Abstract
This study was designed to determine the feasibility and efficacy of long-term nicotine replacement therapy (NRT) in helping persons with schizophrenia remain tobacco-free. Fifty smokers with a diagnosis of schizophrenia or schizo-affective disorder and whose symptoms had been stable for at least two months were enrolled in a program providing group support and NRT (patches) in individually adjusted doses set to maintain baseline nicotine intake. All participants attended weekly group support/motivation sessions. Smoking activity was determined by measuring carbon monoxide levels in expired air. Participants who quit tobacco use completely during the first three months were entered into a single-blind phase in which they received either placebo or active nicotine patches for up to six additional months, along with biweekly group sessions. Sixty days into the open-label phase, 66% of the subjects had reduced their use of tobacco by at least 75%. After 90 days of open-label treatment, 18 subjects (36%) were tobacco-free and qualified to enter the six-month, single-blind phase, eight on placebo and nine on active patches. A significantly greater proportion of those on placebo (8 of 8) compared with those on active patches (3 of 9) relapsed prior to completion of the 6-month period. This difference is statistically significant at the p = 0.009 level. The results of this study indicate that long-term use of NRT is feasible and effective for sustained tobacco-free success and may be an important strategy for reducing health risks due to tobacco use in this special population.
Keywords: schizophrenia, smoking cessation, nicotine replacement
Introduction
The prevalence of tobacco use and nicotine addiction among persons with schizophrenia in America is known to be substantially higher than among the general population. Tobacco use rates of 70%–90% have been reported for persons with schizophrenia, compared with approximately 23% in the overall United States population (Hughes et al 1986; Goff 1992; Lohr and Flynn 1992; Ziedonis and George 1997; Lasser et al 2000; Araki 2002). Unfortunately, persons with schizophrenia are subject to the same tobacco-related health risks as other tobacco users. As a result, tobacco-related death rates among this population reflect its high prevalence of tobacco use (Black 1998; Brown 2000; Ösby et al 2000).
Standard smoking cessation therapies are effective in helping smokers with schizophrenia quit smoking. Motivational group sessions (Addington et al 1998), NRT (Ziedonis and George 1997; George et al 2000), and bupropion (George et al 2002a) all appear to be useful in reducing smoking in this population. However, when these therapies are completed, the relapse rates after six months are significant. Addington et al (1998) reported that six months after the completion of seven weekly group sessions, the “quit” rate had decreased approximately 70%. Following a three-month treatment with NRT, George et al (2000) reported “quit” rates to decrease by 50%–70%. Similarly, George et al (2002a) reported a 60% decrease in quit rates six months following a nine-week treatment with bupropion. Comparable relapse rates in the general population average approximately 35% (Fiore et al 1994).
It has been suggested that nicotine may have a unique role(s) in the brains of persons with schizophrenia (Dalack et al 1998; Simosky et al 2002; Smith et al 2002; McCloughen 2003; Vanable et al 2003; Newhouse 2004) and that this may account for the high prevalence of nicotine use by these persons. The accumulated evidence suggests that successful tobacco cessation programs for persons with schizophrenia may have to consider more than just the addiction.
Hays et al (2001) have demonstrated that in a normal population of smokers, extended treatment of the smoking cessation drug bupropion is effective in relapse prevention compared with placebo. The influence of extended pharmacological treatment on relapse rate in a population of smokers with schizophrenia has not been previously studied. Therefore, we sought to determine the feasibility and efficacy of extended NRT to help persons with schizophrenia remain tobacco-free. Nicotine replacement therapy (NRT) is known as an effective treatment for nicotine addiction (Ziedonis and George 1997; George et al 2000) in persons with schizophrenia. We report here the results of a single-blind study in which continued NRT was compared with placebo for six months after persons with schizophrenia had successfully quit smoking through the use of NRT.
Methods
Fifty volunteers with a diagnosis of schizophrenia or schizo-affective disorder were enrolled in the study. Subjects were recruited through the collaborations with local mental health support providers. Each subject gave written informed consent. In addition to the above diagnoses, each subject had to have stable psychiatric symptoms over the past two months, use tobacco daily, be between 18 and 60 years of age, and be able to participate in all of the study activities. Subjects were not included who had cancer or clinically significant cardiac, renal, gastrointestinal, pulmonary, hepatic, endocrine, hematological, or neurological diseases. Also not included were female subjects who were pregnant or breastfeeding, or any subjects who were judged to be unreliable.
Subjects provided medical and psychiatric histories and a list of concomitant medications. Each subject was given a standard 12-lead electrocardiogram, gave a blood sample for a 24-item clinical chemistry panel, and gave a saliva sample for cotinine determination. Levels of expired carbon monoxide (CO), an indicator of recent smoking, were determined with a Vitalograph-Breath Monitor (Catalog Model #29700, Vitalograph Inc, Lenexa, Kansas, USA). Subjects were administered a Clinical Global Evaluation Scale to determine their relative levels of motivation, cognitive functioning, and schizophrenia symptoms; and they completed a modified Fagerström Test for Nicotine Addiction (Heatherton et al 1991).
Following a review of the screening results by the study staff and psychiatrist, subjects were assigned to groups of no more than 10 individuals per group. The groups met with a health educator weekly for one hour for 12 weeks. During these 12 weeks, subjects were presented with a formal curriculum designed for this population and based on the “strengths model” (Rapp et al 1992). The “strengths model” is designed to help the subjects become more aware of and employ their strengths related to intelligence, competence, motivation, and communication in accomplishing their goal of quitting the use of tobacco. This model emphasizes their successes rather than failures. The health educator was experienced in working with subjects with schizophrenia and was trained in providing tobacco cessation group therapy. During regular office hours, the subjects were able to contact the health educator by phone to ask questions or to seek special assistance. Subjects were encouraged to set a quit date within the first two weeks of their participation. To minimize cravings and/or the emergence of symptoms that may be reduced by nicotine, nicotine patch doses were based on individual cotinine saliva levels. Saliva nicotine and cotinine concentrations are highly correlated with those in plasma (Rose 1993). Subjects were provided with NicoDerm® CQ® patches at doses of 14, 21, or 42 mg/day (Dale et al 1995). Subjects with cotinine levels less than 200 ng/mL were given 14 mg patches; those from 200 to 449 ng/mL received 21 mg patches; those from 450 to 900 ng/mL were given 42 mg (two 21 mg patches); and those above 900 ng/mL received 63 mg (three 21 mg patches). Patches were distributed to subjects at their quit date and instructions were given to refrain from smoking while using NRT. Subjects were encouraged to rotate the site of patch applications. During the first and second months some adjustments of nicotine doses were permitted to accommodate individual experiences with side effects or cravings. No changes in nicotine dosage were permitted beyond that time. At each weekly group session, subjects were monitored for expired carbon monoxide levels, smoking activity, craving levels, and NRT tolerance. Subjects with CO levels of 10 ppm or less were considered non-smokers.
A flow chart defining study phases is shown in Figure 1. Subjects who did not set a “quit” date within two weeks, who did not reduce their tobacco use by 75% after 60 days, or who did not quit 100% after 90 days were discontinued from the study. Those subjects who successfully quit tobacco use after 90 days were randomly assigned (coin flip by blinded third party) to receive either placebo patches (SmithKline Beecham Consumer, Parsippany, NJ, USA) or nicotine patches (Nicoderm® CQ®), with no change in dose for six months. Subjects in this phase were blinded as to the type of patches they received. Subjects attended group sessions no less than every two weeks for informal education/motivational discussions with the health educator. During these sessions subjects were monitored for expired CO levels, smoking activity, craving levels, and NRT tolerance. Subjects with CO levels of less than 10 ppm were considered non-smokers. Subjects with CO levels over 10 ppm for two consecutive weeks were considered relapsed and discontinued from the study.
Figure 1.
Study timeline.
a Smoking quit date due at 2 weeks – subjects discontinued if not set.
b Tobacco use to be reduced by 75% at 60 days – subjects discontinued if not successful.
c Tobacco use to be reduced by 100% at 90 days – subjects discontinued if not successful.
Differences in CO levels between baseline (screening levels) and last observation carried forward were analyzed by comparing means using paired t-tests (2-tailed).
For individuals who quit smoking, group differences in outcome (relapse vs non-relapse) and single-blind treatment type (active nicotine patch vs non-active placebo patch) were analyzed by comparing proportions using Fisher’s exact test (2-tailed). Group differences in outcome (relapse vs non-relapse) and antipsychotic medication (on atypical vs not on atypical antipsychotic) were analyzed by comparing proportions using Fisher’s exact test (2-tailed). In addition, a Kaplan-Meier Survival Analysis was conducted to analyze the relationship of single-blind treatment type and time to relapse. Significance was calculated using log-ranks.
Relationships between smoking-associated variables (pack-years, cotinine, and Fagerström rating) were assessed using Kendall’s correlation coefficient for nonparametric data and Pearson’s correlation coefficient for parametric data. An alpha level of 0.05 was used to designate statistical significance. All data in these analyses were tested for normality. SPSS 11.0.1 for Windows was used for all statistical analyses.
Results
Characteristics of the study subjects are shown in Table 1. The population was nearly equally divided between males and females. All participants smoked cigarettes, the mean pack-years was 40 and the mean Fagerström score was 7.4. The antipsychotic medications and their chlorpromazine equivalents taken by the subjects are listed in Table 2 along with their study outcomes. No discernable pattern is evident. Moreover, there was no significant effect of antipsychotic type on smoking outcome. Thirty-two were taking concomitant antidepressant medication primarily selective serotonin reuptake inhibitors. Four subjects screened were taking bupropion (150 mg/day); one did not return for participation, two did not totally quit smoking and consequently did not participate in the single-blind phase of the study, and the fourth was included in the single-blind nicotine patch group but relapsed after two months.
Table 1.
Subject characteristics
| Mean | SD | Range | Frequencies n = 50 | |
|---|---|---|---|---|
| Age | 42.5 | 10.1 | 21–65 | |
| Sex | 24 (48%) female | |||
| 26 (52%) male | ||||
| Pack years | 39.9 | 28.4 | 1.3–112.0 | |
| Fagerstrom | 7.4 | 1.5 | 4.0–10.0 | |
| Baseline cotinine level | 476.4 | 392.0 | 0.0b–2094.0 | |
| Baseline carbon monoxide level | 29.9 | 21.3 | 1.0–110.0 | |
| Taking antipsychotic?a | 44 (100%) yes | |||
| Taking any Antidepressant?a | 32 (72.7%) yes |
Six cases missing who discontinued participation.
One subject quit smoking shortly before study initiation.
Table 2.
Frequencies of antipsychotic medications by study progress
| Total | Chlorpromazine equivalent: Average mg/day (range) [SD] | Single-blind participants | Reduced smoking by 75%, but failed to reduce by 100% | Failed to reduce smoking by 75% | Discontinued participation | |
|---|---|---|---|---|---|---|
| Risperidone | 18 | 165.2 (50–400) [SD = 99.5] | 4 (22.2%) [3]a | 9 (50.0%) | 2 (11.1%) | 3 (16.7%) |
| Olanzapine | 11 | 266.7 (100–500) [SD = 134.7] | 4 (36.4%) [2]a | 5 (45.5%) | 0 (0.0%) | 2 (18.2%) |
| Clozapine | 10 | 447.3 (60–1000) [SD = 398.7] | 5 (50.0%) [3]a | 1 (10.0%) | 0 (0.0%) | 4 (40.0%) |
| Quetiapine fumarate | 6 | 426.8 (267–667) [SD = 173.8] | 1 (16.7%) [1]a | 3 (50.0%) | 0 (0.0%) | 2 (33.3%) |
| Ziprasidone | 6 | 95.8 (17–133) [SD = 54.8] | 2 (33.3%) [2]a | 1 (16.7%) | 0 (0.0%) | 3 (50.0%) |
| Fluphenazine HCl | 4 | 666.7 (500–1000) [SD = 288.7] | 1 (25.0%) [0]a | 1 (25.0%) | 0 (0.0%) | 2 (50.0%) |
| Haloperidol | 2 | 5000.0 (5000–5000) [SD = 0] | 0 (0.0%) [0]a | 1 (50.0%) | 0 (0.0%) | 1 (50.0%) |
| Chlorpromazine HCl | 2 | 300.0 (300–300) [SD = 0] | 1 (50.0%) [1]a | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) |
| Trifluoperazine HCl | 1 | 1000.0 (1000–1000) [SD = 0] | 1 (100.0%) [1]a | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Aripiprazole | 1 | 300.0 (300–300) [SD = 0] | 1 (100.0%) [0]a | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| None | 3 | – | 2 (66.7%) [1]a | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) |
Indicates number relapsed.
Chlorpromazine equivalents adapted from Lehman and Steinwachs (1998); Woods (2003).
Six subjects were taking an antichloinergic (5 benztropine, 1 trihexyphenidyl). Of these, two were included in the single-blind phase. One subject on placebo relapsed. The other on active patch remained tobacco-free to the conclusion of the study. Of the 50 subjects enrolled: 10 failed to return for group sessions and did not receive NRT; two withdrew from the study for personal reasons prior to starting NRT; four failed to reduce their smoking rate by 75% within 60 days and were discontinued from the study; 16 failed to quit smoking completely by 90 days and were discontinued from the study; and 18 quit smoking and advanced to the single-blind extension (see Table 3 for the characteristics of these groups). None of the subjects who began NRT discontinued the study because of adverse events, and none had any significant problem with skin rash at the patch sites. Of the 42 subjects who used NRT, one used 63 mg, 18 used 42 mg, 16 used 21 mg, and seven used 14 mg doses. Of those who participated in the single-blind phase, four used 42 mg, nine used 21 mg, and three used 14 mg doses. Of the four subjects on 42 mg in single-blind, two relapsed and two remained abstinent.
Table 3.
Smoking characteristics of each study group
| Fagerstrom rating | Pack years | Baseline cotinine level | Baseline carbon monoxide level | Final carbon monoxide level | ||
|---|---|---|---|---|---|---|
| Discontinued participation (n = 12) | Mean | 8.0 | 30.2 | 548.5a | 20.4 | 20.1 |
| SD | 1.2 | 27.4 | 447.3a | 18.4 | 13.6 | |
| Failed to reduce smoking by 75% (n = 4) | Mean | 7.8 | 42.5 | 449.5 | 36.5 | 36.8 |
| SD | 1.5 | 35.2 | 177.0 | 17.6 | 16.1 | |
| Reduced smoking by 75%, but failed to reduce by 100% (n = 16) | Mean | 6.8 | 46.2 | 596.8 | 34.6 | 27.0 |
| SD | 1.4 | 33.9 | 515.4 | 26.0 | 15.1 | |
| Reduced smoking by 100%, but relapsed (n = 11) | Mean | 7.7 | 37.9 | 274.8 | 30.3 | 20.2 |
| SD | 1.5 | 16.9 | 146.9 | 15.1 | 7.6 | |
| Reduced smoking by 100% and maintained non-smoking state (n = 7) | Mean | 7.3 | 44.0 | 420.3 | 30.9 | 1.6 |
| SD | 1.8 | 30.2 | 230.9 | 24.4 | 2.4 | |
| Single-blind subjects receiving nicotine (n = 9) | Mean | 7.8 | 43.0 | 418.1 | 35.9 | 11.1 |
| SD | 1.2 | 25.2 | 201.2 | 23.3 | 14.3 | |
| Single-blind subjects receiving placebo (n = 8) | Mean | 7.8 | 33.8 | 236.8 | 26.1 | 21.1 |
| SD | 1.6 | 18.0 | 152.5 | 11.4 | 15.0 |
One case missing.
The results of the single-blind portion of the study are shown in Figure 2 and Table 4. All eight of the subjects on placebo patches relapsed within six months, while three of nine subjects on nicotine patches relapsed. The difference in smoking status between the placebo and active patch groups was statistically significant at the p = 0.009 level (Fisher 2-tailed test) and p ≤ 0.005 (log ranks). Figure 2 shows a substantial divergence in relapse between the two treatment groups after two months of the single-blind phase. The criterion for relapse was defined as two consecutive measurements of CO above ten parts per million. Table 5 shows the number of months at which this criterion was reached for each of the relapsed subjects and the number of single episodes of CO measurements above 10 ppm prior to reaching the relapse criterion. All of the relapsed subjects showed evidence of intermittent smoking activity prior to relapse while the smoke-free subjects consistently had CO levels below 10 ppm.
Figure 2.
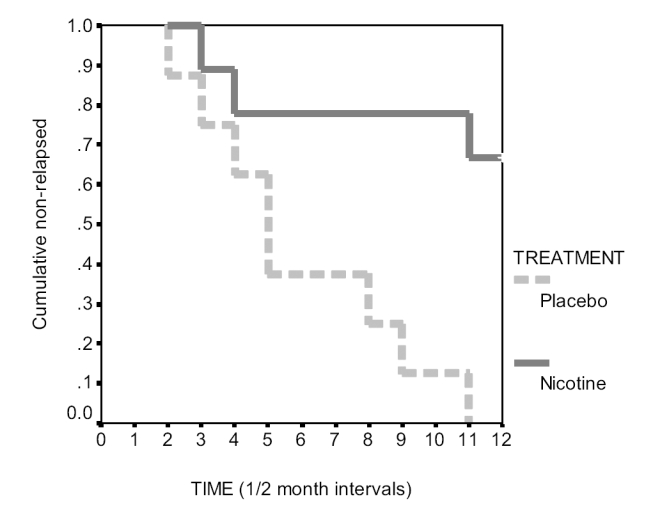
Comparison of time to relapse across treatment groups. The differences seen in Figure 2 illustrate the effect of treatment type on time to relapse. Subjects in the placebo group relapsed sooner than the nicotine group with a 0.0% non-relapse at 6 months, and subjects in the nicotine group had 66.7% non-relapse at 6 months. This effect is statistically significant (logrank = 8.09, df = 1, p < 0.005).
Table 4.
Relationship of nictotine or placebo patch and smoking statusa
| Relapsed | Non-relapse | Total | ||
|---|---|---|---|---|
| Nicotine patch | observed | 3 | 6 | 9 |
| Placebo patch | observed | 8 | 0 | 8 |
| Total | 11 | 6 | 17b |
Fisher’s exact (2-tailed) = 0.009.
One individual who reduced smoking by 100% stopped using any patch, and therefore is not included in either nicotine or placebo group.
Table 5.
Total number of CO measures > 10 in single-blind phase per subject
| Month relapsed (of 6) | #CO’s > 10 | |
|---|---|---|
| Placebo | ||
| 2005 | 5 | 6 |
| 4011 | 3 | 3 |
| 5010 | 2 | 3 |
| 2011 | 4 | 2 |
| 5003 | 1 | 2 |
| 2012 | 2 | 2 |
| 3008 | 6 | 2 |
| 2001 | 3 | 2 |
| Nicotine | ||
| 3003 | – | 0 |
| 5006 | – | 0 |
| 5001 | – | 0 |
| 3005 | – | 0 |
| 4014 | – | 0 |
| 3002 | – | 0 |
| 5009 | 6 | 2 |
| 2009 | 2 | 2 |
| 3009 | 3 | 5 |
Abbreviations: CO, carbon monoxide.
Dashes denote participants who did not relapse.
Five subjects on their own volition attempted to reduce or discontinue the use of patches during the initial phase of the study. One subject remained tobacco-free throughout the study without using patches. That individual was not included in these analyses.
No significant correlation was observed between baseline cotinine levels and Fagerström ratings or pack-years. No significant differences were observed among the groups shown in Table 2 with the exception of the final CO levels that were significantly reduced in the individuals who remained smoke-free throughout the single-blind phase of the study.
Discussion
The results of this pilot study are encouraging. Approximately two-thirds of the subjects reduced their tobacco consumption by 75% within 60 days, and approximately one-third quit completely within 90 days. This compares favorably to quit rates previously reported in subjects with schizophrenia using nicotine patches and group therapies in open label protocols (Addington 1998; George et al 2000). The significant difference in relapse rates between placebo and active patch groups offer particularly promising support for long-term NRT in persons with schizophrenia who have quit using tobacco, although the small sample size requires that our conclusions be considered tentative. With a similarly designed study, Hays et al (2001) have shown that long-term treatment with bupropion after smoking cessation also reduces the rate of relapse in a normal population.
Although this study was conducted in a single blind fashion, the fact that the primary end point, CO levels in expired air, is an objective measure along with the robust response, suggest that the results are worthy of further verification.
The contents of the education/support group sessions were not formally evaluated; however, based on clinical observations they did appear to play a role in the participants’ success by providing opportunities for mutual support and encouragement, and providing focus seemed to contribute to success. Nevertheless, the group sessions alone were not adequate in preventing relapses.
The high prevalence of tobacco use among persons with schizophrenia is widely discussed. Perhaps less appreciated is the high level of addiction as evidenced by high Fagerström nicotine dependence scores. The average Fagerström score found in this study was 7.4. This compares with scores of approximately 2.5–4.5 in nonschizophrenic populations (Fagerström et al 1996; John et al 2003). Scores reported in subjects with schizophrenia range from 5.6 to 9.04 (Addington 1997; George et al 2002b; Patkar et al 2002; Avila et al 2003; Spring 2003). In two studies that compared scores between subjects with and without schizophrenia, those with schizophrenia had significantly higher Fagerström scores (Avila et al 2003; Spring 2003). Another study reported higher scores for smokers with schizophrenia than those without, but the difference was not significant (George et al 2002b). Overall, subjects with schizophrenia appear to have higher than normal Fagerström nicotine dependence scores. In spite of a high dependence level, persons with schizophrenia do have a desire and motivation to give up tobacco (Addington et al 1997), but may require continued smoking cessation therapies to help them achieve long-term success.
The results of this preliminary study suggest additional investigations. First, confirmation of these results in a larger number of subjects and for a longer duration would be useful for determining the practical significance of these findings. Second, information on the cost/benefit of long-term therapy must be evaluated. Is the cost of the program justified by improvements in the health of the individuals with a concomitant reduction in healthcare costs and morbidity?
Acknowledgments
Support for this investigation was provided by The American Legacy Foundation and the Via Christi Foundation. Placebo patches were donated by SmithKline Beecham Consumer, Parsippany, NJ, USA. The authors gratefully acknowledge the following for their excellent assistance and contributions: Cheryl Carmichael for technical and literature support; BJ Kennedy for site coordination; and a special thank you to the staff and management of ComCare of Sedgwick County, Kansas, the Mental Health Association of South Central Kansas, and the Breakthrough Club of Wichita for their cooperation and assistance in recruiting subjects. This study was approved by the Institutional Review Board, Via Christi Regional Medical Center, Wichita, Kansas.
References
- Addington J, el-Guebaly N, Addington D, et al. Readiness to stop smoking in schizophrenia. Can J Psychiatry. 1997;42:49–52. doi: 10.1177/070674379704200107. [DOI] [PubMed] [Google Scholar]
- Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155:974–6. doi: 10.1176/ajp.155.7.974. [DOI] [PubMed] [Google Scholar]
- Araki H, Suemaru K, Gomita Y. Neuronal nicotinic receptor and psychiatric disorders: functional and behavioral effects of nicotine. Jpn J Pharmacol. 2002;88:133–8. doi: 10.1254/jjp.88.133. [DOI] [PubMed] [Google Scholar]
- Avila MT, Sherr JD, Hong E, et al. Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology. 2003;28:2184–91. doi: 10.1038/sj.npp.1300265. [DOI] [PubMed] [Google Scholar]
- Black DW. Iowa record-linkage study: death rates in psychiatric patients. J Affect Disord. 1998;50:277–82. doi: 10.1016/s0165-0327(98)00019-6. [DOI] [PubMed] [Google Scholar]
- Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212–17. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, MeadorWoodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155:1490–501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Dale LC, Hurt RD, Offord KP, et al. High-dose nicotine patch therapy: percentage of replacement and smoking cessation. JAMA. 1995;274:1353–8. [PubMed] [Google Scholar]
- Fagerström KO, Kunze M, Schoberberger R, et al. Nicotine dependence versus smoking prevalence: comparisons among countries and categories of smokers. Tob Control. 1996;5:52–6. doi: 10.1136/tc.5.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Smith SS, Jorenby DE, et al. The effectiveness of the nicotine patch for smoking cessation: a meta-analysis. JAMA. 1994;271:1940–7. [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry. 2002a;52:53–61. doi: 10.1016/s0006-3223(02)01339-2. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002b;26:75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry. 2000;157:1835–42. doi: 10.1176/appi.ajp.157.11.1835. [DOI] [PubMed] [Google Scholar]
- Goff DC, Henderson DC, Amico E. Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry. 1992;149:1189–94. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Rigotti NA, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Ann Intern Med. 2001;135:423–33. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, et al. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–7. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Hapke U, et al. The Fagerström test for nicotine dependence in two adult population samples-potential influence of lifetime amount of tobacco smoked on the degree of dependence. Drug Alcohol Depend. 2003;71:1–6. doi: 10.1016/s0376-8716(03)00038-3. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lehman AF, Steinwachs DM. At issue: translating research into practice: The schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull. 1998;24:1–10. doi: 10.1093/oxfordjournals.schbul.a033302. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Flynn K. Smoking and schizophrenia. Schizophr Res. 1992;8:93–102. doi: 10.1016/0920-9964(92)90024-y. [DOI] [PubMed] [Google Scholar]
- McCloughen A. The association between schizophrenia and cigarette smoking: a review of the literature and implications for mental health nursing practice. Int J Ment Health Nurs. 2003;12:119–29. doi: 10.1046/j.1440-0979.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem. 2004;4:267–82. doi: 10.2174/1568026043451401. [DOI] [PubMed] [Google Scholar]
- Ösby U, Correia N, Brandt L, et al. Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr Res. 2000;45:21–8. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190:604–10. doi: 10.1097/00005053-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Rapp RC, Siegal HA, Fisher JH, et al. A strengths-based model of case management/advocacy: adapting a mental health model to practice work with persons who have substance abuse problems. In: Ashery R, editor. Progress and issues in case management. Rockville: National Institute on Drug Abuse; 1992. pp. 79–91. [PubMed] [Google Scholar]
- Rose JE, Levin ED, Benowitz N. Saliva nicotine as an index of plasma levels in nicotine skin patch users. Ther Drug Monit. 1993;15:431–5. doi: 10.1097/00007691-199310000-00012. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Freedman R. Nicotinic agonists and psychosis. Curr Drug Target CNS Neurol Disord. 2002;1:149–62. doi: 10.2174/1568007024606168. [DOI] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, et al. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–97. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Spring B, Pingitore R, McChargue DE. Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. Am J Psychiatry. 2003;160:316–22. doi: 10.1176/appi.ajp.160.2.316. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Carey MP, Carey KB, et al. Smoking among psychiatric outpatients: relationship to substance use, diagnosis, and illness severity. Psychol Addict Behav. 2003;17:259–65. doi: 10.1037/0893-164X.17.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Ziedonis DM, George TP. Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophr Bull. 1997;23:247–54. doi: 10.1093/schbul/23.2.247. [DOI] [PubMed] [Google Scholar]



