Abstract
Fluvoxamine is a selective-serotonin reuptake inhibitor (SSRI) that has proved effective in large double-blind, randomized, controlled trials involving patients with social anxiety disorder (SAD), obsessive-compulsive disorder (OCD), and panic disorder. Improvements have also been demonstrated in patients with post-traumatic stress disorder, as well as those with a range of obsessive-compulsive spectrum disorders including binge eating disorder, bulimia nervosa, pathological gambling, and body dysmorphic disorder. Several well controlled studies have confirmed the efficacy of fluvoxamine in children and adolescents with OCD, SAD, and other anxiety disorders, and it was the first SSRI to be registered for the treatment of OCD in children. Fluvoxamine is well tolerated. In common with other SSRIs, the most frequently reported adverse event is nausea. Fluvoxamine does not cause sedation or cognitive impairment and is associated with a low risk of sexual dysfunction, suicidality, and withdrawal reactions. It is safe in overdose and has no significant effect on body weight or cardiovascular parameters.
Keywords: fluvoxamine, anxiety disorders, obsessive-compulsive disorder, panic disorder, post-traumatic stress disorder
Introduction
Fluvoxamine was the first of the selective serotonin reuptake inhibitors (SSRIs) that remains in clinical use. Although originally developed as an antidepressant (Wilde et al 1993) (an indication for which it is licensed in many countries, with the exception of the United States), its most widespread application is in the treatment of anxiety disorders, particularly obsessive-compulsive disorder (OCD). It was the first drug licensed for use in adults, and subsequently for children as well, in OCD in the United States. In recent years, there have been a number of studies of fluvoxamine in other anxiety disorders, particularly in social anxiety disorder (SAD) and its eastern equivalent taijin kyofusho (fluvoxamine was the first SSRI to be licensed in Japan).
There have been few recent reviews of fluvoxamine, and the majority of the older reviews focus largely on its use in depression. Whilst fluvoxamine is undoubtedly effective in depression, it is in the context of anxiety disorders that the drug is most frequently encountered and where there is a lack of critical overviews of its utility. This review examines the evidence for efficacy of fluvoxamine in OCD, SAD, obsessive compulsive spectrum disorders, panic disorder, and post-traumatic stress disorder (PTSD). Apart from the SSRIs, treatment options in these disorders have major tolerability disadvantages, with benzodiazepines, tricyclic antidepressants, and neuroleptics being the mainstay of treatment until the advent of the SSRIs. Fluvoxamine has probably been better evaluated in a wider range of anxiety disorders than any of the other SSRIs, and the publication of recent data in this area warrants further review.
Pharmacology
Fluvoxamine is a SSRI antidepressant. It potently inhibits the reuptake of serotonin but has little effect on dopamine and norepinephrine uptake systems. In addition, apart from binding to σ1 receptors, it has a low affinity for neuro-transmitter receptors (Leonard 1992; Hyttel 1993). The affinity of fluvoxamine for σ1 receptors, which may be involved in psychosis and aggression, is greater than that seen with the other SSRIs (Narita et al 1996).
The anxiolytic activity of fluvoxamine has been demonstrated in a number of animal models including the ultrasonic rat pup vocalization test, anticipatory anxiety in mice, and schedule-induced polydipsia in rats (Njung’e and Handley 1991; Olivier et al 1993; Woods et al 1993).
Pharmacokinetics
The key pharmacokinetic properties of fluvoxamine are summarized in Table 1. It is efficiently absorbed after oral administration and its bioavailability is not affected by food. Thereafter it undergoes widespread distribution, although it has lower plasma protein binding than all other SSRIs with the exception of citalopram. Extensive metabolism occurs in the liver, with less than 4% of the dose being excreted unchanged. None of the resulting metabolites have psychotropic activity (Overmars et al 1983). Fluvoxamine has an elimination half-life of approximately 15 hours after a single dose, which increases by 30%–50% after multiple dosing, thus making it suitable for once-daily dosing. Excretion is via the urine. There is minimal excretion of fluvoxamine in breast milk (Piontek et al 2001).
Table 1.
Pharmacokinetic properties of fluvoxamine
| Oral absorption | ≥ 94% |
| Cmax | 31–87 mg/L |
| Tmax | 2–8 hours |
| Time to reach steady-state | ≈ 10 days |
| Absolute bioavailability | ≈ 50% |
| AUC | 927 μg/L~hour |
| Vd | 25 L/kg |
| Plasma protein binding | ≈ 77% |
| t½β | 15 hours after a single dose |
| Route of metabolism | Hepatic oxidation |
| Route of excretion | Urine |
Data adapted from van Harten (1995).
Abbreviations: Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; AUC, area under the plasma concentration-time curve; Vd, volume of distribution; t½β, terminal elimination half-life.
The pharmacokinetic profile of fluvoxamine is unchanged in patients with renal impairment (van Harten 1995), but slower elimination in patients with hepatic impairment may necessitate an initial dose reduction (van Harten et al 1993). Age has little impact (de Vries et al 1992), although slow titration is advised in elderly patients and the recommended maximum dose for children is 200 mg/day.
In contrast to a number of other SSRIs, fluvoxamine is only a weak inhibitor of cytochrome P450 (CYP) 2D6 and is therefore unlikely to result in interactions when used in combination with drugs that are metabolized by this isoenzyme, eg, many antipsychotics (DeVane 1998). Fluvoxamine is, however, a potent inhibitor of CYP1A2 and a moderate inhibitor or CYP3A4 and CYP2C19, and may prolong the elimination of agents metabolized by these isoenzymes, eg, warfarin, theophylline, and some benzodiazepines (DeVane 1998). There are no interactions between fluvoxamine and alcohol (van Harten et al 1992), lithium (Milijkovic et al 1997), or digoxin (Ochs et al 1989).
Clinical studies
Social anxiety disorder
SAD is the most common of the anxiety disorders, after specific phobia, with a lifetime prevalence of at least 10% (Kessler et al 1994; Weiller et al 1996). It is characterized by a fear of embarrassment in social or performance situations, and occurs in either the generalized form, which is associated with a fear of many situations including, for example, speaking, eating, writing in front of others, or socializing in a group; or the nongeneralized form, where the fear occurs in just one or two situations. The symptoms can be severe, particularly in the generalized form, and lead to significant impairment in education, work, social, and family situations. Because onset is usually before or during adolescence, patients can suffer for many years, and a high risk of comorbidity with other psychiatric disorders contributes to further disability.
A number of randomized, double-blind, placebo-controlled studies have confirmed the efficacy of fluvoxamine in improving the symptoms of SAD, as well as reducing the disruption it causes in everyday life (Table 2) (van Vliet et al 1994; Stein et al 1999, 2003; Davidson et al 2004; Westenberg et al 2004). Good evidence also exists for the efficacy of paroxetine and sertraline (MacQueen et al 2001; Wagstaff et al 2002), although fluoxetine showed no significant difference from placebo in the only reported double-blind study (Kobak et al 2002).
Table 2.
Summary of randomized, double-blind, placebo controlled trials with fluvoxamine in social anxiety disorder
| Percentage change in score from baseline at end of study | |||||||
|---|---|---|---|---|---|---|---|
| Study | N | Duration | Regimens | LSAS | CGI severity score | SDS | Response rate |
| van Vliet et al 1994 | 28 | 12 weeks | FLV 150 mg/day | −46% (anxiety)*** | 46%a | ||
| −37% (avoidance) | |||||||
| Placebo | −14% (anxiety) | 7% | |||||
| −20% (avoidance) | |||||||
| Stein et al 1999 | 86 | 12 weeks | FLV 50–300 mg/day | −28%** | −35% (work) | 43%b* | |
| −25% (social) | |||||||
| −28% (family) | |||||||
| Placebo | −9% | +8% (work) | 23% | ||||
| 0% (social) | |||||||
| +22% (family) | |||||||
| Stein et al 2003 | 109 | 24 weeks | FLV CR 100–300 mg/day | −60% | −52%** | −68%* | 80%b |
| Placebo | −51% | −40% | −54% | 74% | |||
| Davidson et al 2004 | 279 | 12 weeks | FLV CR 100–300 mg/day | −30%** | −22%*** | −34%* | 34%b*** |
| Placebo | −15% | −11% | −20% | 17% | |||
| Westenberg et al 2004 | 294 | 12 weeks | FLV CR 100–300 mg/day | −37%* | −31%* | −41%* | 48%b |
| Placebo | −28% | −21% | −32% | 44% | |||
Abbreviations: FLV, fluvoxamine; CR, controlled-release; LSAS, Liebowitz Social Anxiety Scale; SDS, Sheehan Disability Scale; CGI, Clinical Global Impression severity score.
Based on LSAS.
Based on CGI improvement.
p < 0.05 vs placebo
p < 0.01 vs placebo
p < 0.001 vs placebo.
Fluvoxamine was the first SSRI to be evaluated in a randomized, controlled trial for the treatment of SAD (van Vliet et al 1994); the results from this small study showed a significantly greater improvement with fluvoxamine than with placebo on the Liebowitz Social Anxiety Scale (LSAS), Hamilton Anxiety Scale, and the 90-item Symptom Checklist. In a larger study involving 86 patients who had been suffering from SAD for an average of around 25 years, fluvoxamine was significantly superior to placebo on the LSAS, Brief Social Phobia Scale, Social Phobia Inventory, and the Clinical Global Impression (CGI) response rate (Stein et al 1999). Two subsequent studies, each involving more than 250 patients diagnosed specifically with the generalized form of SAD, confirmed that the controlled release formulation of fluvoxamine (100–300 mg/day) was significantly more effective than placebo on the LSAS, CGI severity scale, and the Sheehan Disability Scale (SDS) (Davidson et al 2004; Westenberg et al 2004). Interestingly, fluvoxamine has also shown some efficacy in patients with taijin kyofusho, a disorder thought to be a form of SAD unique to Eastern cultures that is centred on a fear of embarrassing or offending other people (Matsunaga et al 2001).
Because SAD is a chronic disorder, maintenance treatment may be necessary. A 24-week, double-blind extension study, conducted in 109 patients who had experienced at least minimal improvement after 12 initial weeks of treatment with fluvoxamine CR (100–300 mg/day) or placebo (Westenberg et al 2004), showed that patients treated with fluvoxamine continued to show greater improvement than those given placebo, although the magnitude of change was smaller in the extension than in the acute phase (Stein et al 2003).
Fluvoxamine is also effective in children and adolescents with SAD and other anxiety disorders and was the first SSRI to be approved by the Food and Drug Administration for use in children. In a randomized, double-blind study in 128 children and adolescents aged 6–17 years (> 70% aged ≤ 12 years) with SAD, separation anxiety disorder, or generalized anxiety disorder, fluvoxamine (up to 300 mg/day) was significantly more effective than placebo from week 3 onwards on the Pediatric Anxiety Rating Scale (Figure 1) (Research Unit on Pediatric Psychopharmacology Anxiety Study Group 2001). By the end of the 8-week study, the mean score was < 10 in the fluvoxamine group, indicating no more than mild anxiety, whilst it remained high in the placebo group. The response rate (CGI improvement score of < 4) was 76% with fluvoxamine and 29% with placebo (p < 0.001). A 6-month open-label extension of this study showed that anxiety symptoms remained low in 94% of patients who initially responded to fluvoxamine (Walkup et al 2002). Amongst the 48 initial placebo nonresponders, 56% had a clinically significant improvement in anxiety when switched to fluvoxamine; 71% of the 14 initial fluvoxamine nonresponders also showed a significant improvement when switched to fluoxetine.
Figure 1.
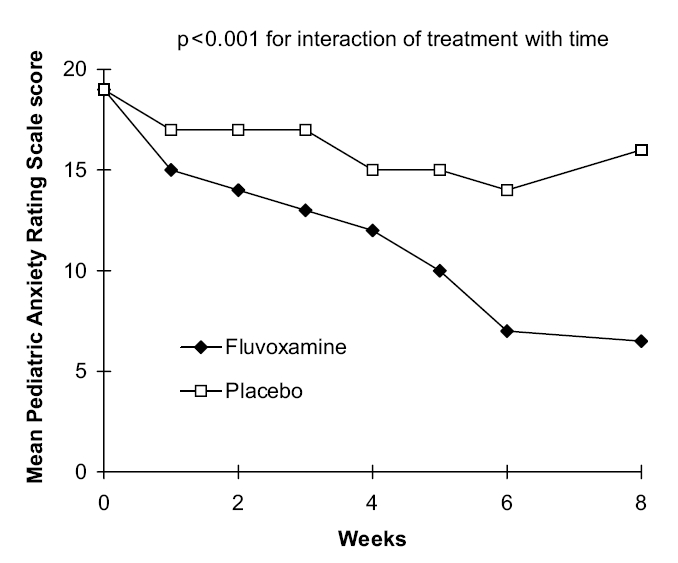
Mean Pediatric Anxiety Rating Scale score in children and adolescents with SAD (separation anxiety disorder) or generalized anxiety disorder treated with fluvoxamine or placebo. Source: Research Unit on Pediatric Psychopharmacology Anxiety Study Group. 2001. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med, 344:1279–85. Copyright © 2001. Reproduced with permission from Massachusetts Medical Society.
Obsessive-compulsive disorder
OCD is characterized by recurrent or persistent obsessions (thoughts) and compulsions (behaviors) that cause marked distress and significantly interfere with everyday functioning. It has a lifetime prevalence of 2%–5% (Karno et al 1988) and a high degree of comorbidity.
The efficacy of fluvoxamine in the management of OCD has been confirmed in a number of randomized, double-blind, controlled studies, and it was the first SSRI to be registered for this indication. Placebo-controlled studies conducted over 6–12 weeks have consistently shown a significantly better response to fluvoxamine (100–300 mg/day) than to placebo as assessed by the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), National Institute of Mental Health Obsessive-Compulsive (NIMH-OC) scale, and the CGI scales (Perse et al 1987; Goodman et al 1989, 1996; Jenike et al 1990; Greist et al 1995; Hollander et al 2003). There is evidence from two placebo-controlled studies to indicate that fluvoxamine significantly augments the efficacy of behavior therapy (Cottraux et al 1993; Hohagen et al 1998).
The majority of double-blind, active treatment-controlled studies have been conducted with the tricyclic antidepressant clomipramine, a serotonin reuptake inhibitor (Table 3) (Freeman et al 1994; Koran et al 1996; Milanfranchi et al 1997; Mundo et al 2000, 2001). These studies all showed that fluvoxamine and clomipramine are equally effective for the treatment of OCD, although fluvoxamine was generally better tolerated. One study compared the effects of fluvoxamine with the tricyclic antidepressant desipramine which, in contrast to clomipramine, is a noradrenaline reuptake inhibitor (Table 3) (Goodman et al 1990). The response rate and the percentage reduction in the Y-BOCS score at the end of the study were both significantly greater with fluvoxamine than with desipramine (p ≤ 0.05).
Table 3.
Summary of randomized, double-blind, active controlled trials with fluvoxamine in obsessive-compulsive disorder
| Percentage change in score from baseline at end of study | |||||||
|---|---|---|---|---|---|---|---|
| Study | N | Duration | Regimens | Y-BOCS | NIMH-OC | CGI severity score | Response rate |
| Versus CMI | |||||||
| Freeman et al 1994 | 64 | 10 weeks | FLV 150–250 mg/day | −68% | −73% | 59%a | |
| CMI 150–250 mg/day | −69% | −73% | 53% | ||||
| Koran et al 1996 | 73 | 10 weeks | FLV 100–300 mg/kg | −30% | 56%b | ||
| CMI 100–250 mg/kg | −30% | 54% | |||||
| Milanfranchi et al 1997 | 25 | 9 weeks | FLV 300 mg/kg | −38% | 85%a | ||
| CMI 300 mg/kg | −40% | 83% | |||||
| Mundo et al 2000 | 128 | 10 weeks | FLV 150–300 mg/kg | −46% | −40% | −32% | 60%a,b |
| CMI 150–300 mg/kg | −50% | −40% | −33% | 67% | |||
| Mundo et al 2001 | 217 | 10 weeks | FLV 150–300 mg/day | −46% | −40% | −32% | 62%b |
| CMI 150–300 mg/kg | −47% | −39% | −33% | 65% | |||
| Versus DMI | |||||||
| Goodman et al 1990 | 40 | 8 weeks | FLV 100–300 mg/kg | −29%* | 52%a* | ||
| DMI 100–300 mg/kg | −1% | 11% | |||||
Abbreviations: FLV, fluvoxamine; CMI, clomipramine; DMI, desipramine; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale; NIMH-OC, National Institute of Mental Health Obsessive-Compulsive scale; CGI, Clinical Global Impression severity score.
Based on CGI improvement.
Based on Y-BOCS.
p ≤ 0.05 vs comparator.
Long-term maintenance treatment with fluvoxamine appears to reduce the risk of relapse, and there is some indication that lower dosages may be effective, although only small studies have been conducted to date (Ravizza et al 1996; Mundo et al 1997).
As with other anxiety disorders, fluvoxamine has proved effective in children and adolescents with OCD. A 10-week, double-blind, placebo-controlled study in 120 children and adolescents (approximately 50% were aged 8–12 years and 50% aged 13–17 years) showed that fluvoxamine (50–200 mg/day) was significantly superior to placebo on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) from week 1 onwards (p < 0.05) (Figure 2) (Riddle et al 2001). Such an early response is not generally observed in studies in adult patients. Statistically significant differences between fluvoxamine and placebo were also seen on the NIMH-OC and CGI scales and a response (≥ 25% reduction in CY-BOCS total score) was achieved in 42% of fluvoxamine patients and 26% of placebo patients. In a 1-year open extension phase involving 98 patients, continuation of fluvoxamine (200 mg/day) resulted in further small beneficial effects, whilst patients switched from placebo to fluvoxamine experienced a marked improvement in CY-BOCS score (Walkup et al 1999).
Figure 2.
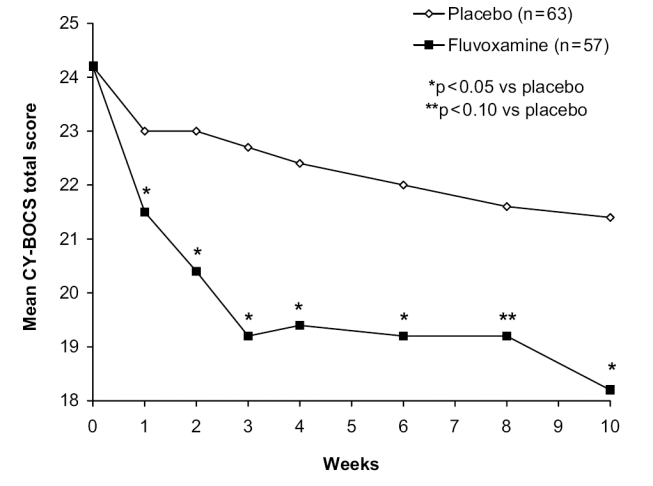
Mean CY-BOCS total score in children and adolescents with OCD treated with fluvoxamine or placebo. Source: Riddle MA, Reeve EA, Yaryura-Tobias J, et al. 2001. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry, 40:222–9. Reproduced with permission from Lippincott Williams & Wilkins.
Obsessive-compulsive spectrum disorders
Obsessive-compulsive spectrum disorders consist of a group of conditions, each of which has distinct diagnostic criteria, which share certain features with OCD (eg, demographics, symptoms, neurobiology, and effective treatments) (Hollander and Wong et al 1995). Fluvoxamine has proved effective in a number of these disorders and is probably one of the most widely studied SSRIs in this area, although many of the studies had an open design and involved only small numbers of patients. Randomized, double-blind, controlled studies have, however, been conducted in patients with binge eating disorder, bulimia nervosa, pathological gambling, compulsive buying, and autism.
Binge eating disorder
Binge eating is one of the common eating disorders and is characterized by recurrent episodes of uncontrolled overeating. Unlike patients with bulimia nervosa, no measures are taken to avoid weight gain. In a randomized, double-blind study in 83 patients, fluvoxamine (50–300 mg/day) was significantly (p < 0.05) more effective than placebo after 9 weeks in terms of rates of reduction in CGI severity score, body mass index, and the frequency of binges, and rate of increase in CGI improvement score and level of response (Hudson et al 1998). The addition of fluvoxamine (300 mg/day), but not fluoxetine (60 mg/day), has also been reported to enhance the efficacy of cognitive behavioral therapy in a 6-month randomized study involving 108 patients (Ricca et al 2001).
Bulimia nervosa
Patients with bulimia nervosa also experience compulsive eating binges, but their fear of loss of control and consequent weight gain leads them to use measures such as vomiting and taking laxatives. Fluvoxamine (100–300 mg/day) proved significantly (p < 0.05) more effective than placebo in preventing relapse in a 12-week, randomized, double-blind study in 72 patients who had already been successfully treated with psychotherapy (Fichter et al 1996, 1997). An earlier open study in 20 patients showed that fluvoxamine (50–150 mg/day) significantly (p < 0.05) reduced various aspects of bulimic behavior (Ayuso-Gutierrez et al 1994).
Pathological gambling
Pathological gambling is a chronic and progressive condition that results in significant impairment in everyday living and a high rate of suicide attempts. Following promising findings with fluvoxamine (100–300 mg/day) in a single-blind crossover study, in which a response was achieved in 7 out of the 10 patients (Hollander et al 1998), a small (n = 10) randomized, double-blind crossover study was conducted by the same group (Hollander et al 2000). Fluvoxamine (100–250 mg/day) treatment for 8 weeks resulted in a significantly greater improvement in overall gambling severity on the Pathological Gambling-CGI scale compared with placebo (41% vs 17%; p < 0.005). Gambling urge and behavior were also significantly improved with fluvoxamine treatment.
Compulsive buying
A response (as assessed by the shopping version of the Y-BOCS) to fluvoxamine (up to 300 mg/day) was reported in 9 out of 10 patients enrolled in a 9-week open study; patients were less preoccupied with shopping and reported spending less money (Black et al 1997). A subsequent 9-week, randomized, double-blind study in 23 patients reported a significantly (p < 0.05) greater improvement with fluvoxamine (up to 300 mg/day) than with placebo on the Maudsley Obsessive-compulsive Inventory (Black et al 2000). However, there were no other statistically significant differences between the two treatment groups.
Autism
The efficacy of fluvoxamine (50–300 mg/day) in autistic adults was demonstrated in a 12-week, randomized, double-blind study involving 30 patients (McDougle et al 1996). Significant (p < 0.05) benefits over placebo were seen in repetitive thoughts and behaviors, maladaptive behavior, and aggression and aspects of social relatedness, notably language use. A response was seen in 53% of fluvoxamine-treated patients compared with none in the placebo group (p < 0.01). Autistic children have also shown a response to fluvoxamine in an open study (Yokoyama et al 2002) and a double-blind, crossover study (Fukuda et al 2001). The latter involved 18 children and showed significant (p < 0.05) improvements in a number of behaviors including eye contact and language use.
Body dysmorphic disorder
Although no double-blind studies have been reported to date, there are considerable data from noncomparative trials to indicate that fluvoxamine is beneficial in patients with body dysmorphic disorder (Hollander et al 1994; Perugi et al 1996; Phillips et al 1998, 2001). Scales used to assess the efficacy of fluvoxamine include the Y-BOCS modified for body dysmorphic behavior, the Brown Assessment of Beliefs Scale, and the CGI. Psychogenic skin excoriation, which is commonly seen in patients with body dysmorphic disorder, has also been shown to respond to fluvoxamine (Arnold et al 1999; O’Sullivan et al 1999).
Other disorders
Other disorders in which fluvoxamine has shown beneficial effects include pervasive developmental disorders (Martin et al 2003), trichotillomania (Stanley et al 1997), and hypochondriasis (Fallon et al 2003).
Panic disorder
Patients with panic disorder suffer from recurrent panic attacks characterized by a range of somatic and cognitive symptoms including sweating, trembling, shortness of breath, palpitations, and fear of dying or losing control. It has a lifetime prevalence of around 3% (Kessler et al 1994), and patients often suffer from comorbid agoraphobia or depression.
Randomized, double-blind, placebo- and active-controlled studies have shown that fluvoxamine is effective in patients with panic disorder (with or without agoraphobia), reducing both the number of panic attacks and the level of anxiety. Three short-term, placebo-controlled studies (one large-scale [n = 179 Asnis et al 2001] and two smaller scale [n = 36 Hoehn-Saric et al 1993; and n = 46 Sandmann et al 1998]) showed that fluvoxamine (up to 300 mg/day) was significantly (p < 0.05) more effective than placebo in reducing the number of panic attacks. In two studies (Hoehn-Saric et al 1993; Asnis et al 2001), the frequency of full panic attacks per week was reduced by 74%–100% with fluvoxamine and 33%–66% with placebo (p < 0.05) at the end of treatment, whilst the third study (Sandmann et al 1998) showed a significant difference only in the frequency of limited symptom attacks (53% vs 31%; p < 0.05). Anxiety, as assessed by the Clinical Anxiety Scale (CAS), was also reduced to a significantly greater extent by fluvoxamine.
Four small, short-term studies have compared fluvoxamine with other active pharmacotherapeutic treatments. In two of these studies, fluvoxamine (up to 150 mg/day) was significantly (p < 0.05) more effective than ritanserin and maprotiline, although neither are indicated for the treatment of panic disorder (den Boer and Westenberg 1988, 1990). Fluvoxamine (up to 300 mg/day) proved significantly (p < 0.01) more effective than imipramine (up to 300 mg/day) in reducing the total number of panic attacks in a placebo-controlled study involving 54 patients with severe panic disorder (Bakish et al 1996). Anxiety was improved to a similar extent in both groups. A lower dose of fluvoxamine (150 mg/day) did not differ significantly from brofaromine (150 mg/day) in reducing the frequency of panic attacks, anxiety, and avoidance behavior in a somewhat smaller study of 30 patients (van Vliet et al 1995).
Four randomized, placebo-controlled studies have assessed the effects of fluvoxamine in comparison with, or in combination with, various behavioral treatments. Fluvoxamine (up to 300 mg/day) proved more effective than cognitive therapy in 55 patients with moderate to severe panic disorder (Black et al 1993). After 8 weeks, the percentage of patients free of panic attacks was 73% in the fluvoxamine group compared with 48% and 25% in the cognitive therapy and placebo groups, respectively. The final CAS score was significantly lower with fluvoxamine than with cognitive therapy or placebo (p < 0.05). In a somewhat larger (n = 149) 12-week study, a lower dose of fluvoxamine (up to 150 mg/day) did not show any significant benefits over cognitive therapy, although an additive effect was observed when the two treatments were used together (Sharp et al 1996). Fluvoxamine (up to 150 mg/day) also enhanced the effects of exposure therapy in a 12-week study in 76 patients (de Beurs et al 1995). A 2-year follow-up showed that the beneficial effects of fluvoxamine plus exposure therapy were maintained and that these patients tended to require less aftercare than those not treated with fluvoxamine (de Beurs et al 1999).
Post-traumatic stress disorder
Patients with post-traumatic stress disorder (PTSD) re-experience previous extreme trauma (eg, rape or combat) in the form of persistent dreams and flashbacks and suffer from hyperarousal and numbing symptoms. Only a minority of patients who survive trauma go on to develop PTSD, thus suggesting that it is not just a normal reaction.
Fluvoxamine has shown efficacy in both civilians and war veterans with PTSD, although only small open, noncomparative studies have been conducted to date. Four studies involved veterans from either World War II (de Boer et al 1992) or the Vietnam War (Marmar et al 1996; Neylan et al 2001; Escalona et al 2002) treated with fluvoxamine (100–300 mg/day) for between 10 and 14 weeks. Improvements were seen in intrusion, avoidance, and hyperarousal symptoms in all four studies, together with a reduction in anxiety. In one study that focused particularly on sleep disturbance, fluvoxamine improved all domains of subjective sleep quality, the greatest effect being seen for reduction of dreams linked to the traumatic experience (Neylan et al 2001). Sleep maintenance insomnia and troubled sleep also showed a large treatment response. A substantial improvement in sleep quality was also noted in the study involving World War II veterans (de Boer et al 1992).
Two studies have assessed fluvoxamine in civilians with PTSD. In a 4-week study in 14 patients who had experienced a variety of traumatic events (mainly traumatic bereavement and childhood sexual trauma), fluvoxamine (50–300 mg/day) significantly (p < 0.05) improved the symptoms of PTSD on a range of efficacy scales, the mean reduction from baseline ranging from 35% to 48% (Davidson et al 1998). Autonomic reactivity (heart rate, blood pressure) to trauma scripts fell significantly (p < 0.05) following 10 weeks of fluvoxamine treatment (100–300 mg/day) in 16 PTSD patients (Tucker et al 2000). Significant improvements were also seen in PTSD symptom clusters and depression.
Tolerability and patient acceptability
Fluvoxamine is generally well tolerated and has proved safe in all age groups from children (Research Unit on Pediatric Psychopharmacology Anxiety Study Group 2001; Riddle et al 2001) to the elderly (Wagner et al 1996; Jaquenoud and Kat et al 1997) and in patients with mild cardiovascular disease or epilepsy (Harmant et al 1990; Präger et al 1991). In common with other SSRIs, nausea is the most frequent adverse event. A review of 66 postmarketing studies conducted worldwide in 34 587 patients (mainly suffering from depression) treated with fluvoxamine (50–300 mg/day; modal dose 100 mg/day) for between 4 to 52 weeks showed that nausea occurred in 15.7% of patients; all other adverse events had an incidence of < 10% (Table 4). Prescription-event monitoring of fluvoxamine in 10 401 patients also showed nausea to be the only event seen in > 10% of patients (Edwards et al 1994). A very low level of suicidality was reported in these studies (Edwards et al 1994; Wagner et al 1994) and this was confirmed in global postmarketing surveillance data obtained over 17 years in an estimated 28 million patients exposed to fluvoxamine (Buchberger and Wagner et al 2002). Switch to mania, serotonin syndrome, and convulsions are rarely reported and, although some patients may experience withdrawal symptoms, they are generally mild and resolve spontaneously. Fluvoxamine is relatively safe in overdose, an important consideration in patients with psychiatric disorders. In a review of 221 cases of overdose (≤ 9 g), the acute toxicity that could be attributed to fluvoxamine alone was rarely severe and cardiotoxicity was rare (Garnier et al 1993).
Table 4.
Incidence of adverse events (≥ 2%) with fluvoxamine in postmarketing studies (n = 34 587)
| Adverse event | Incidence (%) |
|---|---|
| Nausea | 15.7 |
| Somnolence | 6.4 |
| Asthenia | 5.1 |
| Headache | 4.8 |
| Dry mouth | 4.8 |
| Insomnia | 4.0 |
| Dizziness | 3.7 |
| Abdominal pain | 3.5 |
| Vomiting | 3.5 |
| Dyspepsia | 3.2 |
| Constipation | 3.2 |
| Nervousness | 3.2 |
| Tremor | 2.8 |
| Diarrhea | 2.4 |
| Anorexia | 2.2 |
Adapted from Wagner et al (1994).
Fluvoxamine does not significantly change body weight (Harris and Ashford et al 1991) and has few cardiovascular or anticholinergic effects (Präger et al 1991; Flett et al 1992; Wagner et al 1994; Hochberg et al 1995). Studies have shown negligible sedation or impairment in cognitive or psychomotor function (Saletu et al 1983; Hindmarch 1995). A meta-analysis of 73 double-blind, crossover, placebo- or verum-controlled studies assessing the effects of drugs used in the treatment of SAD on objective parameters of cognitive and psychomotor performance in healthy volunteers showed that only fluvoxamine and bupropion were entirely free of impairment in any test (Hindmarch and Trick in press). A total of 1949 individual psychometric tests were evaluated, of which 428 (22%) showed impairment after drug administration. Fluvoxamine does not appear to disrupt sleep (Silvestri et al 2001) and has indeed shown beneficial effects on sleep in patients with PTSD (Neylan et al 2001).
In contrast to many antidepressants, fluvoxamine has a low incidence of sexual dysfunction. Prescription event monitoring and postmarketing studies rarely report sexual problems. However, in studies designed to specifically assess sexual side effects, fluvoxamine appears to have less effect than the other SSRIs on sexual function (Nemeroff et al 1995; Montejo et al 1996, 1997). A 6-week, double-blind study in healthy men with premature ejaculation showed that placebo and fluvoxamine had no effect on ejaculation time whilst paroxetine, fluoxetine, and sertraline all significantly increased ejaculation latency, the greatest effect being seen with paroxetine (Figure 3) (Waldinger et al 1998).
Figure 3.
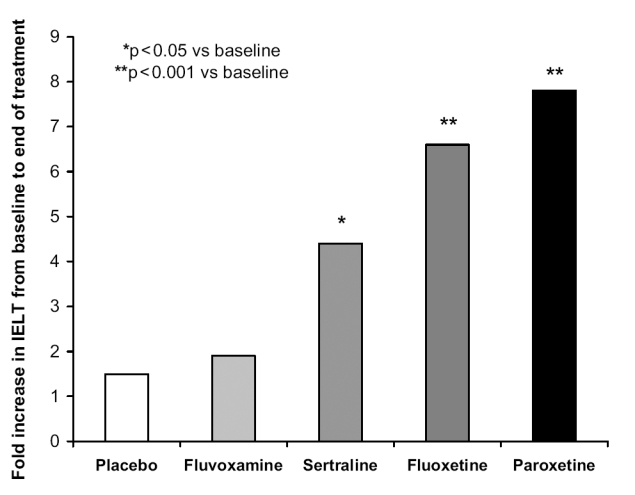
Increase in intravaginal ejaculation latency time (IELT) after 6 weeks in patients with premature ejaculation. Data adapted from Waldinger et al (1998).
Conclusions
Anxiety disorders are extremely common, highly disabling, and associated with considerable comorbidity with depression, substance abuse disorders, and other serious psychiatric illnesses. This category of illness therefore represents an important area of medical need. The well known disadvantages of the benzodiazepines, tricyclic antidepressants, and neuroleptics have resulted in the SSRIs, with their relatively benign adverse event profile, becoming first-line treatment for many anxiety disorders. Among the SSRIs, fluvoxamine has been particularly well studied and was the first to be approved in OCD. Importantly, fluvoxamine has also been fully studied in children and adolescents, since some anxiety disorders (OCD and SAD in particular) have their onset in childhood.
The efficacy of fluvoxamine has been demonstrated by double-blind, randomized, controlled studies in OCD, SAD, and panic disorder. It lacks the common treatment-limiting anticholinergic and cognitive side effects and cardiotoxicity of the tricyclic antidepressants (heretofore clomipramine was the mainstay of OCD treatment), and the cognitive impairment and dependence susceptibility of the benzo-diazepines that are used in a variety of other anxiety disorders. It is also safe in overdose and is associated with a low incidence of suicidality. Compared with the other SSRIs, fluvoxamine has a generally similar tolerability profile, dominated by usually mild and self-limiting gastrointestinal complaints, but appears to cause less sexual side effects and less cognitive disturbance. Unlike paroxetine, it does not induce a significant withdrawal syndrome. Fluvoxamine can therefore be considered a first-line treatment for adults and children with a range of anxiety disorders.
As well as clinical trials, the safety and tolerability of fluvoxamine has been established in extensive, published postmarketing studies, prescription event monitoring, and specific studies in patients with concomitant medical problems such as cardiovascular disease and multi-medicated elderly patients. The safety and tolerability of fluvoxamine in real-world use has thus been widely studied. Whilst the efficacy of fluvoxamine in a variety of anxiety disorders is well established from randomized, clinical studies, there is a relative lack of published large naturalistic efficacy studies. However, there is now considerable clinical experience with fluvoxamine and no reason to believe that the efficacy of fluvoxamine under routine clinical conditions would differ in any significant way from that observed in clinical studies.
Overall, fluvoxamine is an important first-line therapy in the treatment of OCD, SAD, and panic disorder. Its tolerability profile is better than traditional treatments and may offer some advantages over other SSRIs.
References
- Arnold LM, Mutasim DF, Dwight MM, et al. An open clinical trial of fluvoxamine treatment of psychogenic excoriation. J Clin Psychopharmacol. 1999;19:15–18. doi: 10.1097/00004714-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Asnis GM, Hameedi FA, Goddard AW, et al. Fluvoxamine in the treatment of panic disorder: a multi-center, double-blind, placebo-controlled study in outpatients. Psychiatry Res. 2001;103:1–14. doi: 10.1016/s0165-1781(01)00265-7. [DOI] [PubMed] [Google Scholar]
- Ayuso-Gutierrez JL, Palazón JL, Ayuso-Mateos JL. Open trial of fluvoxamine in the treatment of bulimia nervosa. Int J Eating Disord. 1994;15:245–9. doi: 10.1002/1098-108x(199404)15:3<245::aid-eat2260150307>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bakish D, Hooper CL, Filteau MJ, et al. A double-blind, placebo-controlled trial comparing fluvoxamine and imipramine in the treatment of panic disorder with or without agoraphobia. Psychopharmacol Bull. 1996;32:135–41. [PubMed] [Google Scholar]
- Black DW, Gabel J, Hansen J, et al. A double-blind comparison of fluvoxamine versus placebo in the treatment of compulsive buying disorder. Ann Clin Psychiatry. 2000;12:205–11. doi: 10.1023/a:1009030425631. [DOI] [PubMed] [Google Scholar]
- Black DW, Monahan P, Gabel J. Fluvoxamine in the treatment of compulsive buying. J Clin Psychiatry. 1997;58:159–63. doi: 10.4088/jcp.v58n0404. [DOI] [PubMed] [Google Scholar]
- Black DW, Wesner R, Bowers W, et al. A comparison of fluvoxamine, cognitive therapy and placebo in the treatment of panic disorder. Arch Gen Psychiatry. 1993;50:44–50. doi: 10.1001/archpsyc.1993.01820130046008. [DOI] [PubMed] [Google Scholar]
- Buchberger R, Wagner W. Fluvoxamine: safety profile in extensive post-marketing surveillance. Pharmacopsychiatry. 2002;35:101–8. doi: 10.1055/s-2002-31522. [DOI] [PubMed] [Google Scholar]
- Cottraux J, Mollard E, Bouvard M, et al. Exposure therapy, fluvoxamine, or combination treatment in obsessive-compulsive disorder: one-year follow-up. Psychiatry Res. 1993;49:63–75. doi: 10.1016/0165-1781(93)90030-k. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Weisler RH, Malik M, et al. Fluvoxamine in civilians with posttraumatic stress disorder. J Clin Psychopharmacol. 1998;18:93–5. doi: 10.1097/00004714-199802000-00020. [DOI] [PubMed] [Google Scholar]
- Davidson J, Yaryura-Tobias J, Du Pont R, et al. Fluvoxamine-controlled-release formulation for the treatment of generalized social anxiety disorder. J Clin Psychopharmacol. 2004;24:118–25. doi: 10.1097/01.jcp.0000106222.36344.96. [DOI] [PubMed] [Google Scholar]
- de Beurs E, van Balkom AJ, Lange A, et al. Treatment of panic disorder with agoraphobia: comparison of fluvoxamine, placebo, and psychological panic management combined with exposure and of exposure in vivo alone. Am J Psychiatry. 1995;152:683–91. doi: 10.1176/ajp.152.5.683. [DOI] [PubMed] [Google Scholar]
- de Beurs E, van Balkom AJLM, Van Dyck R, et al. Long-term outcome of pharmacological and psychological treatment for panic disorder with agoraphobia: a 2-year naturalistic follow-up. Acta Psychiatr Scand. 1999;99:59–67. doi: 10.1111/j.1600-0447.1999.tb05385.x. [DOI] [PubMed] [Google Scholar]
- De Boer M, Op den Velde W, Falger PJR. Fluvoxamine treatment for chronic PTSD: a pilot study. Psychother Psychosom. 1992;57:158–63. doi: 10.1159/000288593. [DOI] [PubMed] [Google Scholar]
- Den Boer JA, Westenberg HGM. Effect of a serotonin and noradrenaline uptake inhibitor in panic disorders: a double-blind comparative study with fluvoxamine and maprotiline. Int Clin Psychopharmacol. 1988;3:59–74. doi: 10.1097/00004850-198801000-00005. [DOI] [PubMed] [Google Scholar]
- Den Boer JA, Westenberg HGM. Serotonin function in panic disorder: a double-blind placebo controlled study with fluvoxamine and ritanserin. Psychopharmacol. 1990;102:85–94. doi: 10.1007/BF02245749. [DOI] [PubMed] [Google Scholar]
- DeVane CL. Differential pharmacology of newer antidepressants. J Clin Psychiatry. 1998;59(Suppl 20):85–93. [PubMed] [Google Scholar]
- de Vries MH, Raghoebar M, Mathlener IS, et al. Single and multiple oral dose fluvoxamine kinetics in young and elderly subjects. Ther Drug Monit. 1992;14:493–8. doi: 10.1097/00007691-199212000-00010. [DOI] [PubMed] [Google Scholar]
- Edwards JG, Inman WHW, Wilton L, et al. Prescription-event monitoring of 10,401 patients treated with fluvoxamine. Br J Psychiatry. 1994;164:387–95. doi: 10.1192/bjp.164.3.387. [DOI] [PubMed] [Google Scholar]
- Escalona R, Canive JM, Calais LA, et al. Fluvoxamine treatment in veterans with combat-related post-traumatic stress disorders. Depress Anxiety. 2002;15:29–33. doi: 10.1002/da.1082. [DOI] [PubMed] [Google Scholar]
- Fallon BA, Qureshi AI, Schneier FR, et al. An open trial of fluvoxamine for hypochondriasis. Psychosomatics. 2003;44:298–303. doi: 10.1176/appi.psy.44.4.298. [DOI] [PubMed] [Google Scholar]
- Fichter MM, Kruger R, Rief W, et al. Fluvoxamine in prevention of relapse in bulimia nervosa: effects on eating-specific psychopathology. J Clin Psychopharmacol. 1996;16:9–18. doi: 10.1097/00004714-199602000-00003. [DOI] [PubMed] [Google Scholar]
- Fichter MM, Leibl C, Krüger R, et al. Effects of fluvoxamine on depression, anxiety and other areas of general psychopathology in bulimia nervosa. Pharmacopsychiatry. 1997;30:85–92. doi: 10.1055/s-2007-979488. [DOI] [PubMed] [Google Scholar]
- Flett SR, Szabadi E, Bradshaw CM. A comparison of the effects of fluvoxamine and amitriptyline on autonomic functions in healthy volunteers. Eur J Clin Pharmacol. 1992;42:529–33. doi: 10.1007/BF00314863. [DOI] [PubMed] [Google Scholar]
- Freeman CPL, Trimble MR, Deakin JFW, et al. Fluvoxamine versus clomipramine in the treatment of obsessive-compulsive disorder? A multicenter, randomized, double-blind, parallel-group comparison. J Clin Psychiatry. 1994;55:301–5. [PubMed] [Google Scholar]
- Fukuda T, Sugie H, Ito M, et al. Clinical evaluation of treatment with fluvoxamine, a selective serotonin reuptake inhibitor in children with autistic disorder. No To Hattatsu. 2001;33:314–18. [PubMed] [Google Scholar]
- Garnier R, Azoyan P, Chataigner D, et al. Acute fluvoxamine poisoning. J Int Med Res. 1993;21:197–208. doi: 10.1177/030006059302100405. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Kozak MJ, Liebowitz M, et al. Treatment of obsessive compulsive disorder with fluvoxamine: a multicentre double blind placebo controlled trial. Int Clin Psychopharmacol. 1996;11:21–9. [PubMed] [Google Scholar]
- Goodman WK, Price LH, Delgado PL, et al. Specificity of serotonin reuptake inhibitors in the treatment of obsessive-compulsive disorder. Comparison of fluvoxamine and desipramine. Arch Gen Psychiatry. 1990;47:577–85. doi: 10.1001/archpsyc.1990.01810180077011. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, et al. Efficacy of fluvoxamine in obsessive-compulsive disorder: a double-blind comparison with placebo. Arch Gen Psychiatry. 1989;46:36–44. doi: 10.1001/archpsyc.1989.01810010038006. [DOI] [PubMed] [Google Scholar]
- Greist JH, Jenike MA, Robinson D, et al. Efficacy of fluvoxamine in obsessive-compulsive disorder: results of a multicentre, double-blind, placebo-controlled trial. Eur J Clin Res. 1995;7:195–204. [Google Scholar]
- Harmant J, van Rijckevorsel-Harmant K, de Barsy T, et al. Fluvoxamine: an antidepressant with low (or no) epileptogenic effect. Lancet. 1990;2:386. doi: 10.1016/0140-6736(90)91938-7. [DOI] [PubMed] [Google Scholar]
- Harris B, Ashford J. Maintenance antidepressants and weight gain: a comparison of fluvoxamine and amitriptyline. Br J Clin Res. 1991;2:81–8. [Google Scholar]
- Hindmarch I. The behavioural toxicity of the selective serotonin reuptake inhibitors. Int Clin Psychopharmacol. 1995;9(Suppl 4):13–17. doi: 10.1097/00004850-199501004-00002. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Trick L. Behavioural toxicity of pharmacotherapeutic agents used in social anxiety disorder. Hum Psychopharmacol. doi: 10.1111/j.1742-1241.2009.02085.x. In press. [DOI] [PubMed] [Google Scholar]
- Hochberg H, Kanter D, Houser V. Electrocardiographic findings during extended clinical trials of fluvoxamine in depression: one years experience. Pharmacopsychiatry. 1995;28:253–6. doi: 10.1055/s-2007-979612. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Hipsley PA. Effect of fluvoxamine on panic disorder. J Clin Psychopharmacol. 1993;13:321–6. [PubMed] [Google Scholar]
- Hohagen F, Winkelmann G, Rasche-Räuchle H, et al. Combination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo: results of a multicentre study. Br J Psychiatry. 1998;173(Suppl 35):71–8. [PubMed] [Google Scholar]
- Hollander E, Cohen L, Simeon D, et al. Fluvoxamine treatment of body dysmorphic disorder. J Clin Psychopharmacol. 1994;14:75–7. [PubMed] [Google Scholar]
- Hollander E, DeCaria CM, Finkell JN, et al. A randomised, double-blind fluvoxamine/placebo crossover trial in pathologic gambling. Biol Psychiatry. 2000;47:813–17. doi: 10.1016/s0006-3223(00)00241-9. [DOI] [PubMed] [Google Scholar]
- Hollander E, DeCaria CM, Mari E, et al. Short-term single-blind fluvoxamine treatment of pathological gambling. Am J Psychiatry. 1998;155:1781–3. doi: 10.1176/ajp.155.12.1781. [DOI] [PubMed] [Google Scholar]
- Hollander E, Koran LM, Goodman WK, et al. A double-blind, placebo-controlled study of the efficacy and safety of controlled-release fluvoxamine in patients with obsessive-compulsive disorder. J Clin Psychiatry. 2003;64:640–7. doi: 10.4088/jcp.v64n0604. [DOI] [PubMed] [Google Scholar]
- Hollander E, Wong CM. Obsessive-compulsive spectrum disorders. J Clin Psychiatry. 1995;56(Suppl 4):3–6. [PubMed] [Google Scholar]
- Hudson JI, McElroy SL, Raymond NC, et al. Fluvoxamine in the treatment of binge-eating disorder: a multicenter placebo-controlled, double-blind trial. Am J Psychiatry. 1998;155:1756–62. doi: 10.1176/ajp.155.12.1756. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Comparative pharmacology of selective serotonin reuptake inhibitors (SSRIs) Nord J Psychiatry. 1993;47(Suppl 30):5–12. [Google Scholar]
- Jaquenoud E, Kat M. The safety of fluvoxamine in very elderly patients with depression and somatic symptoms. Prim Care Psychiatry. 1997;3:175–81. [Google Scholar]
- Jenike MA, Hyman S, Baer L, et al. A controlled trial of fluvoxamine in obsessive-compulsive disorder: implications for a serotonergic theory. Am J Psychiatry. 1990;147:1209–15. doi: 10.1176/ajp.147.9.1209. [DOI] [PubMed] [Google Scholar]
- Karno M, Golding JM, Sorenson SB, et al. The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry. 1988;45:1094–9. doi: 10.1001/archpsyc.1988.01800360042006. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KC, Zhao S, et al. Lifetime and 12-month prevalence of DSM-IIIR psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kobak KA, Greist JH, Jefferson JW, et al. Fluoxetine in social phobia: a double-blind, placebo-controlled pilot study. J Clin Psychopharmacol. 2002;22:257–62. doi: 10.1097/00004714-200206000-00005. [DOI] [PubMed] [Google Scholar]
- Koran LM, McElroy SL, Davidson JRT, et al. Fluvoxamine versus clomipramine for obsessive-compulsive disorder: a double-blind comparison. J Clin Psychopharmacol. 1996;16:121–9. doi: 10.1097/00004714-199604000-00004. [DOI] [PubMed] [Google Scholar]
- Leonard B. Pharmacological differences of serotonin reuptake inhibitors and possible clinical relevance. Drugs. 1992;43(Suppl 2):3–10. doi: 10.2165/00003495-199200432-00003. [DOI] [PubMed] [Google Scholar]
- MacQueen G, Born L, Steiner M. The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev. 2001;7:1–24. doi: 10.1111/j.1527-3458.2001.tb00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmar CR, Schoenfeld F, Weiss DS, et al. Open trial of fluvoxamine treatment for combat-related posttraumatic stress disorder. J Clin Psychiatry. 1996;57(Suppl 8):66–70. [PubMed] [Google Scholar]
- Martin A, Koenig K, Anderson GM, et al. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Autism Dev Disord. 2003;33:77–85. doi: 10.1023/a:1022234605695. [DOI] [PubMed] [Google Scholar]
- Matsunaga H, Kiriike N, Matsui T, et al. Taijin kyofusho: a form of social anxiety disorder that responds to serotonin reuptake inhibitors? Int J Neuropsychopharmacol. 2001;4:231–7. doi: 10.1017/S1461145701002474. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Naylor ST, Cohen DJ, et al. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry. 1996;53:1001–8. doi: 10.1001/archpsyc.1996.01830110037005. [DOI] [PubMed] [Google Scholar]
- Milanfranchi A, Ravagli S, Lensi P, et al. A double-blind study of fluvoxamine and clomipramine in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol. 1997;12:131–6. doi: 10.1097/00004850-199705000-00002. [DOI] [PubMed] [Google Scholar]
- Milijkovic BR, Pokrajac M, Timotijevic I, et al. The influence of lithium on fluvoxamine therapeutic efficacy and pharmacokinetics in depressed patients on combined fluvoxamine-lithium therapy. Int Clin Psychopharmacol. 1997;12:207–12. [PubMed] [Google Scholar]
- Montejo AI, Llorca G, Izquierdo JA, et al. Sexual dysfunction secondary to SSRIs. A comparative analysis of 308 patients. Actas Luso-Espanolas de Neurologia, Psiquiatria y Ciencias Afines. 1996;24:311–21. [PubMed] [Google Scholar]
- Montejo AL, Llorca G, Izquierdo JA, et al. SSRI-induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J Sex Marital Ther. 1997;23:176–94. doi: 10.1080/00926239708403923. [DOI] [PubMed] [Google Scholar]
- Mundo E, Bareggi SR, Pirola R, et al. Long-term pharmacotherapy of obsessive-compulsive disorder: a double-blind controlled study. J Clin Psychopharmacol. 1997;17:4–10. doi: 10.1097/00004714-199702000-00002. [DOI] [PubMed] [Google Scholar]
- Mundo E, Maina G, Uslenghi C, et al. Multicentre, double-blind comparison of fluvoxamine anpid clomipramine in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol. 2000;15:69–76. doi: 10.1097/00004850-200015020-00002. [DOI] [PubMed] [Google Scholar]
- Mundo E, Rouillon F, Figuera M, et al. Fluvoxamine in obsessive-compulsive disorder: similar efficacy but superior tolerability in comparison with clomipramine. Hum Psychopharmacol. 2001;16:461–8. doi: 10.1002/hup.317. [DOI] [PubMed] [Google Scholar]
- Narita N, Hashimoto K, Tomitaka S, et al. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptor in rat brain. Eur J Pharmacol. 1996;307:117–19. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Ninan PT, Ballenger JC, et al. Double-blind multicenter comparison of fluvoxamine versus sertraline in the treatment of depressed outpatients. Depression. 1995;3:163–9. [Google Scholar]
- Neylan TC, Metzler TJ, Schoenfeld FB, et al. Fluvoxamine and sleep disturbances in posttraumatic stress disorder. J Traum Stress. 2001;14:461–7. doi: 10.1023/A:1011100420978. [DOI] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Effects of 5-HT uptake inhibitors, agonists and antagonists on burying of harmless objects by mice; a putative test for anxiolytic agents. Br J Pharmacol. 1991;104:105–12. doi: 10.1111/j.1476-5381.1991.tb12392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs HR, Greenblatt DJ, Verburg-Ochs B, et al. Chronic treatment with fluvoxamine, clovoxamine and placebo: interaction with digoxin and effects on sleep and alertness. J Clin Pharmacol. 1989;29:91–5. doi: 10.1002/j.1552-4604.1989.tb03243.x. [DOI] [PubMed] [Google Scholar]
- Olivier B, Bosch L, van-Hest A, et al. Preclinical evidence on the psychotropic profile of fluvoxamine. Pharmacopsychiatry. 1993;26(Suppl 1):2–9. doi: 10.1055/s-2007-1014370. [DOI] [PubMed] [Google Scholar]
- O’Sullivan RL, Phillips KA, Keuthen NJ, et al. Near-fatal skin picking from delusional body dysmorphic disorder responsive to fluvoxamine. Psychosomatics. 1999;40:79–81. doi: 10.1016/S0033-3182(99)71276-4. [DOI] [PubMed] [Google Scholar]
- Overmars H, Scherpenisse PM, Post LC. Fluvoxamine maleate: metabolism in man. Eur J Drug Metab Pharmacokinet. 1983;8:269–80. doi: 10.1007/BF03188757. [DOI] [PubMed] [Google Scholar]
- Perse TL, Greist JH, Jefferson JW, et al. Fluvoxamine treatment of obsessive-compulsive disorder. Am J Psychiatry. 1987;144:1543–8. doi: 10.1176/ajp.144.12.1543. [DOI] [PubMed] [Google Scholar]
- Perugi G, Giannotti D, Di Vaio S, et al. Fluvoxamine in the treatment of body dysmorphic disorder. J Clin Psychiatry. 1996;59:165–71. doi: 10.1097/00004850-199612000-00006. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Dwight MM, McElroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. J Clin Psychiatry. 1998;59:165–71. doi: 10.4088/jcp.v59n0404. [DOI] [PubMed] [Google Scholar]
- Phillips KA, McElroy SL, Dwight MM, et al. Delusionality and response to open-label fluvoxamine in body dysmorphic disorder. J Clin Psychiatry. 2001;62:87–91. doi: 10.4088/jcp.v62n0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek CM, Wisner KL, Perel JM, et al. Serum fluvoxamine levels in breastfed infants. J Clin Psychiatry. 2001;62:111–13. doi: 10.4088/jcp.v62n0207. [DOI] [PubMed] [Google Scholar]
- Präger G, Stollmaier W, Parger R, et al. Safety and tolerance of fluvoxamine in cardiac patients. TW Neurologie Psychiatrie. 1991;5:548–62. [Google Scholar]
- Ravizza L, Barzega G, Bellino S. Drug treatment of obsessive-compulsive disorder (OCD): long-term trial with clomipramine and selective serotonin reuptake inhibitors (SSRIs) Psychopharmacol Bull. 1996;32:167–73. [PubMed] [Google Scholar]
- Research Unit on Pediatric Psychopharmacology Anxiety Study Group. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–85. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- Ricca V, Mannucci E, Mezzani B, et al. Fluoxetine and fluvoxamine combined with individual cognitive-behaviour therapy in binge eating disorder: a one-year follow-up study. Psychother Psychosom. 2001;70:298–306. doi: 10.1159/000056270. [DOI] [PubMed] [Google Scholar]
- Riddle MA, Reeve EA, Yaryura-Tobias J, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomised, controlled, multicentre trial. J Am Acad Child Adolesc Psychiatry. 2001;40:222–9. doi: 10.1097/00004583-200102000-00017. [DOI] [PubMed] [Google Scholar]
- Saletu B, Grünberger J, Rajna P. Pharmaco-EEG profiles of antidepressants. Pharmacodynamic studies with fluvoxamine. Br J Clin Pharmacol. 1983;15(Suppl 3):369S–384S. doi: 10.1111/j.1365-2125.1983.tb02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann J, Lörch B, Bandelow B, et al. Fluvoxamine or placebo in the treatment of panic disorder and relationship to blood concentrations of fluvoxamine. Pharmacopsychiatry. 1998;31:117–21. doi: 10.1055/s-2007-979311. [DOI] [PubMed] [Google Scholar]
- Sharp DM, Power KG, Simpson RJ, et al. Fluvoxamine, placebo, and cognitive behaviour therapy used alone and in combination in the treatment of panic disorder and agoraphobia. J Anxiety Disord. 1996;10:219–42. [Google Scholar]
- Silvestri R, Pace-Schott EF, Gersh T, et al. Effects of fluvoxamine and paroxetine on sleep structure in normal subjects: a home-based Nightcap evaluation during drug administration and withdrawal. J Clin Psychiatry. 2001;62:642–52. doi: 10.4088/jcp.v62n0812. [DOI] [PubMed] [Google Scholar]
- Stanley MA, Breckenridge JK, Swann AC, et al. Fluvoxamine treatment of trichotillomania. J Clin Psychopharmacol. 1997;17:278–83. doi: 10.1097/00004714-199708000-00007. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Westenberg HG, Yang H, et al. Fluvoxamine CR in the long-term treatment of social anxiety disorder: the 12- to 24-week extension phase of a multicentre, randomised, placebo-controlled trial. Int J Neuropsychopharmacol. 2003;6:317–23. doi: 10.1017/S146114570300364X. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fyer AJ, Davidson JRT, et al. Fluvoxamine treatment of social phobia (social anxiety disorder): a double-blind, placebo-controlled study. Am J Psychiatry. 1999;156:756–60. doi: 10.1176/ajp.156.5.756. [DOI] [PubMed] [Google Scholar]
- Tucker P, Smith KL, Marx B, et al. Fluvoxamine reduces physiologic reactivity to trauma scripts in posttraumatic stress disorder. J Clin Psychopharmacol. 2000;20:367–72. doi: 10.1097/00004714-200006000-00014. [DOI] [PubMed] [Google Scholar]
- van Harten J. Overview of the pharmacokinetics of fluvoxamine. Clin Pharmacokinet. 1995;29(Suppl 1):1–9. doi: 10.2165/00003088-199500291-00003. [DOI] [PubMed] [Google Scholar]
- van Harten J, Duchier J, Devissaguet J-P, et al. Pharmacokinetics of fluvoxamine maleate in patients with liver cirrhosis after single-dose oral administration. Clin Pharmacokinet. 1993;24:177–82. doi: 10.2165/00003088-199324020-00006. [DOI] [PubMed] [Google Scholar]
- van Harten J, Stevens LA, Raghoebar M, et al. Fluvoxamine does not interact with alcohol or potentiate alcohol-related impairment of cognitive function. Clin Pharmacol Ther. 1992;52:427–35. doi: 10.1038/clpt.1992.166. [DOI] [PubMed] [Google Scholar]
- van Vliet IM, den Boer JA, Westenberg HGM. Psycho-pharmacological treatment of social phobia: a double-blind placebo controlled study with fluvoxamine. Psychopharmacol. 1994;115:128–34. doi: 10.1007/BF02244762. [DOI] [PubMed] [Google Scholar]
- van Vliet IM, den Boer JA, Westenberg HG, et al. A double-blind comparative study of brofaromine and fluvoxamine in outpatients with panic disorder. J Clin Psychopharmacol. 1996;16:299–306. doi: 10.1097/00004714-199608000-00005. [DOI] [PubMed] [Google Scholar]
- Wagner W, Hauser V, Wong LF. The safety profile of fluvoxamine in elderly patients. Hum Psychopharmacol. 1996;11:267–72. [Google Scholar]
- Wagner W, Zaborny BA, Gray TE. Fluvoxamine. A review of its safety profile in world-wide studies. Int Clin Psychopharmacol. 1994;9:222–6. doi: 10.1097/00004850-199400940-00001. [DOI] [PubMed] [Google Scholar]
- Wagstaff AJ, Cheer SM, Matheson AJ, et al. Paroxetine: an update of its use in psychiatric disorders in adults. Drugs. 2002;62:655–703. doi: 10.2165/00003495-200262040-00010. [DOI] [PubMed] [Google Scholar]
- Waldinger MD, Hengeveld MW, Zwinderman AH, et al. The effect of SSRIs on ejaculation: a double-blind, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine and sertraline. J Clin Psychopharmacol. 1998;18:274–81. doi: 10.1097/00004714-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Walkup J, Labellarte M, Riddle MA, et al. Treatment of pediatric anxiety disorders: an open-label extension of the research units on pediatric psychopharmacology anxiety study. J Child Adolesc Psychopharmacol. 2002;12:175–88. doi: 10.1089/104454602760386879. [DOI] [PubMed] [Google Scholar]
- Walkup J, Reeve E, Yaruyura-Tobias J, et al. Fluvoxamine in childhood OCD: long-term treatment. Eur Neuropsychopharmacol. 1999;9:307. [Google Scholar]
- Weiller E, Bisserbe JC, Boyer P, et al. Social phobia in general health care. An unrecognised undertreated disabling disorder. Br J Psychiatry. 1996;168:169–74. doi: 10.1192/bjp.168.2.169. [DOI] [PubMed] [Google Scholar]
- Westenberg HG, Stein DJ, Yang H, et al. A double-blind placebo-controlled study of controlled release fluvoxamine for the treatment of generalized social anxiety disorder. J Clin Psychopharmacol. 2004;24:49–55. doi: 10.1097/01.jcp.0000104906.75206.8b. [DOI] [PubMed] [Google Scholar]
- Wilde MI, Plosker GL, Benfield P. Fluvoxamine: an updated review of its pharmacology and therapeutic use in depressive illness. Drugs. 1993;46:895–924. doi: 10.2165/00003495-199346050-00008. [DOI] [PubMed] [Google Scholar]
- Woods A, Smith C, Szewczak M, et al. Selective serotonin reuptake inhibitors decrease schedule-induced polydipsia in rats: a potential model for obsessive compulsive disorder. Psychopharmacol. 1993;112:195–8. doi: 10.1007/BF02244910. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Hirose M, Haginoya K, et al. Treatment with fluvoxamine against self-injury and aggressive behavior in autistic children. No To Hattatsu. 2002;34:249–53. [PubMed] [Google Scholar]


