Abstract
Objective
To establish the effectiveness of EEG biofeedback using beta training as a relaxation technique and ultimately reducing anxiety levels of patients with confirmed unstable angina or myocardial infarction.
Methodology
Patients with confirmed unstable angina or myocardial infarction referred by cardiologists were recruited 2–3 days after their cardiac event from the cardiology wards. Their initial anxiety scores were determined using the Hospital Anxiety and Depression Scale. Those that returned for therapy underwent instrument feedback training using EEG every two weeks for a total of five sessions. EEG frequencies were measured for all sessions. Dropouts who did not participate in the program agreed to return 3 months later for the second psychological assessment. The study design was uncontrolled.
Results
Subjects had significantly lower anxiety scores at the second screening (p < 0.001), while the dropouts had significantly higher scores (p < 0.001). Beta training was effective in increasing sensory motor rhythm (SMR) waves but no significant effect was present for the alpha waves.
Conclusions
The uncontrolled nature of the study limits firm conclusions. However, the significantly lowered anxiety scores for subjects and enhancing of SMR waves indicate the effectiveness of beta training as a promising approach to EEG biofeedback for anxiety reduction.
Keywords: EEG biofeedback, cardiac events, anxiety, alpha waves, beta waves
Introduction
Appropriate breathing and relaxation techniques decrease the morbidity and mortality of patients with cardiac events especially myocardial infarction (Dixhorn and Duivenvoordeen 1999). Incorporating these stress reduction therapies in the usage of EEG biofeedback proves to be an important entity in cardiac rehabilitation programs involving patients with ischemic heart diseases. EEG biofeedback might benefit patients with cardiac events as it is associated with the central nervous system and reduces anxiety. Anxiety aggravates the heart condition of patients with coronary heart disease through sympathetic nervous system hyperactivity, increased heart rate, diminished autonomic control of the heart, and increased blood pressure variability, which eventually affects coronary endothelium and plaque formation (King 1997). The present study aims to discover the effectiveness of EEG biofeedback in influencing brainwaves by looking at the EEG frequencies and the distribution of alpha and beta waves during therapy as well as its capability to decrease anxiety levels after five concurrent sessions.
EEG biofeedback, or as its more commonly known, neurofeedback, has shown much clinical value in treating neurobiological disorders such as epilepsy (Kotchoubey et al 1999), attention deficit hyperactivity disorder (Masterpasqua and Healey 2003), and substance abuse (Peniston and Kulkosky 1989). Research shows that a number of these neurological and psychological disorders can be characterized by distinctive EEG patterns and that neurofeedback may help clients change their patterns (Masterpasqua and Healey 2003). However, studies have also focused on healthy individuals as EEG biofeedback, like mental skills training and aerobic exercise, was able to improve the performance of musicians (Egner and Gruzelier 2003).
Neurofeedback refers to an operant conditioning paradigm where participants learn to influence the electrical activity of their brain by regulating specific AC frequencies and the slow cortical potentials (SCPs) of the EEG (Vernon et al 2003). The electrical brain activity is used to control a computer, and this type of communication is usually called a brain-computer interface (Neuper et al 2003). Theta training was traditionally viewed as forming part of a relaxation inducing technique and was originally associated with meditation (Kassamatao and Hirai 1969). However, this form of training requires participants to relax with their eyes closed, receiving only auditory feedback. The most common indicators of relaxation are an increase in alpha frequencies and less complexity in EEG signals, and this establishes the positive effect of a mind machine (Stolc et al 2003). This study utilizes the less frequent beta training and its capability to bring about a mental state of brain relaxation as effective as the alpha and theta bands.
Methodology
Participants
Patients with confirmed unstable angina or myocardial infarction referred by the cardiologists were recruited 2–3 days after their cardiac event from the cardiology wards, National University of Malaysia Hospital (HUKM) and the Institut Jantung Negara (IJN). A cardiac event was based on typical clinical symptomatology, identified through ECG evidence and elevated serum levels of myocardial enzymes. These patients were entered into the study irrespective of age, sex, or ethnicity. All patients were given full details of the study at recruitment and were invited to participate. Written consent was obtained.
Psychological assessment
The Hospital Anxiety and Depression Scale (HADS) was administered at initial diagnosis of cardiac events and during follow-up three months later. The cut-off point indicates the range from normal to severe. The normal rate is below 8, while 8–10 indicates mild symptoms, 11–14 moderate symptoms, and a range of 15–21 points to a severe state of anxiety. For this study, severity of anxiety was limited to three groups, normal and severe representing both extremes, while mild and moderate were categorized as one level. The questionnaire was interview-aided and not self-rated, and only one interviewer conducted the questionnaire for all subjects to avoid interviewer bias. It was not a single-blind study as the interviewer was aware of the treatment conditions. Anxiety levels were used in this study to determine the effectiveness of the therapy. The HADS was chosen due to its practicality and expediency (Roberts et al 2001). Its most important feature was its capability to produce a separate score to establish the presence and severity of anxiety and depression simultaneously. The HADS was also practical in this study as the sample size was small and the scale was validated for use in non-psychiatric units, thus making it an acceptable instrument for cardiac patients.
EEG biofeedback training
Participants who returned for therapy received instrument feedback involving beta/sensory motor rhythms (SMR) training using commercially available biofeedback equipment (Procomp+/Biograph programme-Thought Technology Ltd, Montreal, QC, Canada) every two weeks for a total of 5 sessions. The biofeedback instrumentation provided a media of transferring information from these brainwaves. It worked on a reward basis in which positive feedback resulted in accumulated points, and negative feedback resulted in these points being withheld. A computer monitor was placed 150 cm from the patient. Both the visual feedback received through a video game-like display portraying a maze animation and audio feedback with the inclusion of music with changes in frequency, volume, and rhythm enabled the individual to respond by moving toward a better, learned, and voluntary-controlled function as they consciously directed their brainwaves. For feedback control, there were 3 bargraphs; beta (16–40 Hz), theta (4–7 Hz), and EEG (1–40 Hz). Bars were dark when the signal was within acceptable parameters and turned a bright color when they were not, to rapidly indicate which condition was not satisfied. When the beta signal was above threshold, the animation started and a song played while the counter accumulated points. When any of the three signals got out of condition (beta below threshold, theta, or EEG [1–40 Hz] were above threshold), the animation and music stopped. The animation instrument comprising a maze was linked to this beta instrument.
EEG was recorded from channel C3 according to the International 10–20 system for all training. The reference electrode was placed at the left ear lobe and ground electrode at the right ear lobe. The system applied a variety of digital filters to the recorded signal to extract frequency-domain information. The subsets of the whole EEG bandwidth were filtered and the real-time changes in the total power generated by all the frequencies in the subsets were measured. Frequencies above 40 Hz were interpreted as EMG noise from the neighboring muscles. The component brainwaves of the EEG were separated into individual bands with alpha band defined as 8–12 Hz. Beta waves were categorized into low beta or sensory motor rhythm, SMR (12–15 Hz), beta (16–20 Hz), and high beta (21–40 Hz). Minimal configuration for monochannel EEG measurement consisted of 1 active electrode.
Procedure
Subjects received continuous feedback involving a completion of five sessions of EEG biofeedback training in the three months duration. The workings of the feedback loop were explained to all participants before the start of sessions and they were required to relax without falling asleep. In the training sessions, they were instructed on ways of improving their positive feedback and taught simple breathing exercises to use. Subjects were also required to practice the relevant relaxation and breathing exercises that provided positive feedback daily, preferably at a fixed time of day. The subjects underwent the whole length of the experiment period in a quiet but not a soundproof area, with no one present except for the examiner, and these conditions were preserved for all subjects as described by Teplan (2002). Changes in the EEG frequencies were measured by use of a task that required participants to sustain the 3 bar graphs displayed within the expected parameters described in the above section titled EEG biofeedback training. The first session and the last session were assessed to compare the progress made by the participants at the end of the therapy with the time of recruitment. Mean frequencies were compared for the entire session for the first and last session each participant underwent. Those that dropped out earlier did not receive any therapy. Anxiety levels for all patients (subjects and dropouts) were determined twice, once at time of recruitment (first screening) and at the follow-up assessment three months later (second screening). Within-group and between-group statistical comparisons were made using Student’s t-test.
Results
Characteristics of the sample population
All 115 patients recruited into the study went through an initial psychological assessment in the first screening. Fifty (43%) of them returned to participate in the EEG biofeedback therapy. Four percent completed only 3 sessions, and 20% completed 4. The remainder (n = 38, 76%) completed the required number of 5 sessions. Mean time of completion for sessions was 7.20 minutes, with times ranging from 3.01 to 14.27 minutes. Forty-three percent of the sessions were completed within 5 minutes, with 55% exceeding 5 minutes. Only 2 sessions were completed in less than 5 minutes, with one taking 3 minutes and the other 4.
The remaining 65 patients that declined to participate in the program agreed to be reevaluated for the follow-up psychological assessment during the second screening. For the purpose of this paper, the first group of 65 patients that dropped out without any EEG biofeedback intervention will be identified as dropouts. The patients that consented to the therapy will be considered as subjects, with those successfully completing all 5 sessions as subjects 1, and those that dropped out after going through at least 3 sessions of EEG biofeedback sessions as subjects 2 (n = 12). Most of the subjects (subjects 1 and subjects 2) were male (68%), while dropouts had an almost equal distribution of both sexes; males (n = 30, 46%) and females (n = 35, 53%).
Anxiety levels
Most patients (subjects and dropouts) had normal anxiety levels (71%) at initial recruitment. Thirty-four (68%) of the subjects and 73% of the dropouts had normal levels of anxiety scores at first assessment (Figure 1). There was no difference for anxiety scores between subjects and dropouts for the first screening, but dropouts had significantly higher scores compared with subjects at the second screening (p < 0.001). At completion of therapy, subjects had significantly (p < 0.001) lower mean anxiety scores than at screening, while dropouts had significantly (p < 0.001) higher mean anxiety scores at second than at first screening.
Figure 1.
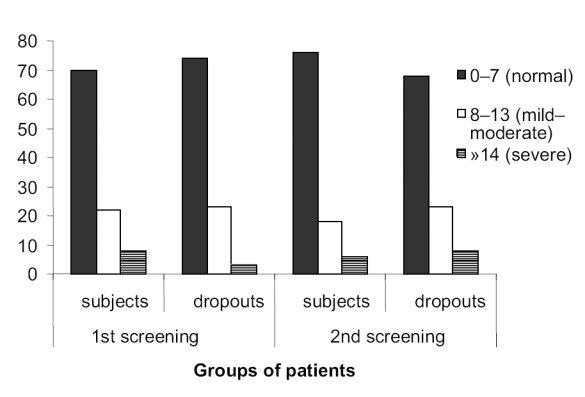
Percentage of patients with different levels of severity for anxiety. Levels of severity for anxiety based on Hospital Anxiety and Depression Scale. First screening is during recruitment; second screening is at follow-up assessment 3 months later. Subjects (n = 50) are those that consented to participate in the EEG biofeedback. Thirty-eight of the subjects completed all 5 sessions, and 12 of them dropped out after 3 or 4 sessions. Dropouts (n = 65) declined to participate in the therapy but agreed to be psychologically assessed.
The dropouts had more than half of those cases (n = 28, 58%) with initial normal range of scores and more than half of the initial mild to severe cases (n = 9, 53%) recording higher scores for the second screening, respectively (Table 1). A mere 14% of the dropouts decreased their anxiety scores. Neither of the subject groups (subjects 1 and subjects 2) had a single case that had increased anxiety scores for the second assessment. More than half of subjects 1 with normal anxiety levels in first screening (n = 16, 59%) decreased their scores while the remainder maintained their scores. Almost half of subjects 1 who initially scored in the mild to severe range lowered their scores (Table 1). Four of the 5 mild to severe cases in the subjects 2 decreased their scores. Mean and maximum value for subjects 2 with initial normal scores succeeded in decreasing their mean and maximum scores but not their maximum value (Table 2). Those who scored mild to severe levels of anxiety scores for dropouts and the subject groups all managed to decrease their minimum scores, but the most drastic change was for subjects 1 that had a minimum score of 0 as compared with 8 in the first screening.
Table 1.
Changes in anxiety scores at second screening as compared with the first screening in subjects 1, subjects 2, and dropouts
| Patients recruited (n = 115) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consented to treatment (n = 50) | ||||||||||||||
| Completed 5 session Subjects 1a (n = 38) |
Completed < 5 sessions Subjects 2b (n = 12) |
Declined treatment Dropoutsc (n = 65) |
||||||||||||
| Initial anxiety levels (based on anxiety scores at first screening)d | ||||||||||||||
| Normal (n = 27) |
Mild to severe (n = 11) |
Normal (n= 7) |
Mild to severe (n = 5) |
Normal (n = 48) |
Mild to severe (n = 17) |
|||||||||
| Changes in anxiety scores at second screening as compared with first screening | ||||||||||||||
| ↓ | No | ↓ | No | ↓ | No | ↓ | No | ↓ | No | ↑ | ↓ | No | ↑ | |
| n | 16 | 11 | 5 | 6 | 3 | 4 | 4 | 1 | 5 | 15 | 28 | 4 | 4 | 9 |
Subjects 1: patients recruited that returned to participate in the therapy and successfully completed all 5 required sessions.
Subjects 2: patients recruited that returned to participate in the therapy and completed at least 3 sessions but not all of the 5 required sessions.
Dropouts: patients recruited that did not return to participate in the therapy although agreed to be assessed for the follow-up psychological assessment at second screening.
Anxiety levels: normal range (< 8) and mild to severe (≥ 8).
NOTE: ↓, those that decreased levels of anxiety scores; No, those with no changes in their anxiety scores; ↑, those that had increased levels of anxiety scores.
Table 2.
Anxiety scores for subjects 1, subjects 2, and dropouts for first and second screening
| Patients recruited (n = 115) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial anxiety levels (based on anxiety scores at first screening) | ||||||||||||
| Normal (n = 82) | Mild to severe (n = 33) | |||||||||||
| Dropoutsa (n = 48) | Subjects 1b (n = 27) | Subjects 2c (n = 7) | Dropouts (n = 17) | Subjects 1 (n = 11) | Subjects 2 (n = 5) | |||||||
| Screening | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd |
| Anxiety scores | ||||||||||||
| Mean | 4 | 5 | 4 | 2 | 4 | 3 | 11 | 11 | 10 | 8 | 13 | 10 |
| Minimum | 0 | 1 | 0 | 0 | 1 | 0 | 8 | 5 | 8 | 0 | 8 | 6 |
| Maximum | 7 | 12 | 7 | 6 | 6 | 6 | 15 | 15 | 19 | 15 | 19 | 15 |
Dropouts: patients recruited that did not return to participate in the therapy although agreed to be assessed for the follow-up psychological assessment at second screening.
Subjects 1: patients recruited that returned to participate in the therapy and successfully completed all 5 required sessions.
Subjects 2: patients recruited that returned to participate in the therapy and completed at least 3 sessions but not all of the 5 required sessions.
EEG frequencies for alpha and beta waves
To describe the performance over the training period, the online results of training sessions were shown as mean frequencies of both alpha and beta waves. Nineteen (70%) of the subjects that scored normal scores had high initial alpha waves for the first and last sessions. The normal initial scorers maintained their high alpha frequencies in the last session and those that did not have high initial frequencies were still unable to increase their frequencies. Nevertheless, 73% of those that scored mild to severe anxiety levels in the first screening also had high initial alpha waves. Furthermore, these participants had 1 person for subjects 1 and 2 persons for subjects 2 that succeeded in increasing their alpha frequencies by their final session (Table 3).
Table 3.
EEG frequencies for alpha and beta waves in subjects 1 and subjects 2 for the first and last session
| Patients consented to treatment (n = 50) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Initial anxiety levels (based on anxiety scores at first screening)a | ||||||||
| Normal (n = 34) |
Mild to severe (n = 16) |
|||||||
| Subjects 1b (n = 27) |
Subjects 2c (n = 7) |
Subjects 1 (n = 11) |
Subjects 2 (n = 5) |
|||||
| Session | 1st | last | 1st | last | 1st | Last | 1st | last |
| Alpha frequencies | ||||||||
| (≥ 8 Hz), n | 19 | 19 | 4 | 4 | 8 | 9 | 2 | 3 |
| AMF, Hz | ||||||||
| Mean | 12.8 | 15.5 | 10.3 | 14.2 | 16.1 | 21.5 | 7.8 | 9.0 |
| Minimum | 4.8 | 5.1 | 4.1 | 5.0 | 2.5 | 7.2 | 6.4 | 7.7 |
| Maximum | 27.2 | 28.9 | 24.5 | 26.8 | 27.3 | 28.9 | 8.7 | 11.0 |
| Beta frequencies (16–40 Hz), n | 6 | 11 | 1 | 2 | 5 | 8 | – | – |
| BMF, Hz | ||||||||
| Mean | 8.8 | 10.9 | 7.1 | 9.8 | 11.6 | 14.7 | 4.5 | 5.3 |
| Minimum | 3.5 | 3.7 | 2.3 | 4.1 | 2.2 | 4.2 | 4.1 | 4.5 |
| Maximum | 19.3 | 20.1 | 17.6 | 19.6 | 19.3 | 19.2 | 4.7 | 6.5 |
Anxiety levels: normal ranges (< 8) and mild–severe (≥ 8)
Subjects 1: patients recruited that returned to participate in the therapy and successfully completed all 5 required sessions
Subjects 2: patients recruited that returned to participate in the therapy and completed at least 3 sessions but not all of the 5 required sessions
NOTE: Dashes denote data are not suitable for beta frequencies.
Abbreviations: AMF, mean alpha frequencies; BMF, mean beta frequencies.
None of the subjects recorded high beta waves and instead most had very low beta frequencies of SMR. A mere 6 participants of subjects 1 with normal levels of anxiety scores recorded moderate levels of beta waves (16–40 Hz) at their first session. Nevertheless, 5 of the subjects 1 group and 1 of the subjects 2 group managed to increase beta waves from low frequency SMR waves to moderate beta frequencies. Three of the subjects 1 group with initial mild to severe anxiety scores increased their SMR waves to the preferred beta frequencies. All of these 3 lowered their anxiety scores to normal levels by the second assessment.
Discussion
As anxiety remains prevalent in patients after their cardiac events (Goodacre et al 2001), progress was indicated in the participants by their significantly reduced anxiety scores after five sessions of beta training. The ability to generate alpha brainwaves has been associated with the self-regulation of stress (Wacker 1996). Previous studies specifically linked anxiety and relaxation with EEG recordings (Isotani et al 2001) and found that an increase in alpha frequencies in the frontal scalp area is an indication of positive relaxation training effects of audiovisual stimulation (Teplan et al 2003) and is neuroprotective (Sterman et al 1970).
As a whole, most participants had normal anxiety levels and this probably had some effect on the results of the EEG recordings particularly the alpha waves. Although alpha changes are tightly linked to anxiety changes, this is only evident in high-anxiety subjects as discovered by Hardt and Kamiya (1978). Their study showed that some people with high levels of anxiety have low alpha waves, and EEG alpha increase is beneficial only for patients who exhibit this low amplitude alpha. Patients with anxiety who show abnormally high levels of alpha at baseline readings do not respond as effectively to alpha increase biofeedback. Another important factor is that alpha waves attenuation is no longer evident with open eyes (Nakagome 2000), and our subjects were instructed to keep their eyes open throughout their sessions. Another study also found no stimulation on the alpha band for participants that were asked to remain awake but with their eyes closed (Schutter et al 2001).
Generally, patients undergoing beta training would have initial high beta frequencies of 22–40 Hz to be used for baseline comparisons. However, the beta bands for all our subjects during the recruitment stage were in the low SMR frequencies, which were below normal beta frequencies. This also suggests that these participants were not highly stressed at the initial diagnosis of their cardiac event during recruitment as excessive beta waves are associated with anxiety (Kiloh et al 1981).
The significant difference in the mean anxiety scores between the participants and the control group for the second assessment suggests that EEG biofeedback can effectively lower mild to moderate levels of anxiety, thus making it ideal to be used in nonpsychiatric units, ie, cardiac settings. Biofeedback as a low arousal training methodology has been previously efficient in treating anxiety disorders (Rice et al 1993). Inconsistencies in the different studies concerning anxiety reduction could be attributed to the way anxiety was assessed in individual patients and the actual length of treatment with longer periods of treatment reducing anxiety more effectively.
Detailed evaluation of each patient undergoing treatment would be a better evaluation of the characteristics and efficiency of the treatment. The Biograph program used for our study only required an electrode to be placed on only one active site in the scalp (C3) for EEG recordings. Most of the other similar studies recorded EEG from more than one active site according to the International 10–20 system. Furthermore, any increase in arousal affects the EEG frequencies in the entire region of the scalp and not just an isolated area (Barry et al 2004). Changes in arousal levels are linked with global activity while specific regional activity is linked with processing. A comprehensive EEG recording from the global activity might give more details on the effect of EEG beta waves training on anxiety levels.
Although we used an open and uncontrolled design, our study has a reasonably large sample size compared with some previous neurotherapy studies (Tebecis et al 1976; Bird et al 1978; Lubar JO and Lubar JF 1984; Wacker 1996; Miner et al 1998; Kim et al 2002), all of which had less than 20 participants. Nevertheless, criticisms of the small number of subjects have resulted in bigger sample sizes for more recent studies (Kaiser and Othmer 2000; Putnam 2000; Joyce and Siever 2001).
While the number of subjects participating was sufficient, the initial high dropout rate makes it difficult to conduct a double-blind study in which the one group of subjects are randomly assigned to EEG biofeedback and the other group to another type of biofeedback. Nevertheless, our study of 5 sessions of operant training of EEG activity was sufficient to produce significant changes in anxiety scores. Even in the subjects 2 group, those that dropped out by the third or fourth session were able to reduce their anxiety scores. A more detailed assessment would be to see the long-term effect of therapy, and evaluate whether a 3-month program could still have positive influence on anxiety 6 months or one year after completion of treatment.
Clinical implications
EEG biofeedback using beta training appeared useful in the treatment of anxiety based on the psychological assessment. Even so, the absence of significant associations between anxiety scores and EEG frequencies for either alpha or beta waves suggests that the improvement may be due to a placebo effect (Passini et al 1977). Although training involving EEG activation must be within a consistent therapy situation (Rosenfeld et al 1996), it is difficult to distinguish the effects of EEG training from confounding conditions like drowsiness, medications, caffeinated drinks, changes in emotional state/arousal, artifact from eye movements, time of day, and state of alertness. Therefore, these factors should be taken into consideration to eliminate the possibility of their influence on the positive effects resulting from intervention.
Nevertheless, EEG biofeedback provides accumulated benefits for some participants. The learning gained by the subjects during their training can be applied in their everyday life, as participants had their eyes open and learnt to stay relaxed while remaining alert and reducing their tendency to fall asleep. Biofeedback may also indirectly assist the participant to be better focused, more in control, and to feel clinically better.
The open and uncontrolled nature of our study militates against firm conclusions. However, the significant reduction in anxiety scores could lead to a big improvement in the morbidity and mortality rate of these patients as a further reduction was seen even in those scoring normal level scores during initial recruitment. The increase in SMR waves by the end of the therapy suggested a higher level of concentration and alertness for participants as they underwent their relaxation and biofeedback therapy. This was evident in another study which showed that enhancing SMR frequencies into a relaxed state had been linked with significant behavioral and cognitive changes (Ramirez et al 2001). Further research, however, is warranted to evaluate beta training as an effective EEG biofeedback in producing positive results as an adjunct technique to improve the various relaxation techniques. In summary, beta training could be proposed as a promising approach.
Acknowledgments
The research in this article was made possible by the IRPA 06-02-02-0121 project grant and the Ethical Committee, Faculty of Medicine, Universiti Kebangsaan Malaysia. We also thank Associate Professor Dr Abdul Aziz Jemain from the Faculty of Science and Technology, Universiti Kebangsaan, Malaysia for his statistical assistance.
References
- Barry RJ, Rushby JA, Johnstone SJ, et al. Event-related potentials in the auditory oddball as a function of EEG alpha phase at stimulus onset. Clin Neurophysiol. 2004;115:2593–601. doi: 10.1016/j.clinph.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bird BL, Newton FA, Sheer DE, et al. Behavioral and electroencephalographic correlates of 40-Hz EEG biofeedback training in humans. Biofeedback Self Regul. 1978;3(1):13–28. doi: 10.1007/BF00998560. [DOI] [PubMed] [Google Scholar]
- Dixhoorn JJV, Duivenvoorden HJ. Effect of relaxation therapy on cardiac events after myocardial infarction. J Cardiopulm Rehabil. 1999;19:178–85. doi: 10.1097/00008483-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Egner T, Gruzelier JH. Ecological validity of neurofeedback: modulation of slow wave EEG enhances musical performance. Neuroreport. 2003;14:1221–4. doi: 10.1097/01.wnr.0000081875.45938.d1. [DOI] [PubMed] [Google Scholar]
- Goodacre S, Mason S, Arnold J, et al. Psychologic morbidity and health-related quality of life of patients assessed in a chest pain observation unit. Ann Emerg Med. 2001;38:369–79. doi: 10.1067/mem.2001.118010. [DOI] [PubMed] [Google Scholar]
- Hardt JV, Kamiya J. Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science. 1978;201:79–81. doi: 10.1126/science.663641. [DOI] [PubMed] [Google Scholar]
- Isotani T, Tanaka H, Lehmann D, et al. Source location of EEG activity during hypnotically induced anxiety and relaxation. Inter J Psychophysiol. 2001;41:143–53. doi: 10.1016/s0167-8760(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Joyce M, Siever D. Audio-visual entrainment program as a treatment for behavior disorders in a school setting. J Neurotherapy. 2001;4(2) [Google Scholar]
- Kaiser J, Othmer S. Effect of neurofeedback on variables of attention in a large multi-center trial. J Neurotherapy. 2000;4(1) [Google Scholar]
- Kassamatoa A, Hirai T. An electroencephalic study of Zen meditation. Psychologia. 1969;12:205–25. [Google Scholar]
- Kiloh LG, McComas AJ, Osselton JW, et al. Clinical electroencephalography. London: Butterworths; 1981. [Google Scholar]
- Kim YY, Choi JM, Kim SY, et al. Changes in EEG of children during brain respiration-training. Am J Chin Med. 2002;30:405–17. doi: 10.1142/S0192415X02000272. [DOI] [PubMed] [Google Scholar]
- King KB. Psychologic and social aspects of cardiovascular disease. Ann Behav Med. 1997;19:264–70. doi: 10.1007/BF02892290. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B, Busch S, Strehl U, et al. Changes in EEG power spectra during biofeedback of slow cortical potentials in epilepsy. Appl Psychophysiol Biofeedback. 1999;24:213–33. doi: 10.1023/a:1022226412991. [DOI] [PubMed] [Google Scholar]
- Lubar JO, Lubar JF. Electroencephalographic biofeedback of SMR and beta for treatment of attention deficit disorders in a clinical setting. Biofeedback Self Regul. 1984;9(1):1–23. doi: 10.1007/BF00998842. [DOI] [PubMed] [Google Scholar]
- Masterpasqua F, Healey KN. Neurofeedback in psychological practice. Professional psychology. Res Pract. 2003;34:652–6. [Google Scholar]
- Miner LA, McFarland DJ, Wolpaw JR. Answering questions with an electroencephalogram-based brain-computer interface. Arch Phys Med Rehabil. 1998;79:1029–33. doi: 10.1016/s0003-9993(98)90165-4. [DOI] [PubMed] [Google Scholar]
- Nakagome K. Clinical application and limitations of electroencephalography (EEG) J Jpn Med Assoc. 2000;123:543–50. [Google Scholar]
- Neuper C, Muller GR, Kubler A, et al. Clinical application of an EEG-based brain-computer interface: a case study in a patient with severe motor impairment. Clin Neurophysiol. 2003;114:399–409. doi: 10.1016/s1388-2457(02)00387-5. [DOI] [PubMed] [Google Scholar]
- Passini FT, Watson CG, Dehnel L, et al. Alpha wave biofeedback training therapy in alcoholics. J Clin Psychol. 1977;33:292–9. doi: 10.1002/1097-4679(197701)33:1+<292::aid-jclp2270330166>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Peniston EG, Kulkosky PJ. Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcohol Clin Exp Res. 1989;13:271–9. doi: 10.1111/j.1530-0277.1989.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Putnam J. The effects of brief, eyes-open alpha brain wave training with audio and video relaxation induction on the EEG of 77 army reservists. J Neurotherapy. 2000;4(1) [Google Scholar]
- Ramirez PM, Desantis D, Opler LA. EEG biofeedback treatment of ADD. A viable alternative to traditional medical intervention? Ann N Y Acad Sci. 2001;931:342–58. [PubMed] [Google Scholar]
- Rice KM, Blanchard EB, Purcell M. Biofeedback treatments of generalized anxiety disorder: preliminary results. Biofeedback Self Regul. 1993;18:93–104. doi: 10.1007/BF01848110. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Bonnici DM, Mackinnon AJ, et al. Psychometric evaluation of the Hospital Anxiety and Depression Scale (HADS) among female cardiac patients. Br J Health Psychol. 2001;6:373–83. doi: 10.1348/135910701169278. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JP, Baehr E, Baehr R, et al. Preliminary evidence that daily changes in frontal alpha asymmetry correlate with changes in affect in therapy sessions. Inter J Psychophysiol. 1996;23:137–41. doi: 10.1016/0167-8760(96)00037-2. [DOI] [PubMed] [Google Scholar]
- Schutter DJLG, Honk JV, d’Alfonso AAL, et al. Effect of slow rTMS at the right dorsolateral prefrontal cortex on EEG asymmetry and mood. Neuroreport. 2001;12(35) doi: 10.1097/00001756-200103050-00005. [DOI] [PubMed] [Google Scholar]
- Sterman MB, Howe RD, Macdonald LR. Facilitation of spindle burst sleep by conditioning of electroencephalographic activity while awake. Science. 1970;167:1146–8. doi: 10.1126/science.167.3921.1146. [DOI] [PubMed] [Google Scholar]
- Stolc S, Krakovska A, Teplan M. Audiovisual stimulation of human brain. Linear and nonlinear measures. Meas Sci Rev. 2003;3(2) [Google Scholar]
- Tebecis AK, Ohno Y, Matsubara H, et al. A longitudinal study of some physiological parameters and autogenic training. Psychother Psychosom. 1976;27(1):8–17. doi: 10.1159/000286991. [DOI] [PubMed] [Google Scholar]
- Teplan M. Fundamentals of EEG measurement. Meas Sci Rev. 2002;2(2) [Google Scholar]
- Teplan M, Krakovska A, Stolc S. EEG in the context of audiovisual stimulation. Meas Sci Rev. 2003;3(2) [Google Scholar]
- Vernon D, Egner T, Cooper N, et al. The effect of training distinct neurofeedback protocols on aspect of cognitive performance. Int J Psychophysiol. 2003;47:75–85. doi: 10.1016/s0167-8760(02)00091-0. [DOI] [PubMed] [Google Scholar]
- Wacker MS. Alpha brainwave training and perception of time passing: preliminary findings. Biofeedback Self Regul. 1996;21:303–9. doi: 10.1007/BF02214430. [DOI] [PubMed] [Google Scholar]


