Abstract
While skeletal muscle injuries can induce chronic pain, the underlying mechanism is unknown. One possible cause has been suggested to be an increased sensitivity to inflammatory mediators. We demonstrate that self-limited inflammatory hyperalgesia induced by intramuscular carrageenan (lasting ~5 days) results in a state of chronic-latent hyperalgesia, revealed by injection of prostaglandin E2 (PGE2) 10 days after carrageenan at the same site. In carrageenan-pretreated muscle, PGE2 produced hyperalgesia that was unattenuated even 14 days after injection, markedly longer than the 4-h hyperalgesia induced by PGE2 in naïve rats. This chronic-latent hyperalgesia was reversed as well as prevented by spinal intrathecal injection of oligodeoxynucleotide antisense to protein kinase Cε, a second messenger implicated in long-lasting plasticity in cutaneous nociceptors.
Perspective
We describe a novel experimental model for chronic muscle pain, produced by mild acute muscle inflammation, that has clinical significance since it has the potential to reveal cellular processes by which acute inflammation or muscle trauma underlies chronic muscle pain.
Introduction
Chronic muscle pain is a constellation of symptoms that develops after trauma or in association with repetitive strain. It is believed to be dependent, at least in part, on muscle inflammation,6; 38; 39 as it responds to non-steroidal anti-inflammatory drugs19 and cytokine levels are increased in the symptomatic muscle.21; 33 While symptoms may improve over time, they return with the use of the involved muscle, even years later.15; 20; 35 Unfortunately, little is known about the cellular mechanisms underlying chronic muscle pain 8, in particular the mechanism mediating the transition from acute to chronic pain.
Recently, we established a model of chronic latent inflammatory pain in cutaneous tissues. In this model, a single exposure to the inflammogen, carrageenan (a classic agent for the induction of experimental inflammation and inflammatory pain that is relevant to clinical inflammatory pain states 9; 10; 14), produces a prolonged hypersensitivity to subsequent exposure of hyperalgesic agents 4; 12; 27; 28. In this “hyperalgesic priming” or chronic-latent hyperalgesia, the inflammatory mediator prostaglandin (PG) E2 and other inflammatory agents that act directly on nociceptors (e.g. 5-hydroxtryptamine and adenosine) produce an enhanced and markedly prolonged hyperalgesia (>24 hr compared to <4 hr in naïve rats) when injected several weeks after the initial response to carrageenan has resolved, a plastic change in nociceptor function mediated by PKCε. Since chronic muscle pain is believed to be dependent, at least in part, on muscle inflammation 6; 38; 39, we investigated whether chronic hyperalgesia develops in muscle following recovery from transient inflammation.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (250–400 g) were housed in the Animal Care Facility at UCSF, under environmentally controlled conditions (7 am to 7 pm light cycles; 21–23°C) with food and water available ad libitum. Care and use of rats conformed to National Institutes of Health guidelines, and was approved by the UCSF Institutional Animal Care and Use Committee. Effort was made to minimize the number of animals used and their suffering.
Measurement of hyperalgesia
Mechanical nociceptive thresholds were quantified using a digital force transducer (Chatillon DFI2, Amtek Inc., Largo, FL) with a custom-made 6 mm diameter probe attached to the transducer. Rats were lightly restrained in a Plexiglas holder that allows for easy access to the hind limb and application of the transducer probe to the belly of the gastrocnemius muscle. The nociceptive threshold was defined as the force, in Newtons, required to produce a flexion reflex in the hind leg. Baseline withdrawal threshold was defined as the mean of 3 readings taken at 5-min intervals. Each hind limb was treated as an independent measure and each experiment was performed on a separate group of rats, and animals were followed over time. All behavioral testing was done between 10 am and 4 pm.
Intramuscular injections
Rats were briefly anesthetized with 3% isoflurane and vehicle (0.9% saline) or drug was injected into the belly of the gastrocnemius muscle in one hind limb (in a volume of 10 μl); the skin over the injection sites were marked with indelible pen so that the same area can be repeatedly tested for mechanical nociceptive threshold.
Carrageenan hyperalgesia
Carrageenan (100 μg in 10 μl 0.9% saline) was injected into the belly of the gastrocnemius muscle. In our previous studies on carrageenan-induced nociceptive priming in cutaneous tissue we administered 50 μg in 5 μl 4; 28, i.e. half the dose used in the current muscle nociceptive priming study The 100 μg/10 μl dose of carrageenan was determined in pilot studies as sufficient to produce robust muscle primary mechanical hyperalgesia, equivalent to that seen in the cutaneous model with 50 μg/5 μl. It is likely that this dose/volume difference is due to the greater volume of distribution at the muscle injection site compared to intradermal injections.
Antisense oligodeoxynucleotide (ODN) preparation
Since priming develops over a period of several days, continuous inhibition of PKCε by administration of antagonists (which have a relatively short half-life) into muscle would be technically challenging. Therefore, to address the role of PKC&epsi in muscle afferents we employed antisense oligonucleotides to PKCε to reduce expression of this enzyme, thereby producing a functional block of neuronal PKCε activity.
The 20-mer PKCε antisense ODN sequence, 5′-GCC AGC TCG ATC TTG CGC CC-3′, was directed against a unique sequence of rat PKCε. The corresponding GenBank accession number and ODN position within the cDNA sequence are XM345631 and 226–245, respectively. The mismatch ODN sequence, 5′-GCC AGC GCG ATC TTT CGC CC-3′, corresponds to the PKCε subunit antisense sequence with 2 bases mismatched (in bold typeface). We have previously shown that this antisense ODN against PKCε decreases PKCε protein in dorsal root ganglia.11
For intrathecal injection of ODN, rats were briefly anesthetized with 3% isoflurane, a 30-guage needle inserted into the subarachnoid space on the midline between the L4 and L5 vertebrae and ODN (80 μg in 10 μl) slowly injected. Control animals received injections of mismatch ODN.1–3; 13; 17; 18; 26–28
Experimental groups
Experiment 1 — Carrageenan hyperalgesia
We tested the effects of carrageenan on nociceptive threshold injection by measuring basal nociceptive mechanical threshold of the gastrocnemius muscle then administering a single injection of carrageenan (100 μg in 10 μl, n=3–6) and then testing mechanical nociceptive threshold at 30, 60 and 120 min and 1, 2, 3, 4 and 7 days after injecting carrageenan. A control group received intramuscular injection of 0.9% saline (10 μl) instead of the carrageenan (n=3–6).
Experiment 2 — Chronic latent hyperalgesia
To determine whether a prior administration of carrageenan can induce a state of chronic latent hyperalgesia, a single carrageenan injection (100 μg in 10 μl, n=3–6) was administered to the gastrocnemius muscle. Ten days after this injection, nociceptive thresholds were assessed to confirm that they had returned to pre-carrageenan injection levels and then the inflammatory mediator PGE2 (1 μg) was then injected into the gastrocnemius muscle. Nociceptive threshold was then re-assessed 1 and 4 h and again 1, 2 and 7 days after PGE2 administration.
Experiment 3 — PKCε-dependence
To determine whether PKCε contributes to hyperalgesic priming in muscle we first tested whether attenuating PKCε prior to exposure to carrageenan can prevent hyperalgesic priming. PKCε ODN (80 μg/20 μl, n=6) was administered intrathecally in one group of rats, once daily for 3 days prior to carrageenan and then daily for 5 days after carrageenan. Ten days after carrageenan injection, nociceptive thresholds were assessed (they had returned to pre-carrageenan injection levels) and the inflammatory mediator PGE2 (1 μg) injected into the gastrocnemius muscle. Nociceptive threshold was then re-assessed 1 and 4 h and again 1, 3, 9 and 14 days after PGE2 administration. In order to determine whether attenuating PKCε can reverse hyperalgesia priming, in a different group of rats PKCε ODN was administered intrathecally once daily for 3 days beginning 5 days after carrageenan (to test reversal). Nociceptive thresholds were determined prior to carrageenan administration and again 2 and 5 days after injection (to determine acute carrageenan hyperalgesia and recovery of nociceptive threshold to pre-carrageenan levels). Eight days after carrageenan injection, PGE2 (1 μg) was injected into the gastrocnemius muscle, and nociceptive threshold assessed 1 h, 4 h and 1, and 4 days after PGE2 administration.
Statistics
Group data are expressed as mean ± SEM of n observations in hind limbs. Statistical comparisons were made by using repeated measures ANOVA, with Bonferroni post hoc test, using StatView statistical software.
Results
Carrageenan hyperalgesia
The mechanical threshold to elicit leg withdrawal decreased by ~60% within 2 h of the intramuscular injection of carrageenan (100 μg) and remained at approximately this level at least 2 days (saline, open circles vs. carrageenan, filled circles, P<0.0001 repeated measures ANOVA, Figure 1). By day 4, nociceptive thresholds had returned to baseline. Control animals injected with 0.9% saline vehicle (open circles) exhibited no significant change in nociceptive threshold.
Figure 1. Carrageenan muscle hyperalgesia.
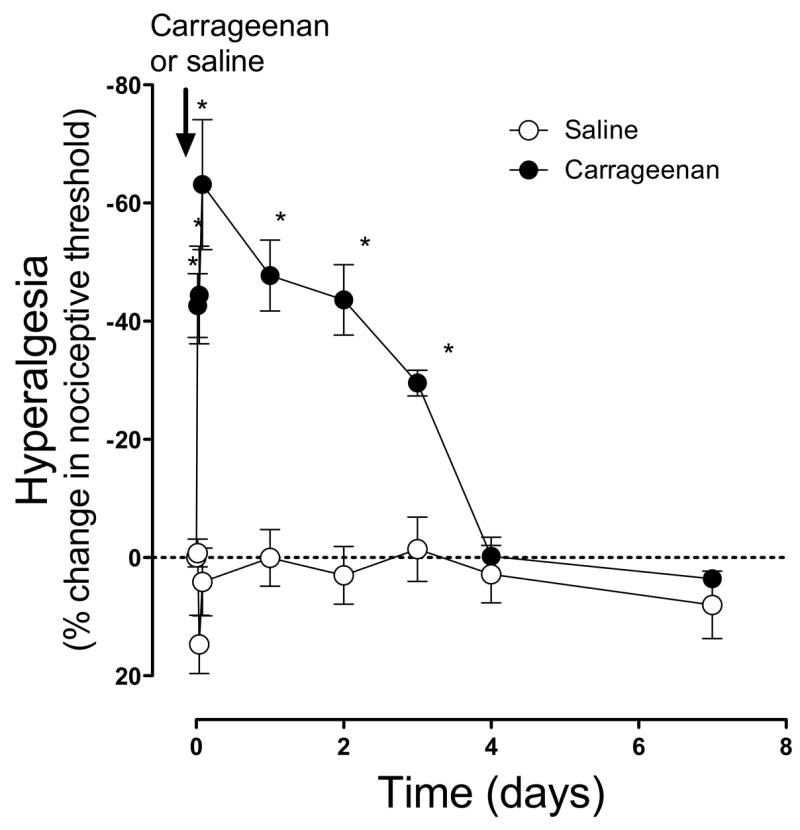
Carrageenan (1%; 30 μl, filled circles n=3–6), injected into the gastrocnemius muscle of adult male rats decreased nociceptive threshold by ~60% within one day, and nociceptive threshold was lower than saline-injected muscle for 3 days. By day 4, nociceptive threshold was not significantly different from baseline (and saline-treated) values. Control animals injected with saline (0.9% NaCl, 30 μl, open circles n=3–6) exhibited no significant change in threshold.
Chronic-latent hyperalgesia
Following complete recovery from the acute hyperalgesia induced by intramuscular carrageenan (which occurs ~4 days after carrageenan administration, see Figure 1), the response to a new inflammatory challenge was assessed. Ten days after carrageenan administration, after verifying the return to pre-carrageenan baseline withdrawal threshold, the inflammatory mediator PGE2 (1 μg) was injected into the gastrocnemius muscle (time 0). In saline pretreated control animals (open circles), PGE2 induced a short-lived hyperalgesia that completely resolved within 4 hours. In contrast, in the carrageenan-primed muscle (filled circles) the duration of PGE2-induced hyperalgesia was significantly and markedly prolonged remaining undiminished at least 1 week days post PGE2 administration (2-way ANOVA, P<0.001, Figure 2).
Figure 2. Chronic-latent muscle hyperalgesia.
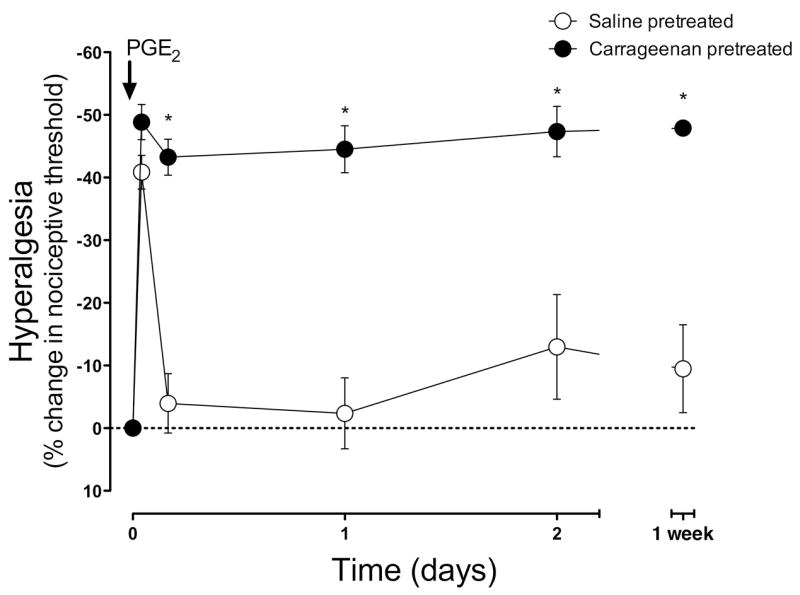
Ten days after carrageenan (1%; 30μl), PGE2 (100 ng) was injected into the muscle. In saline pretreated rats (open circles, n=3–6), PGE2-induced hyperalgesia had completely resolved within 3 hours, but in the carrageenan-pretreated muscle (filled circles, n=6), the duration of hyperalgesia was greatly enhanced, remaining undiminished 1 week after PGE2 admininstration.
PKCε dependence
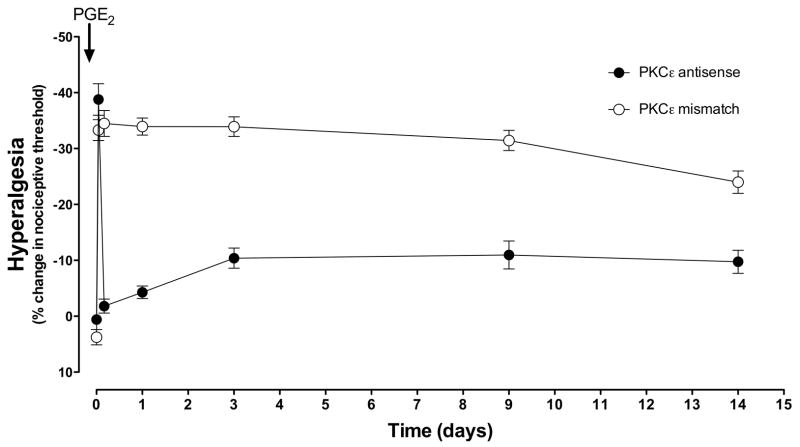
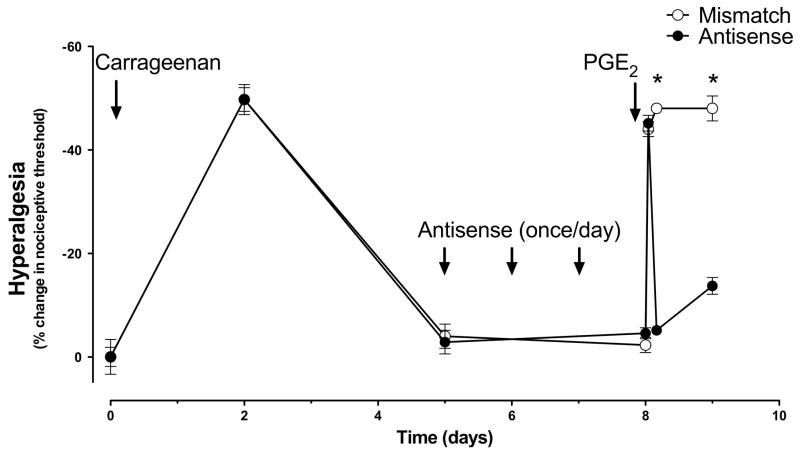
To determine whether PKCε contributes to hyperalgesic priming in muscle, as it does in skin4, we determined if PKCε ODN could prevent the development of and/or reverse chronic latent hyperalgesia. PKCε ODN (80 μg/20 μl) was administered intrathecally once daily for 3 days prior to carrageenan and then daily for 5 days after carrageenan (to test prevention), or daily for 3 days beginning 5 days after carrageenan (to test reversal). PGE2 hyperalgesia was evaluated 6 days after the last PKCε ODN administration (i.e. 11 days after carrageenan) to test for prevention, or 1 day after the last PKCε ODN administration (i.e. 8 days after carrageenan) to test for reversal. PKCε ODN (filled circles, n=6) treatment completely prevented the development of carrageenan chronic-latent hyperalgesia (mismatch vs. antisense P<0.0001, repeated measures ANOVA, Figure 3), while not affecting the magnitude or duration of the acute phase of PGE2 hyperalgesia. PKCε ODN (filled circles, n=6) treatment also reversed hyperalgesic priming when injected after recovery from carrageenan hyperalgesia (mismatch vs. antisense P<0.0001, repeated measures ANOVA, Figure 4).
Figure 3. PKCε antisense prevents chronic-latent hyperalgesia.
Three days before and 5 days after intramuscular carrageenan (1%; 30 μl in gastrocnemius), PKCε mismatch ODN (open circles, n=6) or antisense (filled circles, n=6), was injected intrathecally once daily. Six days after the final mismatch ODN, PGE2 (100 ng, injected into the gastrocnemius) produced a decrease in nociceptive threshold that lasted at least 14 d. In contrast, PGE2 hyperalgesia in PKCε antisense ODN-treated rats (filled circles, n=6) was significantly shorter, and similar in duration to saline-pretreated rats (cf. open circles Figure 2).
Figure 4. PKCε antisense reverses chronic-latent hyperalgesia.
PKCε mismatch ODN (open circles, n=6) or antisense (filled circles, n=6) was injected intrathecally daily for 3 days, 5 days after intramuscular carrageenan (1%; 30μl in gastrocnemius) when nociceptive thresholds had returned to baseline. One day after the final ODN injection, PGE2 (100 ng, injected into the gastrocnemius) was injected into the muscle. In PKCε antisense ODN-injected rats (filled circles, n=6), PGE2 hyperalgesia was present 1 h post-PGE2, but by 4 h nociceptive threshold had returned to baseline, similar to the effect in saline pretreated rats (see open circles Figure 2). In contrast, in PKCε mismatch ODN administered rats, nociceptive threshold was significantly decreased even 24 h post PGE2 administration.
Discussion
Chronic muscle pain is a major health problem,22; 40 due in part to its long-term persistence and in part to its recurrence after resolution of acute symptoms, following resumption of precipitating activity (e.g. musicians or athletes resuming their occupation15; 20; 35). This clinical picture suggests that in these patients there exists a latent hyperalgesic state that may be unmasked following an innocuous triggering event (e.g. minor mechanical insult) to produce moderate to severe pain. In the current study we have demonstrated that intramuscular carrageenan induces a state of chronic-latent mechanical hyperalgesia. This state can be demonstrated, following recovery from acute carrageenan-induced muscle hyperalgesia (which lasts ~5 days), when PGE2 is injected at the same intramuscular site to produce a markedly prolonged hyperalgesic response that lasts at least 2 days, compared to ~3 h in control animals. We have previously described a similar phenomenon in cutaneous nociceptors, a phenomenon that we have termed hyperalgesic priming. An important feature of hyperalgesic priming is a switch in the second messenger for inflammatory mediator-induced hyperalgesia. Thus, while PGE2 hyperalgesia in the skin is normally PKC-independent, during hyperalgesic priming, PGE2 hyperalgesia is now mediated by the novel PKC isoform, PKCε.4; 28 The development of hyperalgesic priming can be prevented by attenuation of PKCε in the primary afferent nociceptor using spinal intrathecal administration of ODN antisense to PKCε. Similar to what we observed in the skin, chronic-latent hyperalgesia in muscle was prevented following pretreatment with PKCε antisense. We also observed that hyperalgesic priming could be reversed when PKCε antisense is administered after carrageenan administration.
While we cannot exclude a contribution from central/spinal sites to chronic latent muscle hyperalgesia, we have previously shown that intrathecally administered PKCε ODN antisense significantly reduces PKCε expression in peripheral dorsal root ganglion neurons. Furthermore, in rats treated with PKCε ODN antisense, a specific peptide inhibitor of PKCε (εV1–2 peptide) that attenuates peripherally-mediated hyperalgesia induced by epinephrine in naïve or PKCε ODN mismatch treated rats, was no longer effective 28. Thus, while it is possible that central nociceptive circuitry could also be affected by PKCε, the available data indicates a critical role for PKCε in peripheral nerve endings.
We observed that following recovery from a short-lived hyperalgesia produced by 100 μg carrageenan, we observed a chronic-latent hyperalgesia, characterized by a dramatically prolonged PGE2–induced hyperalgesia. Previous studies have shown that carrageenan induces muscle hyperalgesia when given at much higher doses 16; 21; 31, but at these doses administration of carrageenan into rat gastrocnemius muscle produces myonecrosis (at 3 mg) 31, and myositis (at 15 mg) 7; 34, it did not produce mechanical hyperalgesia when given at 300 μg 31. Our ability to detect acute carrageenan mechanical hyperalgesia at a much lower dose (100 μg) may be dependent on our use of 6 mm diameter probe, which is likely to activate many more muscle C-fiber afferents than the von Frey hairs (diameter ~1 mm) used in previous studies.
There have been several attempts to develop appropriate animal models for muscle pain, for example by intramuscular injection of inflammatory agents such as carrageenan (4 mg) 16 capsaicin 36 or formalin 33. However, at the doses used in these studies, these agents produce overt tissue damage and immune cell infiltration 33, while other models using intramuscular injection of TNFα or acidic saline have been show to produce muscle pain without causing damage to the muscle tissue or immune cell recruitment 33; 37. However these approaches have been criticized as not adequately modeling tonic or persistent type of muscle pain syndromes 32 and acidic saline produces a bilateral hyperalgesia following a unilateral injection, suggesting a central sensitization, contrasting with clinical muscle pain following injury or in myofascial pain syndromes wherein pain tends to be localized to specific muscles rather than a more generalized pain syndrome 23. Our animal model of chronic muscle pain, using very low-dose carrageenan as the initiating inflammatory stimulus, is likely to be useful for future studies designed to determine the underlying mechanisms of chronic muscle pain.
Clinically, one of the most important aspects of inflammatory pain is the development of chronic pain following acute inflammation, e.g. as produced in repetitive strain disorders.24 We have hypothesized that this process involves cellular mechanisms different from those of acute inflammation and that after the resolution of a transient inflammatory event, a long-lasting state of enhanced responsiveness to subsequent hyperalgesic stimuli can exist. In this study we describe a novel experimental model for muscle pain produced by mild acute muscle inflammation. This model has clinical significance since it tracks the transition from acute to chronic latent peripheral muscle hyperalgesia, and has the potential to reveal cellular processes by which acute inflammation or muscle trauma can create a state of enhanced susceptibility to inflammatory mediators or subsequent mechanical stimulation. These finding have begun to clarify mechanisms underlying chronic muscle pain; this model has the potential to provide information for future strategies for the prevention and treatment of chronic musculoskeletal pain. Of particular importance in this regard, is whether muscle pain resulting from eccentric activity, repetitive ergonomic strain and/or vibration (all of which are believed to involve an inflammatory component 5; 25; 29; 30) also produce a state of chronic latent hyperalgesia. We are currently developing models of these work-related musculoskeletal pain syndromes to test this hypothesis.
Acknowledgments
This research was supported by grants from NIAMS (AR054635, to JDL) and TRDPR (15RT-0032, to PGG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessandri Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 3.Aley KO, Levine JD. Multiple receptors involved in peripheral alpha 2, mu, and A1 antinociception, tolerance, and withdrawal. J Neurosci. 1997;17:735–744. doi: 10.1523/JNEUROSCI.17-02-00735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbe MF, Barr AE. Inflammation and the pathophysiology of work-related musculoskeletal disorders. Brain Behav Immun. 2006;20:423–429. doi: 10.1016/j.bbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr AE, Barbe MF. Pathophysiological tissue changes associated with repetitive movement: a review of the evidence. Phys Ther. 2002;82:173–187. doi: 10.1093/ptj/82.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berberich P, Hoheisel U, Mense S. Effects of a carrageenan-induced myositis on the discharge properties of group III and IV muscle receptors in the cat. J Neurophysiol. 1988;59:1395–1409. doi: 10.1152/jn.1988.59.5.1395. [DOI] [PubMed] [Google Scholar]
- 8.Bongers PM, de Vet HC, Blatter BM. Repetitive strain injury (RSI): occurrence, etiology, therapy and prevention. Ned Tijdschr Geneeskd. 2002;146:1971–1976. [PubMed] [Google Scholar]
- 9.Dawson J, Sedgwick AD, Edwards JC, Lees P. A comparative study of the cellular, exudative and histological responses to carrageenan, dextran and zymosan in the mouse. International journal of tissue reactions. 1991;13:171–185. [PubMed] [Google Scholar]
- 10.Di Rosa M. Biological properties of carrageenan. J Pharm Pharmacol. 1972;24:89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- 11.Dina OA, Hucho T, Yeh J, Malik-Hall M, Reichling DB, Levine JD. Primary afferent second messenger cascades interact with specific integrin subunits in producing inflammatory hyperalgesia. Pain. 2005;115:191–203. doi: 10.1016/j.pain.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Dina OA, McCarter GC, de Coupade C, Levine JD. Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron. 2003;39:613–624. doi: 10.1016/s0896-6273(03)00473-2. [DOI] [PubMed] [Google Scholar]
- 13.Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci. 2004;19:634–642. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- 14.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nature medicine. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 15.Humphries D, Jamison M. Clinical and magnetic resonance imaging features of cricket bowler’s side strain. Br J Sports Med. 2004;38:E21. doi: 10.1136/bjsm.2003.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- 17.Khasar SG, Gold MS, Dastmalchi S, Levine JD. Selective attenuation of mu-opioid receptor-mediated effects in rat sensory neurons by intrathecal administration of antisense oligodeoxynucleotides. Neurosci Lett. 1996;218:17–20. doi: 10.1016/0304-3940(96)13111-6. [DOI] [PubMed] [Google Scholar]
- 18.Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- 19.Kvien TK, Viktil K. Pharmacotherapy for regional musculoskeletal pain. Best Pract Res Clin Rheumatol. 2003;17:137–150. doi: 10.1016/s1521-6942(02)00102-x. [DOI] [PubMed] [Google Scholar]
- 20.Lederman RJ. Neuromuscular problems in musicians. Neurologist. 2002;8:163–174. doi: 10.1097/00127893-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain. 2007;8:127–136. doi: 10.1016/j.jpain.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Luo X, Pietrobon R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine. 2004;29:79–86. doi: 10.1097/01.BRS.0000105527.13866.0F. [DOI] [PubMed] [Google Scholar]
- 23.Maquet D, Croisier JL, Demoulin C, Crielaard JM. Pressure pain thresholds of tender point sites in patients with fibromyalgia and in healthy controls. Eur J Pain. 2004;8:111–117. doi: 10.1016/S1090-3801(03)00082-X. [DOI] [PubMed] [Google Scholar]
- 24.Melhorn JM. Cumulative trauma disorders and repetitive strain injuries. The future. Clin Orthop. 1998:107–126. [PubMed] [Google Scholar]
- 25.Mense S. Mechanisms of transition from acute to chronic muscle pain. Orthopade. 2004;33:525–532. doi: 10.1007/s00132-003-0611-2. [DOI] [PubMed] [Google Scholar]
- 26.Papir-Kricheli D, Frey J, Laufer R, Gilon C, Chorev M, Selinger Z, Devor M. Behavioural effects of receptor-specific substance P agonists. Pain. 1987;31:263–276. doi: 10.1016/0304-3959(87)90041-8. [DOI] [PubMed] [Google Scholar]
- 27.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 28.Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 29.Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev. 2005;11:64–85. [PubMed] [Google Scholar]
- 30.Peate WF. Occupational musculoskeletal disorders. Prim Care. 1994;21:313–327. [PubMed] [Google Scholar]
- 31.Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104:567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ro JY, Capra NF, Lee JS, Masri R, Chun YH. Hypertonic saline-induced muscle nociception and c-fos activation are partially mediated by peripheral NMDA receptors. Eur J Pain. 2007;11:398–405. doi: 10.1016/j.ejpain.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 34.Schomburg ED, Steffens H, Maznychenko AV, Pilyavskii AI, Hellstrom F, Kostyukov AI, Maisky VA. Acute muscle inflammation enhances the monosynaptic reflexes and c-fos expression in the feline spinal cord. Eur J Pain. 2007;11:579–586. doi: 10.1016/j.ejpain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Sherry MA, Best TM. A comparison of 2 rehabilitation programs in the treatment of acute hamstring strains. J Orthop Sports Phys Ther. 2004;34:116–125. doi: 10.2519/jospt.2004.34.3.116. [DOI] [PubMed] [Google Scholar]
- 36.Sluka KA. Stimulation of deep somatic tissue with capsaicin produces long-lasting mechanical allodynia and heat hypoalgesia that depends on early activation of the cAMP pathway. J Neurosci. 2002;22:5687–5693. doi: 10.1523/JNEUROSCI.22-13-05687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stauber WT. Factors involved in strain-induced injury in skeletal muscles and outcomes of prolonged exposures. J Electromyogr Kinesiol. 2004;14:61–70. doi: 10.1016/j.jelekin.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. Jama. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]