller sample size for this group.
DISCUSSION
We explored the relation between disadvantage and chronic kidney disease burden to determine whether disadvantaged groups should be targeted for health promotion, screening, and early intervention. On crude analysis, we found large and consistent effects of disadvantage on chronic kidney disease prevalence across the 3 countries. Following adjustment for age and gender, disadvantage was associated with higher prevalence of chronic kidney disease in the US population. Income quartile had the strongest effect among the White population, whereas employment status had the strongest effect among the non-Hispanic Black and Mexican American populations.
These results are consistent with previous findings for the US population.9 To find prevalent chronic kidney disease, disadvantaged groups in the United States are an excellent group to study. Moreover, adjusting for known chronic kidney disease risk factors did not alter these findings; hence, disadvantage affects chronic kidney disease prevalence in the United States via mechanisms independent of the clustering of risk factors in groups by SES. By contrast, the crude associations observed for the Australian and Thai populations disappeared after adjustment for age and gender, and we cannot definitively conclude on the basis of our results that there is a consistent international association between being in the most disadvantaged socioeconomic group and higher chronic kidney disease prevalence.
It is postulated that education, income, and employment have an impact on health via such mechanisms as deprivation in infancy and childhood, poor diet and nutrient intake, fewer leisure-time activities, lack of social support, and housing and monetary difficulties.32 Other factors relevant to chronic kidney disease prevalence may include exposure to infection, environmental toxins, and poor fetal nutrition influencing kidney development and subsequent function.33 Differences in access to health care and health insurance may also play a role, possibly explaining the high residual risks of chronic kidney disease among unemployed non-Hispanic Black and Mexican American participants. SES affects health through complex pathways. Why income and education have significant effects on chronic kidney disease prevalence in the US non-Hispanic White population but not among the Australian population is not clear. Factors relating to differences in health care systems or issues of access to health care and primary prevention may be involved.
Elevated Burden of Chronic Kidney Disease in Thailand
After adjustment for age and gender, there were no significant associations for the Thai population. Profound social and economic changes accompanied by rapidly changing patterns of diet and exercise are promoting a rising burden of chronic, noncommunicable disease in developing countries.34 Large-scale urbanization of the rural poor has also contributed to escalating rates of cardiovascular disease, diabetes, and other chronic diseases.35 Cardiovascular diseases are a leading cause of death in Thailand. Major vascular risk factors, including elevated blood pressure and serum total cholesterol, diabetes, obesity, and smoking, are highly prevalent in Thailand, especially in the urban population.14 Chronic kidney disease is inextricably interrelated with chronic vascular diseases and shares risk factors. We believe the interaction between environmental and socioeconomic circumstances generates an excess burden of chronic kidney disease in Southeast Asia.
In Thailand, infectious diseases, exposure to plant and animal toxins, kidney stones causing obstruction to urine flow, and traditional medicines are relatively common causes of chronic kidney disease in rural areas, whereas in larger urban centers, the causes of chronic kidney disease resemble those reported for the United States and Australia, with diabetic nephropathy in particular a significant and growing cause.36 We were unable to detect a robust association between relative disadvantage and prevalence of chronic kidney disease in Thailand, perhaps because escalating rates of vascular risk factors are leading to an elevated burden of chronic kidney disease among the less disadvantaged urban population and infectious and other causes are leading to a high burden of chronic kidney disease among the more disadvantaged rural population.
A number of other factors may have contributed to the null result for the Thai population. First, income, years of education, and employment status are likely to be distributed differently in Thailand than in the United States and Australia. Second, although we defined categories of education and income designed to identify groups of relative disadvantage specific to each country, this relative disadvantage may not have the same effect in Thailand in terms of societal participation, access to services including health care, and detrimental exposure to disadvantage in early life. Although the majority of the Thai population had 4 years of education, this is unlikely to signify the same level of disadvantage in early life, opportunity in later life, or ability to process health-related information as do 12 years of education in the United States or Australia. Third, income, educational attainment, and employment status, when used in cross-national comparisons, may be poorer markers of a person’s place in Thai society. Last, urban or rural area of residence, a factor included in the multivariate model, may be a more important determinant of access to employment, income, and educational opportunities and of access to health care services in a country with less well-developed health and social infrastructures.
Limitations
Our analysis is subject to several limitations. First, our data are cross-sectional. Chronic kidney disease develops slowly, and social position may affect its progression across the life course.37 In each study, serum creatinine was measured on only one occasion; whether kidney damage was persistent was not assessed. The cross-sectional nature of the data introduces the possibility of reverse causality whereby poor physical and mental functioning because of chronic kidney disease and associated comorbidities may limit overall prospects for employment and income. However, because we were interested in earlier stages of chronic kidney disease, which is often asymptomatic, reverse causality is less of a concern.
Second, the need to define measures of SES that were consistent with the available data necessitated broad categorizations of education, income, and employment. Third, GFR-estimating formulae have not been validated for Asian populations, making the accuracy of values estimated for the Thai participants uncertain. A single study among Chinese patients with chronic kidney disease showed some bias in the use of GFR-estimating formulae when compared with gold standard measures of kidney function.38 This would affect estimates of chronic kidney disease prevalence but not necessarily the association with SES. Finally, we considered only individual-level socioeconomic factors and did not account for the possibility that living in a poorer area exerts an independent effect on health.8
Conclusions
Epidemiological studies examining the relation between SES and kidney disease have focused primarily on the link between SES and treated ESKD.39–42 To our knowledge, our study is the first population-representative analysis of the effect of SES on chronic kidney disease prevalence in the Australian and Thai populations and the first use of international data to directly compare the magnitude and consistency of the effects. Data from the InterASIA study indicate a heavy burden of chronic kidney disease in Thailand, with 13.9% of the population 35 years and older having an eGFR at less than 60 mL/min/1.73 m2 compared with 10% in Australia and 6.6% in the United States. Preventative approaches to chronic kidney disease are particularly critical in low- and middle-income countries such as Thailand because of the extremely high burden of cardiovascular morbidity and mortality associated with chronic kidney disease and, for a smaller number of patients, the enormous resource, infrastructure, and personnel requirements of dialysis and transplant programs.36,43
Our results indicated neither a detrimental effect of being in the group at greatest disadvantage nor a detrimental effect of being at the greatest advantage in terms of chronic kidney disease prevalence among Thai adults. This suggests that the advantaged and the disadvantaged share somewhat equally in the large burden of chronic kidney disease in the Thai population, although likely through different causal pathways. American clinical practice guidelines identify those of low SES as a population group susceptible to chronic kidney disease, and our results are consistent with this. In the United States, chronic kidney disease prevention and management strategies should take particular account of the higher likelihood of disease among low-income non-Hispanic Whites and unemployed non-Hispanic Black and Mexican American individuals. It is less clear whether disadvantaged Australians would benefit from targeted intervention. More research is needed to determine which population groups in Thailand experience the greatest risk of chronic kidney disease and what is driving the large burden of chronic kidney disease in this country.
Acknowledgments
S. White was supported by an Australian Postgraduate Award.
Peer Reviewed
Contributors S.L. White was involved in the conception of the analyses, drew together the results of the analyses, interpreted the data, and led the writing of the article. K. McGeechan conducted the analyses. M. Jones was involved in the conception of the analyses and the acquisition of data and supervised the data analysis. A. Cass was involved in the conception of the analyses and writing. S.J. Chadban was involved in the conception of the analyses and writing. K. R. Polkinghorne was involved in the conception of the analyses and the acquisition of data and assisted in data analysis. V. Perkovic was involved in the conception of the analyses and writing and the acquisition of data. P.J. Roderick was involved in the conception of the analyses and writing and the acquisition of data. All authors helped in the conception and design of the article and interpretation of the data, all critically revised drafts of the article, and all approved the final version for publication.
Human Participant Protection No protocol approval was needed for this study.
References
- 1.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339: 1448–1456. [DOI] [PubMed] [Google Scholar]
- 2.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DW. Evidence-based guide to slowing the progression of early renal insufficiency. Intern Med J. 2004;34:50–57. [DOI] [PubMed] [Google Scholar]
- 4.Kunst AE, Groenhof F, Mackenbach JP, Health EW. Occupational class and cause specific mortality in middle aged men in 11 European countries: comparison of population based studies. EU Working Group on Socioeconomic Inequalities in Health. BMJ. 1998;316: 1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch JW, Kaplan GA, Cohen RD, Tuomilehto J, Salonen JT. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality, and acute myocardial infarction? Am J Epidemiol. 1996;144:934–942. [DOI] [PubMed] [Google Scholar]
- 6.van Rossum CT, Shipley MJ, van de Mheen H, Grobbee DE, Marmot MG. Employment grade differences in cause specific mortality. A 25-year follow up of civil servants from the first Whitehall study. J Epidemiol Community Health. 2000;54:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fored CM, Ejerblad E, Fryzek JP, et al. Socioeconomic status and chronic renal failure: a population-based case-control study in Sweden. Nephrol Dial Transplant. 2003;18:82–88. [DOI] [PubMed] [Google Scholar]
- 8.Merkin SS, Coresh J, Roux AV, Taylor HA, Powe NR. Area socioeconomic status and progressive chronic kidney disease: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2005;46:203–213. [DOI] [PubMed] [Google Scholar]
- 9.Martins D, Tareen N, Zadshir A, et al. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2006;47:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis. 2003;42:677–684. [DOI] [PubMed] [Google Scholar]
- 11.Barsoum R. End-stage renal disease in North Africa. Kidney Int Suppl. 2003;63(suppl 83):S111–S114. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104: 2746–2753. [DOI] [PubMed] [Google Scholar]
- 13.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;32:1–407. [PubMed] [Google Scholar]
- 14.InterASIA Collaborative Group. Cardiovascular risk factor levels in urban and rural Thailand—The International Collaborative Study of Cardiovascular Disease in Asia (InterASIA). Eur J Cardiovasc Prev Rehabil. 2003;10:249–257. [DOI] [PubMed] [Google Scholar]
- 15.Dunstan DW, Zimmet PZ, Welborn TA, et al. The Australian Diabetes, Obesity and Lifestyle Study (Aus-Diab)—methods and response rates. Diabetes Res Clin Pract. 2002;57:119–129. [DOI] [PubMed] [Google Scholar]
- 16.Dirks JH, de Zeeuw D, Agarwal SK, et al. Prevention of chronic kidney and vascular disease: toward global health equity—the Bellagio 2004 Declaration. Kidney Int Suppl. 2005;68(suppl 98):S1–S6. [DOI] [PubMed] [Google Scholar]
- 17.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. [DOI] [PubMed] [Google Scholar]
- 18.United States Renal Data System, USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 2006.
- 19.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Greene T, Kusek JW, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000; 11:A0828. [Google Scholar]
- 22.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the Aus-Diab kidney study. J Am Soc Nephrol. 2003;14(suppl 2): S131–S138. [DOI] [PubMed] [Google Scholar]
- 23.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39: 920–929. [DOI] [PubMed] [Google Scholar]
- 24.Förster MF, Mira d’Ercole M. Income Distribution and Poverty in OECD Countries in the Second Half of the 1990s. Paris, France: Organisation for Economic Co-operation and Development; 2005. OECD Social, Employment and Migration Working Paper, No. 22.
- 25.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291: 844–850. [DOI] [PubMed] [Google Scholar]
- 26.Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341: 1127–1133. [DOI] [PubMed] [Google Scholar]
- 27.Banks J, Marmot M, Oldfield Z, Smith JP. Disease and disadvantage in the United States and in England. JAMA. 2006;295:2037–2045. [DOI] [PubMed] [Google Scholar]
- 28.Levin A, Stevens L, McCullough PA. Cardiovascular disease and the kidney. Tracking a killer in chronic kidney disease. Postgrad Med. 2002;111:53–60. [DOI] [PubMed] [Google Scholar]
- 29.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. [DOI] [PubMed] [Google Scholar]
- 30.The World Bank Group. Education Attainment in the Adult Population (Barro-Lee Dataset). Available at: http://go.worldbank.org/5Y76FNJ0I0. Accessed December 1, 2006.
- 31.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluations, classifications, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 32.Marmot MG, Smith GD, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. [DOI] [PubMed] [Google Scholar]
- 33.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994;23:171–175. [PubMed] [Google Scholar]
- 34.Popkin BM. Urbanization, lifestyle changes and the nutrition transition. World Dev. 1999;27: 1905–1916. [Google Scholar]
- 35.World Health Organization. The World Health Report 2002. Geneva, Switzerland: World Health Organization; 2002.
- 36.Sitprija V. Nephrology in South East Asia: fact and concept. Kidney Int Suppl. 2003;63(suppl 83): S128–S130. [DOI] [PubMed] [Google Scholar]
- 37.Shoham DA, Vupputuri S, Kshirsagar AV. Chronic kidney disease and life course socioeconomic status: a review. Adv Chronic Kidney Dis. 2005;12:56–63. [DOI] [PubMed] [Google Scholar]
- 38.Zuo L, Ma YC, Zhou YH, Wang M, Xu GB, Wang HY. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45:463–472. [DOI] [PubMed] [Google Scholar]
- 39.Young EW, Mauger EA, Jiang KH, Port FK, Wolfe RA. Socioeconomic status and end-stage renal disease in the United States. Kidney Int. 1994;45:907–911. [DOI] [PubMed] [Google Scholar]
- 40.Byrne C, Nedelman J, Luke RG. Race, socioeconomic status, and the development of end-stage renal disease. Am J Kidney Dis. 1994;23:16–22. [DOI] [PubMed] [Google Scholar]
- 41.Cass A, Cunningham J, Wang Z, Hoy W. Social disadvantage and variation in the incidence of end-stage renal disease in Australian capital cities. Aust N Z J Public Health. 2001;25:322–326. [DOI] [PubMed] [Google Scholar]
- 42.Perneger TV, Whelton PK, Klag MJ. Race and end-stage renal disease. Socioeconomic status and access to health care as mediating factors. Arch Intern Med. 1995; 155:1201–1208. [PubMed] [Google Scholar]
- 43.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]


