Abstract
We have previously reported the existence of multiple isoforms of human Nedd4-2 (AJP Renal 2003, 285: F916). When overexpressed in M-1 collecting duct epithelia, full length Nedd4-2 (Nedd4-2), Nedd4-2 lacking the N-terminal C2 domain (Nedd4-2ΔC2) and Nedd4-2 lacking WW domains 2 and 3 (Nedd4-2ΔWW2,3) variably reduce benzamil-sensitive Na+ transport. We investigated the effect of each of the Nedd4-2 isoforms on cell-surface expression and ubiquitination of ENaC subunits. We find that αENaC when transfected alone or with β and γENaC is expressed at the cell surface and this membrane expression is variably reduced by co-expression with each of the Nedd4-2 isoforms. Nedd4-2 reduces the half-life of ENaC subunits and enhances the ubiquitination of α, β and γ ENaC subunits when expressed alone or together suggesting that each subunit is a target for Nedd4-2 mediated ubiquitination. As has been reported recently, we confirm that the surface expressed pool of ENaC is multi-ubiquitinated. Inhibitors of the proteasome increase ubiquitination of ENaC subunits and stimulate Na+ transport in M-1 cells consistent with a role for the ubiquitin-proteasome pathway in regulating Na+ transport in the collecting duct.
Keywords: sodium transport, collecting duct, ENaC, hypertension
Introduction
Amiloride-sensitive Na+ reabsorption occur via epithelial Na+ channel (ENaC) in the transporting epithelia of the connecting tubules and collecting ducts of the kidney. ENaC exists as a heteromultimeric protein complex at the apical membrane of the epithelial cells and consist of three structurally related subunits, α, β, and γ. Aldosterone is one of the more important hormones that regulate distal nephron Na+ transport and its actions appear to be mediated via ENaC. The identification of ENaC mutations that lead to severe inherited hypertension from enhanced Na+ reabsorption or to profound hypotension from salt wasting underscores the importance of the ENaC in the distal nephron in extracellular fluid volume homeostasis and the control of blood pressure (7, 15, 27).
Each of the α, β, and γ subunits of ENaC contain a PY motif in its C-terminus which serves as an endocytosis motif. Heterozygous mutation of the PY motif of β or γ ENaC leads to persistence of ENaC at the cell surface and increased Na+ reabsorption and hypertension in Liddle’s syndrome, an autosomal dominant form of severe hypertension (7, 15, 27). In more recent studies, the PY motif of ENaC was shown to interact with WW domains of the HECT domain-type E3 ubiquitin ligases. Several members of this broad family including Nedd4, Nedd4-2, WWP1 and WWP2 have been shown to inhibit ENaC function in heterologous expression systems (12, 28, 32). Inhibition of Nedd4-2 enhances ENaC activity in a lung epithelial cell line, H441 and when ENaC is overexpressed, in a rat thyroid epithelial cell line, FRT (30). Nedd4-2 has emerged as a likely ubiquitin ligase that regulates ENaC in the collecting duct as well since Nedd4-2 can be phosphorylated and inhibited by Sgk1, a kinase that may mediate some of the actions of aldosterone (6, 29). Furthermore, in the collecting duct and connecting tubule, dietary Na+ intake and aldosterone reduce the abundance of Nedd4-2 and induce some isoforms of 14-3-3, a group of regulatory proteins that reduces Nedd4-2 interaction with ENaC, positioning Nedd4-2 as a central player in the regulation of ENaC in the collecting duct and connecting tubule (3, 10, 17, 18).
In our previous studies we reported on the existence of many isoforms of human Nedd4-2 that arise from alternate transcription and translation and also from variable splicing of some internal exons (11, 12). In the present study we further analyze Na+ transport with each of three hNedd4-2 isoforms in a collecting duct epithelial cell line, M-1. We examine the impact of hNedd4-2 isoforms on the kinetics, the cell-surface expression and the ubiquitination of ENaC.
Materials and methods
Materials
Dexamethasone, selenium, transferrin, triiodothyronine, epidermal growth factor, chloroquine, D-glucose, N-acetyl-Leu-Leu-norleucinal (ALLN), Z-Leu-Leu-Leu-al (MG132) were all purchased from Sigma-Aldrich (St. Louis, MO) and cycloheximide was from EMD Chemicals (San Diego, CA). All cell culture media were obtained from Invitrogen Life Technologies (Gaithersburg, MD). Anti-FLAG M2 and anti-actin were obtained from Sigma Aldrich; anti-V5, anti-Myc and anti-Xpress from Invitrogen; anti-FK1 from Biomol (Plymouth Meeting, PA); anti-P4D1 and HRP-conjugated goat anti-rabbit IgG from Cell Signaling Technology (Danvers, MA), anti-HA from US Biological (Swampscott, MA) anti-α tubulin and HRP-conjugated goat anti-mouse IgG and anti-mouse IgM were from Santa Cruz Biotechnology (Santa Cruz, CA).
Transfection
Human embryonic kidney cell line HEK293 was cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin. Human α, β and γ ENaC cDNAs were subcloned in pcDNA3 (Invitrogen) with C-terminal FLAG, V5 and myc epitopes respectively. Coding regions of all three hNedd4-2 isoforms were subcloned into expression vectors as described previously (12), and an HA-tagged ubiquitin expression vector was a gift from Dr. Dirk Bohmann. Nedd4-2CS was generated by mutating cysteine to alanine at position 942 of hNedd4-2 using QuikChange™ Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). HEK 293 cells were transiently transfected with α, β and γ ENaC, hNedd4-2 and/or HA-ubiquitin using Lipofectamine 2000 (Invitrogen) as per manufacturer’s instructions for adherent cells. Twenty four hr following transfection, cells were directly lysed for western blotting or immunoprecipitation or first used for cell surface biotinylation assays and then lysates prepared. In some experiments, cells were treated with MG132 (1μM), a membrane-permeable inhibitor of the proteasome, for 4 hours prior to lysis/biotinylation. In other experiments, cycloheximide (20 μg/ml), an inhibitor of eukaryotic translation, was added to transfected cells in serum free media for various times prior to cell lysis.
Western blot analysis and immunoprecipitation
Transfected cells were washed twice in phosphate buffered saline (PBS) followed by lysis in 150mM NaCl, 50mM Tris, pH 7.4, 1% Triton X-100, 10μM N-acetyl-Leu-Leu-norleucinal (ALLN) and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) at 4ºC. Protein concentrations were determined using BCA™ protein assay kit (Pierce, Rockford, IL). Equal amounts of protein were diluted in SDS-PAGE sample loading buffer (30% glycerol, 50mM Tris-Cl, pH 6.8, 7.5 % SDS, 200 mM dithiothreitol and bromophenol blue), boiled, separated on a 7% gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA). Membrane was blocked with PBS-Tween 20 (PBST, 0.05% Tween-20 in PBS) containing 5% milk for one hour followed by overnight incubation in 5% milk-PBST with appropriate primary antibody (anti-FLAG 1:3000; anti-actin 1:1000; anti-Xpress 1:5000; anti-HA 1:3000; anti-FK1 1:1000; anti-P4D1 1:1000 or anti-α tubulin 1;1000). Membranes were then washed with PBST and incubated with HRP-conjugated secondary antibody (1:5000) for one hour followed by several washings with PBST. Chemiluminescence detection of proteins was done using Supersignal West Pico or Femto chemiluminescent substrate (Pierce) and the image captured using VisionWorksLS Image Acquisition and Analysis Software and the EC3 imaging system (UVP, LLC, Upland, CA). Stripping and reprobing of membranes with different antibodies was performed with Restore™ Western Blot Stripping Buffer (Pierce) as per manufacturer’s instructions.
For immunoprecipitation 1 mg of protein lysates were incubated overnight with the appropriate antibody in 1:250 dilutions with end-over-end rotation at 4ºC. The cell lysate/antibody mixture was incubated with 80 μl (40ul bead bed volume per sample) of protein G Sepharose (GE Healthcare, Piscataway, NJ) for 1 hour at 4ºC followed by several washings with cold PBS and immunoprecipitates were eluted in 50–80 μl immunoPure® non-reducing sample buffer (Pierce). Eluted proteins were separated on SDS-PAGE, transferred to membranes and blotted as described above.
Cell surface Biotinylation
Transfected HEK 293 cells were surface biotinylated 24 hrs after transfection by Pinpoint™ Cell Surface Protein Isolation Kit (Pierce) as per manufacturer’s protocol. In some experiments after surface biotinylation, HEK cells were lysed in IP buffer and 2–3 mg of proteins were incubated at 4ºC overnight with the appropriate antibody followed by further incubation with protein G Sepharose and PBS washings as described earlier. Immunoprecipitates were eluted from beads in elution buffer (2% SDS, 50mM Tris, 150 mM NaCl, pH 7.5) at 80ºC for 10 min, diluted in IP buffer and subjected to Neutravidin precipitation and analyzed by SDS-PAGE as described above.
Measurement of short-circuit current
M-1 cells were cultured in DMEM/F-12 containing 10% FBS and 1% penicillin-streptomycin. For transport studies, M-1 cells were grown on filters and short circuit currents (Isc) and transepithelial voltage and resistance were measured at 37ºC in Ussing chambers as described previously (12). In some experiments, cells on filters were treated with MG132 (1μM) or chloroquine (100 μM) for varying time periods prior to measurement of Isc.
Adenovirus transduction
Recombinant adenovirus expressing Nedd4-2 and Nedd4-2ΔC2 were constructed as described previously (12). Adenoviral vectors expressing Nedd4-2CS and Nedd4-2ΔWW2, 3 were generated by the vector core facility at the University of Iowa. M-1 cells were transduced with adenovirus as previously described (12).
Statistical analysis
The results are presented as the means ± SD (or SE) with comparison between groups performed by one way analysis of variance and student t test. Significant values were considered at p < 0.05 (SigmaStat, SPSS Chicago, IL).
Results
Nedd4-2 isoforms differentially reduces Na+ transport in collecting duct epithelia
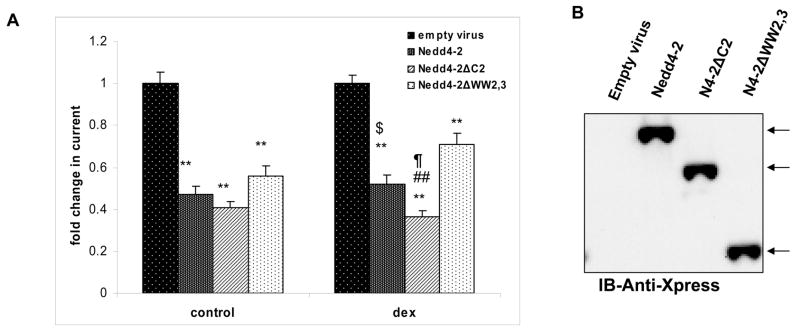
We have identified several hNedd4-2 isoforms including the full length protein that has a C2 domain and all 4 WW domains (Nedd4-2), an isoform lacking a C2 domain (Nedd4-2ΔC2) and an isoform lacking 2 WW domains (Nedd4-2ΔWW2,3) and had begun to examine their function in Xenopus oocytes and in M-1, collecting duct epithelial cells. Transient transfection and expression of Nedd4-2ΔC2 in M-1 cells reduced basal benzamil-sensitive short circuit current (Isc) whereas Nedd4-2ΔWW2,3 was without apparent effect although these were not directly compared (12). To improve expression of Nedd4-2 isoforms without disrupting monolayer integrity on filters, we constructed recombinant adenoviral vectors expressing various hNedd4-2 isoforms. Short circuit currents were measured 24 hr post viral transduction and again following dexamethasone stimulation. Our results indicate that Nedd4-2 isoforms inhibit Na+ transport and that there are differences between these isoforms which are more pronounced and significant following dexamethasone treatment (Fig. 1A). As we have seen in Xenopus oocytes previously, Nedd4-2ΔC2 is the most potent inhibitor of Na+ transport with Nedd4-2ΔWW2,3 being the least effective (12). A Western blot analysis performed on transduced M-1 lysates using an anti-Xpress antibody demonstrated equal expression of all three isoforms confirming that differences between isoforms could not be explained by variations in Nedd4-2 expression (Fig. 1B).
Figure 1. Nedd4-2 isoforms differentially regulate ENaC activity in M-1 cells.
Panel A. M-1 cells transduced with adenovirus expressing Nedd4-2, Nedd4-2ΔC2, Nedd4-2ΔWW2,3 or empty virus and Isc measured in Ussing chambers under control conditions and after treatment with 100 nM dexamethasone (dex) for 24 hr. M-1 cells overexpressing all 3 isoforms had significantly lower currents as compared to empty virus (**P < 0.001; Tukey’s pairwise multiple comparison, SigmaStat; n = 15; mean ± SE). Following steroid treatment, currents were significantly different in between each of the Nedd4-2 isoforms (## P < 0.001 compared to Nedd4-2ΔWW2,3; $P < 0.05 compared to Nedd4-2ΔWW2,3; ¶P < 0.05 compared to Nedd4-2; Tukey’s pairwise multiple comparison, SigmaStat; n = 15; mean ± SE). Panel B. Representative western blot of transduced M-1 cells demonstrate relatively equal expression of each of the Nedd4-2 isoforms. Full length Nedd4-2 migrates at ~116 kDa; Nedd4-2ΔC2 (N4-2ΔC2) migrates at ~101 kDa and Nedd4-2ΔWW2,3 (N4-2ΔWW2,3) migrates at ~83 kDa.
Nedd4-2 isoforms reduces surface expression of αENaC in HEK293 cells
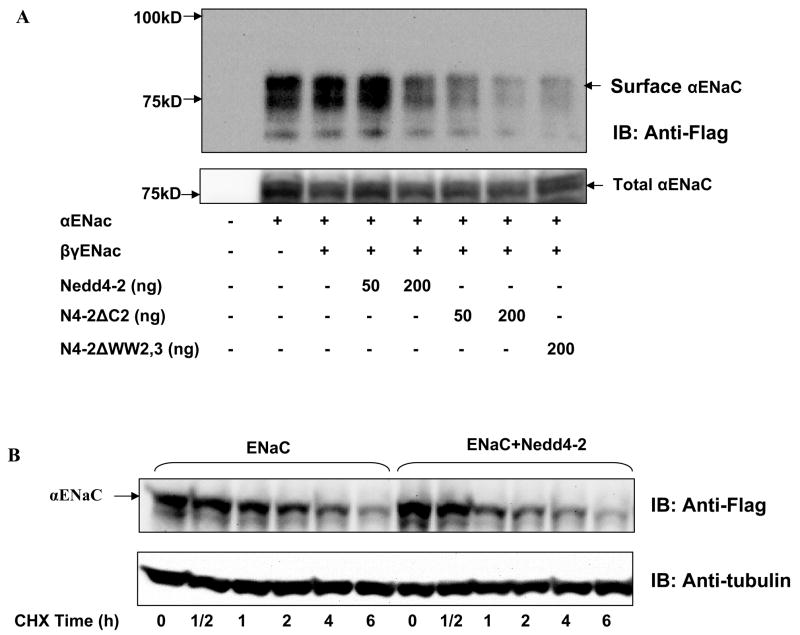
To understand the role of Nedd4-2 isoforms in regulating ENaC, we overexpressed Nedd4-2 isoforms in HEK293 along with single or multiple subunits of individually tagged ENaC subunits. αENaC expressed at the cell membrane was detected by affinity purification of biotinylated surface proteins. Interestingly, αENaC when expressed alone was just as abundant at the cell surface as when expressed with β and γ ENaC, suggesting that at least in this heterologous expression system, αENaC does not require coexpression with β and γ ENaC subunits to traffic to or from the cell surface (Fig. 2A). We observed a dramatic reduction in cell surface expression of ENaC with each of the Nedd4-2 isoforms and in a dose dependent manner, with Nedd4-2ΔC2 being more potent than Nedd4-2 (Fig. 2A). These results are consistent with a role for Nedd4-2 in ubiquitination and degradation of ENaC.
Figure 2. Surface expression and the half-life of ENaC is greatly reduced by Nedd4-2.
Panel A: Nedd4-2 isoforms or a control empty vector was transfected with ENaC subunits as indicated into HEK293 cells and biotinylated surface proteins (panel above) and total cellular lysates (panel below) were blotted with an anti-FLAG antibody, the epitope carried by the αENaC subunit. Each of the Nedd4-2 isoforms reduces surface expression of αENaC in a dose dependent manner with Nedd4-2ΔC2 being most potent. Representative of at least two separate experiments. Panel B: ENaC subunits were cotransfected with Nedd4-2 in HEK 293 cells. Cells were treated with 20 μg/ml cycloheximide to arrest translation and at indicated time points lysates prepared and immunoblotted with an anti-FLAG antibody and after stripping re-probed with anti-α tubulin antibody. Overexpression of Nedd4-2 decreases αENaC stability.
Nedd4-2 overexpression decreases ENaC stability
To determine if Nedd4-2 increases degradation of ENaC, we examined the effect of Nedd4-2 on steady-state levels of αENaC in HEK293 cells after arresting protein translation with cycloheximide. We demonstrate that the half-life of αENaC is shorter when Nedd4-2 is overexpressed compared to its absence confirming that Nedd4-2 increases ENaC turnover (Fig. 2B).
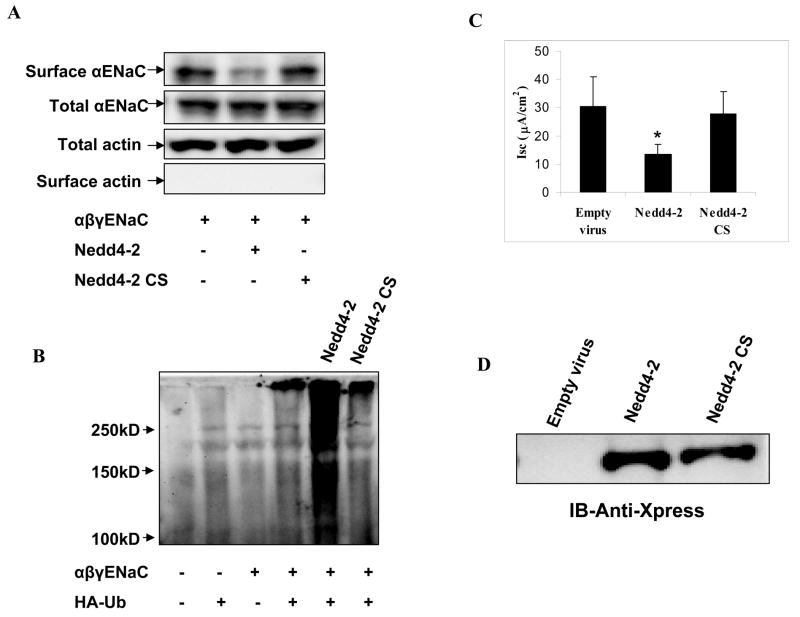
Inactive Nedd4-2 does not reduce surface expression or function of αENaC
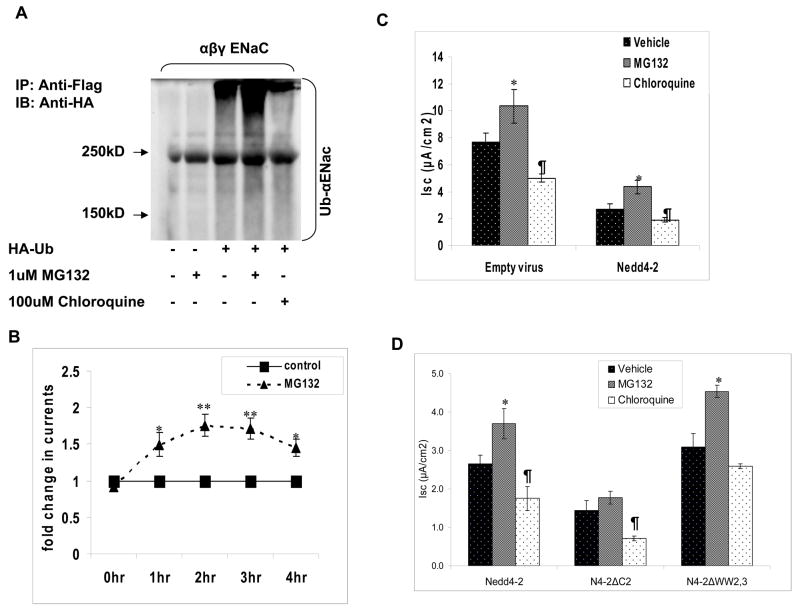
To determine if the ubiquitin ligase function of Nedd4-2 was necessary for the effect of Nedd4-2 on membrane expression of αENaC, we created a catalytically inactive Nedd4-2 by mutating a HECT domain cysteine residue at position 942 to serine (Nedd4-2CS). When compared to wild type Nedd4-2, ubiquitin ligase deficient Nedd4-2 had no effect on surface expression of αENaC in HEK293 cells (Fig. 3A). To validate the surface biotinylation assay, we reprobed surface blots for actin, an abundant intracellular cytoskeletal protein and demonstrated its absence from the biotinylated fraction of cellular proteins. We then transfected HEK 293 cells with tagged αβγENaC subunits, HA-tagged ubiquitin and Nedd4-2 or Nedd4-2 CS to test the effect of Nedd4-2 on ubiquitination of αβγENaC. Immunoprecipitation of ENaC followed by immunoblotting for HA-tagged proteins demonstrated the characteristic high molecular mass smear of ubiquitinated proteins. The data shows that Nedd4-2 robustly stimulates ubiquitination of the ENaC multimer while Nedd4-2CS did not appear to increase ubiquitination (Fig 3B).
Figure 3. Inactivation of the catalytic domain of Nedd4-2 abolishes its effect on ENaC.
Panel A. Active or inactive Nedd4-2 (Nedd4-2CS) or a control empty vector was transfected with ENaC subunits as indicated into HEK293 cells and biotinylated surface proteins and total cellular lysates were blotted with an anti-FLAG antibody, or an actin antibody. The data demonstrates that Nedd4-2 CS does not affect surface expression of ENaC. Actin was immunoblotted in total and surface fractions to confirm that intracellular proteins are excluded in affinity purified surface protein preparations. Panel B. Active or inactive Nedd4-2 (Nedd4-2CS) or a control empty vector were transfected with ENaC subunits and HA-tagged ubiquitin (HA-Ub) as indicated into HEK293 cells. ENaC subunits were immunoprecipitated with an anti-FLAG antibody and immunoblotted with anti-HA to detect ubiquitinated ENaC. Ubiquitinated proteins appear as high molecular mass smear. Compared to active Nedd4-2, Nedd4-2CS has no effect on ubiquitination of ENaC in HEK293 cells. Panel C: Adenoviral vectors expressing active or inactive Nedd4-2 (Nedd4-2CS) or a control empty virus were transduced into M-1 cells. Compared to active Nedd4-2, Nedd4-2CS has no effect on amiloride-sensitive Isc in M-1 cells. (*P < 0.05; Dunn’s pairwise multiple comparison, SigmaStat; n = 6; mean ± SD). Panel D. Representative western blot of transduced M-1 cells demonstrate relatively equal expression of the Nedd4-2 isoforms.
We then generated an adenoviral vector expressing Nedd4-2 CS, expressed this in M-1 cells and measured amiloride-sensitive Isc. Compared to an adenoviral vector expressing Nedd4-2, Nedd4-2 CS mutant had no significant effect on ENaC-mediated Na+ transport although both Nedd4-2 forms were equally expressed when analyzed by western blotting (Fig. 3C and D). The lack of effect of a ubiquitin-ligase deficient Nedd4-2 on Na+ transport in M-1 cells correlated with preserved surface expressed αENaC in HEK cells.
Each of the Nedd4-2 isoforms enhances ubiquitination
Since the ubiquitin ligase function of Nedd4-2 appeared to be required for its effect on Na+ transport, we studied the effect of Nedd4-2 isoforms on ubiquitination of ENaC subunits. Immunoprecipitation of ENaC followed by immunoblotting for ubiquitinated proteins demonstrated that ubiquitination of ENaC subunits can be detected even in the absence of overexpressed Nedd4-2 and that αENaC is ubiquitinated even when expressed alone. Importantly, ubiquitination of the αβγ complex increased in the presence of each of the Nedd4-2 isofoms although we could not detect a noticeable difference among 3 isoforms (Fig. 4).
Figure 4. Each of the Nedd4-2 isoforms, enhances the ubiquitination of ENaC.
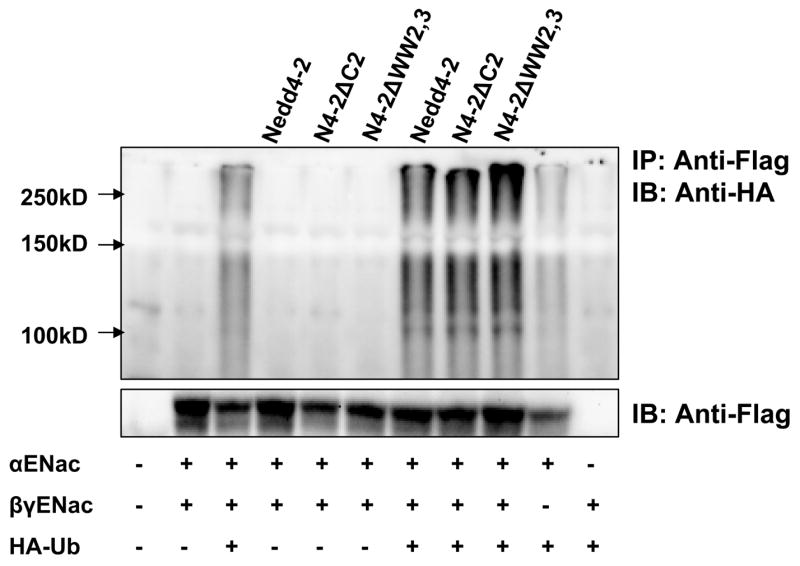
Nedd4-2 isoforms or a control empty vector were transfected with ENaC subunits and HA-tagged ubiquitin (HA-Ub) as indicated into HEK293 cells. ENaC subunits were immunoprecipitated with an anti-FLAG antibody and immunoblotted with anti-HA to detect ubiquitinated ENaC. Total cellular lysates were immunoblotted with anti-FLAG antibody. Ubiquitinated proteins appear as high molecular mass smear. αENaC when expressed alone can be ubiquitinated and ubiquitination of the ENaC complex is increased in the presence of each Nedd4-2 isoform.
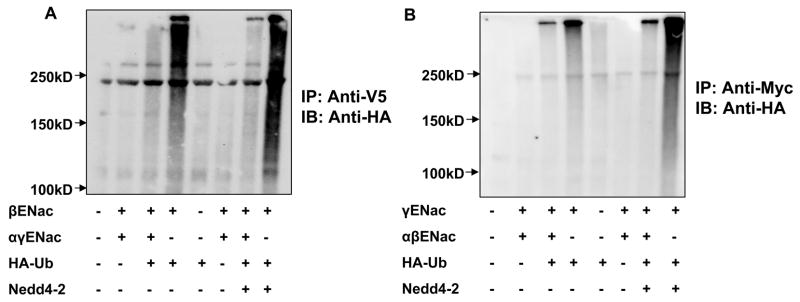
Each subunit of ENaC can be ubiquitinated which is enhanced by Nedd4-2
We next addressed the question of whether the β and γENaC subunits can be ubiquitinated and if this ubiquitination was also enhanced by Nedd4-2. Flag-tagged αENaC, V5-tagged βENaC and myc-tagged γENaC subunits were expressed together or individually along with HA-tagged ubiquitin in the presence and absence of Nedd4-2 in HEK 293 cells and ubiquitinated proteins detected by immunoprecipitation and blotting. We observed that both β and γENaC subunits can be ubiquitinated when expressed alone and that this was further stimulated by Nedd4-2 (Fig. 5A and B). Together, the data in figures 4 and 5 clearly show that each of the three ENaC subunits can be ubiquitinated and that this can occur even when these subunits are expressed alone and that in each case ubiquitination is enhanced by overexpression of Nedd4-2.
Figure 5. The accessory ENaC subunits β and γ are also targets for ubiquitination.
Tagged ENaC subunits were transfected alone or together with or without Nedd4-2 and HA-tagged ubiquitin (HA-Ub) as indicated into HEK293 cells. ENaC subunits were immunoprecipitated with an anti-V5 (β) or anti-myc (γ) antibody and immunoblotted with anti-HA to detect ubiquitinated ENaC subunits. Panel A. ENaC subunit β is ubiquitinated when expressed alone or together and when βENaC is expressed alone this ubiquitination is enhanced by co-expression of Nedd4-2. Panel B. ENaC subunit γ is ubiquitinated when expressed alone or together and as with βENaC this is enhanced by co-expression of Nedd4-2. Similar results were observed from at least three independent experiments for each subunit.
Nedd4-2 induces ubiquitination of cell-surface expressed αENaC
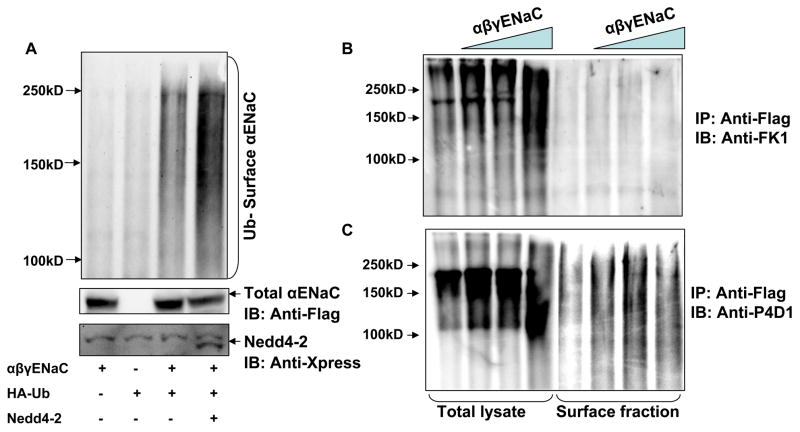
Since Nedd4-2 isoforms increased ubiquitination of ENaC and since co-expression of the oligomeric complex did not seem to be required for ubiquitination we wondered if a surface expressed pool of ENaC was being ubiquitinated by Nedd4-2. To detect ubiquitinated ENaC at the surface, tagged αβγENaC, HA-ubiquitin and Nedd4-2 were co-expressed in HEK 293 cells. Following treatment with 1 μM of MG132 for 3–4 hours to inhibit proteasomal degradation, membrane proteins were biotinylated and sequentially immunoprecipitated with an anti-FLAG antibody, then affinity purified with Neutravidin, and immunoblotted for ubiquitinated ENaC. We detected ubiquitination of αENaC at the cell surface which is further enhanced by Nedd4-2 suggesting that at least some part of the ubiquitinated ENaC complex is found at the cell surface (Fig. 6A). Our experiments do not clarify if ubiquitination occurs while ENaC is at the cell surface or prior to trafficking to the cell membrane.
Figure 6. ENaC subunits at the cell surface contain monoubiquitin but not polyubiquitin chains.
Panel A. Nedd4-2 or a control empty vector was transfected with ENaC subunits and HA-tagged ubiquitin as indicated into HEK293. Biotinylated surface proteins (panel above) were affinity purified and then immunoprecipitated with an anti-FLAG antibody and blotted with an anti-HA antibody (upper panel). Total cellular lysates were also immunoblotted with an anti-FLAG antibody (middle panel) and anti-Xpress antibody (bottom panel), the epitope tag attached to Nedd4-2. Panel B. and C. HEK 293 cells were co-transfected with increasing amounts of ENaC subunits (0, 5, 10, 15 μg of each subunit). The cells were treated with 1μM MG132 for 4 hours and biotinylated surface proteins were affinity purified and then immunoprecipitated with an anti-FLAG antibody and blotted with an anti-FK1 antibody (B) or anti-P4D1 (C). Total cellular lysates were also immunoblotted with these antibodies. The anti-polyubiquitin antibody FK1 detects ubiquitin chains only in cellular lysate and not in surface fractions whereas anti-P4D1 which identifies both monoubiquitin and polyubiquitin chains identifies ubiquitin in surface fractions.
Cell surface fraction of expressed αENaC is monoubiquitinated
We then performed experiments to determine if αENaC at the cell surface is modified by the addition of monoubiquitin chains at multiple residues (multiubiquitination) or by the addition of polyubiquitin chains. HEK293 cells were co-transfected with increasing amounts of ENaC subunits and twenty four hr post-transfection cells were treated with MG132, then surface proteins biotinylated and cell lysates prepared. Expressed cellular αENaC was isolated from the total protein by immunoprecipitation with anti-FLAG antibody and the cell surface-specific fraction of αENaC was captured by Neutravidin affinity purification. Total as well as surface fractions of αENaC were separated by SDS-PAGE, blotted and probed first with an anti-polyubiquitin antibody (FK1) and after stripping, reprobed with P4D1, an antibody that detects polyubiquitin as well as monoubiquitin chains attached to substrate proteins. We show the characteristic high molecular mass smear of ubiquitinated proteins captured by FK1 antiserum in whole cell lysates but not in surface fractions, whereas reblotting the same membrane with P4D1 shows the high molecular mass smear in both the fractions (Fig. 6B and C). This result indicates that αENaC when expressed in HEK293 cells is monoubiquitinated but not polyubiquitinated at the cell surface, confirming results recently reported by others (33).
Effect of Nedd4-2 isoforms on ubiquitination of total and surface ENaC subunits
To determine if there are qualitative differences in the ability of Nedd4-2 isoforms to ubiquitinate individual ENaC subunits, we expressed individual subunits with each of the Nedd4-2 isoforms. Each of the subunits were ubiquitinated but we were unable to demonstrate a meaningful difference in ubiquitination of total cellular ENaC between each of the Nedd4-2 isoforms (Fig. 7A). We also examined the effect of Nedd4-2 isoforms on ubiquitination of individual ENaC subunits at the cell surface and demonstrated that each of the Nedd4-2 isoforms were able to ubiquitinate surface α (Fig. 7B) β and γENaC (data not shown). Finally we confirmed that each of the Nedd4-2 isoforms reduced surface expression of αENaC when expressed alone (Fig. 7C).
Figure 7.
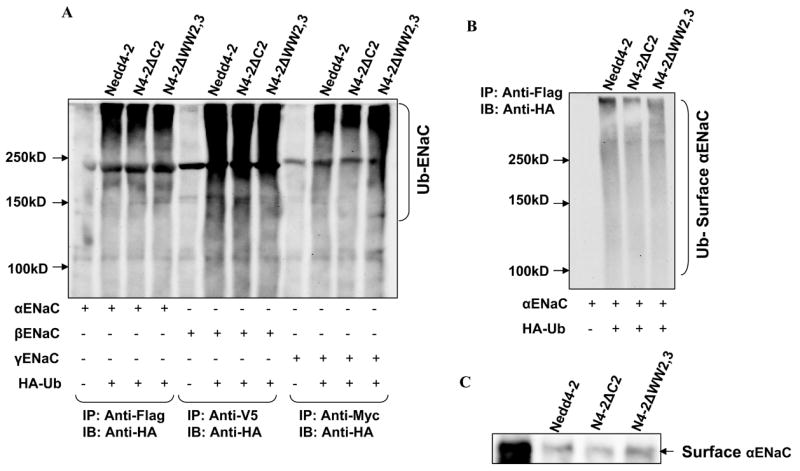
Effect of Nedd4-2 isoforms on ubiquitination of individual ENaC subunits: Panel A: Each of the Nedd4-2 isoforms were transfected with individual ENaC subunits and HA-tagged ubiquitin (HA-Ub) as indicated into HEK293 cells. ENaC subunits were immunoprecipitated with an anti-FLAG/V5/Myc antibody and immunoblotted with anti-HA to detect ubiquitinated α, β and γENaC respectively. Ubiquitinated proteins appear as high molecular mass smear. Each of the ENaC subunits when expressed alone can be ubiquitinated in the presence of each Nedd4-2 isoform. Panel B: αENaC was transfected with each of Nedd4-2 isoforms into HEK293 cells. Then biotinylated surface proteins were affinity purified and then immunoprecipitated with an anti-FLAG antibody and blotted with an anti-HA antibody. Each Nedd4-2 isoform ubiquitinates a surface expressed fraction of αENaC. Panel C: αENaC was transfected with each of Nedd4-2 isoforms into HEK293 cells and biotinylated surface proteins were blotted with an anti-FLAG antibody. Each of the Nedd4-2 isoforms reduces surface expression of αENaC correlating with ubiquitination seen in panel A and B.
ENaC is target for proteosomal degradation
Ubiquitinated ENaC is internalized and is thought to be targeted to the lysosome or proteasome for degradation. To determine if ENaC is a target for proteasomal or lysosomal degradation, we overexpressed tagged ubiquitin with αβγENaC subunits in HEK293 cells in the presence and absence of MG132 or chloroquine, followed by immunoprecipitation and immunoblotting (Fig. 8A). We demonstrate that ubiquitinated αENaC is increased in the presence of MG132 but not with chloroquine suggesting that ENaC is targeted to the proteasome for degradation. To determine if inhibition of the proteasomal degradation pathway would have an impact on Na+ transport, we then tested the effect of MG132 in M-1 cells. Following MG132 treatment, Na+ transport increased significantly as early as one hour and reached a peak ( ~ two fold) between 2–3 hours (Fig. 8B). Due to a significant drop in resistance in MG132-treated monolayers, we could not study the effect of MG132 beyond 4 hours. To determine if Nedd4-2 targets ENaC to the proteasome we overexpressed Nedd4-2 in M-1 cells and then examined the effect of MG132 on Na+ transport. As described earlier, Nedd4-2 reduced basal Isc as compared to empty virus. Treatment with MG132 was able to partially reverse the effect of Nedd4-2 on Na+ transport in M-1 cells suggesting that Nedd4-2 reduces Na+ transport by targeting ENaC subunits to the proteasome (Fig. 8C). Interestingly, chloroquine appeared to reduce Na+ transport in M-1 cells.
Figure 8.
The effect of Nedd4-2 isoforms and proteasomal inhibitors on ENaC function: Panel A. ENaC subunits were expressed with and without HA-tagged Ubiquitin (HA-Ub) in HEK293 cells and cells were treated with or with-out 1μM MG132 or 100 μM chloroquine for 4 hours as indicated. Ubiquitinated ENaC subunits were detected by immunoprecipitation with anti-Flag followed by immunoblotting with anti-HA antibody. There appeared to be more ubiquitinated ENaC detectable in the presence of MG132. Panel B. M-1 cells grown in filters were treated with or without 1μM MG132 and Isc measured in Ussing chambers. Isc was expressed as fold change compared to the control value corresponding to the same time point. Currents increase significantly within an hr of MG132 and reaches a peak between 2-3 hr (**P < 0.001; *P < 0.05 Tukey’s pairwise multiple comparison, SigmaStat; n = 8 from three different experiment; means ± SE). Panel C. M-1 cells were transduced with adenovirus expressing Nedd4-2 or empty virus and Isc measured in Ussing chambers under control conditions and after treatment with MG132 or chloroquine for 2 hr. M-1 cells overexpressing Nedd4-2 had significantly lower currents as compared to empty virus. MG132 but not chloroquine partially reverses the effect of Nedd4-2 on Na+ transport (*P < 0.05 between vehicle and MG132 ; ¶P < 0.05 between vehicle and chloroquine; Tukey’s pairwise multiple comparison, SigmaStat; n = 12 from 3 experiments; mean ± SE).
Panel D. M-1 cells were transduced with adenovirus expressing each of the Nedd4-2 isoforms and Isc measured in Ussing chambers under control conditions and after treatment with MG132 or chloroquine for 2 hr. M-1 cells overexpressing Nedd4-2ΔC2 had lower currents compared to Nedd4-2 and Nedd4-2ΔWW2,3. MG132 partially reverses the effect of Nedd4-2 and Nedd4-2ΔWW2,3, but not Nedd4-2ΔC2, on Na+ transport (*P < 0.05 between vehicle and MG132 ; ¶P < 0.05 between vehicle and chloroquine; Tukey’s pairwise multiple comparison, SigmaStat; n = 16 from 3 experiments; mean ± SE).
Finally, we tested the effect of MG132 or chloroquine on Na+ transport after expression of each of the Nedd4-2 isoforms. As shown earlier, Nedd4-2ΔC2 had a larger effect on inhibition of Na+ transport compared to Nedd4-2 and to Nedd4-2ΔWW2,3 (Fig. 8D). Compared to Nedd4-2 and to Nedd4-2ΔWW2,3, MG132 was unable to significantly increase Na+ transport in cells expressing Nedd4-2ΔC2. Together, the data in M-1 and HEK 293 cells suggest that ENaC is variably regulated by individual Nedd4-2 isoforms, and targeted to the proteasome for degradation.
Discussion
Nedd4-2 (also referred to as Nedd4L), is a member of the Nedd4/Rsp5 family of E3 ubiquitin protein ligases with a catalytic HECT domain (homologous to E6-AP carboxyl terminus) and was originally identified as a developmentally regulated gene highly expressed in the mouse central nervous system (16). These are modular proteins that contain multiple WW domains, which are protein-protein interaction domains, a carboxyl-terminal HECT domain which carries the ubiquitin ligase function and some members of this family also contain an amino-terminal C2 domain, which is a Ca2+/lipid-binding domain (1, 26). Members of Nedd4/Rsp5 family are evolutionarily highly conserved and are required for the ubiquitination of a large number of cellular targets and regulate diverse cellular processes including signaling, trafficking of plasma membrane proteins and degradation of misfolded proteins.
In previous studies we reported the existence of multiple isoforms of hNedd4-2 that arise from alternate transcription and translation and from variable splicing of some internal exons (11). This leads to Nedd4-2 isoforms with and without a C2 domain and isoforms that vary in the number of WW domains. In vitro binding studies suggested that different WW domains have variable affinities against PY motif of ENaC (2, 8, 9, 11, 14). When co-expressed with ENaC in Xenopus oocytes, we found that Nedd4-2 with and without a C2 domain robustly reduced Na+ transport although a Nedd4-2 isoform without WW domains 2 and 3 resulted in a smaller fall in Na+ transport that was not statistically significant (12). The effects of Nedd4-2 isoforms on Na+ transport correlated with the strength of their interaction when heterologously expressed in COS-7 cells.
In the present study we extend these observations by examining the effect of Nedd4-2 isoforms on surface expression of ENaC and on ubiquitination and degradation of ENaC. We first tested the effect of Nedd4-2 isoforms expressed with recombinant adenoviral vectors on Na+ transport in M-1 cells. We found that each of the three isoforms substantially inhibited Na+ transport although Nedd4-2 ΔWW2,3 was the weakest inhibitor (Fig. 1). The prior data in Xenopus oocytes may reflect differences in the model system wherein both Nedd4-2 and ENaC were heterologously expressed and reflect inefficiencies in plasmid-based gene transfer while simultaneously maintaining monolayer integrity in M-1 cells (12). We find that adenoviral transduction of M-1 cells leads to high level gene expression with little effects on transcellular resistance in Ussing chambers. The inhibition of Na+ transport with Nedd4-2 correlated with reduction in cell surface expression of ENaC when each of the Nedd4-2 isoforms was expressed. As has been previously reported, the effect of Nedd4-2 on Na+ transport and on surface expression appeared to require its ubiquitin ligase domain (Fig. 3). However, while others have reported a dominant negative effect of ENaC at least in Xenopus oocytes we do not see such an effect in M-1 cells (4, 14, 24). This may mean that another ubiquitin ligase that interacts with and has a higher affinity for ENaC may be naturally expressed in M-1 cells.
We examined membrane expression of ENaC using a surface biotinylation assay. We demonstrate that αENaC can efficiently traffic to the cell surface even when expressed without β and γ subunits of ENaC (Fig. 2). These studies would indicate that single subunits are processed and delivered to the cell surface although these experiments do not help us determine if the αENaC subunit can be selectively trafficked to the apical membrane of polarized cells. Others have reported that individual ENaC subunits form homomultimers and can traffic to COS-7 and HEK293 plasma membranes with high efficiency, although the cell surface subunits appears to be minimally glycosylated and present in detergent insoluble complexes (25). Whether individual ENaC subunits have any physiological function are not known although when expressed alone, the αENaC subunit but not the β and γENaC subunit, can support small amiloride-sensitive currents (5, 23).
We compared each of three hNedd4-2 isoforms in their ability to reduce cell surface expression of αENaC in heterologous expression system (Fig. 2). We observe that all three isoforms are able to downregulate cell surface expression of αENaC without any significant change in total αENaC. Nedd4-2ΔC2 is most competent in reducing surface expression of αENaC. Previous patch clamp experiments using Xenopus laevis expression system demonstrated that removal of C2 domain of hNedd4 led to an enhanced ability to decrease sodium channels at the cell surface suggesting that the C2 domain may interfere with Nedd4 function (14). As described by others, the C2 domain of Nedd4-2, in contrast, does not appear to significantly alter the effect of Nedd4-2 on ENaC expression or function (14). We compared each of the three hNedd4-2 isoforms in their ability to ubiquitinate the αβγENaC complex. We were unable to demonstrate any differences between these isoforms (Figure 4). This may be because ubiquitinated ENaC in an over-expression system may represent the composite of that ubiquitinated intra-cellularly during processing and that ubiquitinated at the cell surface in response to expressed Nedd4-2. Second, differences in ubiquitination patterns between these isoforms may have differing effects on internalization of ENaC or vary the fate of ubiquitinated ENaC between the degradative and recyling pathways and measurments of total or surface ubiquitinated ENaC can mask real differences in the fate of ENaC. Our results show that there are measurable differences in the ability of these isoforms to reduce surface expression of ENaC and to modulate Na+ transport, although we know little about the relative contribution of these alternate forms to Na+ transport in the native collecting duct.
Our studies demonstrate that each of the Nedd4-2 isoforms reduces surface expression of αENaC and that this is mediated via its ubiquitin ligase activity (Fig. 2 and 3). Ubiquitination is an important mechanism for the degradation of intracellular as well as cell surface proteins including ion channels. The interaction of the WW domains of Nedd4-2 with the PY motif of ENaC is thought to bring the ubiquitin-protein ligase domain into close proximity with lysine residues in the N-terminus of the channel subunits, leading to ubiquitination of the subunits. In previous studies the α and γ subunits, but not the β subunit, was considered to be targets for ubiquitination although this was not consistently examined (3, 10, 31). We demonstrate that ENaC subunits can be ubiquitinated when expressed as a multimeric protein (Fig. 4) as has been demonstrated very recently by others (34). The FLAG epitope on αENaC was used to immunoprecipitate αENaC and as β and γENaC subunits interacting with α these subunits are also likely to be present in this complex, the demonstrated ubiquitination likely corresponds to the sum of the ubiquitination of all ENaC subunits (Figure 4). To confirm that Nedd4-2 could modify each ENaC subunit we also examined ubiquitination of individual subunits with each of the Nedd4-2 isoforms (Fig. 7).
ENaC can be modified by the addition of monoubiquitin at multiple lysine residues to form a multiubiquitinated complex or by addition of polyubiquitin chains to a single lysine residue (13, 21). Some proteins are subject to both forms of post-translational modification. While polyubiquitinated proteins are typically targeted to the proteasome, monoubiquitinated proteins may be targeted to the lysosome or simply be tagged for recyling endosomes. As has been recently reported, we demonstrate that while the cytosolic ENaC is polyubiquitinated, the small fraction of ENaC that is expressed at the cell surface is multiubiquitinated (33).
Although ubiquitination of ENaC is now well recognized, neither the intracellular location nor the fate of ubiquitinated ENaC is well established. Misfolded or unassembled proteins in the endoplasmic reticulum are generally removed by the proteasomal pathway as part of the quality control machinery of the cell. However, the synthesis of αβγENaC subunits are not coordinately regulated with αENaC being regulated by corticosteroids in the connecting tubule and collecting duct while βγ subunits are regulated by corticosteroids in the colon. Under salt replete conditions, there is little detectable αENaC in the distal nephron although there is abundant β and γENaC in an intracellular location that appears to be protected from proteasomal degradation despite the absence of αENaC in the protein complex. Upon salt depletion or under conditions of aldosterone excess, αENaC is rapidly synthesized and the fully assembled αβγ heteromultimer now moves to the apical membrane (19, 20). It would appear therefore that under physiological conditions the role of ubiquitin ligases may be primarily to regulate the abundance of ENaC that reaches the cell surface.
Our data suggests that the proteasomal pathway regulates ENaC abundance in M-1 cells and in HEK 293 cells. We also demonstrate that inhibition of the proteasomal pathway, but not the lysosomal pathway, increases Na+ transport in M-1 cells. Similar results have been reported in A6 cells, a distal nephron cell line derived from Xenopus kidney (22).
In summary, we find that α, β and γ subunits of ENaC can be ubiquitinated when expressed alone as well as when expressed as a complex. The cell surface expression of ENaC and the function of ENaC is reduced by each of the Nedd4-2 isoforms, with Nedd4-2 ΔC2 having the strongest effect. Interaction with Nedd4-2 and the subsequent ubiquitination of ENaC subunits appear to target these proteins for proteasomal degradation.
Acknowledgments
We thank the University of Iowa DNA Core and Vector Core for services provided. We thank Dr. Dirk Bohmann for the HA-tagged ubiquitin plasmid. This work was supported in part by USPHS grant HL71664 and by A VA Merit Review Award.
References
- 1.Ardley HC, Robinson PA. E3 ubiquitin ligases. Essays Biochem. 2005;41:15–30. doi: 10.1042/EB0410015. [DOI] [PubMed] [Google Scholar]
- 2.Asher C, Sinha I, Garty H. Characterization of the interactions between Nedd4-2, ENaC, and sgk-1 using surface plasmon resonance. Biochim Biophys Acta. 2003;1612:59–64. doi: 10.1016/s0005-2736(03)00083-x. [DOI] [PubMed] [Google Scholar]
- 3.Bhalla V, Daidie D, Li H, Pao AC, LaGrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D. SGK1 regulates ubiquitin ligase Nedd4-2 by inducing interaction with 14-3-3. Mol Endocrinol. 2005:me.2005–0193. doi: 10.1210/me.2005-0193. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated Kinase Inhibits the Epithelial Na+ Channel through Functional Regulation of the Ubiquitin Ligase Nedd4-2. J Biol Chem. 2006;281:26159–26169. doi: 10.1074/jbc.M606045200. [DOI] [PubMed] [Google Scholar]
- 5.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 6.Deboneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger J-D, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellison DP, Thomas CP. Hereditary disorders of connecting tubule and collecting duct sodium and potassium transport. In: Mount DB, Pollak MR, editors. Molecular and Genetic Basis of Renal Disease. Philadelphia: Elsevier Saunders; 2007. pp. 251–268. [Google Scholar]
- 8.Fotia AB, Dinudom A, Shearwin KE, Koch JP, Korbmacher C, Cook DI, Kumar S. The role of individual Nedd4-2 (KIAA0439) WW domains in binding and regulating epithelial sodium channels. Faseb J. 2003;17:70–72. doi: 10.1096/fj.02-0497fje. [DOI] [PubMed] [Google Scholar]
- 9.Henry PC, Kanelis V, O'Brien MC, Kim B, Gautschi I, Forman-Kay J, Schild L, Rotin D. Affinity and Specificity of Interactions between Nedd4 Isoforms and the Epithelial Na+ Channel. J Biol Chem. 2003;278:20019–20028. doi: 10.1074/jbc.M211153200. [DOI] [PubMed] [Google Scholar]
- 10.Ichimura T, Yamamura H, Sasamoto K, Tominaga Y, Taoka M, Kakiuchi K, Shinkawa T, Takahashi N, Shimada S, Isobe T. 14-3-3 Proteins Modulate the Expression of Epithelial Na+ Channels by Phosphorylation-dependent Interaction with Nedd4-2 Ubiquitin Ligase. J Biol Chem. 2005;280:13187–13194. doi: 10.1074/jbc.M412884200. [DOI] [PubMed] [Google Scholar]
- 11.Itani OA, Campbell JR, Herrero J, Snyder PM, Thomas CP. Alternate promoters and variable splicing lead to hNedd4-2 isoforms with a C2 domain and varying number of WW domains. Am J Physiol Renal Physiol. 2003;285:F916–929. doi: 10.1152/ajprenal.00203.2003. [DOI] [PubMed] [Google Scholar]
- 12.Itani OA, Stokes JB, Thomas CP. Nedd4-2 isoforms differentially associate with ENaC and regulate its activity. Am J Physiol Renal Physiol. 2005:00394.02004. doi: 10.1152/ajprenal.00394.2004. [DOI] [PubMed] [Google Scholar]
- 13.Kamynina E, Staub O. Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na+ transport. Am J Physiol Renal Physiol. 2002;283:F377–387. doi: 10.1152/ajprenal.00143.2002. [DOI] [PubMed] [Google Scholar]
- 14.Kamynina E, Tauxe C, Staub O. Distinct characteristics of two human Nedd4 proteins with respect to epithelial Na+ channel regulation. Am J Physiol Renal Physiol. 2001;281:F469–477. doi: 10.1152/ajprenal.2001.281.3.F469. [DOI] [PubMed] [Google Scholar]
- 15.Kellenberger S, Schild L. Epithelial Sodium Channel/Degenerin Family of Ion Channels: A Variety of Functions for a Shared Structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Tomooka Y, Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992;185:1155–1161. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- 17.Liang X, Peters KW, Butterworth MB, Frizzell RA. 14-3-3 Isoforms Are Induced by Aldosterone and Participate in Its Regulation of Epithelial Sodium Channels. J Biol Chem. 2006;281:16323–16332. doi: 10.1074/jbc.M601360200. [DOI] [PubMed] [Google Scholar]
- 18.Loffing-Cueni D, Flores SY, Sauter D, Daidie D, Siegrist N, Meneton P, Staub O, Loffing J. Dietary Sodium Intake Regulates the Ubiquitin-Protein Ligase Nedd4-2 in the Renal Collecting System. J Am Soc Nephrol. 2006;17:1264–1274. doi: 10.1681/ASN.2005060659. [DOI] [PubMed] [Google Scholar]
- 19.Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol. 2000;279:F252–258. doi: 10.1152/ajprenal.2000.279.2.F252. [DOI] [PubMed] [Google Scholar]
- 20.Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol. 2001;280:F675–682. doi: 10.1152/ajprenal.2001.280.4.F675. [DOI] [PubMed] [Google Scholar]
- 21.Malik B, Price SR, Mitch WE, Yue Q, Eaton DC. Regulation of epithelial sodium channels by the ubiquitin-proteasome proteolytic pathway. Am J Physiol Renal Physiol. 2006;290:F1285–1294. doi: 10.1152/ajprenal.00432.2005. [DOI] [PubMed] [Google Scholar]
- 22.Malik B, Schlanger L, Al-Khalili O, Bao H-F, Yue G, Price SR, Mitch WE, Eaton DC. ENaC Degradation in A6 Cells by the Ubiquitin-Proteosome Proteolytic Pathway. J Biol Chem. 2001;276:12903–12910. doi: 10.1074/jbc.M010626200. [DOI] [PubMed] [Google Scholar]
- 23.McDonald FJ, Price MP, Snyder PM, Welsh MJ. Cloning and expression of the beta and gamma subunits of the human epithelial sodium channel. Am J Physiol. 1995;267:C1157–C1163. doi: 10.1152/ajpcell.1995.268.5.C1157. [DOI] [PubMed] [Google Scholar]
- 24.Michlig S, Harris M, Loffing J, Rossier BC, Firsov D. Progesterone Down-regulates the Open Probability of the Amiloride-sensitive Epithelial Sodium Channel via a Nedd4-2-dependent Mechanism. J Biol Chem. 2005;280:38264–38270. doi: 10.1074/jbc.M506308200. [DOI] [PubMed] [Google Scholar]
- 25.Prince LS, Welsh MJ. Cell surface expression and biosynthesis of epithelial Na+ channels. Biochem J. 1998;336(Pt 3):705–710. doi: 10.1042/bj3360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J Membr Biol. 2000;176:1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- 27.Schafer JA. Abnormal regulation of ENaC: syndromes of salt retention and salt wasting by the collecting duct. Am J Physiol Renal Physiol. 2002;283:F221–235. doi: 10.1152/ajprenal.00068.2002. [DOI] [PubMed] [Google Scholar]
- 28.Snyder PM. Minireview: Regulation of Epithelial Na+ Channel Trafficking. Endocrinology. 2005;146:5079–5085. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- 29.Snyder PM, Olson DR, Thomas BC. SGK modulates Nedd4-2-mediated inhibition of ENaC. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- 30.Snyder PM, Steines JC, Olson DR. Relative contribution of Nedd4 and Nedd4-2 to ENaC regulation in epithelia determined by RNA interference. J Biol Chem. 2004;279:5042–5046. doi: 10.1074/jbc.M312477200. [DOI] [PubMed] [Google Scholar]
- 31.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staub O, Verrey F. Impact of Nedd4 Proteins and Serum and Glucocorticoid-Induced Kinases on Epithelial Na+ Transport in the Distal Nephron. J Am Soc Nephrol. 2005;16:3167–3174. doi: 10.1681/ASN.2005050454. [DOI] [PubMed] [Google Scholar]
- 33.Wiemuth D, Ke Y, Rohlfs M, McDonald FJ. Epithelial sodium channel (ENaC) is multi-ubiquitinated at the cell surface. Biochem J. 2007;405:147–155. doi: 10.1042/BJ20060747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou R, Patel SV, Snyder PM. Nedd4-2 Catalyzes Ubiquitination and Degradation of Cell Surface ENaC. J Biol Chem. 2007;282:20207–20212. doi: 10.1074/jbc.M611329200. [DOI] [PubMed] [Google Scholar]