Abstract
Combined deficiency of factor V and factor VIII (F5F8D) is caused by mutations in one of 2 genes, either LMAN1 or MCFD2. Here we report the identification of mutations for 11 additional F5F8D families, including 4 novel mutations, 2 in MCFD2 and 2 in LMAN1. We show that a novel MCFD2 missense mutation identified here (D81Y) and 2 previously reported mutations (D89A and D122V) abolish MCFD2 binding to LMAN1. Measurement of platelet factor V (FV) levels in 7 F5F8D patients (4 with LMAN1 and 3 with MCFD2 mutations) demonstrated similar reductions to those observed for plasma FV. Combining the current data together with all previous published reports, we performed a genotype-phenotype analysis comparing patients with MCFD2 mutations with those with LMAN1 mutations. A previously unappreciated difference is observed between these 2 classes of patients in the distribution of plasma levels for FV and factor VIII (FVIII). Although there is considerable overlap, the mean levels of plasma FV and FVIII in patients with MCFD2 mutations are significantly lower than the corresponding levels in patients with LMAN1 mutations. No differences in distribution of factor levels are observed by sex. These data suggest that MCFD2 may play a primary role in the export of FV and FVIII from the ER, with the impact of LMAN1 mediated indirectly through its interaction with MCFD2.
Introduction
Combined deficiency of factor V and factor VIII (F5F8D) is an autosomal recessive disorder characterized by simultaneous reduction in the levels of factor V (FV) and factor VIII (FVIII) activity and antigen and mild-to-moderate bleeding symptoms.1 The reported levels of FV and FVIII in F5F8D patients range from as low as 1% to as high as 46% of normal, but generally fall between 5% and 30%.1 Positional cloning identified 2 genes that are associated with the disorder.2,3 Mutations in LMAN1 account for approximately 70% of F5F8D families, while mutations in MCFD2 account for the remaining 30%.4–6 LMAN1 is a type-1 transmembrane protein that cycles between the endoplasmic reticulum (ER) and the ER-Golgi intermediate compartment (ERGIC).7,8 It contains a mannose-specific carbohydrate recognition domain on the ER lumenal side and ER exit and retrieval motifs on the cytoplasmic side.9 MCFD2 is an EF-hand domain protein that interacts with LMAN1 in a Ca2+-dependent manner.3 The LMAN1-MCFD2 protein complex functions as a cargo receptor that facilitates the transport of FV and FVIII from the ER to the Golgi apparatus.3,10
All the LMAN1 mutations reported to date are null mutations with the exception of a cysteine-to-arginine mutation that disrupts a disulfide bond that is required for its oligomerization and also destabilizes the protein.6 In contrast, both null mutations and missense mutations have been identified in MCFD2. All 4 MCFD2 missense mutations reported to date change highly conserved amino acid residues in the EF hand domains,3,6,11 and 2 have been shown to abolish LMAN1 binding,3 indicating that LMAN1 and MCFD2 must function as a unit to transport FV and FVIII. Although MCFD2 appears to directly interact with FVIII,10 it is unclear whether LMAN1 binds directly to FV/FVIII or indirectly via MCFD2.
Here we report identification of mutations in MCFD2 and LMAN1 in an additional 11 F5F8D families. With the availability of these and other published data on a large number of patients with known mutations and the corresponding FV/FVIII levels, we also address the question of whether there is a phenotypic difference between patients with LMAN1 mutations and those with MCFD2 mutations, and whether FV in plasma and FV in platelets are similarly affected.
Methods
Patients and mutation analysis
Table 1 lists the 15 affected individuals studied from 11 new families with affected individuals. Peripheral blood samples were obtained with written informed consent from probands and family members after diagnosis of F5F8D in accordance with the Declaration of Helsinki. FV and FVIII activities were measured at local clinical laboratories using one-stage assays based on prothrombin time (for FV) and activated partial thromboplastin time (for FVIII). For families B19 to B22 and B26 to B28, amplification by polymerase chain reaction (PCR) of exons and intron-exon junctions of the LMAN1 and MCFD2 genes and DNA sequencing were performed as reported previously.2,3 For families B23 to B25, the presence of previously described mutations in Tunisian and Middle Eastern Jewish populations was confirmed by PCR amplification and restriction analysis as previously reported.12
Table 1.
LMAN1 and MCFD2 mutation analyses, geographic origins, sex, and FV/FVIII levels in new F5F8D patients included in the current study
| Patient | Origin | Sex | FV level | FVIII level | LMAN 1 mutation | MCFD2 mutation |
|---|---|---|---|---|---|---|
| B19 | Afro-Caribbean | F | 4 | 9 | N/D | c.374-375insGA |
| B20-1 (c) | Turkey | M | 35 | 15 | c.795delC | None |
| B20-2 (c) | Turkey | M | 25 | 20 | c.795delC | None |
| B21-1 | Iraq | F | 12 | 18 | c.1356delC | None |
| B21-2 | Iraq | M | 11 | 16 | c.1356delC | None |
| B22-1 | Saudi Arabia | F | 8 | 6 | N/D | c.241G>T (D81Y) |
| B22-2 | Saudi Arabia | F | 13 | 12 | N/D | c.241G>T (D81Y) |
| B23 (c) | Tunisian Jewish | M | 11 | 22 | c.1149+2T>G | N/D |
| B24 (c) | Iranian Jewish | F | 13 | 16 | c.89-90insG | N/D |
| B25 | Iranian Jewish | M | 8 | 5 | c.89-90insG | N/D |
| B26-1 | Italy | F | 12 | 12 | c.2T>C | None |
| B26-2 | Italy | M | 11 | 18 | c.2T>C | None |
| B27-1 | Italy | F | 11 | 9 | None | c.149+5G>A |
| B27-2 | Italy | M | 16 | 11 | None | c.149+5G>A |
| B28 | Italy | M | 15 | 29 | No detectable protein | None |
All mutations are homozygous in affected individuals, except B28 whose mutation has not been identified.
(c) indicates known consanguinity; N/D, not done.
Construction of MCFD2 expression vectors
Cloning of wild-type MCFD2 and MCFD2D129E into the pcDNA3.1-myc-his expression vector was described previously.3 The Stratagene mutagenesis II kit (La Jolla, CA) was used to introduce individual point mutations into the wild-type MCFD2 expression vector. The mutagenesis primers were as follows (only the sense primers are listed): D81Y, CAGCTCCATTACTTCAAAATGCATTATTATGATGGCAATAATTTGCTTG; D89A, GATGGCAATAATTTGCTTGATGGCTTAGAACTCTCCACA; and D122V, AAGATGAACTGATTAACATAATAGTTGGTGTTTTGAGAGATGATGAC. The presence of the desired mutation and the absence of a second mutation were verified by DNA sequencing.
Metabolic labeling and immunoprecipitation
COS-1 cells were transfected with the wild-type and different mutant MCFD2 expression vectors and labeled with 35S methionine-cysteine (TRAN35S LABEL; MP Biomedicals, Solon, OH) at 28 hours after transfection as previously described.3 Immunoprecipitation with antimyc, anti-MCFD2, and anti-LMAN1 antibodies was performed as previously described.10 Proteins were separated in 4% to 12% Criterion Bis-tris gels run with the Mes running buffer (Bio-Rad, Hercules, CA) and visualized by exposing to a Kodak Bio-Max film (Rochester, NY) using a Kodak Biomax Transcreen LE.
Immunofluorescence staining
Immunofluorescence staining of HeLa cells transfected with different MCFD2 expression vectors was previously described.3 Images were viewed on a Leica DMRXE confocal microscope (Wetzlar, Germany) using a 40× oil-immersion objective with a 1.25 numeric aperture. Confocal images were acquired using the Leica Confocal Software, version 2.61.
Genotyping of the O blood type
The ABO glycosyltransferase gene around the common O1 allele (c.261delG) was amplified using primers MO-46 and MO-57 as described.13 The region around the rare O2 allele (c.G802A) was amplified using the following primers: O2-S, AGATCCTGACTCCGCTGTTC; O2-AS, CACAAGTACTCGGGGGAGAG. The presence of a homozygous O allele, as detected by DNA sequencing, was scored as blood type O. Other genotypes were not distinguished further and scored as non-O. In our patient samples, no O2 genotype was observed.
Isolation of platelets and platelet FV assay
Washed human platelets were obtained from 7 individuals from 5 Italian families (Table 4), with mutations in LMAN1 or MCFD2, and from 15 healthy subjects, as previously described.14 Briefly, blood was collected in ACD (85 mM trisodium citrate, 71 mM citric acid, and 111 mM dextrose, pH 4.5) and centrifuged at 400g for 15 minutes at 20°C to obtain platelet-rich-plasma (PRP). After removal of platelet-poor plasma, platelet pellets were washed 3 times with Tyrode-17.5% albumin solution with 1 μM PGE1 as an inhibitor of platelet activation and then resuspended at 109/mL in the same buffer supplemented with 1 μM PGE1 and 1 μL/mL apyrase (kindly provided by Dr R. L. Kinlough-Rathbone, McMaster University, Hamilton, ON) and 1 mM PMSF (phenylmethanesulphonyl fluoride). FV antigen levels were determined in both plasma and platelet lysates by an enzyme-linked immunosorbent assay (ELISA) using a sheep anti-FV antibody (Affinity Biologicals, Ancaster, ON). Plasma FV and platelet FV were normalized to the hematocrit and platelet counts, respectively, to compare the relative reduction of FV in each pool among F5F8D patients and controls. Of note, the size of the platelet (plt) FV pool in our study (∼ 3.7% of total circulating FV, or ∼ 637 ng/109 plts in healthy controls) is significantly smaller than in previous reports (2500-7730 ng/109 plts by a radioimmunoassay15 and 900-1300 ng/109 plts by a polyclonal antisera-based ELISA16). This discrepancy could be due to intrinsic differences in antigenicity between platelet and plasma FV. Consistent with this notion, FV has been shown to be substantially modified in platelets.17,18
Table 4.
FV levels in plasma and platelets, the means and the ratios between patients and healthy controls.
| Patient | Plate count, ×106 plts/mL | Plasma FV, ng/mL | Plasma FV, ng/mL blood | Platelet FV, ng/109 plts | Platelet FV, ng/mL blood | Platelet FV/total FV, % |
|---|---|---|---|---|---|---|
| Control group | ||||||
| Control 1 | 427 | 7242 | 4251 | 778 | 332 | 7.2 |
| Control 2 | 331 | 8000 | 4424 | 661 | 219 | 4.7 |
| Control 3 | 186 | 6708 | 3542 | 545 | 101 | 2.9 |
| Control 4 | 319 | 10600 | 5183 | 670 | 214 | 4.0 |
| Control 5 | 246 | 9100 | 5305 | 461 | 113 | 2.1 |
| Control 6 | 218 | 5950 | 3403 | 860 | 187 | 5.2 |
| Control 7 | 286 | 7300 | 4190 | 294 | 84 | 2.0 |
| Control 8 | 187 | 7733 | 3952 | 536 | 100 | 2.5 |
| Control 9 | 324 | 8205 | 4201 | 654 | 212 | 6.6 |
| Control 10 | 232 | 8845 | 5316 | 507 | 118 | 2.8 |
| Control 11 | 267 | 10120 | 5991 | 820 | 219 | 2.0 |
| Control 12 | 288 | 7497 | 4506 | 579 | 167 | 3.6 |
| Control 13 | 205 | 7250 | 4401 | 681 | 140 | 3.1 |
| Control 14 | 254 | 12129 | 6174 | 864 | 219 | 3.4 |
| Control 15 | 239 | 8329 | 4389 | 596 | 142 | 3.1 |
| Mean (±SD) | 267 (±65) | 8334 (±1615) | 4615 (±815) | 634 (±158) | 171 (±67) | 3.7 (±1.6) |
| LMAN1 group | ||||||
| B26-1 | 210 | 1050 | 609 | 75 | 16 | 2.5 |
| B26-2 | 130 | 789 | 633 | 62 | 13 | 2.6 |
| B28 | 211 | 1150 | 447 | 128 | 17 | 2.8 |
| A24 | 258 | 1850 | 1073 | 172 | 44 | 4.0 |
| Mean (±SD) | 202 (±53) | 1210 (±453) | 690 (±268) | 109 (±51) | 23 (±15) | 3.0 (±0.7) |
| MCFD2 group | ||||||
| B27-1 | 193 | 570 | 335 | 54 | 10 | 3.0 |
| B27-2 | 238 | 790 | 466 | 67 | 16 | 3.3 |
| Zhang7-2 | 206 | 580 | 319 | 54 | 11 | 3.4 |
| Mean (±SD) | 212 (±23) | 647 (±124) | 373 (±81) | 58 (±7) | 13 (±3) | 3.2 (±0.2) |
| Ratios | ||||||
| LMAN1/Wt. | 0.15 | 0.13 | ||||
| MCFD2/Wt. | 0.08 | 0.08 |
Plasma and platelet FV antigen levels were measured by ELISA. Plasma FV and platelet FV were normalized to the hematocrit and platelet counts, respectively, to determine the amount of FV of each pool in whole blood.
plts indicates platelets.
Statistical analysis
We compiled a list of the current and all previously reported patients with known mutations in either LMAN1 or MCFD2 and their corresponding FV and FVIII levels (Tables 2,3). In cases where FV and FVIII levels were measured multiple times, only the means are listed. Two-tailed Student t test for independent samples, assuming equal variance, was used to determine differences between the mean activity levels. The relationship between FV and FVIII was measured using the Pearson correlation test.
Table 2.
All reported F5F8D patients with MCFD2 mutations, their geographic origins, sex, and FV/FVIII levels
| Patient | Origin | Sex | ABO | FV | FVIII | Mutation | Mutation effect | Ref no. | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | B3/5 | Italy (c) | M | Non-O | 2.3 | 6.3 | c.149+5G>A | Splicing defect | 5, 6 |
| 2 | B4-1/6 | Italy | M | O | 7.5 | 6 | c.149+5G>A | Splicing defect | 5, 6 |
| 3 | B4-2/6 | Italy | F | 10 | 10 | c.149+5G>A | Splicing defect | 5, 6 | |
| 4 | B5/8 | Italy | F | Non-O | 11 | 23 | c.149+5G>A | Splicing defect | 5, 6 |
| 5 | B10 | Serbia | F | O | 7 | 7 | c.149+5G>A | Splicing defect | 5, 6 |
| 6 | Zhang10-1/19 | United States | M | Non-O | 4 | 12 | c.149+5G>A | Splicing defect | 3 |
| 7 | Zhang10-2/19 | United States | M | Non-O | 8 | 14 | c.149+5G>A | Splicing defect | 3 |
| 8 | Zhang12-1/A32 | Swiss | M | Non-O | 7 | 10 | c.149+5G>A | Splicing defect | 3, 25 |
| 9 | Zhang12-2/A32 | Swiss | M | 8 | 9 | c.149+5G>A | Splicing defect | 3, 25 | |
| 10 | Mohanty-2a | India | M | 5 | 1.2 | c.149+5G>A | Splicing defect | 23 | |
| 11 | Mohanty-2b | India | F | 6.6 | 4 | c.149+5G>A | Splicing defect | 23 | |
| 12 | Zhang1-4/A5 | Iran | F | Non-O | 5 | 10 | c.309+1G>A | Splicing defect | 3, 4 |
| 13 | Zhang5-1/16 | Turkey (c) | M | Non-O | 7.5 | 10 | c.309+1G>A | Splicing defect | 3, 5 |
| 14 | Zhang5-2/16 | Turkey (c) | M | Non-O | 7 | 7 | c.309+1G>A | Splicing defect | 3, 5 |
| 15 | Zhang5-4/16 | Turkey (c) | F | Non-O | 10 | 8 | c.309+1G>A | Splicing defect | 3, 5 |
| 16 | Zhang6-3 | Turkey (c) | F | Non-O | 9.5 | 9 | c.249delT | Frameshift | 3 |
| 17 | Zhang6-4 | Turkey (c) | M | Non-O | 9.5 | 9 | c.249delT | Frameshift | 3 |
| 18 | Zhang6-6 | Turkey (c) | M | Non-O | 9.5 | 9 | c.249delT | Frameshift | 3 |
| 19 | B9 | Kosovo | F | Non-O | 4.5 | 1.5 | c.407T>C | p.I136T | 6 |
| 20 | Zhang9-1/14 | Venezuela | M | O | 9.4 | 9 | c.407T>C | p.I136T | 3, 5 |
| 21 | Zhang9-2/14 | Venezuela | F | O | 4.3 | 7.4 | c.407T>C | p.I136T | 3, 5 |
| 22 | Zhang9-5/14 | Venezuela | F | O | 6.2 | 8.2 | c.407T>C | p.I136T | 3, 5 |
| 23 | Zhang4-1/13 | Venezuela | M | Non-O | 9 | 7 | c.387C>G | p.D129E | 3, 5 |
| 24 | Zhang2-1/A7 | Iran (c) | M | O | 7 | 7 | unknown | Unknown | 3, 4 |
| 25 | B6/A14-III3 | Iran (c) | M | 10 | 6 | c.-6-1G>A | Splicing defect | 6, 24 | |
| 26 | B6/A14-III4 | Iran (c) | F | 10 | 13.5 | c.-6-1G>A | Splicing defect | 6, 24 | |
| 27 | B6/A14-III8 | Iran (c) | F | 10 | 6 | c.-6-1G>A | Splicing defect | 6, 24 | |
| 28 | B6/A14-III16 | Iran (c) | F | 10 | 21 | c.-6-1G>A | Splicing defect | 6, 24 | |
| 29 | Zhang7-1/A21 | Italy | M | O | N/A | N/A | c.103delC | Frameshift | 3, 4 |
| 30 | Zhang7-2/A21 | Italy | M | O | 10 | 11 | c.103delC | Frameshift | 3, 4 |
| 31 | Zhang11/A29 | S. Africa | F | O | 11 | 22 | c.263-270del8nt | Frameshift | 3, 4 |
| 32 | India-1 | India (c) | F | 12.6 | 13.7 | c.365A>C | p.D122V | 11 | |
| 33 | India-2 | India (c) | M | 19.3 | 8.3 | c.149+5G>A | Splicing defect | 11 | |
| 34 | India-4 | India (c) | F | 22.4 | 27.1 | c.149+5G>A | Splicing defect | 11 | |
| 35 | India-5 | India | M | 14.1 | 12.3 | c.149+5G>A | Splicing defect | 11 | |
| 36 | India-6 | India (c) | F | 13.5 | 10.7 | c.149+5G>A | Splicing defect | 11 | |
| 37 | India-7 | India (c) | F | 5.6 | 7.8 | c.149+5G>A | Splicing defect | 11 | |
| 38 | India-8 | India (c) | M | 18.9 | 7.2 | c.211-244del | Frameshift | 11 | |
| 39 | India-9 | India (c) | F | 16.2 | 16.7 | c.211-244del | Frameshift | 11 | |
| 40 | Nyfeler | S. America | F | 11.5 | 12 | c.[431C>G]+[del8.5kb] | p.S144X+ | 27 | |
| Promoter deletion | |||||||||
| 41 | B18 | Greece | F | Non-O | 7 | 5 | c.266A>C | p.D89A | 6 |
| 42 | B19 | Afro-Caribbean | F | Non-O | 4 | 9 | c.374-375insGA | Frameshift | This study |
| 43 | B22-1 | Saudi Arabia | F | O | 8 | 6 | c.241G>T | p.D81Y | This study |
| 44 | B22-2 | Saudi Arabia | F | O | 13 | 12 | c.241G>T | p.D81Y | This study |
| 45 | B27-1 | Italy | F | 11 | 9 | c.149+5G>A | Splicing defect | This study | |
| 46 | B27-2 | Italy | M | 16 | 11 | c.149+5G>A | Splicing defect | This study |
In cases where 2 different numbers were used to designate the same patient/family in different papers, both are indicated in the second column (separated by a slash).
ABO indicates ABO blood group; Ref, reference; (c), known consanguinity in the family; N/A, information not available.
Table 3.
All reported F5F8D patients with LMAN1 mutations, their geographic origins, sex, and FV/FVIII levels
| Patient | Origin | Sex | ABO | FV | FVIII | Mutation | Mutation effect | Ref no. | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Japan | M | Non-O | 12 | 18 | c.604C>T | p.R202X | 5 |
| 2 | 2 | Japan | F | Non-O | 13 | 12 | c.422delC | Frameshift | 5 |
| 3 | 4 | Italy | F | O/O | 4.6 | 9.5 | c.2T>C | p.M1T | 5 |
| 4 | 7-1 | Italy | M | Non-O | 8 | 16 | c.2T>C | p.M1T | 5 |
| 5 | 7-2 | Italy | M | Non-O | 1.7 | 22 | c.2T>C | p.M1T | 5 |
| 6 | 9 | Italy | F | Non-O | 15 | 15 | c.2T>C | p.M1T | 5 |
| 7 | 10 | Italy | M | O/O | 13 | 15 | c.2T>C | p.M1T | 5 |
| 8 | 12-1 | Venezuela | F | O/O | 4 | 8 | c.720-735del16bp | Frameshift | 5 |
| 9 | 12-2 | Venezuela | F | O/O | 16 | 6.6 | c.720-735del16bp | Frameshift | 5 |
| 10 | 12-3 | Venezuela | F | 7 | 10 | c.720-735del16bp | Frameshift | 5 | |
| 11 | 12-4 | Venezuela | F | 40 | 20 | c.720-735del16bp | Frameshift | 5 | |
| 12 | 12-5 | Venezuela | M | O/O | 17 | 10 | c.720-735del16bp | Frameshift | 5 |
| 13 | 15-1 | France | F | Non-O | 25 | 10 | c.904A>T | p.K302X | 5 |
| 14 | 15-2 | France | F | O/O | 5 | 20 | c.904A>T | p.K302X | 5 |
| 15 | 17 | US Armenian | N/A | N/A | N/A | c.1519delA | Frameshift | 5 | |
| 16 | 18 | United States | N/A | N/A | N/A | c.1109-1121delTC | Frameshift | 5 | |
| 17 | B11-1 | Italy | M | Non-O | 26 | 23 | c.2T>C | p.M1T | 6 |
| 18 | B11-2 | Italy | F | Non-O | 13 | 20 | c.2T>C | p.M1T | 6 |
| 19 | A1-IV1 | Iran (c) | M | 8 | 5.5 | c.912-913insA | Frameshift | 4 | |
| 20 | A1-IV4 | Iran (c) | F | 7 | 14 | c.912-913insA | Frameshift | 4 | |
| 21 | A1-IV6 | Iran (c) | F | 11 | 9 | c.912-913insA | Frameshift | 4 | |
| 22 | A2-IV1 | Iran (c) | F | Non-O | 7.5 | 7 | c.912-913insA | Frameshift | 4 |
| 23 | A3-IV4 | Iran (c) | F | Non-O | 20 | 16 | c.[912-913insA]+[89-90insG] | Frameshift | 4 |
| 24 | A4-IV7 | Iran (c) | M | 10.5 | 11 | c.1149+2T>G | Splicing defect | 4 | |
| 25 | A6-III1 | Iran (c) | F | 12 | 12.5 | c.822-1G>A | Splicing defect | 4 | |
| 26 | A6-III2 | Iran (c) | M | 12 | 14 | c.822-1G>A | Splicing defect | 4 | |
| 27 | A8-III2 | Iran (c) | M | 5 | 6 | c.1214-1218delAAATG | Frameshift | 4 | |
| 28 | A9-III2 | Iran (c) | M | 18 | 8 | c.822-1G>A | Splicing defect | 4 | |
| 29 | A10-III1 | Iran (c) | F | 12 | 15 | c.23delG | Frameshift | 4 | |
| 30 | A10-III4 | Iran (c) | M | 19 | 16 | c.23delG | Frameshift | 4 | |
| 31 | A11-IV4 | Iran (c) | M | O/O | 18 | 17 | c.89-90insG | Frameshift | 4 |
| 32 | A12-III3 | Iran (c) | M | Non-O | 2.5 | 2.2 | c.822-1G>A | Splicing defect | 4 |
| 33 | A16-II7 | Iran (c) | M | 9 | 13.5 | c.604C>T | p.R202X | 4 | |
| 34 | A17-III1 | Iran (c) | M | 5 | 7.5 | c.604C>T | p.R202X | 4 | |
| 35 | A17-IV1 | Iran (c) | F | 34 | 14.5 | c.604C>T | p.R202X | 4 | |
| 36 | A17-IV2 | Iran (c) | M | 10 | 8 | c.604C>T | p.R202X | 4 | |
| 37 | A18-II3 | Pakistan (c) | F | Non-O | 10 | 15 | c.904A>T | p.K302X | 4 |
| 38 | A19-II1 | Pakistan (c) | F | Non-O | 14 | 14 | c.1366C>T | p.R456X | 4 |
| 39 | A19-II2 | Pakistan (c) | M | 14 | 14 | c.1366C>T | p.R456X | 4 | |
| 40 | A20-1 | Pakistan (c) | M | 8 | 6 | c.904A>T | p.K302X | 4 | |
| 41 | A20-2 | Pakistan (c) | F | 11 | 7.7 | c.904A>T | p.K302X | 4 | |
| 42 | A22 (B7) | Italy | M | Non-O | 8 | 24 | exon4 skipping† | no protein | 4 |
| 43 | A24 | Italy | F | O/O | 17 | 26 | c.2T>C | p.M1T | 4 |
| 44 | A25-1 | Italy | M | Non-O | 9 | 10 | c.2T>C | p.M1T | 4 |
| 45 | A25-2 | Italy | F | N/A | N/A | c.2T>C | p.M1T | 4 | |
| 46 | A26-1 | Pakistan | M | 18 | 18 | c.904A>T | p.K302X | 4 | |
| 47 | A26-2 | Pakistan | M | 18 | 18 | c.904A>T | p.K302X | 4 | |
| 48 | A27 | China | M | 7 | 9 | c.1366C>T | p.R456X | 4 | |
| 49 | A30 | Pakistan (c) | F | 6 | 3 | c.904A>T | p.K302X | 4 | |
| 50 | A31 | Pakistan (c) | M | 14 | 18 | c.904A>T | p.K302X | 4 | |
| 51 | A34 | Italy | M | 9 | 27 | c.639+1G>T | Splicing defect | 4 | |
| 52 | A35-1 | Italy | F | 6 | 23 | c.639+1G>T | Splicing defect | 4 | |
| 53 | A35-2 | Italy | F | 8 | 25 | c.639+1G>T | Splicing defect | 4 | |
| 54 | A36 | Italy | M | 14 | 27 | c. 1208-1209insT | Frameshift | 4 | |
| 55 | A37 | China | F | 17 | 19 | c.1366C>T | p.R456X | 4 | |
| 56 | B1 | Algeria | M | N/A | N/A | c.31delG | Frameshift | 4 | |
| 57 | B12-1 | Austria | M | O/O | 15 | 12 | c.780delT | Frameshift | 6 |
| 58 | B12-2 | Austria | F | O/O | 24 | 13 | c.780delT | Frameshift | 6 |
| 59 | B13 | Iraq | F | O/O | 14 | 25 | c.961G>T | p.E321X | 6 |
| 60 | B14 | Poland (c) | M | O/O | 16 | 23 | c.839delA | Frameshift | 6 |
| 61 | B15 | Belgium | N/A | Non-O | 11 | 35 | c.822+1G>A | Splicing defect | 6 |
| 62 | B16 | United States | F | Non-O | 23 | 24 | c.822+33-34insGGTT*† | Exon 8 skipping | 6 |
| 63 | B17 | Argentina | F | Non-O | 18 | 23 | c1423T>C† | p.C475R | 6 |
| 64 | Dansako-1 | Japan | N/A | 12 | 12 | c.604C>T | p.R202X | 19 | |
| 65 | Thai | Thailand | F | 10 | 12.5 | c.[1366C>T]+[823-1G>C] | p.R456X+splicing | 26 | |
| 66 | India-3 | India (c) | F | 9.5 | 16.1 | c.813-822 + 62del72 | Frameshift | 11 | |
| 67 | Farah | Lebanon | F | 13.7 | 8 | c.[1138C>T]+[1270delG] | p.Q380X+frameshift | 21 | |
| 68 | Mohanty-3 | India | F | 7 | 2.2 | c.340G>T | p.G114X | 23 | |
| 69 | D'Ambrosio | Italy | M | 8.7 | 13.7 | c.2T>C | p.M1T | 19 | |
| 70 | Nichols-1 | Tunisian Jewish (c) | M | 5.7 | 13.7 | c.1149+2T>G | Splicing defect | 2 | |
| 71 | Nichols-5a | Tunisian Jewish (c) | F | 11.7 | 23.7 | c.1149+2T>G | Splicing defect | 2 | |
| 72 | Nichols-5b | Tunisian Jewish (c) | M | 23 | 22 | c.1149+2T>G | Splicing defect | 2 | |
| 73 | Nichols-6 | Tunisian Jewish (c) | F | 9 | 24 | c.1149+2T>G | Splicing defect | 2 | |
| 74 | Nichols-8 | Tunisian Jewish (c) | M | 14.5 | 9.5 | c.1149+2T>G | Splicing defect | 2 | |
| 75 | Nichols-9a | Tunisian Jewish (c) | F | 14.3 | 17.2 | c.1149+2T>G | Splicing defect | 2 | |
| 76 | Nichols-9b | Tunisian Jewish (c) | M | 20 | 32 | c.1149+2T>G | Splicing defect | 2 | |
| 77 | Nichols-2a | Iraqi Jewish (c) | F | 23 | 10 | c.89-90insG | Frameshift | 2 | |
| 78 | Nichols-2b | Iraqi Jewish (c) | F | 23 | 13 | c.89-90insG | Frameshift | 2 | |
| 79 | Nichols-2c | Iraqi Jewish (c) | F | 14 | 13 | c.89-90insG | Frameshift | 2 | |
| 80 | Nichols-2d | Iraqi Jewish (c) | F | 28 | 46 | c.89-90insG | Frameshift | 2 | |
| 81 | Nichols-2e | Iraqi Jewish (c) | M | 24 | 24.5 | c.89-90insG | Frameshift | 2 | |
| 82 | Nichols-3a | Iranian Jewish (c) | F | 13.7 | 19.3 | c.89-90insG | Frameshift | 2 | |
| 83 | Nichols-3b | Iranian Jewish (c) | M | 23 | 22 | c.89-90insG | Frameshift | 2 | |
| 84 | 11 | Iranian Jewish | F | Non-O | 8.3 | 21.7 | c.89-90insG | Frameshift | 5 |
| 85 | Nichols-4a | Iraqi Jewish (c) | M | 11 | 19 | c.89-90insG | Frameshift | 2 | |
| 86 | Nichols-4b | Iraqi Jewish (c) | F | 15 | 14 | c.89-90insG | Frameshift | 2 | |
| 87 | Nichols-7a | Egyptian Jewish (c) | F | 12.8 | 15.6 | c.89-90insG | Frameshift | 2 | |
| 88 | Nichols-7b | Egyptian Jewish (c) | M | 10.4 | 25.25 | c.89-90insG | Frameshift | 2 | |
| 89 | Ma-1 | China | M | 12.4 | 8.2 | c.[949C>T]+[1366C>T] | p.[Q317X]+[R456X] | 22 | |
| 90 | Ma-2 | China | F | 13.5 | 11.3 | c.[949C>T]+[1366C>T] | p.[Q317X]+[R456X] | 22 | |
| 91 | B21-1 | Turkey (c) | M | Non-O | 35 | 15 | c.795delC | Frameshift | This study |
| 92 | B21-2 | Turkey (c) | M | Non-O | 25 | 20 | c.795delC | Frameshift | This study |
| 93 | B22-1 | Iraq | F | Non-O | 12 | 18 | c.1356delC | Frameshift | This study |
| 94 | B22-2 | Iraq | M | Non-O | 11 | 16 | c.1356delC | Frameshift | This study |
| 95 | B23 | Tunisian Jewish (c) | M | Non-O | 11 | 22 | c.1149+2T>G | Splicing defect | This study |
| 96 | B24 | Iranian Jewish (c) | F | Non-O | 13 | 16 | c.89-90insG | Frameshift | This study |
| 97 | B25 | Iranian Jewish | M | Non-O | 8 | 5 | c.89-90insG | Frameshift | This study |
| 98 | B28-1 | Italy | F | Non-O | 12 | 12 | c.2T>C | M1T | This study |
| 99 | B28-2 | Italy | M | Non-O | 12 | 12 | c.2T>C | M1T | This study |
| 100 | B29 | Italy | M | 15 | 29 | Unknown | No protein | This study |
In cases where 2 different numbers were used to designate the same patient/family in different publications, both are indicated in the second column (separated by a slash). Additional clinical data on previously unreported siblings have been added into certain families. Patient A30 appears to also have von Willebrand disease, and therefore is excluded from the statistical analysis.
ABO indicates ABO blood group; Ref, reference; (c), known consanguinity in the family; and N/A indicates information not available.
Causative relationship has not been established for this mutation/polymorphism.
Compound heterozygote with no mutation detected on the second allele.
Results
Mutation analysis in additional F5F8D patients
We analyzed 15 patients with F5F8D from 11 previously unreported families. Table 1 summarizes the factor V and factor VIII levels of the affected individuals. The proband in family B19 is of Afro-Caribbean origin. Family B20 is a consanguineous Turkish family with 2 affected siblings. Family B21 is an Iraqi Chaldean family. Family B22 is from Saudi Arabia. Families B23 to B25 are 3 unrelated Iranian Jewish families. Families B26 to B28 are from Italy. A novel homozygous MCFD2 mutation was identified in family B19 (c.374-375insGA). This mutation occurs in exon 3 and leads to frameshift and a premature stop of translation. A novel homozygous MCFD2 missense mutation (c.241G>T) was identified in family B22. This mutation causes a substitution of tyrosine for a highly conserved aspartic acid residue at amino acid position 81 (D81Y). Novel homozygous LMAN1 mutations (c.795delC and c.1356delC) were identified in both affected siblings in families B20 and B21, respectively. A previously reported mutation (c.149+5G>A) was identified in both affected siblings of family B27. The LMAN1 mutations in families B20 and B21 are single-nucleotide deletions in exon 8 and exon 11 that both result in a frameshift and a premature stop of translation. Analysis of families B23 to B25 identified homozygosity in all affected individuals for 2 previously reported mutations (c.1149+2T>G and c.89–90insG).2 The proband of family B26 carries a mutation (c.2T>C) commonly found in patients of Italian origin.19 Although no mutations were identified by DNA sequencing of the MCFD2 and LMAN1 genes in family B28, Western blot analysis demonstrated the absence of detectable LMAN1 protein (data not shown), suggesting an LMAN1 regulatory or splice mutation missed by the exonic sequence analysis.
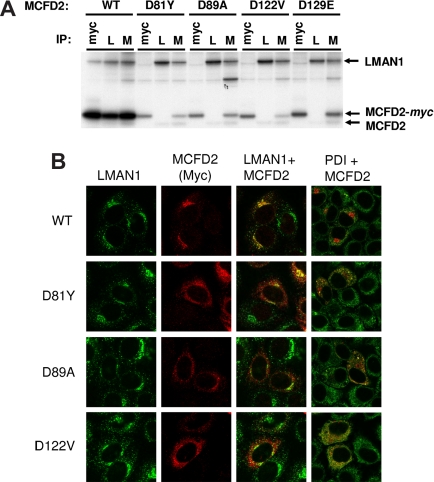
All known missense mutations in MCFD2 abolish LMAN1 binding
The D81Y mutation is one of 5 missense mutations identified in MCFD2 to date, all of which result in an amino acid substitution at a highly conserved amino acid residue in one of the 2 EF hand domains. Two of these missense mutations (D129E and I136T), both located in the second EF hand domain, have been shown to disrupt LMAN1 binding.3 To test the effect of the remaining 3 missense mutations (D81Y and 2 previously reported mutations, D89A6 and D122V11), each was expressed as a myc-tagged fusion protein and transfected into COS-1 cells. Antimyc antibody was used to specifically identify the missense mutant proteins. All 3 missense mutants failed to coimmunoprecipitate with LMAN1 (Figure 1A). In contrast, the wild-type MCFD2 (both the endogenous and the myc-tagged proteins) are readily immunoprecipitated in complex with LMAN1, as detected by both anti-LMAN1 and anti-MCFD2 antibodies (Figure 1A). By immunofluorescence staining, all 3 missense mutant MCFD2 proteins are detected by anti-myc antibody in transfected cells, but fail to colocalize with LMAN1 (Figure 1B), in contrast to wild-type MCFD2. The staining pattern for all 3 missense MCFD2 mutations resembles that of protein disulfide isomerase (PDI), an ER marker (Figure 1B), similar to the pattern previously observed for the D129E and I136T mutations.3
Figure 1.
Characterization of MCFD2 missense mutations. (A) MCFD2 missense mutants fail to coimmunoprecipitate with LMAN1. COS1 cells were transfected with myc-tagged WT or mutant MCFD2, metabolically labeled, and immunoprecipitated with the indicated antibodies: myc indicates antimyc; L, anti-LMAN1; and M, anti-MCFD2. (B) MCFD2 missense mutants are mislocalized in cells. HeLa cells were transfected with myc-tagged WT or mutant MCFD2 and stained with rabbit anti-LMAN1, monoclonal antimyc, or monoclonal anti-PDI, and secondary antibodies conjugated with Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen, Carlsbad, CA).
Correlation of genotype with FV and FVIII levels
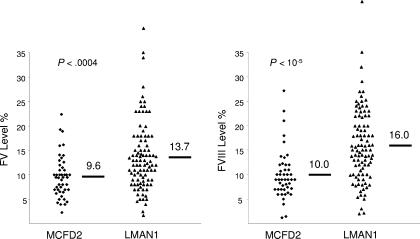
To date, at least 15 MCFD2 mutations and 32 LMAN1 mutations have been reported, including the 4 new mutations identified here. In addition, unidentified mutations may exist in regulatory regions of LMAN1 that result in no mRNA accumulation.6 Data on FV and FVIII levels are available for a total of 46 patients with MCFD2 mutations and a total of 96 patients with LMAN1 mutations (Table 2–3).2–6,11,19–27 Figure 2 shows a comparison of FV and FVIII levels between patients with MCFD2 mutations and patients with LMAN1 mutations. We observed a small but statistically significant difference in the distribution of FV and FVIII levels between these 2 classes of patients, with lower levels for both factors in the patients with MCFD2 mutations compared with those with LMAN1 mutations (mean values of 9.6 vs 13.7 for FV [P < .001] and 10.0 vs 16.0 for FVIII [P < .001]). FVIII levels also exhibit a wider distribution, which is consistent with previous observations of healthy subjects and LMAN1 carriers.28 In contrast, no significant differences were observed between male and female patients for either FV or FVIII levels (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This includes analyses of the whole patient population, as well as subgroups divided by gene mutations (LMAN1 versus MCFD2). Although averages are slightly higher in all female groups, this trend is not statistically significant.
Figure 2.
Correlation of genotypes and levels of FV and FVIII in F5F8D patients. Distribution of FV levels and FVIII levels in patients with LMAN1 mutations and MCFD2 mutations. The short bars and numbers indicate the average values. To meet the assumptions of normality, before the Student t test analysis, the FV and FVII data were cube-root transformed.
FVIII, but not FV, is known to be affected by ABO blood type, with lower levels in blood group O individuals thought to result from reduction in plasma von Willebrand factor.29,30 We thus tested the possibility that differences in ABO blood group might partially explain the distribution of FVIII levels observed in Figure 2. For all patients listed in Tables 2–3 for whom DNA samples were available, genotyping was performed for the 2 most common O genotypes (O1 and O2, together accounting for > 99% of group O31) to distinguish blood group O from non-O types. No significant difference in FV and FVIII levels was observed in patients with blood group O compared with non-O blood groups (Figure S2).
Correlation of FV levels with FVIII levels
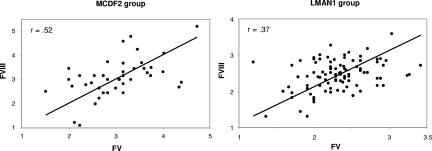
If the observed variation in factor levels is the result of biologic differences in the effect of loss of function for MCFD2 versus LMAN1, then plasma FV levels might be expected to correlate with FVIII levels for each individual patient. To test this hypothesis, we performed a Pearson correlation analysis on the FV/FVIII levels. Moderate correlation was observed in both patients with MCFD2 mutations (r = 0.53, P = .001) and those with LMAN1 mutations (r = 0.322, P = .001; Figure 3), suggesting that deficiencies in LMAN1 or MCFD2 exert a similar impact on FV and FVIII. A nonparametric Spearman correlation analysis yielded similar results (data not shown).
Figure 3.
Correlation of FV levels and FVIII levels. Shown are Pearson correlation analysis on FV and FVIII levels (cube-root transformed) for each individual patient in (A) patients with MCFD2 mutations (P = .001) and (B) patients with LMAN1 mutations (P < .001).
Correlation between platelet and plasma FV
Circulating FV exists in both plasma and platelets. The platelet FV pool has been shown to originate from endocytosis of plasma FV in humans18,32; although in mice, platelet FV is derived exclusively from biosynthesis within the megakaryocytes.33,34 To address the question of whether LMAN1 and MCFD2 mutations exert differential effects between these 2 pools, we measured platelet FV antigen levels in 4 patients with LMAN1 mutations and 3 patients with MCFD2 mutations, as well as 15 healthy controls. Platelet FV levels are reduced to the same extent as FV levels in plasma, for both the LMAN1 group (15% of control for plasma and 13% for platelets) and the MCFD2 group (8% of control for both plasma and platelets; Table 4).
Discussion
Here we report 4 MCFD2 and 5 LMAN1 mutations. The mutation in family B19 appears to be the result of a duplication event, with insertion of a GA dinucleotide in a region that normally contains a string of 3 GAs. This mutation results in a frameshift that deletes both EF hand domains of MCFD2. The mutation in family B22 (D81Y) is the fifth missense mutation identified in MCFD2, and the second identified in the first EF hand domain. In the current report, we demonstrate that this mutation, as well as 2 other missense mutations identified previously, disrupts the binding to LMAN1 (Figure 1). Taken together with results of previously reported D129E and I136T mutations, all missense mutations identified to date are located in the EF hand domains and result in loss of LMAN1 binding. The 2 mutations identified in families B23 to B25 are consistent with the previously reported founder effect.2 To date, all F5F8D patients in Jewish families have been found to carry either the c.1149+2T>G (Tunisian Jews) or the c.89-90insG mutation (Middle Eastern Jews), with the combined prevalence of one of these mutations in these populations estimated at 1:100 000.12,28 The c.2T>C mutation in LMAN1 identified in family B26 has been reported only in patients of Italian origin; therefore, it likely represents a founder allele in the Italian population. The mutation in family B27 (c.149+5G>A) is thus far the most frequently identified mutation in MCFD2. Previous studies have shown that this is a recurring mutation that arose independently in different geographic regions.6,11
The difference observed between LMAN1 and MCFD2 patients in the distribution of plasma levels for FV and FVIII is unlikely due to a systematic error in measurement as these data were collected at multiple centers around the world. However, a consistent trend toward high or low values from one of the centers contributing a relatively large group of patients could potentially bias the results, particularly given the smaller sample size for the MCFD2 group. Of note, most of the patients in a recent report from India (included in our analysis) have MCFD2 mutations,11 with measurements clustered at the high end of the range for both FV and FVIII. However, if data from these patients are removed, the difference in levels between the MCFD2 and LMAN1 groups increases even further in significance (mean values of 8.4 vs 13.7 for FV [P < .001] and 9.3 vs 16.0 for FVIII [P < .001]). A recent report of an additional 9 F5F8D patients from 5 families is notable for levels in the lower end of the range, particularly for FVIII (at 1% or lower for 6 of 9 patients).35 Mutations have not been identified in these patients. In addition to laboratory-specific bias in factor activity measurements and biologic differences between MCFD2 and LMAN1, other factors may contribute to variations in plasma FV and FVIII levels among certain groups of patients, including common genetic modifiers or environmental factors unique to the corresponding population. However, sex and ABO blood type do not appear to be important modifying factors (Figure 3). Interestingly, FVIII levels in F5F8D patients with type O blood are not further reduced compared with non-O blood types.
Platelet FV levels are reduced to the same extent as plasma FV levels, for both the LMAN1 group and the MCFD2 group (Table 4). A prior study on 2 F5F8D patients is consistent with our results, demonstrating reduced factor Xa binding in platelet releasates to approximately 40% of the healthy control.36 The platelet FV pool in humans is derived via endocytosis of plasma FV,18,32 whereas in the mouse, platelet FV is synthesized de novo in the megakaryocytes.33,34 Recent studies suggest that endocytosis of FV is a specific, clathrin-dependent, and probably receptor-mediated mechanism.37 Our observations of similarly reduced FV levels in platelets and plasma of F5F8D patients suggest that the receptor for uptake of plasma FV into megakaryocytes is not saturated at physiologic concentrations of plasma FV. Alternatively, a mechanism may exist that regulates the rate of FV uptake into megakaryocytes to match the plasma FV level. Consistent with either of these models, the amount of intracellular FV in cultured megakaryocytes has been shown to be dependent on the exogenous factor V concentrations in the culture media.38 Our results would predict that other conditions that alter plasma FV level should also alter the platelet level, including severe liver diseases or autoimmune clearance of plasma FV. Although a number of patients with FV inhibitor antibodies have been described,39–42 bleeding symptoms are highly variable, and direct measurement of platelet FV antigen in this setting has not been reported.
The finding that MCFD2 mutations are generally associated with lower levels of FV and FVIII suggests that MCFD2 may play a more direct role in transporting FV and FVIII, perhaps initiating the interaction with these 2 cargo proteins in the ER and/or recruiting them to ER exit sites. Consistent with this hypothesis, MCFD2 with a missense mutation (D129E) that disrupts the LMAN1-MCFD2 interaction can still be cross-linked to FVIII.10 The lectin activity of LMAN1 may function to further stabilize the receptor-cargo complex by binding to the sugar residues of FV and FVIII. Alternatively, LMAN1 may merely function to carry MCFD2 and FV/FVIII cargo to the ER exit sites for packaging into COPII vesicles. In this model, LMAN1 deficiency would act only through the resulting secondary MCFD2 deficiency and higher levels of FV/FVIII in LMAN1−/− patients could reflect FV/FVIII transport by the small, residual pool of intracellular MCFD2. A recent study suggests that MCFD2 is dispensable for the interaction of LMAN1 with the lysosomal enzymes cathepsin C and cathepsin Z.43 Thus, MCFD2 could function as a selective cargo adaptor for FV and FVIII, with LMAN1 deficiency resulting in subclinical deficiencies for additional cargo proteins that directly interact with LMAN1 or are linked to adaptors other than MCFD2. This hypothesis awaits experimental confirmation.
Supplementary Material
Acknowledgments
The authors thank Dr MariaTeresa Bajetta and Dr Rossana Lombardi for their assistance during the experiments performed in Milan, and the Genomic Medicine Biorepository at Cleveland Clinic Foundation for processing some patient samples.
This work was partially supported by grants from the National Institutes of Health (PO1 HL057346 [D.G., R.J.K., B.Z.], R37 HL039693 [D.G.], and HL052173 [R.J.K.]), a Career Development Award from the National Hemophilia Foundation (B.Z.), Fondazione Italo Monzino, Milan, Italy (POC(2005-2007)BS-1/2005), and Telethon Foundation, Italy (grant nos. GGP030261 and GGP06155). R.J.K. and D.G. are investigators of the Howard Hughes Medical Institute.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.Z., M.S., C.Z., and A.Y. performed experiments; B.Z., F.P., and D.G. designed the research and analyzed results; B.Z. made the figures; P.P. performed statistical analyses; B.Z., M.S., F.P., and D.G. wrote the paper; other authors provided vital new reagents and clinical data, and critical review of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bin Zhang, Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH 44195; e-mail: zhangb@ccf.org.
References
- 1.Zhang B, Ginsburg D. Familial multiple coagulation factor deficiencies: new biologic insight from rare genetic bleeding disorders. J Thromb Haemost. 2004;2:1564–1572. doi: 10.1111/j.1538-7836.2004.00857.x. [DOI] [PubMed] [Google Scholar]
- 2.Nichols WC, Seligsohn U, Zivelin A, et al. Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell. 1998;93:61–70. doi: 10.1016/s0092-8674(00)81146-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B, Cunningham MA, Nichols WC, et al. Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nat Genet. 2003;34:220–225. doi: 10.1038/ng1153. [DOI] [PubMed] [Google Scholar]
- 4.Neerman-Arbez M, Johnson KM, Morris MA, et al. Molecular analysis of the ERGIC-53 gene in 35 families with combined factor V factor VIII deficiency. Blood. 1999;93:2253–2260. [PubMed] [Google Scholar]
- 5.Nichols WC, Terry VH, Wheatley MA, et al. ERGIC-53 gene structure and mutation analysis in 19 combined factors V and VIII deficiency families. Blood. 1999;93:2261–2266. [PubMed] [Google Scholar]
- 6.Zhang B, McGee B, Yamaoka JS, et al. Combined deficiency of factor V and factor VIII is due to mutations in either LMAN1 or MCFD2. Blood. 2006;107:1903–1907. doi: 10.1182/blood-2005-09-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kappeler F, Klopfenstein DR, Foguet M, Paccaud JP, Hauri HP. The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J Biol Chem. 1997;272:31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- 8.Nufer O, Kappeler F, Guldbrandsen S, Hauri HP. ER export of ERGIC-53 is controlled by cooperation of targeting determinants in all three of its domains. J Cell Sci. 2003;116:4429–4440. doi: 10.1242/jcs.00759. [DOI] [PubMed] [Google Scholar]
- 9.Baines AC, Zhang B. Receptor-mediated protein transport in the early secretory pathway. Trends Biochem Sci. 2007;32:381–388. doi: 10.1016/j.tibs.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Kaufman RJ, Ginsburg D. LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway. J Biol Chem. 2005;280:25881–25886. doi: 10.1074/jbc.M502160200. [DOI] [PubMed] [Google Scholar]
- 11.Jayandharan G, Spreafico M, Viswabandya A, et al. Mutations in the MCFD2 gene are predominant among patients with hereditary combined FV and FVIII deficiency (F5F8D) in India. Haemophilia. 2007;13:413–419. doi: 10.1111/j.1365-2516.2007.01477.x. [DOI] [PubMed] [Google Scholar]
- 12.Segal A, Zivelin A, Rosenberg N, et al. A mutation in LMAN1 (ERGIC-53) causing combined factor V and factor VIII deficiency is prevalent in Jews originating from the island of Djerba in Tunisia. Blood Coagul Fibrinolysis. 2004;15:99–102. doi: 10.1097/00001721-200401000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Olsson ML, Chester MA. A rapid and simple ABO genotype screening method using a novel B/O2 versus A/O2 discriminating nucleotide substitution at the ABO locus. Vox Sang. 1995;69:242–247. doi: 10.1111/j.1423-0410.1995.tb02602.x. [DOI] [PubMed] [Google Scholar]
- 14.Cattaneo M, Bettega D, Lombardi R, Lecchi A, Mannucci PM. Sustained correction of the bleeding time in an afibrinogenaemic patient after infusion of fresh frozen plasma. Br J Haematol. 1992;82:388–390. doi: 10.1111/j.1365-2141.1992.tb06434.x. [DOI] [PubMed] [Google Scholar]
- 15.Tracy PB, Eide LL, Bowie EJW, Mann KG. Radioimmunoassay of Factor V in human plasma and platelets. Blood. 1982;60:59–63. [PubMed] [Google Scholar]
- 16.Hayward CP, Rivard GE, Kane WH, et al. An autosomal dominant, qualitative platelet disorder associated with multimerin deficiency, abnormalities in platelet factor V, thrombospondin, von Willebrand factor, and fibrinogen and an epinephrine aggregation defect. Blood. 1996;87:4967–4978. [PubMed] [Google Scholar]
- 17.Gould WR, Silveira JR, Tracy PB. Unique in vivo modifications of coagulation factor V produce a physically and functionally distinct platelet-derived cofactor: characterization of purified platelet-derived factor V/Va. J Biol Chem. 2004;279:2383–2393. doi: 10.1074/jbc.M308600200. [DOI] [PubMed] [Google Scholar]
- 18.Gould WR, Simioni P, Silveira JR, et al. Megakaryocytes endocytose and subsequently modify human factor V in vivo to form the entire pool of a unique platelet-derived cofactor. J Thromb Haemost. 2005;3:450–456. doi: 10.1111/j.1538-7836.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 19.D'Ambrosio R, Santacroce R, Di Perna P, et al. A new case of combined factor V and factor VIII deficiency further suggests that the LMAN1 M1T mutation is a frequent cause in Italian patients. Blood Coagul Fibrinolysis. 2007;18:203–204. doi: 10.1097/MBC.0b013e328012b615. [DOI] [PubMed] [Google Scholar]
- 20.Dansako H, Ishimaru F, Takai Y, et al. Molecular characterization of the ERGIC-53 gene in two Japanese patients with combined factor V-factor VIII deficiency. Ann Hematol. 2001;80:292–294. doi: 10.1007/s002770000283. [DOI] [PubMed] [Google Scholar]
- 21.Farah RA, de Moerloose P, Bouchardy I, et al. Combined factor V-factor VIII deficiency (F5F8D): compound heterozygosity for two novel truncating mutations in LMAN1 in a consanguineous patient. Thromb Haemost. 2006;95:893–895. [PubMed] [Google Scholar]
- 22.Ma ES, Wong CL, Lam HY, Wang CL, Ma SY. Combined factors V and VIII deficiency (F5F8D) in a Chinese family due to compound heterozygosity for nonsense mutations of the LMAN1 gene. Br J Haematol. 2007;139:509–511. doi: 10.1111/j.1365-2141.2007.06809.x. [DOI] [PubMed] [Google Scholar]
- 23.Mohanty D, Ghosh K, Shetty S, et al. Mutations in the MCFD2 gene and a novel mutation in the LMAN1 gene in Indian families with combined deficiency of factor V and VIII. Am J Hematol. 2005;79:262–266. doi: 10.1002/ajh.20397. [DOI] [PubMed] [Google Scholar]
- 24.Neerman-Arbez M, Antonarakis SE, Blouin J-L, et al. The locus for combined factor V-factor VIII deficiency (F5F8D) maps to 18q21, between D18S849 and D18S1103. Am J Hum Genet. 1997;61:143–150. doi: 10.1086/513897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oeri J, Matter M, Isenschmid H, Hauser F, Koller F. Angeborener mangel an faktor V (parahaemophilie) verbunden mit echter haemophilie A bein zwei brudern. Med Probl Paediatr. 1954;1:575–588. [PubMed] [Google Scholar]
- 26.Sirachainan N, Zhang B, Chuansumrit A, et al. Combined factor V and factor VIII deficiency in a Thai patient: a case report of genotype and phenotype characteristics. Haemophilia. 2005;11:280–284. doi: 10.1111/j.1365-2516.2005.01092.x. [DOI] [PubMed] [Google Scholar]
- 27.Nyfeler B, Kamiya Y, Boehlen F, et al. Deletion of 3 residues from the C-terminus of MCFD2 affects binding to ERGIC-53 and causes combined factor V and factor VIII deficiency. Blood. 2008;111:1299–1301. doi: 10.1182/blood-2007-09-112854. [DOI] [PubMed] [Google Scholar]
- 28.Seligsohn U, Zivelin A, Zwang E. Combined factor V and factor VIII deficiency among non-Ashkenazi Jews. N Engl J Med. 1982;307:1191–1195. doi: 10.1056/NEJM198211043071907. [DOI] [PubMed] [Google Scholar]
- 29.Gallinaro L, Cattini MG, Sztukowska M, et al. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood. 2008;111:3540–3545. doi: 10.1182/blood-2007-11-122945. [DOI] [PubMed] [Google Scholar]
- 30.Haberichter SL, Balistreri M, Christopherson P, et al. Assay of the von Willebrand factor (VWF) propeptide to identify patients with type 1 von Willebrand disease with decreased VWF survival. Blood. 2006;108:3344–3351. doi: 10.1182/blood-2006-04-015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsson ML, Chester MA. Polymorphism and recombination events at the ABO locus: a major challenge for genomic ABO blood grouping strategies. Transfus Med. 2001;11:295–313. doi: 10.1046/j.1365-3148.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 32.Camire RM, Pollak ES, Kaushansky K, Tracy PB. Secretable human platelet-derived factor V originates from the plasma pool. Blood. 1998;92:3035–3041. [PubMed] [Google Scholar]
- 33.Sun H, Yang TL, Yang A, Wang X, Ginsburg D. The murine platelet and plasma factor V pools are biosynthetically distinct and sufficient for minimal hemostasis. Blood. 2003;102:2856–2861. doi: 10.1182/blood-2003-04-1225. [DOI] [PubMed] [Google Scholar]
- 34.Yang TL, Pipe SW, Yang A, Ginsburg D. Biosynthetic origin and functional significance of murine platelet factor V. Blood. 2003;102:2851–2855. doi: 10.1182/blood-2003-04-1224. [DOI] [PubMed] [Google Scholar]
- 35.Shetty S, Madkaikar M, Nair S, et al. Combined factor V and VIII deficiency in Indian population. Haemophilia. 2000;6:504–507. doi: 10.1046/j.1365-2516.2000.00421.x. [DOI] [PubMed] [Google Scholar]
- 36.Miletich JP, Majerus DW, Majerus PW. Patients with congenital factor V deficiency have decreased factor Xa binding sites on their platelets. J Clin Invest. 1978;62:824–831. doi: 10.1172/JCI109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouchard BA, Williams JL, Meisler NT, Long MW, Tracy PB. Endocytosis of plasma-derived factor V by megakaryocytes occurs via a clathrin-dependent, specific membrane binding event. J Thromb Haemost. 2005;3:541–551. doi: 10.1111/j.1538-7836.2005.01190.x. [DOI] [PubMed] [Google Scholar]
- 38.Suehiro Y, Veljkovic DK, Fuller N, et al. Endocytosis and storage of plasma factor V by human megakaryocytes. Thromb Haemost. 2005;94:585–592. [PubMed] [Google Scholar]
- 39.Ajzner E, Balogh I, Haramura G, et al. Anti-factor V auto-antibody in the plasma and platelets of a patient with repeated gastrointestinal bleeding. J Thromb Haemost. 2003;1:943–949. doi: 10.1046/j.1538-7836.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 40.Nesheim ME, Nichols WL, Cole TL, et al. Isolation and study of an acquired inhibitor of human coagulation factor V. J Clin Invest. 1986;77:405–415. doi: 10.1172/JCI112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perdekamp MT, Rubenstein DA, Jesty J, Hultin MB. Platelet factor V supports hemostasis in a patient with an acquired factor V inhibitor, as shown by prothrombinase and tenase assays. Blood Coagul Fibrinolysis. 2006;17:593–597. doi: 10.1097/01.mbc.0000245297.64644.ee. [DOI] [PubMed] [Google Scholar]
- 42.Wiwanitkit V. Spectrum of bleeding in acquired factor V inhibitor: a summary of 33 cases. Clin Appl Thromb Hemost. 2006;12:485–488. doi: 10.1177/1076029606293438. [DOI] [PubMed] [Google Scholar]
- 43.Nyfeler B, Zhang B, Ginsburg D, Kaufman RJ, Hauri HP. Cargo selectivity of the ERGIC-53/MCFD2 transport receptor complex. Traffic. 2006;7:1473–1481. doi: 10.1111/j.1600-0854.2006.00483.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.