Abstract
Aminoglycoside uptake in the inner ear remains poorly understood. We subcutaneously injected a fluorescently-conjugated aminoglycoside, gentamicin–Texas Red (GTTR), to investigate the in vivo uptake of GTTR in the inner ear of several vertebrates, and in various murine sensory cells using confocal microscopy.
In bullfrogs, GTTR uptake was prominent in mature hair cells, but not in immature hair cells. Avian hair cells accrued GTTR more rapidly at the base of the basilar papilla. GTTR was associated with the hair bundle; and, in guinea pigs and mice, somatic GTTR fluorescence was initially diffuse before punctate (endosomal) fluorescence could be observed. A baso-apical gradient of intracellular GTTR uptake in guinea pig cochleae could only be detected at early time points (<3 h). In 21−28 day mice, cochlear GTTR uptake was greatly reduced compared to guinea pigs, 6-day-old mice, or mice treated with ethacrynic acid. In mice, GTTR was also rapidly taken up, and retained, in the kidney, dorsal root and trigeminal ganglia. In linguinal and vibrissal tissues rapid GTTR uptake cleared over a period of several days.
The preferential uptake of GTTR by mature saccular, and proximal hair cells resembles the pattern of aminoglycoside-induced hair cell death in bullfrogs and chicks. Differences in the degree of GTTR uptake in hair cells of different species suggests variation in serum levels, clearance rates from serum, and/or the developmental and functional integrity of the blood–labyrinth barrier. GTTR uptake by hair cells in vivo suggests that GTTR has potential to elucidate aminoglycoside transport mechanisms into the inner ear, and as a bio-tracer for in vivo pharmacokinetic studies.
Keywords: Inner ear, Gentamicin, Hair cells, Blood–labyrinth barrier
1. Introduction
Aminoglycoside antibiotics are powerful anti-Gram negative bacterial drugs that bind to mammalian RNA (Myrdal et al., 2005b), bacterial ribosomes and inhibit protein synthesis (Zierhut et al., 1979). However, the clinical use of aminoglycosides is limited to life-threatening infections because of serious ototoxic (4−14% of patients receiving aminoglycosides) and nephrotoxic (9−14%) side-effects (Kahlmeter and Dahlager, 1984). In mammals, including humans, ototoxicity is a serious, and mostly permanent, side-effect of aminoglycoside therapy, whereas nephrotoxicity is often acute and reversible. Kidney tubule epithelia retain their ability to undergo cellular proliferation following aminoglycoside toxicity, unlike sensory epithelia in the mammalian inner ear (Chen and Segil, 1999; Nonclercq et al., 1992; Xie et al., 2001).
The degree of aminoglycoside-induced hair cell death appears to be dependent on the degree of intracellular drug uptake, and both decrease along a baso-apical gradient in the cochlea (Beaubien et al., 1991; Hackney et al., 1990; Hiel et al., 1992a; Hiel et al., 1992b). Physiologically, the cationic aminoglycosides reversibly abolish the transduction current in hair cells, and are permeant blockers of the transduction channels (Kroese et al., 1989; Marcotti et al., 2005). Aminoglycosides rapidly increase intracellular levels of Ca++ and reactive oxygen species (Clerici et al., 1996; Hirose et al., 1999; Staecker et al., 1997), induce loss of calcium-binding proteins and cytoskeletal organization (Hackney et al., 1990; Imamura and Adams, 2003b), and initiate a variety of cell death processes in vivo and in vitro (Ding et al., 2002; Jiang et al., in press; Mangiardi et al., 2004; Ylikoski et al., 2002).
Early autoradiographic studies described an intracellular and nuclear distribution of aminoglycosides in the organ of Corti within 2 h (Portmann et al., 1974; von Ilberg et al., 1971). Subsequent studies confirmed the rapid uptake of aminoglycosides into the organ of Corti and endocochlear fluids within 2 h following a single injection (Tran Ba Huy et al., 1986; Tran Ba Huy et al., 1983). More recent studies have used fluorescent probes, immunohistochemistry, autoradiography and electron microscopy to localize aminoglycosides in hair cells up to six months after administration (de Groot et al., 1990; Dulon et al., 1993; Hashino et al., 1997; Imamura and Adams, 2003a; Richardson et al., 1997). Subcellular localization of aminoglycosides in hair cells is greatest below the cuticular plate: in lysosomes, multi-vesicular bodies, small tubules and vesicles, suggestive of endocytotic uptake across the apical membrane of hair cells that is bathed in endolymph (de Groot et al., 1990; Hashino and Shero, 1995a; Hashino et al., 2000; Hayashida et al., 1989; Hiel et al., 1992a; Richardson et al., 1997).
Dulon et al. (1989) administered fluorescein-conjugated gentamicin to isolated outer hair cells, but observed only binding to the cell surface. Recently, we used gentamicin conjugated to Texas Red (GTTR) that revealed intracellular localization in endosomes, nuclei, mitochondria, Golgi bodies, endoplasmic reticulum of hair cells, and also diffusely throughout the cytoplasm of both hair cells and kidney tubule cells (Myrdal and Steyger, 2005a; Myrdal et al., 2005b; Steyger et al., 2003). These in vitro studies corroborated a variety of previous cochlear studies (above, see also: (Ding et al., 1995; Portmann et al., 1974; von Ilberg et al., 1971)).
The present study was conducted to determine the utility of GTTR for in vivo studies. Following injection, the comparative distribution of GTTR was analyzed using confocal microscopy in the inner ears of bullfrogs, chicks, guinea pigs, neonatal and post-natal mice. In addition, we localized GTTR fluorescence in other sensory, neural and renal cells elsewhere in the body. The results reveal subtle differences in the uptake of GTTR in the inner ear epithelia of different species, and in other sensory cell types known for aminoglycoside toxicity. Thus, GTTR has the potential to characterize drug transport and uptake mechanisms that lead to toxicity.
2. Materials and methods
2.1. Gentamicin–Texas Red conjugation
Gentamicin (GT) sulfate (Sigma, MO; MW = 449−477; 50 mg/ml in 100 mM K2CO3, pH 9) and succinimidyl esters of Texas Red (Molecular Probes, OR; MW = 817; 2 mg/ml in dimethyl formamide) were agitated together at 4 °C for several days to produce a gentamicin–Texas Red conjugate (GTTR). Typically, 4.4 ml of 50 mg/ml (final volume) GT was mixed with 0.6 ml of 2 mg/ml Texas Red esters (TR) to produce an approximately 300:1 molar ratio of GT:GTTR. The conjugation procedure reduces the polycationic charge of GT by 1 for each amine group conjugated to TR, proportionately increasing its hydrophobicity. Thus, the high molar ratio of GT to TR esters (300:1) typically produces a conjugation ratio of one TR molecule to a single GT molecule (Sandoval et al., 1998).
2.2. Animals
Adult bullfrogs (>3 in., approx 200 g), White Leghorn chicks (14 day), juvenile pigmented guinea pigs (200−250 g, >4 weeks) and C57/BL6 mice (6 day and 21−28 day old) were used in this study. The Institutional Animal Care and Use Committees of Oregon Health and Science University, and the Children's Hospital, Boston approved this study.
Chicks, guinea pigs and mice were injected subcutaneously, while bullfrogs were injected in the pelvic lymphatic sac, with a single 300 mg/kg dose of the 300:1 molar ratio GT/GTTR solution, then allowed to recover for 0.5, 1, 2, 3, 6, 7.5, 9 or 24 h (n ≥ 3 for each time/data point). In some cases, guinea pigs received a single injection of 150 mg/kg gentamicin and allowed to recover for 3 h. Several mice received a subsequent GT/GTTR injection 24 and 48 h after the initial injection and allowed to recover for a further 24 h.
In control animals, hydrolyzed TR treated identically to the conjugation mixture, or vehicle alone, were injected at the same volumes and concentrations equivalent to experimental animals, and the animals allowed to recover for equivalent times.
2.3. Tissue fixation and microscopy
At specific time-points (0.5, 1, 2, 3, 6, 7.5, 9, 24, 48, 72 h) following the initial injection, animals were deeply anesthetized (frogs by immersion in chilled 0.2% MS-222; chicks with 1.5−3 ml/kg Beuthanasia D; mice and guinea pigs using 100 mg/kg ketamine), and inner ear organs were excised and fixed in 4% formaldehyde overnight as described previously (Steyger et al., 2003). Also, at specific time-points (1, 3, 24 h, 3 and 7 days) following injection, additional murine tissues were retrieved from anesthetized mice, including: dorsal root and trigeminal ganglia, kidneys, tongue and muzzle skin were excised and fixed in 4% formaldehyde overnight.
After washing in PBS, fixed tissues were permeabilized using ice-cold acetone, and if necessary, subsequently labeled with Alexa-488-conjugated phalloidin for 1 h to localize filamentous actin. Solvents like acetone and methanol unmask cytoplasmic GTTR fluorescence while retaining punctate (endosomal) fluorescence that can be washed away by ionic detergents like Triton X-100 (Myrdal et al., 2005b). Excised inner ear epithelia, and vibrotomed sections (10 μm) of kidney and neuronal tissues were whole-mounted in VectaShield (Vector Labs) and observed using a Bio-Rad MRC 1024 ES laser scanning confocal system attached to a Nikon Eclipse TE300 inverted microscope. Images were collected using 1024 × 1024 pixel box size using either (i) a 4x (N.A.: 0.25) or (ii) a 60x lens (N.A.: 1.4) with a resolution of 230 nm in the xy-axis and 440 nm in the xz-axis (Steyger et al., 2003). Images were post-processed using the Bio-Rad LaserSharp imaging software. Wholemounted chick cochleae were examined on a Leica TCS SP confocal laser-scanning microscope (Leica Microsystems Heidelberg GmbH, Heidelberg, Germany) using a 20x (N.A.: 0.7) or a 40x (N.A.: 1.25) oil immersion objective digitally zoomed to 2x, and acquired sequentially. Confocal imaging of control sensory epithelia was performed at the same laser intensity and gain settings used for sensory epithelia from GT/GTTR-treated animals. All images are representative examples of data collected from ≥3 animals, and were prepared for publication using PhotoShop software.
3. Results
3.1. Controls
Confocal imaging of bullfrog saccules, chick basilar papillae, or rodent organs of Corti from animals that received GT/GTTR revealed extensive GTTR uptake throughout the sensory epithelium (Fig. 1A–D). Using the same confocal intensity, gain, iris and black level settings used for GTTR-labeled tissues, confocal imaging of sensory epithelia from animals that received hydrolyzed TR revealed negligible fluorescence at all time points tested (Fig. 1E–H). FITC-phalloidin labeling did not induce cross-talk (and therefore false-positive labeling) in bullfrog, guinea pig or murine inner ear tissues in the Texas Red channel when illuminated by the 568 nm laser. However, in chicks, weak TR-like fluorescence was visible in chick stereociliary bundles, due to cross-talk from FITC-phalloidin-labeled stereocilia (Fig. 1F). Injections of hydrolyzed TR with 300 mg/kg GT, or vehicle alone also did not result in TR fluorescence in inner ear epithelia (data not shown).
Fig. 1.
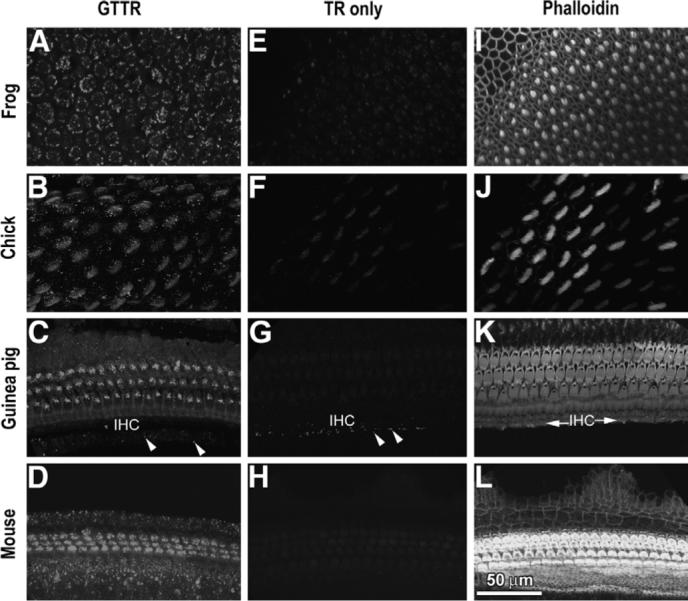
GTTR fluorescence in: (A) bullfrog saccule, (B) chick basilar papilla, (C) guinea pig cochlea and (D) 6-day-old murine cochlea 24 h after GT/GTTR injection (chick, 9 h). Panels (E–H) show negligible TR fluorescence in bullfrog saccule, chick basilar papilla, guinea pig and mouse cochleae, respectively, at equivalent time points and identical laser settings. (F) Weak, non-specific fluorescence is visible in chick stereociliary bundles, due to crosstalk from FITC-phalloidin-labeled stereocilia. (G) Weak autofluorescence (or aldehyde-induced fluorescence; arrowheads) is present in the inner sulcus region of the guinea pig, adjacent to the inner hair cell region (IHC in C, K). (I–L) Phalloidin-labeled sensory epithelia imaged in panels E–H, showing actiniferous regions at the lumenal surface. Scale bar applies to all images.
In 3−4 week guinea pigs that received vehicle alone, some autofluorescence (or aldehyde-induced fluorescence) could be determined in the inner sulcus region adjacent to the inner hair cells (IHCs; as shown in Fig. 1C and G). Weak, punctate TR-like autofluorescence (or aldehyde-induced fluorescence) was detected in the bullfrog saccule at elevated laser intensity settings, and in apical coils of 21−28 day old murine cochleae after the vehicle alone was administered (data not shown). This auto-fluorescence was attributed to age-related pigments (lipofuscin) that accumulate in post-mitotic cells, with an emission peak at 600 nm (Porta, 2002).
3.2. Adult bullfrog saccule
Within 30 min after GT/GTTR injection, weak GTTR fluorescence was initially detected in mature hair cells in the central region of the saccule (not shown). The intensity of both diffuse and punctate fluorescence in the hair cell cytoplasm was increased at 1 h (Fig. 2A), and at 3 h (Fig. 2B). Hair cells appeared to retain the intensity of the punctate GTTR fluorescence 24 h after injection. In mature hair cells, GTTR fluorescence was associated with the hair bundle (Fig. 2, inset in H). Immature hair cells at the saccular periphery frequently has much less GTTR fluorescence than mature hair cells at all time points examined, although some had intense punctate labeling (Fig. 2).
Fig. 2.
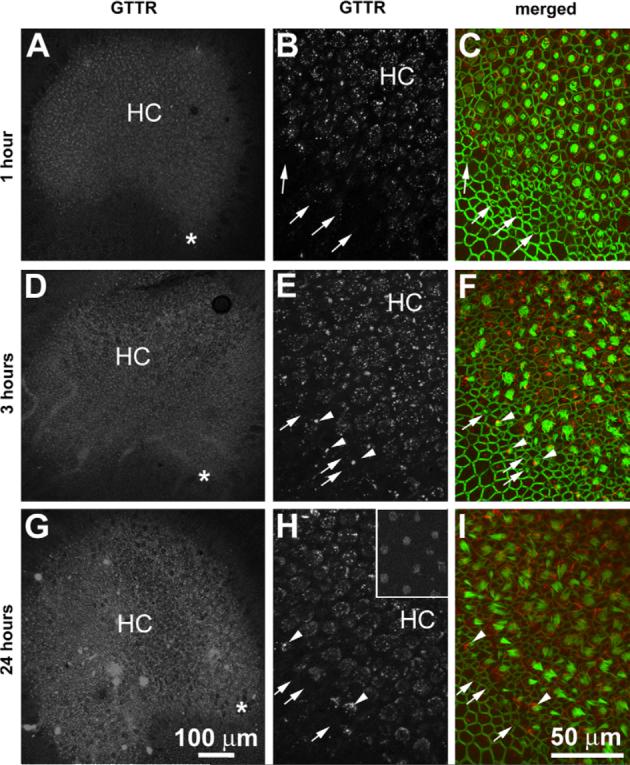
Low resolution images of the bullfrog saccule and growth zones (*; A, D, G), and higher resolution images of immature and mature hair cells (B, C, E, F, H, I) following lymphatic sac injection of GT/GTTR. (A, B, D, E, G, H) GTTR fluorescence is more prominent in mature hair cells (HC) in the central regions of the saccule. Immature hair cells at the saccular periphery (arrows) typically display less GTTR fluorescence than central mature hair cells (HC). Some peripheral, immature hair cells (arrowheads) display intense punctate fluorescence. (Inset in H) GTTR fluorescence is also associated with the hair bundles of mature hair cells. Scale bar in G applies to A and D. Scale bar in I also applies to B, C, E, F, H and inset in H.
3.3. Chick
GTTR was initially detected in chick hair cells 6 h after GT/GTTR injection, particularly in hair bundles in the proximal region of the basilar papilla (Figs. 1B; 3B), and less intensely in hair bundles in distal regions (Fig. 3A). Punctate GTTR fluorescence could be clearly determined in the cell bodies of proximal hair cells (Fig. 3B), and only sparsely in distal hair cells on the neural side of the basilar papilla (Fig. 3A). At later time points (7.5 and 9 h following injection), Hair bundles were more distinctly labeled with GTTR, and the cell bodies, of proximal hair cells than distal hair cells on the neural side of the basilar papilla. Punctate GTTR labeling was more frequent in both proximal and particularly, distal hair cells than at earlier time points (Fig. 3C and D, respectively).
Fig. 3.
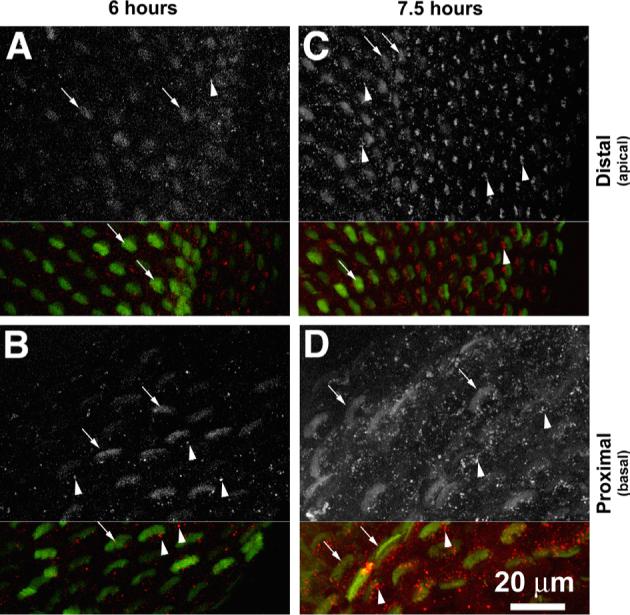
GTTR fluorescence hair cells of chick basilar papilla. (A) Six hours after GT/GTTR injection, weak stereociliary GTTR fluorescence (arrows) sparse, punctate GTTR fluorescence (arrowhead) occurs in distal hair cells compared to the more intense stereociliary and more abundant punctate GTTR fluorescence in proximal hair cells (B). (C, D) 7.5 h after injection, distinct punctate GTTR fluorescence (arrowheads) can be seen in both proximal and distal hair cells. Hair cells closest to the neural edge are o the left in (A, C). Scale bar applies to all images.
3.4. Guinea pigs
Three hours after GT/GTTR injection, GTTR fluorescence can be clearly detected in the stereociliary bundles of guinea pig cochlear hair cells, particularly outer hair cells (OHCs, Figs. 4A and B; 6A). Apical OHC stereociliary bundles are larger, and therefore more distinctly labeled than the smaller basal cochlear OHCs bundles. Diffuse GTTR fluorescence was observed in the infra-cuticular cytoplasm of OHCs after 3 h (Figs. 4A, B and 6A), and also in the soma of some IHCs (Figs. 4A and 6E). A distinct annular band of GTTR fluorescence was associated with the lateral membranes of OHCs (Fig. 6E). Throughout the cochlea, punctate GTTR fluorescence in the subcuticular plate region of hair cells could be first observed only at 6 h (inset in Fig. 4B), but with increased frequency and intensity at 24 h after injection, most prominently at the base of the cochlea (Fig. 4C and D). Typically, OHCs accrued more GTTR fluorescence than IHCs (Fig. 4).
Fig. 4.
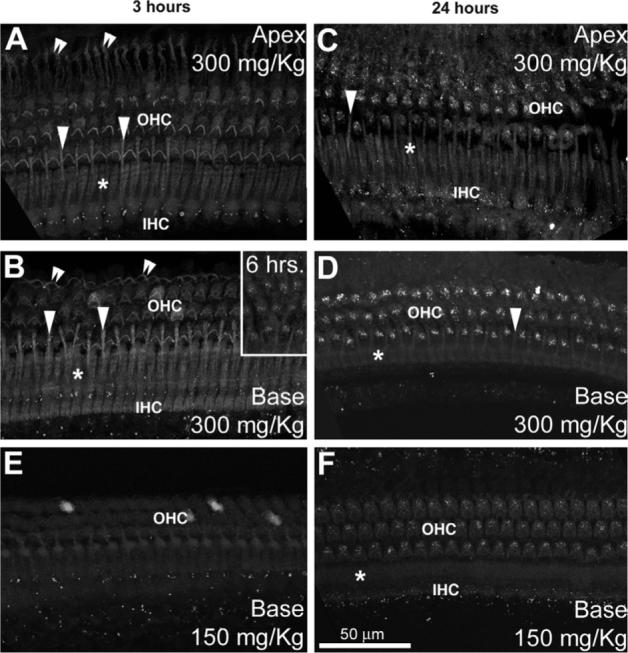
Guinea pig organ of Corti examined at 3 (A, B, E), and 24 h (C, D, F) after 300 mg/kg (A–D) or 150 mg/kg GT/GTTR injection (E, F). Three hours after injection, hair cells at the base of the cochlea (B) display slightly greater intensities of GTTR fluorescence compared to apical hair cells (A). This apparent gradient had disappeared 24 h post-injection (C, D). OHCs have greater fluorescence than IHCs. Note that at 3 h (A, B, E), only diffuse GTTR fluorescence can be observed in the organ of Corti (except for the punctate autofluorescence adjacent to IHCs). Sparse, punctate labeling is first seen in basal cochlear OHCs 6 h post-injection (B, inset), and all OHCs after 24 h (D, F). Note that the outer pillar cell phalanges (arrowheads) between the first row of OHCs, in the Deiters' cell phalanges of the third row (double arrowheads) and the inner pillar cell phalanges (*) are also diffusely labeled. Scale bar applies to all images.
Fig. 6.
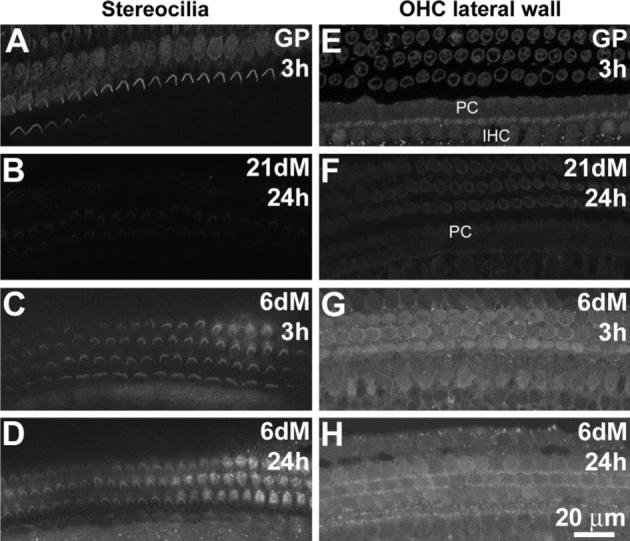
Comparative levels of GTTR fluorescence in stereocilia and baso-lateral membranes. (A) GTTR fluorescence is associated with the stereociliary bundles of guinea pig (GP) hair cells within 3 h. Note the diffuse fluorescence in the infracuticular cytoplasm of OHCs of the third row. (B) In 21−28 day mice (21dM), only weak or negligible GTTR fluorescence is present in OHC stereocilia 24 h post-injection. In 6-day mice (6dM), GTTR fluorescence associated with stereocilia at 3 h (C) or 24 h (D) after injection, is more intense than in 21−28 day mice (B). Annular GTTR fluorescence is localized in the lateral membrane region of OHCs in (E) guinea pigs within 3 h, and (F) far less intensely in 21−28 day old mice 24 h post-injection. GTTR fluorescence is also present in pillar cells (PC) and IHC bodies in guinea pig and murine cochleae (E, F). (G) Annular GTTR fluorescence in the OHC lateral membrane region is also observed in 6-day mice after 3 h, above the diffuse somatic GTTR fluorescence. (H) After 24 h, the lateral membrane GTTR fluorescence cannot be distinguished from somatic fluorescence in 6-day mice. Images A–H acquired and post-processed identically. Scale bar applies to all images.
In supporting cells of the organ of Corti, GTTR fluorescence could not be distinctly observed in the cell bodies of Deiters' cells, nor the majority of other supporting cells in the organ of Corti. However, diffuse GTTR fluorescence was detected in the pillar cell bodies, the inner pillar cell phalanges overlying the head of the outer pillar cell, and in the outer pillar cell phalange interdigitating between individual OHCs of the first row, and in the Deiters' cell phalanges of the third row at all time points (Fig. 4).
Guinea pigs dosed with 150 mg/kg GT/GTTR were compared with animals that received 300 mg/kg GT/GTTR. (Both groups received equal molar ratios of GT:GTTR, 300:1.) Guinea pigs that received the lower dose of GT/GTTR had a similar distribution of GTTR fluorescence in the organ of Corti (Fig. 4E and F) as at the higher dose. However, at the lower dose, GTTR fluorescence was weaker, yet still visible, throughout the organ of Corti at both 3 and 24 h (Fig. 4E and F) compared to the higher dose (Fig. 4B and D)
3.5. Mice
In 21−28 day mice, only faint diffuse GTTR fluorescence was observed in the organ of Corti 3 and 24 h after GT/GTTR injection (Fig. 5A). After 24 h, weak diffuse and punctate fluorescence could be observed in the cell bodies of both IHCs and OHCs (Fig. 5B). At 72 h after the initial injection (with subsequent injections at 24 and 48 h after the initial injection, respectively), both diffuse and punctate GTTR fluorescence was marginally increased in intensity in these same locations (Fig. 5C). Little GTTR fluorescence was observed in many of the surrounding supporting cells (Deiters' cells, Hensen's cells, interdental cells) in the basal region of the cochlea, even after 3 daily GT/GTTR injections; although, some GTTR fluorescence could be seen in pillar cell phalanges (Fig. 5).
Fig. 5.
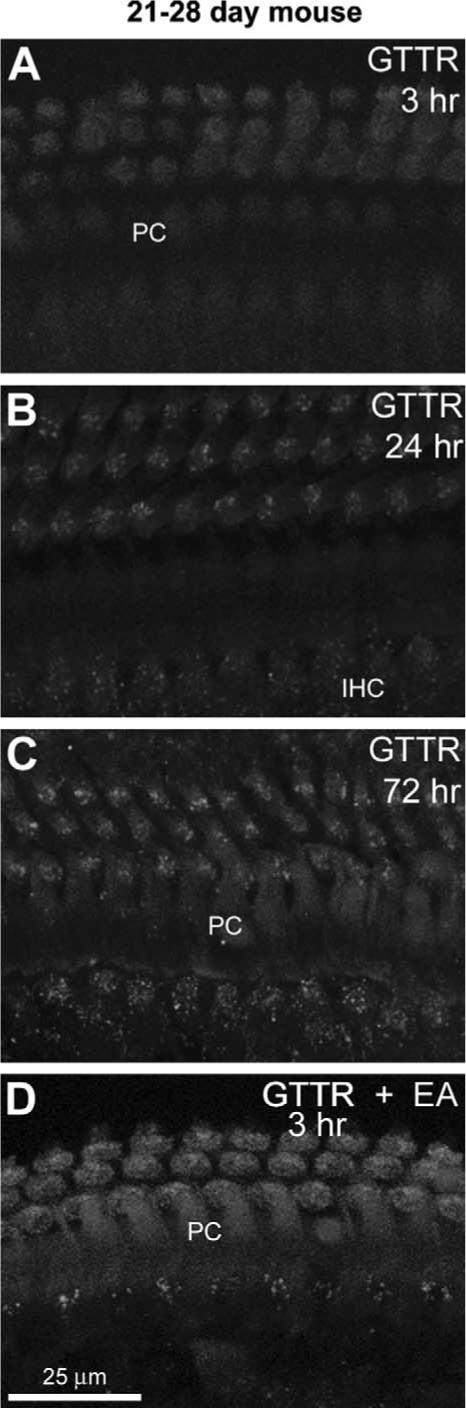
GTTR fluorescence in the 21−28 day old murine cochleae examined at 3 (A, D), 24 (B) and 72 h (C) after GT/GTTR injection. (A) Weak, diffuse GTTR fluorescence in hair cells and pillar cells (PC) 3 h post-injection. (B) Both diffuse and punctate GTTR fluorescence are present in IHCs and OHCs hair cells 24 h post-injection. (C) Punctate and diffuse GTTR fluorescence in the organ of Corti (after 3 daily injections of GT/GTTR), 72 h after the initial injection. OHCs have greater intensity of GTTR fluorescence than IHCs. (D) Three hours after GT/GTTR injection, and 2 h after ethacrynic acid injection, increased diffuse GTTR fluorescence occurred in OHCs compared to OHCs from animals treated without ethacrynic acid (A, B, C). All images acquired and post-processed identically. Scale bar applies to all images.
When 21−28 day cochlear surface preparations were examined using the same confocal settings as for guinea pigs, negligible GTTR fluorescence could be observed in the hair bundles (Fig. 6B), with only faint fluorescence in the OHC lateral wall (24 h after injection) when compared to the robust labeling intensities in guinea pig hair cells 3 h post-injection (Fig. 6A and E). To determine if this drop in GTTR fluorescence in 21−28 day murine hair cells (compared to guinea pig hair cells) was a result of a newly-established murine blood–labyrinth barrier, formed around day 16, (Ehret, 1976; Shnerson and Pujol, 1981), we injected 6-day-old mice with GT/GTTR. At 3, and 24 h after injection, using the same confocal settings, more intense GTTR fluorescence was observed in OHC and IHC stereocilia (Fig. 6C and D) and cell bodies (Fig. 6G and H) compared to 21−28 day old mice (Fig. 6B and F).
To further test the hypothesis that the blood–labyrinth barrier could restrict GTTR uptake in 21−28 day old mice in vivo, we treated mice with ethacrynic acid (EA) to disrupt the blood–labyrinth barrier (Ding et al., 2003). In EA-treated mice, GTTR fluorescence in the organ of Corti (Fig. 5D) and capillaries of the stria vascularis (Fig. 7B) was much greater 3 h after GT/GTTR injection (Fig. 5D) than in mice not treated with EA (Figs. 5A, 7A). GTTR labeling of the stria vascularis and lateral wall could not be observed at later time-points (data not shown).
Fig. 7.
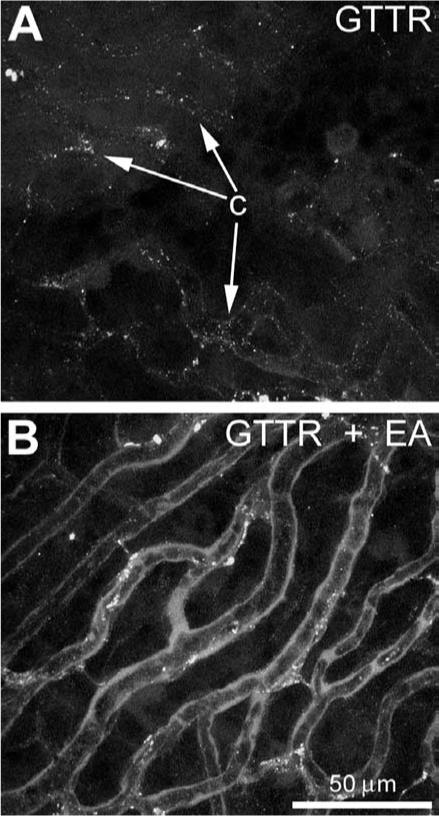
GTTR fluorescence in the stria vascularis of 21−28 day old murine cochleae (A) 3 h after GT/GTTR injection, or (B) 3 h after GT/GTTR injection, and 2 h after ethacrynic acid injection. (A) Punctate GTTR fluorescence in tissues surrounding capillaries (c) within the stria vascularis. (B) Three hours after GT/GTTR injection, and 2 h after ethacrynic acid injection, increased GTTR fluorescence in tissues surrounding capillaries compared to those from animals treated without ethacrynic acid (A). Images acquired and post-processed identically. Scale bar applies to both images.
3.6. GTTR uptake in sensory neurons
GTTR was barely be detected in the neuronal soma of the dorsal root ganglion (DRG) or trigeminal ganglion (TG) 3 h after injection (not shown). At 24 h post-injection, GTTR uptake was clearly detected in the majority of neuronal soma in the DRG and TG, peaking in intensity at 3 days (Fig. 8). At 7 days post-injection, GTTR fluorescence had barely diminished (Fig. 8C and G).
Fig. 8.
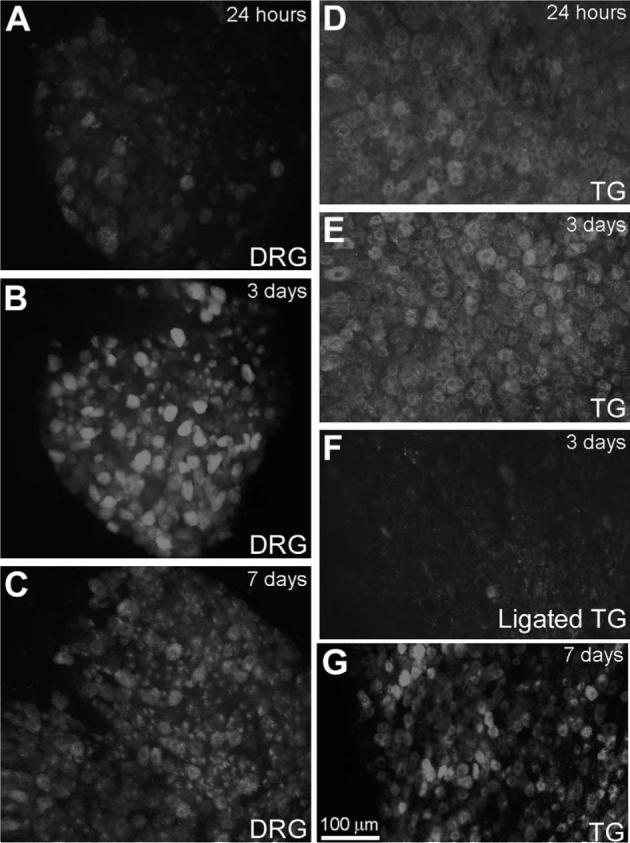
GTTR uptake in sensory neurons. In the DRG or TG, GTTR fluorescence is present in neuronal cell bodies 24 h post-injection (A, D), peaking at 3 days (B, E). At 7 days post-injection, GTTR fluorescence is still retained in neuronal soma (C, G). (F) When infra-orbital dendritic processes of the TG are cut and ligated, diminished GTTR fluorescence occurred in TG neuronal soma compared to sham-operated animals (E). Scale bar applies to all images.
To determine if the delay in GTTR fluorescence in soma of these ganglia was due to retrograde transport from the sensory endings of these neurons in the neuromuscular junctions (DRG) and facial regions (TG) (Meyers et al., 2003), we lacerated and ligated the infra-orbital nerve—a branch of the TG—in 21−28 day mice. Examination of ipsi-lateral TG soma 3 days after injection revealed diminished GTTR fluorescence (Fig. 8F) compared to sham-operated animals (Fig. 8E) or contra-lateral TG (data not shown).
3.7. GTTR uptake in tongue and vibrissae
In the tongue, GTTR rapidly accrued in filiform and fungiform papillae (including a ring of gustatory sensory neurons) within 3 h after injection (Fig. 9A). This uptake of GTTR began to clear within 24 h in fungiform papillae, and by 3 days post-injection, linguinal tissues only weakly retained GTTR fluorescence (Fig. 9C), barely above control levels (Fig. 9D).
Fig. 9.
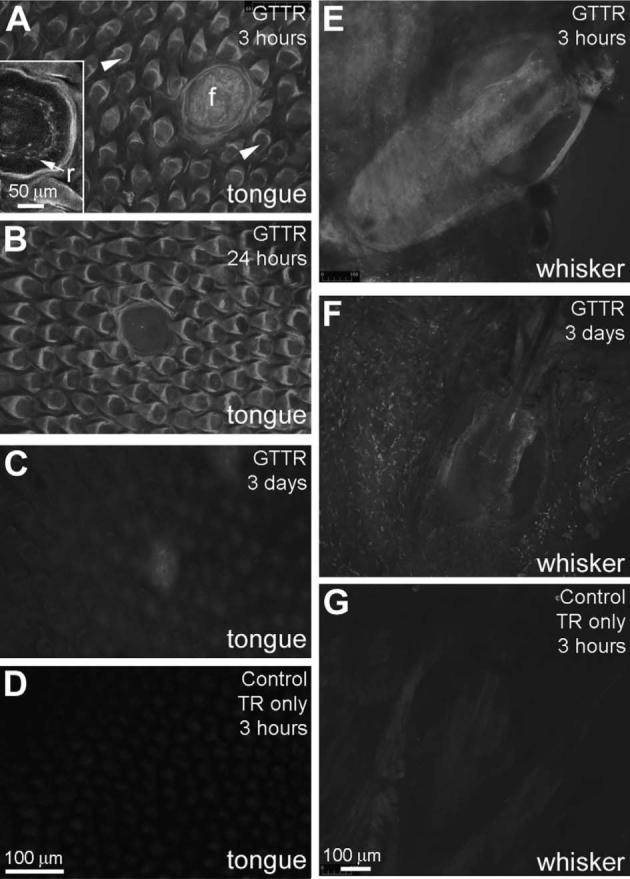
GTTR uptake in tongue and vibrissae. (A) In the tongue, GTTR fluorescence is present in filiform (sh=rtrif) and fungiform (f) papillae (the latter has a ring of labeled gustatory neurons; r in inset) within 3 h of GT/GTTR injection. (B) Within 24 h, GTTR fluorescence is diminished, particularly in fungiform papillae. By 3 days post-injection, linguinal tissues only weakly retain GTTR fluorescence (C) above background, non-specific fluorescence levels (D). (E) The tissues surrounding the vibrissae on the muzzle are rapidly infiltrated with GTTR fluorescence within 3 h, but this had largely cleared 3 days later (F). (G) Vibrissa displaying weak, non-specific fluorescence 3 h after injection of hydrolyzed TR only. Scale bar in D applies to A–C. Scale bar in G applies to E and F.
Similar data was obtained from the vibrissae on the muzzle (Fig. 9E and F). GTTR rapidly infiltrated in the tissues surrounding the whisker bulb-root within 3 h after injection (Fig. 9E). However, this fluorescence began to clear within 24 h, and by 3 days post-injection, tissues surrounding the whisker bulb-root only weakly retained GTTR fluorescence (Fig. 9F). At 7 days post-injection (not shown), GTTR fluorescence was barely above control levels (Fig. 9G), which showed some weak autofluorescence in the whisker bulb root region.
3.8. Uptake of GTTR in the kidney
Vibrotomed sections of murine kidneys were double-labeled with phalloidin-Alexa 488 to locate prominent brush border lining the lumenal space of proximal tubule cells. Strong diffuse and punctate GTTR fluorescence was present in the epithelial cells lining the proximal tubule 3 h after injection (Fig. 10A–C). Negligible fluorescence was seen in the distal tubules, and glomerulus (Fig. 10A–C). Control kidneys, fixed 3 h after injection with hydrolyzed TR only, also displayed negligible fluorescence (Fig. 10D). Punctate GTTR fluorescence in kidney proximal tubule epithelia slowly dissipated over time, although it was still present 3 days and 7 days after injection, together with a diffuse distribution of fluorescence (Fig. 10E and F).
Fig. 10.
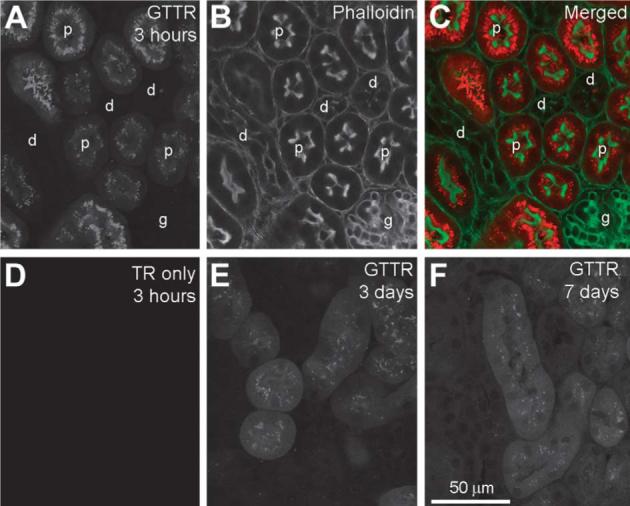
GTTR uptake in the kidney. (A) Three hours after injection, strong GTTR fluorescence occurred in proximal tubule cells (p), but not in glomerular (g) or distal tubule cells (d) as identified by phalloidin labeling (B), and merged images of A and B (C). (D) Kidney tissue 3 h after injection with hydrolyzed TR only revealed negligible fluorescence. (E) Punctate GTTR fluorescence in proximal tubule cells is less intense and less extensive than at 3 days (E) and at 7 days (F) after injection. Note the diffuse cytoplasmic GTTR fluorescence present in proximal tubule cells 3 and 7 days post-injection. Scale bar applies to all images.
4. Discussion
Vertebrate inner ear cells take up and retain GTTR in specific cell types in vivo following a single injection, similar to that revealed by immunocytochemical and autoradiographic methods (Balogh et al., 1970; Imamura and Adams, 2003a; von Ilberg et al., 1971). We also observed uptake, retention and clearance of GTTR at other anatomical locations. No indications of cellular damage or cell death could be detected using the fluorescent probes (GTTR or Alexa-488-phalloidin) used in this acute in vivo study. Indeed, aminoglycosides are detected in hair cells before functional changes can be measured (Aran et al., 1999; Hiel et al., 1992a).
4.1. Specificity of GTTR for gentamicin-binding sites
Previous studies have shown that the intracellular distribution of GTTR is almost identical to that of immunolabeled gentamicin in both kidney cells and bullfrog saccular hair cells (Myrdal et al., 2005b; Steyger et al., 2003). Those studies also revealed that gentamicin immunolabeling often only labeled a subset of gentamicin binding sites, as gentamicin bound to nucleolar RNA is typically not labeled by antibodies. Furthermore, GTTR binding can be competed away by unconjugated gentamicin, demonstrating that GTTR (and gentamicin) uptake and binding is saturable; this is a hallmark for the bio-relevance of any biological tracer (Myrdal et al., 2005b). Nonetheless, the tracer may have different pharmacokinetic properties compared to the native molecule. Hydrolyzed Texas Red was not observed in any of the tissues examined; and faint autofluorescence observed in specified locations were omitted from descriptions of specific GTTR uptake.
4.2. Bullfrog saccule
Hair cells in the central regions of the saccule are typically functionally mature. At the saccular periphery, there is a perimeter of 2−3 rows of immature hair cells, considered to be precursors of the more central mature hair cells (Li and Lewis, 1979). The distribution of GTTR in mature hair cells in vivo resembled that seen in saccular explants (Steyger et al., 2003). The appearance (<30 min) of punctate (endosomal) fluorescence in mature bullfrog hair cells was more rapid than in either avian or mammalian hair cells (≥6 h), reflecting either weak blood–labyrinth barrier (BLB) function, and/or high serum levels of GTTR.
In vivo, GTTR uptake was more prominent in mature hair cells, mirroring the pattern of more rapid aminoglycoside-induced cell death by mature hair cells compared to immature hair cells (Baird et al., 1996). The weaker uptake of GTTR in many peripheral, immature hair cells in vivo may also account for enhanced resistance of regenerating/immature hair cells to aminoglycoside toxicity (Baird et al., 1996; Hashino and Salvi, 1996). In explants, punctuate GTTR fluorescence in peripheral hair cells is much more intense compared to mature hair cells (Steyger et al., 2003). This may be due to increased endocytotic activity in following explantation and immersion in culture media, as observed in other explantation protocols (Stanislawski et al., 1997).
4.3. Chicks
In chicks, a descending proximo-distal gradient of GTTR uptake, particularly in hair cells along the neural edge, is similar to the pattern of avian hair cell death following aminoglycoside treatment (Bhave et al., 1995; Cotanche et al., 1994; Hashino et al., 1995b). Recent studies show that noise-trauma can induce avian hair cell death processes within 3−6 h of insult (Mangiardi et al., 2004). However, aminoglycosides also induce these same hair cell death processes 9−12 h after a single injection (Mangiardi et al., 2004). GTTR was first detected in the chick basilar papilla only 6 h post-injection. Thus, aminoglycoside-induced avian hair cell death processes are activated in a similar time frame after its initial appearance of the ototoxin in the inner ear to that seen for noise trauma (3−6 h). This time-delay between injection and initial detection of GTTR in avian hair cells may reflect phylogenetic differences in serum levels and clearance of aminoglycosides, drug transport, the inner ear vasculature, and/or the integrity of the blood–labyrinth barrier of chicks compared to amphibians and mammals.
4.4. Guinea pigs
Diffuse GTTR fluorescence in hair cells and pillar cells was observed with a slight decreasing baso-apical gradient in guinea pig cochleae 3 h post-injection. However, this apparent fluorescent gradient was not discernable after 24 h, corroborating immunoenzymatic studies (Imamura and Adams, 2003a). This distribution is contrary to other reports that used multiple injections of gentamicin, e.g. (Hiel et al., 1992a), that might alter the dynamics of gentamicin uptake following successive doses (Imamura and Adams, 2003a), for example increasing cation channel expression (Kitahara et al., 2005) that could facilitate drug uptake.
At early time points (<3 h), in basal coils of guinea pig (and murine) cochleae, intracellular GTTR fluorescence was typically diffuse, particularly in OHCs, suggestive of nonendocytotic uptake (Myrdal et al., 2005b; Steyger et al., 2003; Wedeen et al., 1983). Although the molecular weight of GTTR is approximately 1100 MW, other large organic and fluorescent molecules have been shown to permeate cation channels and enter a variety of mechanosensory cells, including the mechano-electrical transduction channel at the tips of stereocilia (Gale et al., 2001; Marcotti et al., 2005; Meyers et al., 2003). GTTR also enters kidney cells via nonendocytotic mechanisms (Myrdal and Steyger, 2005a; Myrdal et al., 2005b).
Diffuse GTTR fluorescence was also visible in hair cells at later time points, along with intense punctate labeling in the cell body 6 h after injection. It is possible that endocytosed GTTR may be present at earlier time points at sub-resolution and at intensities below that of diffuse cytoplasmic fluorescence. However, it is more likely that the appearance of punctate fluorescence is a time-dependent event via endocytosis (Myrdal et al., 2005b). The subcompartments represented by the punctate GTTR labeling are likely to include endosomes, lysosomes, mitochondria, ER, endosomes, and Golgi bodies (Ding et al., 1995; Hashino et al., 1997; Sandoval et al., 2000; Steyger et al., 2003).
Initial studies of tritiated aminoglycoside uptake showed rapid uptake in endocochlear tissues, cells, and fluids within 2 h (Balogh et al., 1970; Tran Ba Huy et al., 1986; Tran Ba Huy et al., 1983; von Ilberg et al., 1971). Tritiated aminoglycosides cannot distinguish between cytoplasmic and organelle uptake, except by electron microscopy (Hashino et al., 1997; Wedeen et al., 1983), precluding the analysis of subcellular distribution of aminoglycoside uptake in large numbers of individual cell types. Thus, high resolution confocal microscopy of GTTR fluorescence in wholemounted cochlear surface preparations may offer greater sensitivity for differentiating between cytoplasmic and endocytotic aminoglycoside uptake in large numbers of cells compared to autoradiography of resin-embedded, sectioned material.
GTTR is associated with the hair bundles of all species examined, and the lateral membranes of guinea pig and murine OHCs. Confocal microscopy is unable to resolve whether this GTTR labeling is binding to the glycocalyx or other outer membranous structures of the cell membrane from that within the inner leaflet of the cell membrane, or cortical cytoplasm (Au et al., 1987; Marche et al., 1987; Richardson et al., 1989). Aminoglycosides are known to bind strongly to phosphoinositides (e.g., PIP2, etc.) that are constitutive components of all membranes (Schacht, 1979). Thus GTTR binding to membranous-rich structures of hair cells like the hair bundle, and potentially the OHC sub-surface cisternae along the lateral membrane is not surprising.
4.5. Mice
Intensity differences between basal and apical cochlear surface preparations of 21−28 day mice were obscured by autofluorescence in apical OHCs, which is attributed to age-related pigments (lipofuscin; emission peak at ∼600 nm) that accumulate in post-mitotic cells (Porta, 2002), close to that of Texas Red. At later time points (>24 h), intensity differences between apical and basal coils were not distinguishable, as with guinea pig cochleae using GTTR or immuno-enzymatic methods (Imamura and Adams, 2003a).
Wu et al. (2001) demonstrated that higher doses of aminoglycosides (per unit bodyweight) are required to induce ototoxicity in mice compared to other rodents. They attributed this to increased pharmacokinetics of aminoglycoside elimination from serum compared to guinea pigs. In addition, in mice, higher doses did not lead to increased serum levels compared to other rodents (Wu et al., 2001).
We found that several daily doses of gentamicin were required to observe even moderate levels of GTTR fluorescence in hair cells of 21−28-day mice (compared to guinea pigs). This may be due to differences in levels of, or clearance of, aminoglycosides in serum. When the murine blood–labyrinth barrier was either immature (6-day mice) or compromised by ethacrynic acid, we saw elevated levels of GTTR uptake in hair cells compared to those from (untreated) 21−28 day mice. This suggests that a mature, and intact blood–labyrinth barrier is effective at reducing the uptake of aminoglycosides by hair cells in adult mice. Nonetheless, aminoglycosides were still able to cross the intact blood–labyrinth barrier in vivo and enter both cytoplasmic and endocytotic domains of adult cochlear hair cells, unlike FM1−43 (Meyers et al., 2003).
4.6. Non-sensory cells in the cochlea
Deiter's cell phalanges and pillar cells did take up diffuse GTTR fluorescence within 3 h, corroborating earlier studies (de Groot et al., 1990; Imamura and Adams, 2003a; von Ilberg et al., 1971). In guinea pigs, pillar cells are more susceptible to aminoglycoside toxicity than other supporting cells (Ryan et al., 1980; Steyger, 1991). The diffuse GTTR labeling in supporting cell phalanges is also co-located with actiniferous phalloidin labeling. However, these actiniferous structures, and others like stereocilia or tight junctions, do not show artefactual fluorescence in the Texas Red channel when labeled with fluorescent phalloidin in control tissues (see Fig. 1). This may indicate an interaction between GTTR and aminoglycoside-binding components that can regulate the dynamics of these actiniferous structures, e.g. PIP2 (Lee and Rhee, 1995; Slepecky and Chamberlain, 1983; Suchy and Nussbaum, 2002).
GTTR generally did not rapidly infiltrate the remaining supporting cells (compared to Deiter's, pillar and hair cells). This is probably due to a general paucity of endocytotic activity and/or cation channels. Alternatively, these supporting cells may have an as-yet-unidentified drug clearance mechanism to pump GTTR out of the cytoplasm, similar to p-glycoprotein product of the multi-drug resistance gene (Zhang et al., 2000).
GTTR uptake in the stria vascularis at early bit not later time points also corroborated previous reports (Balogh et al., 1970; Imamura and Adams, 2003a), suggesting either low levels of uptake or rapid extrusion. Although not considered a primary target of aminoglycosides, the lateral wall and stria vascularis is subject to cytotoxicity only during chronic gentamicin treatment (Forge and Fradis, 1985; Forge et al., 1987). Ethacrynic acid greatly enhanced uptake of GTTR around the strial capillaries, and hair cells, correlating with reports of enhanced aminoglycoside-induced hair cell death after ethacrynic acid treatment in vivo (Brummett, 1981; Fox and Brummett, 1979; Rybak, 1982). The mechanism for this enhanced GTTR uptake remains to be determined (Ding et al., 2003; Tran Ba Huy et al., 1983), as the physical integrity of the BLB remains intact following loop diuretic treatment (Duvall and Robinson, 1989; Naito and Watanabe, 1997; Syka and Melichar, 1985).
4.7. Kidney
Rapid and sustained GTTR uptake in proximal tubule cells (<3 h), but not in glomerular or distal tubule cells, also corroborated previous in vivo studies (Dunn et al., 2002). In vitro, both proximal and distal tubule cells take up GTTR (Myrdal and Steyger, 2005a; Myrdal et al., 2005b). The paucity of GTTR labeling in distal tubule cells in vivo suggests either that: (i) GTTR uptake by proximal tubule cells reduces the availability of GTTR for distal tubule cells, or (ii) distal tubule cells are able to rapidly clear (remove) GTTR from its cytoplasmic domains, unlike proximal tubule cells which retain GTTR, or (iii) the electro-physiological conditions in the nephron lumen changes between the proximal and distal sectors and may no longer favor GTTR uptake by distal tubule cells (Goodman et al., 2001).
4.8. Other sensory locations and mechanisms of uptake
GTTR uptake in linguinal taste buds, and vibrissae corroborate FM-143 studies (Meyers et al., 2003). Like kidney cells and hair cells, linguinal taste buds also express a variety of TRP channels (Hofmann et al., 2003; Macpherson et al., 2005) that may be aminoglycoside-permissive. However, GTTR fluorescence cleared over time, almost to background levels by 7 days, unlike FM1−43 studies, indicating an unidentified aminoglycoside clearance mechanism. In contrast, hair cells, kidney cells, and in the neuronal cell bodies of the TG and DRG all retained GTTR for several days. Indeed, aminoglycosides are retained by outer hair cells for 6 months or longer (Aran et al., 1999; Dulon et al., 1993; Imamura and Adams, 2003a).
Neurons in the TG extend dendrites to sensory endings in the facial areas, including the vibrissae. Three hours after injection, little labeling is seen in these neuronal cell bodies, but by 24 and 72 h after injection, these same soma contain and retain GTTR fluorescence. This time-course is similar to that of FM1−43 in the TG, and is suggestive of retrograde transport from sensory endings (Meyers et al., 2003). When the infra-orbital nerve (a branch of the TG) was cut and ligated, little GTTR fluorescence can be seen in the TG, further supporting a retrograde transport hypothesis.
GTTR uptake in neurons of different diameters in the DRG also corroborated FM1−43 studies (Meyers et al., 2003). Small diameter neurons in the DRG (and TG) with thermosensitive and nocioreceptive sensory modalities express TRPV1 (Caterina et al., 1997). However, many large diameter neurons, with proprioreceptive sensory modalities, also take up GTTR, and FM1−43, and probably express other TRP channels, e.g., TRPV3 (Meyers et al., 2003; Xu et al., 2002), that may also be aminoglycoside-permissive. Electrophysiological studies also report aminoglycoside blockade of DRG neurons that may account for cases of aminoglycoside-induced analgesia of pain receptors (Raisinghani and Premkumar, 2005; Zhou and Zhao, 2002; Zhou et al., 2001). Hair cells and kidney cells also express a variety of transient receptor potential (TRP) channels which allow the uptake of large organic molecules, including aminoglycosides (Corey et al., 2004; Hellwig et al., 2004; Liedtke et al., 2000; Marcotti et al., 2005; Meyers et al., 2003; Raisinghani and Premkumar, 2005; Strotmann et al., 2000; Xu et al., 2002; Zheng et al., 2003). Thus, the uptake of GTTR into cells through aminoglycoside-permissive cation channels is likely to lead to diffuse cytoplasmic labeling. Such cytoplasmic uptake would be in addition to endocytotic uptake (Hashino et al., 1997; Myrdal et al., 2005b; Sandoval et al., 2000).
Following sub-cutaneous injection, GTTR, a fluorescently-tagged aminoglycoside, is transported through the body and is taken up and retained by a variety of vertebrate sensory, kidney and neuronal cells in vivo hair cells. The in vivo distribution of GTTR is similar to that of FM1−43 (Meyers et al., 2003), which is rapidly taken up through mechanosensitive channels, particularly TRP channels. In addition, GTTR is able to traverse the blood–labyrinth barrier for uptake by hair cells in the adult (murine) cochlea, unlike FM1−43, and thus may be useful for pharmacokinetic and fluorescent microscopic studies of aminoglycoside entry into cochlear fluids, tissues and cells.
Acknowledgements
Funded by NIDCD 04555 (PSS); NIDCD 01689 and DRF (DAC). The authors are grateful to Dennis Trune for discussions related this manuscript.
Abbreviations
- EA
ethacrynic acid
- FA
4% formaldehyde
- GT
gentamicin
- GTTR
gentamicin conjugated to Texas Red
- IHC(s)
inner hair cell(s)
- N.A.
numerical aperture
- OHC(s)
outer hair cell(s)
- PBS
phosphate buffered saline
- PC
pillar cells
- PIP2
phosphatidylinositol-4, 5-bisphosphate
- RNA
ribonucleic acid
- TR
Texas Red
References
- Aran JM, Erre JP, Lima da Costa D, Debbarh I, Dulon D. Acute and chronic effects of aminoglycosides on cochlear hair cells. Ann. NY Acad. Sci. 1999;884:60–68. doi: 10.1111/j.1749-6632.1999.tb08636.x. [DOI] [PubMed] [Google Scholar]
- Au S, Weiner ND, Schacht J. Aminoglycoside antibiotics preferentially increase permeability in phosphoinositide-containing membranes: a study with carboxyfluorescein in liposomes. Biochim. Biophys. Acta. 1987;902:80–86. doi: 10.1016/0005-2736(87)90137-4. [DOI] [PubMed] [Google Scholar]
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann. NY Acad. Sci. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- Balogh K, Jr., Hiraide F, Ishii D. Distribution of radioactive dihydrostreptomycin in the cochlea. An autoradiographic study. Ann. Otol. Rhinol. Laryngol. 1970;79:641–652. doi: 10.1177/000348947007900329. [DOI] [PubMed] [Google Scholar]
- Beaubien AR, Ormsby E, Bayne A, Carrier K, Crossfield G, Downes M, Henri R, Hodgen M. Evidence that amikacin ototoxicity is related to total perilymph area under the concentration-time curve regardless of concentration. Antimicrob. Agents Chemother. 1991;35:1070–1074. doi: 10.1128/aac.35.6.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave SA, Stone JS, Rubel EW, Coltrera MD. Cell cycle progression in gentamicin-damaged avian cochleas. J. Neurosci. 1995;15:4618–4628. doi: 10.1523/JNEUROSCI.15-06-04618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett RE. Effects of antibiotic–diuretic interactions in the guinea pig model of ototoxicity. Rev. Infect. Dis. 1981;3(suppl):S216–S223. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear. Res. 1996;98:116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Geleoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Lee KH, Stone JS, Picard DA. Hair cell regeneration in the bird cochlea following noise damage or ototoxic drug damage. Anat. Embryol. 1994;189:1–18. doi: 10.1007/BF00193125. [DOI] [PubMed] [Google Scholar]
- de Groot JC, Meeuwsen F, Ruizendaal WE, Veldman JE. Ultrastructural localization of gentamicin in the cochlea. Hear. Res. 1990;50:35–42. doi: 10.1016/0378-5955(90)90031-j. [DOI] [PubMed] [Google Scholar]
- Ding D, Jin X, Zhao J. Accumulation sites of kanamycin in cochlear basal membrane cells. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1995;30:323–325. [PubMed] [Google Scholar]
- Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear. Res. 2002;164:115–126. doi: 10.1016/s0378-5955(01)00417-8. [DOI] [PubMed] [Google Scholar]
- Ding D, McFadden SL, Browne RW, Salvi RJ. Late dosing with ethacrynic acid can reduce gentamicin concentration in perilymph and protect cochlear hair cells. Hear. Res. 2003;185:90–96. doi: 10.1016/s0378-5955(03)00258-2. [DOI] [PubMed] [Google Scholar]
- Dulon D, Zajic G, Aran JM, Schacht J. Aminoglycoside antibiotics impair calcium entry but not viability and motility in isolated cochlear outer hair cells. J. Neurosci. Res. 1989;24:338–346. doi: 10.1002/jnr.490240226. [DOI] [PubMed] [Google Scholar]
- Dulon D, Hiel H, Aurousseau C, Erre JP, Aran JM. Pharmacokinetics of gentamicin in the sensory hair cells of the organ of Corti: rapid uptake and long term persistence. C. R. Acad. Sci. III. 1993;316:682–687. [PubMed] [Google Scholar]
- Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am. J. Physiol. Cell Physiol. 2002;283:C905–C916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- Duvall AJ, 3rd, Robinson KS. Cochlear vessel permeability to horseradish peroxidase after diuretic administration in the chinchilla. Acta Otolaryngol. 1989;108:397–403. doi: 10.3109/00016488909125545. [DOI] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus). J. Am. Audiol. Soc. 1976;1:179–184. [PubMed] [Google Scholar]
- Forge A, Fradis M. Structural abnormalities in the stria vascularis following chronic gentamicin treatment. Hear. Res. 1985;20:233–244. doi: 10.1016/0378-5955(85)90028-0. [DOI] [PubMed] [Google Scholar]
- Forge A, Wright A, Davies SJ. Analysis of structural changes in the stria vascularis following chronic gentamicin treatment. Hear. Res. 1987;31:253–265. doi: 10.1016/0378-5955(87)90195-x. [DOI] [PubMed] [Google Scholar]
- Fox KE, Brummett RE. The relationship between the cytotoxicity of kanamycin and ethacrynic acid for mammalian cells in vitro and their ototoxicity in vivo. Acta Otolaryngol. (Stockh) 1979;87:72–78. doi: 10.3109/00016487909126389. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1−43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LS, Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman's the pharmacological basis of therapeutics. 10th ed. McGraw-Hill; New York: 2001. [Google Scholar]
- Hackney CM, Furness DN, Steyger PS. Dallos P, Geisler CD, Matthews JW, Ruggero M, Steele CR, editors. Structural abnormalities in inner hair cells following kanamycin-induced outer hair cell loss. Mechanics and Biophysics of Hearing, Lecture Notes in Biomathematics. 1990. pp. 10–17.
- Hashino E, Shero M. Endocytosis of aminoglycoside antibiotics in sensory hair cells. Brain Res. 1995a;704:135–140. doi: 10.1016/0006-8993(95)01198-6. [DOI] [PubMed] [Google Scholar]
- Hashino E, Salvi RJ. Regenerated hair cells exhibit a transient resistance to aminoglycoside toxicity. Brain Res. 1996;720:172–182. doi: 10.1016/0006-8993(95)01467-5. [DOI] [PubMed] [Google Scholar]
- Hashino E, TinHan EK, Salvi RJ. Base-to-apex gradient of cell proliferation in the chick cochlea following kanamycin-induced hair cell loss. Hear. Res. 1995b;88:156–168. doi: 10.1016/0378-5955(95)00109-h. [DOI] [PubMed] [Google Scholar]
- Hashino E, Shero M, Salvi RJ. Lysosomal targeting and accumulation of aminoglycoside antibiotics in sensory hair cells. Brain Res. 1997;777:75–85. doi: 10.1016/s0006-8993(97)00977-3. [DOI] [PubMed] [Google Scholar]
- Hashino E, Shero M, Salvi RJ. Lysosomal augmentation during aminoglycoside uptake in cochlear hair cells. Brain Res. 2000;887:90–97. doi: 10.1016/s0006-8993(00)02971-1. [DOI] [PubMed] [Google Scholar]
- Hayashida T, Hiel H, Dulon D, Erre JP, Guilhaume A, Aran JM. Dynamic changes following combined treatment with gentamicin and ethacrynic acid with and without acoustic stimulation. Cellular uptake and functional correlates. Acta Otolaryngol. (Stockh) 1989;108:404–413. doi: 10.3109/00016488909125546. [DOI] [PubMed] [Google Scholar]
- Hellwig N, Plant TD, Janson W, Schafer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J. Biol. Chem. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- Hiel H, Bennani H, Erre JP, Aurousseau C, Aran JM. Kinetics of gentamicin in cochlear hair cells after chronic treatment. Acta Otolaryngol. 1992a;112:272–277. doi: 10.1080/00016489.1992.11665417. [DOI] [PubMed] [Google Scholar]
- Hiel H, Schamel A, Erre JP, Hayashida T, Dulon D, Aran JM. Cellular and subcellular localization of tritiated gentamicin in the guinea pig cochlea following combined treatment with ethacrynic acid. Hear. Res. 1992b;57:157–165. doi: 10.1016/0378-5955(92)90148-g. [DOI] [PubMed] [Google Scholar]
- Hirose K, Westrum LE, Stone JS, Zirpel L, Rubel EW. Dynamic studies of ototoxicity in mature avian auditory epithelium. Ann. NY Acad. Sci. 1999;884:389–409. doi: 10.1111/j.1749-6632.1999.tb08657.x. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr. Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Imamura S, Adams JC. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J. Assoc. Res. Otolaryngol. 2003a;4:176–195. doi: 10.1007/s10162-002-2036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S, Adams JC. Changes in cytochemistry of sensory and nonsensory cells in gentamicin-treated cochleas. J. Assoc. Res. Otolaryngol. 2003b;4:196–218. doi: 10.1007/s10162-002-2037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death and Differentiation. doi: 10.1038/sj.cdd.4401706. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlmeter G, Dahlager JI. Aminoglycoside toxicity—a review of clinical studies published between 1975 and 1982. J. Antimicrob. Chemother. 1984;13(Suppl A):9–22. doi: 10.1093/jac/13.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Li HS, Balaban CD. Changes in transient receptor potential cation channel superfamily V (TRPV) mRNA expression in the mouse inner ear ganglia after kanamycin challenge. Hear. Res. 2005;201:132–144. doi: 10.1016/j.heares.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Kroese AB, Das A, Hudspeth AJ. Blockage of the transduction channels of hair cells in the bullfrog's sacculus by aminoglycoside antibiotics. Hear. Res. 1989;37:203–217. doi: 10.1016/0378-5955(89)90023-3. [DOI] [PubMed] [Google Scholar]
- Lee SB, Rhee SG. Significance of PIP2 hydrolysis and regulation of phospholipase C isozymes. Curr. Opin. Cell Biol. 1995;7:183–189. doi: 10.1016/0955-0674(95)80026-3. [DOI] [PubMed] [Google Scholar]
- Li CW, Lewis ER. Structure and development of vestibular hair cells in the larval bullfrog. Ann. Otol. Rhinol. Laryngol. 1979;88:427–437. doi: 10.1177/000348947908800323. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Mangiardi DA, McLaughlin-Williamson K, May KE, Messana EP, Mountain DC, Cotanche DA. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J. Comp. Neurol. 2004;475:1–18. doi: 10.1002/cne.20129. [DOI] [PubMed] [Google Scholar]
- Marche P, Olier B, Girard A, Fillastre JP, Morin JP. Aminoglycoside-induced alterations of phosphoinositide metabolism. Kidney Int. 1987;31:59–64. doi: 10.1038/ki.1987.9. [DOI] [PubMed] [Google Scholar]
- Marcotti W, van Netten S, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters hair cells through the mechano-electrical transducer channels. J. Physiol. 2005 doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1−43 loading of sensory cells through nonselective ion channels. J. Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrdal SE, Steyger PS. TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hear. Res. 2005a;204:170–182. doi: 10.1016/j.heares.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrdal SE, Johnson KC, Steyger PS. Cytoplasmic and intranuclear binding of gentamicin does not require endocytosis. Hear. Res. 2005b;204:156–169. doi: 10.1016/j.heares.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito H, Watanabe K. Alteration in capillary permeability of horseradish peroxidase in the stria vascularis and movement of leaked horseradish peroxidase after administration of furosemide. ORL J. Otorhinolaryngol. Relat. Spec. 1997;59:248–257. doi: 10.1159/000276948. [DOI] [PubMed] [Google Scholar]
- Nonclercq D, Wrona S, Toubeau G, Zanen J, Heuson-Stiennon JA, Schaudies RP, Laurent G. Tubular injury and regeneration in the rat kidney following acute exposure to gentamicin: a time-course study. Ren. Fail. 1992;14:507–521. doi: 10.3109/08860229209047660. [DOI] [PubMed] [Google Scholar]
- Porta EA. Pigments in aging: an overview. Ann. NY Acad. Sci. 2002;959:57–65. doi: 10.1111/j.1749-6632.2002.tb02083.x. [DOI] [PubMed] [Google Scholar]
- Portmann M, Darrouzet J, Coste C. Distribution within the cochlea of dihydrostreptomycin injected into the circulation. An autoradiographic and electron microscopic study. Arch Otolaryngol. 1974;100:473–475. doi: 10.1001/archotol.1974.00780040487014. [DOI] [PubMed] [Google Scholar]
- Raisinghani M, Premkumar LS. Block of native and cloned vanilloid receptor 1 (TRPV1) by aminoglycoside antibiotics. Pain. 2005;113:123–133. doi: 10.1016/j.pain.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Richardson GP, Russell IJ, Wasserkort R, Hans M. Amino-glycoside antibiotics and lectins cause irreversible increases in the stiffness of cochlear hair-cell stereocilia. In: Wilson JP, Kemp DT, editors. Cochlear Mechanisms—Structure, Function and Models. Plenum Press; New York: 1989. pp. 566–578. [Google Scholar]
- Richardson GP, Forge A, Kros CJ, Fleming J, Brown SD, Steel KP. Myosin VIIA is required for aminoglycoside accumulation in cochlear hair cells. J. Neurosci. 1997;17:9506–9519. doi: 10.1523/JNEUROSCI.17-24-09506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AF, Woolf NK, Bone RC. Ultrastructural correlates of selective outer hair cell destruction following kanamycin intoxication in the chinchilla. Hear. Res. 1980;3:335–351. doi: 10.1016/0378-5955(80)90027-1. [DOI] [PubMed] [Google Scholar]
- Rybak LP. Pathophysiology of furosemide ototoxicity. J. Otolaryngol. 1982;11:127–133. [PubMed] [Google Scholar]
- Sandoval R, Leiser J, Molitoris BA. Aminoglycoside antibiotics traffic to the Golgi complex in LLC-PK1 cells. J. Am. Soc. Nephrol. 1998;9:167–174. doi: 10.1681/ASN.V92167. [DOI] [PubMed] [Google Scholar]
- Sandoval RM, Dunn KW, Molitoris BA. Gentamicin traffics rapidly and directly to the Golgi complex in LLC-PK(1) cells. Am. J. Physiol. Renal Physiol. 2000;279:F884–F890. doi: 10.1152/ajprenal.2000.279.5.F884. [DOI] [PubMed] [Google Scholar]
- Schacht J. Isolation of an aminoglycoside receptor from guinea pig inner ear tissues and kidney. Arch. Otorhinolaryngol. 1979;224:129–134. doi: 10.1007/BF00455236. [DOI] [PubMed] [Google Scholar]
- Shnerson A, Pujol R. Age-related changes in the C57BL/6J mouse cochlea. I. Physiological findings. Brain Res. 1981;254:65–75. doi: 10.1016/0165-3806(81)90059-6. [DOI] [PubMed] [Google Scholar]
- Slepecky N, Chamberlain SC. Distribution and polarity of actin in inner ear supporting cells. Hear. Res. 1983;10:359–370. doi: 10.1016/0378-5955(83)90098-9. [DOI] [PubMed] [Google Scholar]
- Staecker H, Dazert S, Malgrange B, Lefebvre PP, Ryan AF, Van de Water TR. Transforming growth factor alpha treatment alters intracellular calcium levels in hair cells and protects them from ototoxic damage in vitro. Int. J. Dev. Neurosci. 1997;15:553–562. doi: 10.1016/s0736-5748(96)00110-4. [DOI] [PubMed] [Google Scholar]
- Stanislawski L, Carreau JP, Pouchelet M, Chen ZH, Goldberg M. In vitro culture of human dental pulp cells: some aspects of cells emerging early from the explant. Clin. Oral. Investig. 1997;1:131–140. doi: 10.1007/s007840050024. [DOI] [PubMed] [Google Scholar]
- Steyger PS. Ph.D. Thesis. Keele University; U.K: 1991. Ultrastructural and immunohistochemical studies of cytoskeletal features in the guinea pig organ of Corti. [Google Scholar]
- Steyger PS, Peters SL, Rehling J, Hordichok A, Dai CF. Uptake of gentamicin by bullfrog saccular hair cells in vitro. J. Assoc. Res. Otolaryngol. 2003;4:565–578. doi: 10.1007/s10162-003-4002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell. Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Suchy SF, Nussbaum RL. The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am. J. Hum. Genet. 2002;71:1420–1427. doi: 10.1086/344517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J, Melichar I. The effect of loop diuretics upon summating potentials in the guinea pig. Hear. Res. 1985;20:267–273. doi: 10.1016/0378-5955(85)90031-0. [DOI] [PubMed] [Google Scholar]
- Tran Ba Huy P, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. J. Clin. Invest. 1986;77:1492–1500. doi: 10.1172/JCI112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Ba Huy P, Manuel C, Meulemans A, Sterkers O, Wassef M, Amiel C. Ethacrynic acid facilitates gentamicin entry into endolymph of the rat. Hear. Res. 1983;11:191–202. doi: 10.1016/0378-5955(83)90078-3. [DOI] [PubMed] [Google Scholar]
- von Ilberg C, Spoendlin H, Arnold W. Autoradiographical distribution of locally applied dihydrostreptomycin in the inner ear. Acta Otolaryngol. 1971;71:159–165. doi: 10.3109/00016487109125345. [DOI] [PubMed] [Google Scholar]
- Wedeen RP, Batuman V, Cheeks C, Marquet E, Sobel H. Transport of gentamicin in rat proximal tubule. Lab. Invest. 1983;48:212–223. [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear. Res. 2001;158:165–178. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- Xie Y, Nishi S, Iguchi S, Imai N, Sakatsume M, Saito A, Ikegame M, Iino N, Shimada H, Ueno M, Kawashima H, Arakawa M, Gejyo F. Expression of osteopontin in gentamicininduced acute tubular necrosis and its recovery process. Kidney Int. 2001;59:959–974. doi: 10.1046/j.1523-1755.2001.059003959.x. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos- Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear. Res. 2002;163:71–81. doi: 10.1016/s0378-5955(01)00380-x. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Saito T, Kimura Y, Sugimoto C, Ohtsubo T, Saito H. Disruption of mdr1a p-glycoprotein gene results in dysfunction of blood–inner ear barrier in mice. Brain Res. 2000;852:116–126. doi: 10.1016/s0006-8993(99)02223-4. [DOI] [PubMed] [Google Scholar]
- Zheng J, Dai C, Steyger PS, Kim Y, Vass Z, Ren T, Nuttall AL. Vanilloid receptors in hearing: altered cochlear sensitivity by vanilloids and expression of TRPV1 in the organ of corti. J. Neurophysiol. 2003;90:444–455. doi: 10.1152/jn.00919.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhao ZQ. Effects of neomycin on high-threshold Ca(2+) currents and tetrodotoxin-resistant Na(+) currents in rat dorsal root ganglion neuron. Eur. J. Pharmacol. 2002;450:29–35. doi: 10.1016/s0014-2999(02)02050-2. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhou ZS, Zhao ZQ. Neomycin blocks capsaicin-evoked responses in rat dorsal root ganglion neurons. Neurosci. Lett. 2001;315:98–102. doi: 10.1016/s0304-3940(01)02356-4. [DOI] [PubMed] [Google Scholar]
- Zierhut G, Piepersberg W, Bock A. Comparative analysis of the effect of aminoglycosides on bacterial protein synthesis in vitro. Eur. J. Biochem. 1979;98:577–583. doi: 10.1111/j.1432-1033.1979.tb13219.x. [DOI] [PubMed] [Google Scholar]


