Abstract
Cultured primary neurons are a well established model for the study of neuronal function in vitro. Here we demonstrated that stable isotope labeling by amino acids in cell culture (SILAC) can be applied to a differentiated, non-dividing cell type such as primary neurons, and we applied this technique to assess changes in the neuronal phosphotyrosine proteome in response to stimulation by brain-derived neurotrophic factor (BDNF), an important molecule for the development and regulation of neuronal connections. We found that 13 proteins had SILAC ratios above 1.50 or below 0.67 in phosphotyrosine immunoprecipitations comparing BDNF-treated and control samples, and an additional 18 proteins had ratios above 1.25 or below 0.80. These proteins include TrkB, the receptor tyrosine kinase for BDNF, and others such as hepatocyte growth factor-regulated tyrosine kinase substrate and signal-transducing adaptor molecule, which are proteins known to regulate intracellular trafficking of receptor tyrosine kinases. These results demonstrate that the combination of primary neuronal cell culture and SILAC can be a powerful tool for the study of the proteomes of neuronal molecular and cellular dynamics.
Primary culture usually refers to differentiated cells that are harvested from a living organism and maintained in culture (1). Dissociated embryonic neuronal cell culture is one of many primary culture systems researchers use to create model systems that more closely resemble in vivo biology, cellular makeup, and complexity than do immortalized cell lines (2). Compared with the intact organ or slice cultures, dissociated primary neuronal cell cultures represent a simplified model that allows evaluation, in a specific cell type, of features such as cell morphology, energy metabolism, signal transduction, and neurotransmitter release (2–4).
In combination with mass spectrometry, SILAC1 can be an effective means for characterization of cellular signaling and protein-protein interactions (5–7). In a SILAC experiment, cells representing different biological conditions are grown in media supplemented with either “light” or “heavy” isotope-containing amino acids. Metabolic incorporation of labeled amino acids into all proteins from cells of one population and subsequent combination of differentially labeled samples in equal ratios enables quantification of proteins from each sample based on the intensities of the corresponding differentially labeled peptides. In the same mass spectrometric experiment, MS/MS can be carried out to obtain sequence information for protein identification (for a review, see Ref. 7).
The advantages of combining primary neuronal culture and SILAC (primary neuronal SILAC) are significant because this approach allows the expansion to a proteomics scale of established biochemical and cell biological experiments that are frequently used to address neuroscience problems. In a “standard” SILAC experiment, labeling of cells over five or more cell divisions ensures that even very stable proteins achieve nearly complete (97% or more) incorporation of labeled amino acids (8, 9). To assure that SILAC could be applied to non-dividing neurons, which do not have the advantage of label dilution effects enjoyed by dividing cells, we tracked the levels of label incorporation for individual proteins and showed that the majority of proteins do indeed achieve significant levels of stable isotope labeling. Here we demonstrate the first application of this approach, and use it to assess BDNF-dependent phosphotyrosine-associated signaling events in dissociated neuronal cultures prepared from embryonic rat cortex and hippocampus.
BDNF is a member of the neurotrophin family of protein growth factors. These proteins function as survival factors that ensure a match between surviving neurons and appropriate targets during development of the mammalian nervous system (10). They also regulate many other aspects of neural function such as cell fate decisions, axon growth, dendrite pruning, long term potentiation, synaptic plasticity, and the expression of proteins crucial for normal neuronal function, such as neurotransmitters and ion channels (10–13). BDNF-dependent phosphotyrosine-associated signaling events are primarily transmitted through the TrkB receptor tyrosine kinase (14). To assess BDNF-dependent phosphotyrosine-associated signaling events, we performed an LC-MS/MS analysis of Tyr(P) IPs from combined lysates of BDNF-treated and control neurons (Fig. 1). These experiments resulted in the identification of 13 proteins that were differentially immunoprecipitated (SILAC ratios above 1.5 or below 0.67) and 18 potential changers (SILAC ratios above 1.25 and below 0.80), suggesting BDNF-dependent changes in tyrosine phosphorylation state or association with a tyrosine phosphorylated protein. Proteins with changing abundances included a number of known and potentially novel pathway participants. We validated the SILAC results for six of these proteins with Western blot time course experiments. Two proteins with increasing abundance in Tyr(P) IPs after BDNF stimulation, Hrs and STAM, were further investigated through biochemical and cell biological approaches. The results suggested a role for the Hrs/STAM-mediated RTK degradative sorting pathway in neurons.
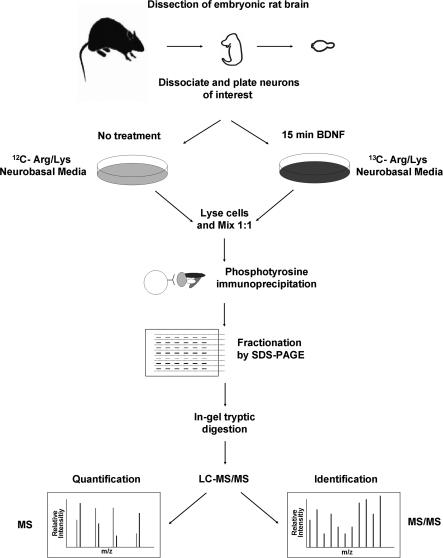
Fig. 1.
Primary neuronal SILAC experimental design. Embryos were removed at DIV 18, their brains were dissected, and the cortexes and/or hippocampi were removed. Neurons were then dissociated and plated in control or labeling medium and allowed to incorporate label for 10 days. Cells were then treated with ligand for 15 min and mixed 1:1 with untreated control cells from the same dissection, and Tyr(P) IPs were performed. Tyr(P) IP eluates were fractionated via SDS-PAGE and digested with trypsin in gel, and the tryptic digests were analyzed by LC-MS/MS to both identify and quantify those proteins present.
EXPERIMENTAL PROCEDURES
Cell Culture and Metabolic Labeling—
Preparation of cortical and hippocampal cell cultures has been described previously (15, 16) (refer to supplemental experimental procedures section for details). Cells were cultured in Neurobasal medium (Invitrogen) supplemented with B27 (Invitrogen) and 0.5 mm l-glutamine (Invitrogen) or an identical medium formulated without l-arginine and l-lysine (Specialty Media, Philipsburg, NJ) supplemented with either heavy 13C isotope-containing amino acids (Cambridge Isotope Laboratories, Andover, MA) or isotopically normal amino acids (Sigma-Aldrich). Culture media were refreshed every 3 days by removing half of the volume present on each plate and replacing it with fresh medium.
BDNF Stimulation, Cell Lysis, and Immunoprecipitation—
After culturing cells for 10 days in vitro, BDNF (PeproTech, Rocky Hill, NJ) was added to neuronal cultures at a concentration of 25 ng/ml for 2, 5, 15 (for SILAC experiments), 30, and 45 min, and cells were washed with ice-cold PBS and immediately placed on ice in ice-cold lysis buffer containing 1.0% Nonidet P-40 (Sigma-Aldrich), 150 mm NaCl, 20 mm Tris-HCl, pH 8, 0.2 mm EDTA, 2 mm Na3VO4, 2 mm NaF, and protease inhibitors (Complete tablet, Roche Applied Science). Lysates for Tyr(P) IP experiments were either combined directly with agarose-conjugated anti-phosphotyrosine antibody pY99 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) with 20 μl of beads/ml of lysate for overnight incubation at 4 °C or for SILAC experiments were first mixed in a 1:1 stimulated:control total protein ratio (based on Bradford assay) after which beads were washed four times with lysis buffer. Immunoprecipitated proteins were eluted by boiling in Laemmli SDS-PAGE reducing buffer (Bio-Rad) for 5 min. In a separate experiment to assess incorporation of stable isotope-labeled amino acids, hippocampal neurons were grown in labeling medium for up to 28 days, and whole cell lysates (lysis conditions described above) were combined with 2× Laemmli reducing buffer.
SDS-PAGE and In-gel Tryptic Digestion—
All samples were separated by SDS-PAGE using 10% Tris-HCl gels (Bio-Rad). Gels were stained with Coomassie Brilliant Blue (Bio-Rad), and gel lanes were cut horizontally into 23 sections for Tyr(P) IP eluates and 14 sections for measurements of label incorporation in lysates. Excised gel bands were cut into small pieces and destained in 25 mm ammonium bicarbonate, 50% acetonitrile; dehydrated with acetonitrile; and dried. The gel pieces were rehydrated with 10 ng/μl trypsin solution in 25 mm ammonium bicarbonate and incubated overnight at 37 °C. Peptides were extracted twice with 5% formic acid, 50% acetonitrile followed by a final extraction with acetonitrile (17). Extracts were pooled, dried by vacuum centrifugation, and reconstituted in 5 μl of 0.1% formic acid, 2% acetonitrile for HPLC sample injection.
Mass Spectrometry and Protein Identification—
The peptide mixtures resulting from tryptic in-gel digestions were analyzed using nanoflow LC-MS/MS. Analysis of samples from label efficiency experiments was performed in the same manner as described previously using CapLC (Waters, Milford, MA) HPLC systems coupled directly to Q-TOF Micro and Q-TOF 1 mass spectrometers (Micromass, Manchester, UK) (18). For analysis of BDNF-induced phosphotyrosine proteome changes, experiments were performed using a nanoACQUITY Ultra Performance Liquid Chromatography system (Waters) coupled directly to a Q-TOF Premier mass spectrometer (Micromass). Raw mass spectrometry data were processed using ProteinLynxGlobalServer 2.2 software (Waters). Proteins were identified using Mascot software (version 2.1, Matrix Science, London, UK) and parsed using ProteinCenter (Proxeon, Odense, Denmark) (refer to supplemental experimental procedures for detailed description).
Quantification of Label Incorporation and SILAC Ratios—
MS spectra of labeled and non-labeled peptide pairs were tracked in the raw LC-MS/MS files, and percent label incorporation for individual proteins was calculated manually for labeling efficiency experiments (supplemental Equation 1). Experimental Tyr(P) IP SILAC ratio quantification was carried out using the open source software MSQuant (kindly provided by Peter Mortensen and Matthias Mann (SourceForge, Inc.). As an additional measure to achieve proper quantification, weighted mean ratios were calculated manually for proteins observed in more than one band and in replicate experiments (supplemental Equation 2). As a control to measure the label incorporation of proteins identified in Tyr(P) IPs, parallel experiments were performed in cells grown in labeling medium only and stimulated with BDNF in the same manner as described above. Ratios were calculated using MSQuant for proteins that had peptide signals with observable unlabeled components. Ratios of less than 10:1 (the dynamic range limit of MSQuant for our data) were corrected for labeling efficiency.
Western Blot Analysis—
Cell culture, cell treatment, and immunoprecipitation were the same as described above. Precipitated proteins were separated by SDS-PAGE and transferred to PVDF membrane. Membranes were blocked in Tris-buffered saline, pH 7.5, 0.05% Tween 20 (Fisher) (TBS-T) containing 5% BSA or skim milk; incubated with the corresponding primary antibodies (pY99, 1:5000; Trk C-14, 1:500; PLCγ E-12, 1:500; Shp2 N-16, 1:500; focal adhesion kinase C-20, 1:500 (all from Santa Cruz Biotechnology, Inc.), Hrs (19), STAM (20) (both kind gifts from Dr. Harald Stenmark), and Nck2, 1:500 (Sigma)) and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.) 1:7500; and detected with ECL (Pierce).
Immunofluorescence and Confocal Microscopy—
Cells were fixed in 4% paraformaldehyde in PBS for 15 min at 4 °C; permeabilized with 0.1% Triton X-100 in PBS for 2 min; washed with TBS, 0.25% fish skin gelatin; blocked in 2% BSA, 10% normal goat serum, 0.25% fish skin gelatin in TBS for 30 min; and then incubated with the relevant antibodies (anti-Hrs, 1:300; anti-EEA1 (BD Biosciences), 1:500; secondary antibodies, 1:500) for 30 min at room temperature in blocking solution. Cells were postfixed for 5 min, washed, and incubated with AlexaFluor555-conjugated activated, tyrosine phosphorylated TrkB (pTrkB) antibody (antibody labeling kit, Molecular Probes/Invitrogen) overnight at 4 °C. Cells were washed three times with PBS, mounted in Mowiol, and imaged using an LSM 510 laser-scanning confocal microscope equipped with a 40×, Plan Neofluor numerical aperture 1.3 differential interference contrast oil immersion objective (both Carl Zeiss MicroImaging, Thornwood, NY). Images were processed using LSM 510 software and National Institutes of Health ImageJ.
HA-Hrs Transient Transfection—
Hippocampal neurons at DIV 8 were transfected with 0.5 μg of HA-Hrs plasmid (a kind gift from Dr. Francis Lee) and 0.5 μl of Lipofectamine 2000 (Invitrogen) per coverslip in Neurobasal medium for 45 min and transferred back to their original media overnight. Cells were then starved in minimum essential medium (Invitrogen), 0.37% glucose (Invitrogen), 100 μm MK801 (Sigma) for 5 h; stimulated with 25 ng/ml BDNF for the indicated times; fixed; stained; and imaged as described above.
RESULTS
Labeling of Neuronal Proteins—
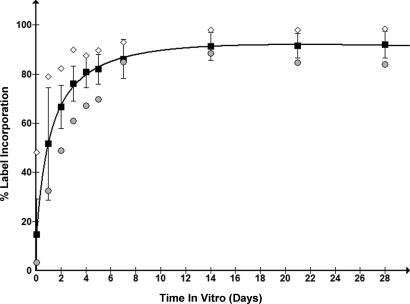
Several proteins from hippocampal neuronal cultures were tracked for efficiency of incorporation of stable isotope-labeled amino acids for a month. All proteins identified from whole cell lysates demonstrated significant (greater that 80%) levels of label incorporation by 14 days in vitro (Fig. 2). This analysis was limited to proteins containing at least one peptide that could be tracked through nine of the 10 time points (supplemental Table 1). These results indicated that proteins from a differentiated, non-dividing cell population such as cultured primary neurons can be labeled by amino acids in culture. At early time points (5 days and less) proteins exhibited a number of unique label incorporation profiles, reflecting distinct combinations of protein synthesis, turnover, and degradation, and demonstrated that primary neuronal SILAC can be used to observe such phenomena.
Fig. 2.
Percent label incorporation of neuronal proteins. Proteins from neuronal whole cell lysates were assessed for label incorporation at several time points (1 h and 1, 2, 3, 4, 5, 7, 14, 21, and 28 days). Circles (•) represent the protein with the lowest percent label incorporation observed, diamonds (♦) represent the highest, and squares (▪) represent the mean with error bars indicating the S.D. for all measurements at each time point. The line is a logarithmic best fit to the average percent label incorporation. All proteins quantified exhibited >70% labeling after 1 week in vitro and >80% by 2 weeks.
BDNF-dependent Tyr(P) IP Protein Abundance Changes—
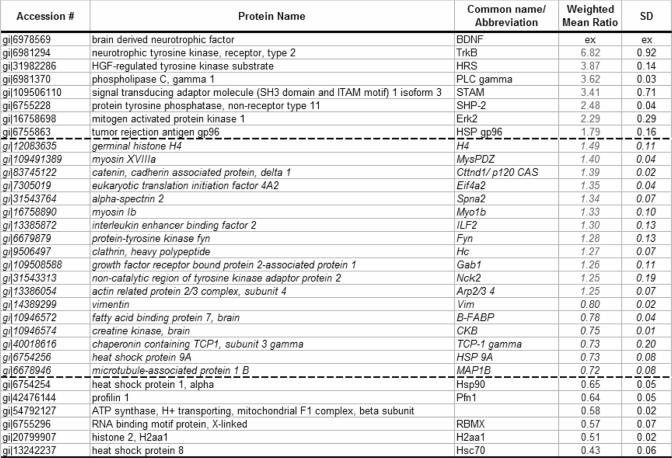
We used SILAC to study the intracellular signaling events initiated by BDNF stimulation in primary cultures of cortical/hippocampal neurons. We performed one preliminary experiment (100 million neurons per condition) and two larger scale replicate experiments (200 million neurons per condition) in which Tyr(P) IPs of combined lysates from stimulated and control neurons were fractionated by SDS-PAGE and then analyzed by LC-MS/MS. Labeling conditions were reversed between replicates to rule out the possibility of any differences arising from SILAC labeling. Proteins were identified using the Mascot search engine, quantified using MSQuant, and corrected for incomplete labeling when necessary. We identified 187 proteins in total with a false positive rate of 0.5% as estimated by the number of hits against the reverse sequences/total hits (supplemental Table 2). Of these proteins, 158 were considered Tyr(P) IP-specific, including proteins identified by only one peptide sequence but in both label states (two isotopic forms of the peptides). Twenty-nine proteins were likely exogenous contaminants such as keratin isoforms, IgG, and albumin and were not reported. Proteins identified by only one unique peptide were also excluded. We obtained quantitation data for 135 proteins with label efficiency measurements available for 65% of this subset. After correcting for incomplete incorporation of isotopic label into individual proteins in these non-dividing neurons, 13 proteins demonstrated protein abundance ratios (stimulated:control states) above 1.50 or below 0.67, and an additional 18 proteins differed by more than three average standard deviations (i.e. 3 × 0.08 = 0.24) from the mean ratio (1.01) of all quantified proteins (Table I). The mean relative S.D. of ratios obtained from replicate experiments was 0.21, indicating good agreement between experiments. Proteins demonstrating differential abundance in Tyr(P) IPs after BDNF treatment included the known receptor for BDNF, TrkB (with a ratio of 6.82 stimulated to control), and other proteins such as Hrs and STAM (ratios of 3.87 and 3.41, respectively). These results demonstrated that primary neuronal SILAC can be a powerful tool for the proteomics study of intracellular signaling in neurons.
Table I.
Proteins of changed abundance in Tyr(P) IPs from primary Neuronal cultures after 15 min of BDNF treatment
Italicized proteins and ratios between the dashed lines (ratios from 1.50 to 1.25 and 0.60 to 0.65) are considered borderline/potential changers. The symbol “ex” stands for known exogenous protein.
Western Blot Validation—
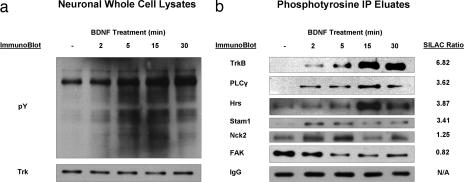
To further confirm the SILAC ratios we observed by MS, several proteins in Table I were selected for Western blot analysis based on availability of antibodies. Western blots and IPs using the pY99 antibodies as well as Western blots for previously known and potentially novel BDNF signaling participants were performed on lysates of cortical/hippocampal neuronal cultures taken from the same dissection as those used for SILAC experiments after treatment with BDNF for 0, 2, 5, 15, or 30 min. The selected proteins included the known BDNF signaling participants, TrkB receptor, PLCγ, and Nck2, and three novel effectors, Hrs, STAM, and focal adhesion kinase. As shown in Fig. 3, Western blotting analysis performed on Tyr(P) IPs and neuronal whole cell lysate using both Tyr(P) and Trk antibodies verified robust receptor activation and downstream tyrosine phosphorylation after stimulation by BDNF. These results are consistent with previous observations that the TrkB receptor and PLCγ are quickly activated after BDNF treatment (11–13). These blots also demonstrated increasing levels of tyrosine phosphorylated proteins upon ligand treatment with equal protein loading demonstrated by a consistent level of Trk receptor (Fig. 3a). To produce consistent loading of gel lanes with parallel Tyr(P) IPs, four replicate Tyr(P) IPs were performed for each time point, eluates were pooled, and the same material was used for all blots. As an additional loading control, the signal intensity for IgG light chain from the immunoprecipitating antibody was monitored for those blots that demonstrated immunoreactivity for this band, i.e. those that used an anti-mouse secondary antibody. Western blot intensities agreed well with their SILAC ratios for all proteins monitored, providing additional evidence for SILAC quantification (Fig. 3b).
Fig. 3.
Western blot validation. Cortical/hippocampal neuronal cultures, taken from the same dissection as those used for SILAC experiments, were treated with BDNF for various times. a, pY99 and Trk Western blots performed on whole cell lysates. b, pY99 IPs of lysates shown in a followed by specific Western blots for both known and potentially novel TrkB signaling participants. SILAC ratios of Tyr(P) IPs from stimulated:unstimulated cells shown for comparison were measured 15 min after BDNF stimulation. FAK, focal adhesion kinase; Hc, heavy chain; pY, phosphotyrosine.
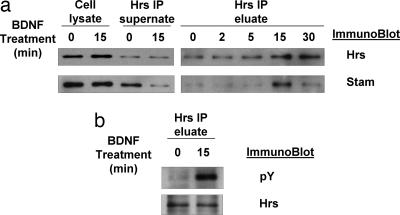
BDNF-dependent Hrs-STAM Interaction, Hrs Tyrosine Phosphorylation, and Co-localization of Phosphorylated TrkB and Hrs in Endosomal Vesicles—
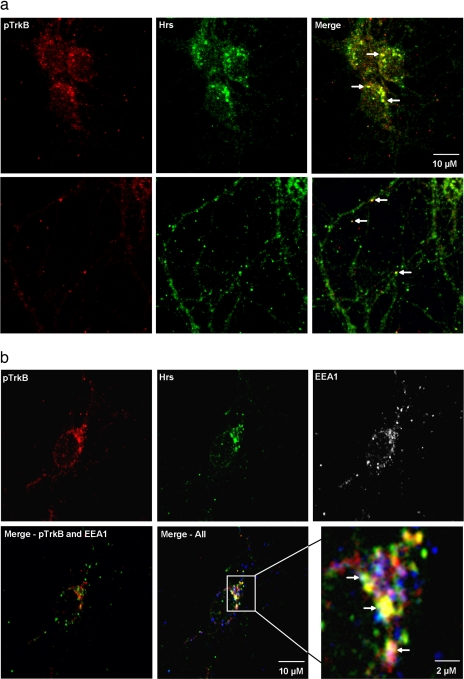
Neuronal whole cell lysates treated with BDNF for various times (control and 2, 5, 15, and 30 min) as described above were used for Hrs IPs followed by Western blotting to detect Hrs and STAM. These blots demonstrated a BDNF-dependent increase in the interaction between Hrs and STAM (Fig. 4a). This was observed as an increase in immunoreactivity for STAM in Hrs IPs after BDNF treatment and as a depletion of the STAM signal from Hrs IP supernatant fluids. PY Western blotting of an Hrs IP also demonstrated that Hrs is tyrosine phosphorylated upon BDNF treatment (Fig. 4b). Additional experiments using two-dimensional blue native PAGE and Hrs Western blotting also showed the movement of Hrs into a large >400-kDa complex upon BDNF treatment (supplemental Fig. 1). Immunofluorescence and confocal microscopy showed Trk and Hrs localizing to discrete vesicle-like puncta, many of which contain both proteins, after 15 min of BDNF treatment (Fig 5a). Co-localization appears to occur in both the cell body and in neuronal processes (indicated by white arrows). Additional staining demonstrated co-localization of these proteins with EEA1, suggesting that Trk and Hrs can co-localize in early endosomes after 15 min of BDNF treatment (Fig. 5b).
Fig. 4.
Enhanced Hrs-STAM interaction and Hrs phosphorylation. a, Hrs IPs of cortical/hippocampal neuronal cultures treated with BDNF were probed for Hrs and STAM by Western blot. These results demonstrated a BDNF-dependent increase in association between Hrs and STAM. b, BDNF-dependent phosphorylation of Hrs was monitored by Tyr(P) Western blotting of Hrs IP eluates. pY, phosphotyrosine.
Fig. 5.
Co-localization of Hrs and pTrkB. Immunofluorescence and confocal microscopy of neuronal cultures demonstrated co-localization of Hrs and pTrkB in vesicular structures after 15 min of BDNF treatment (a). This co-localization can be observed in both the cell body (top panels) and in neurite processes (bottom panels). White arrows indicate examples of instances of co-localizations. b, additional immunofluorescence demonstrated co-localization of Hrs, pTrkB, and EEA1 (a marker for early endosomes) after 15 min of BDNF treatment. White arrows in the enlarged image (bottom right panel) indicate locations of triple co-localization.
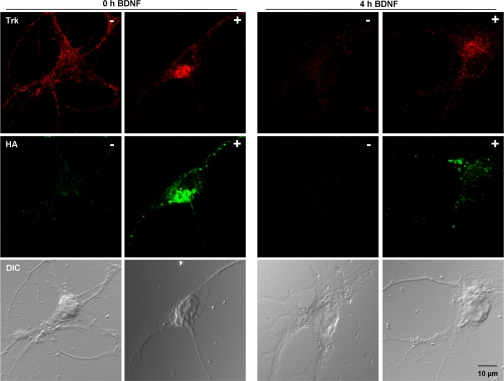
Hrs Overexpression Disrupts Trk Degradation—
Hippocampal neurons (DIV 8) were transfected with a construct expressing HA-Hrs. One day after transfection, cells were starved for 5 h followed by BDNF treatment for 4 h. Neurons were fixed and stained for HA and total Trk (Fig. 6). Transfected and non-transfected imaged cells were present on the same slide with separate slides for control and BDNF-stimulated conditions. Four hours of BDNF treatment after starvation led to down-regulation of Trk receptors in non-transfected control cells (Fig. 6, panels designated with “−” symbols). Cells overexpressing HA-Hrs (transfected) had higher levels of Trk (panels designated with “+” symbols) indicating that receptor degradation upon BDNF treatment was disrupted by overexpression of HA-Hrs.
Fig. 6.
Overexpression of Hrs. Immunofluorescence and confocal microscopy of neuronal cultures either transiently transfected with HA-Hrs (+ panels) or not (− panels) were performed. Starved cells were imaged after (left two rows of panels) or without (right two rows of panels) 4 h of BDNF treatment. Transfected and non-transfected imaged cells were present on the same slide with separate slides for control and BDNF-stimulated conditions. Decreased staining for total Trk (red) after 4 h of BDNF treatment can be observed in control cells (4 h BDNF − panel). Overexpression of HA-Hrs (green) leads to disrupted degradation of Trk (4 h BDNF + panel). DIC, differential interference contrast.
DISCUSSION
Here we describe, to the best of our knowledge, the first application of SILAC to the characterization of cell signaling in non-dividing primary cell cultures such as the primary neurons we used in our studies. The primary neuronal cellular model system has been used for decades in neuroscience and is thus compatible with a large number of established experimental paradigms. The use of these paradigms in combination with SILAC-based methodologies can offer a broader view of cellular function than traditional protein-by-protein assessment. This method has several advantages over previously described cell line-based SILAC experiments in assessing neuronal cellular events. Most importantly, it provides a system that more closely represents the proper in vivo intracellular context, which is a critical factor in evaluating signal transduction events. It has been observed that many RTKs transmit signals through shared pathways but generate distinct biological outputs. This may be attributed to cell-specific context restriction of responses (available downstream components) or to localization of signaling proteins in specific intracellular sites or to a specific combination of signals produced by receptor activation (21–23). This idea reinforces the need to assess downstream events in the proper cellular context and for researchers to be cognizant of this when interpreting data from immortalized cell lines that may more closely represent cancer cells than the original source tissue.
Our results have also dispelled the well accepted belief that SILAC experiments are limited to dividing cell culture systems because of a requirement for five to six population doublings to achieve sufficient labeling. It is indeed possible to label a non-dividing, differentiated cell population such as primary neurons with high efficiency (Fig. 2). The properties of primary neurons such as substantial development; neurite outgrowth, which requires substantial new protein synthesis; and relatively long term survival in vitro contribute significantly to the ability to label them. However, other primary cells with similar properties would also be candidates for SILAC experiments. We also found that the rate of SILAC label incorporation can be monitored over relatively short periods of time (1 h to 5 days) in labeling medium (data not shown). With such measurements, protein half-life has been calculated on a proteomics scale in other systems (24, 25). Such an approach could be useful for addressing questions fundamental to the understanding of neuronal function, such as activity-dependent protein turnover at synapses.
We used primary neuronal SILAC to study neuronal signal transduction resulting from BDNF stimulation of dissociated cultured cortical/hippocampal neurons. After stimulation with BDNF, 13 proteins had SILAC ratios of greater than 1.50 or less than 0.67, and an additional 18 proteins had ratios greater that 1.25 or less than 0.80 in Tyr(P) IPs (Table I) including a number of possible novel participants in BDNF signaling. Also among this list of proteins were known components of the BDNF pathway such as TrkB (14), PLCγ, SHP-2, Gab-1, Erk2 (10, 11, 26), Nck2 (27), and Fyn (16, 28), which increased our confidence that other changes we observed may be relevant to BDNF signaling. We also found motor and junctional proteins, as well as suggestions of interesting phenomena such as regulation of specific cytoskeletal components and differntial regulation of specific histone isoforms. We conducted additional experiments to verify the involvement of Hrs and STAM, which represent interesting downstream effectors that have not been reported previously for TrkB.
We found ratios of 3.87, 3.41, and 1.27 for Hrs, STAM and clathrin heavy chain, respectively, in Tyr(P) IPs from cultured neurons after BDNF treatment. Several studies have demonstrated that activated RTKs are removed from the cell surface by clathrin-mediated endocytosis (29). Receptor trafficking dynamics can influence signal output (30, 31), and this is an area of particular interest in Trk signaling where different Trk family members have shown distinct trafficking behaviors (32). Hrs is believed to couple the concentration of ubiquitinated receptors to recruitment of an extensive machinery for multivesicular body formation (33). Indeed it has recently been shown that TrkA and TrkB receptors are differentially regulated by ubiquitination to modulate the survival of neurons (34).
Hrs and STAM form a heterodimeric complex that associates with endosomal membranes, and Hrs is tyrosine phosphorylated in response to a variety of growth factors and cytokines (35, 36). Hrs has been shown to associate with STAM (20, 37, 38), which undergoes tyrosine phosphorylation in parallel with Hrs (39). Both hepatocyte growth factor- and epidermal growth factor-dependent phosphorylation of Hrs requires a functional endocytic pathway and coincident localization of activated receptor and Hrs in early endosomes (39, 40). To date, no role for this degradative pathway has been observed in neurons, and our results suggest that TrkB may be involved in a similar process. The observed SILAC ratios for Hrs, STAM, and clathrin heavy chain in Tyr(P) IPs after BDNF stimulation (Table I and Fig. 3b) are consistent with increased tyrosine phosphorylation of one or more of these proteins in response to BDNF stimulation. Hrs and STAM appear to increase their affinity for each other in a BDNF-dependent manner as demonstrated by co-immunoprecipitation (Fig 4a), and Hrs is tyrosine phosphorylated in response to BDNF treatment (Fig. 4b). pTrkB was co-localized with Hrs after BDNF treatment (Fig. 5a). Furthermore this co-localization can be observed in EEA1-positive puncta, suggesting that some proportion of co-localized pTrkB and Hrs is in early endosomes (Fig. 5b). Hrs overexpression disrupts its function in a manner similar to Hrs knockdown (41–43). Cells overexpressing HA-Hrs had higher levels of Trk and reduced receptor degradation upon BDNF treatment, suggesting that overexpression of Hrs has a dominant negative effect on Trk degradation (Fig. 6). TrkB receptors have been shown to sort predominantly to a degradation pathway in contrast to TrkA, which is recycled to the cell surface after stimulation, and these divergent trafficking pathways have been linked to alternate biological responses (32). It will be interesting to determine what role the Hrs-STAM complex might play in this divergent behavior of Trk family members. We believe our data provide a strong foundation for a more thorough assessment of intracellular trafficking of TrkB in neuronal cells.
CONCLUSIONS
We have demonstrated that it is possible to label with high efficiency a non-dividing, differentiated cell population such as primary neurons with stable isotope-containing amino acids. The properties of primary neurons such as substantial development and relatively long term survival in vitro contribute significantly to the high labeling efficiency, and other cells with similar properties have potential for use in SILAC experiments. We successfully used primary neuronal SILAC to study signal transduction downstream of BDNF stimulation of dissociated cultured cortical/hippocampal neurons. Proteins demonstrating changing SILAC ratios in Tyr(P) IP after BDNF stimulation include a number of known and possible novel downstream components of the TrkB pathway. The role of some of these proteins in the apparent ability of the receptor to down-regulate its own surface localization after ligand treatment is of particular interest. We believe primary neuronal SILAC represents a powerful tool for the assessment of neuronal function and cellular biology on a proteomics scale and will find numerous applications in neuroscience research.
Supplementary Material
Acknowledgments
We thank Dr. Harald Stenmark for the kind gift of Hrs and STAM antibodies and Dr. Francis Lee for the HA-Hrs plasmid. We also thank Dr. Rithwick Rajagopal and Mercedes Benya for expert technical assistance in establishing primary neuronal cultures and Drs. Helene Cardasis, Guoan Zhang, and David Fenyo for helpful discussions.
Footnotes
Published, MCP Papers in Press, February 6, 2008, DOI 10.1074/mcp.M700387-MCP200
The abbreviations used are: SILAC, stable isotope labeling by amino acids in cell culture; BDNF, brain-derived neurotrophic factor; IP, immunoprecipitation; RTK, receptor tyrosine kinase; Hrs, hepatocyte growth factor-regulated tyrosine kinase substrate; STAM, signal-transducing adaptor molecule; PLCγ, phospholipase C γ; HA, hemagglutinin; DIV, day in vitro; pTrkB, activated, tyrosine phosphorylated TrkB.
This work was supported, in whole or in part, by National Institutes of Health Grant P30 NS050276 (NINDS) and National Institutes of Health Shared Instrumentation Grant RR017990 (to T. A. N.). This work was also supported by the European Molecular Biology Organization (to K. D.), the New York University and National Institutes of Health Graduate Partnership Program in Structural Biology (to D. S. S.), and NIH Grants NS21072 and HD23315 (to M. V. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
REFERENCES
- 1.Alberts, B. ( 2002) Molecular Biology of the Cell, 4th Ed., p. 472, Garland Science, New York
- 2.Silva, R. F., Falcao, A. S., Fernandes, A., Gordo, A. C., Brito, M. A., and Brites, D. ( 2006) Dissociated primary nerve cell cultures as models for assessment of neurotoxicity. Toxicol. Lett. 163, 1–9 [DOI] [PubMed] [Google Scholar]
- 3.Matteoli, M., Verderio, C., Krawzeski, K., Mundigl, O., Coco, S., Fumagalli, G., and De Camilli, P. ( 1995) Mechanisms of synaptogenesis in hippocampal neurons in primary culture. J. Physiol. ( Paris) 89, 51–55 [DOI] [PubMed] [Google Scholar]
- 4.Lindsley, T. A., Kerlin, A. M., and Rising, L. J. ( 2003) Time-lapse analysis of ethanol's effects on axon growth in vitro. Brain Res. Dev. Brain Res. 147, 191–199 [DOI] [PubMed] [Google Scholar]
- 5.Ong, S. E., Blagoev, B., Kratchmarova, I., Kristensen, D. B., Steen, H., Pandey, A., and Mann, M. ( 2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 [DOI] [PubMed] [Google Scholar]
- 6.Blagoev, B., Kratchmarova, I., Ong, S. E., Nielsen, M., Foster, L. J., and Mann, M. ( 2003) A proteomics strategy to elucidate functional protein-protein interactions applied to EGF signaling. Nat. Biotechnol. 21, 315–318 [DOI] [PubMed] [Google Scholar]
- 7.Mann, M. ( 2006) Functional and quantitative proteomics using SILAC. Nat. Rev. Mol. Cell Biol. 7, 952–958 [DOI] [PubMed] [Google Scholar]
- 8.Ong, S. E., and Mann, M. ( 2006) A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC). Nat. Protoc. 1, 2650–2660 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt, F., Strozynski, M., Salus, S. S., Nilsen, H., and Thiede, B. ( 2007) Rapid determination of amino acid incorporation by stable isotope labeling with amino acids in cell culture (SILAC). Rapid Commun. Mass Spectrom. 21, 3919–3926 [DOI] [PubMed] [Google Scholar]
- 10.Huang, E. J., and Reichardt, L. F. ( 2001) Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichardt, L. F. ( 2006) Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAllister, A. K., Katz, L. C., and Lo, D. C. ( 1999) Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 22, 295–318 [DOI] [PubMed] [Google Scholar]
- 13.Poo, M. M. ( 2001) Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2, 24–32 [DOI] [PubMed] [Google Scholar]
- 14.Bothwell, M. ( 1995) Functional interactions of neurotrophins and neurotrophin receptors. Annu. Rev. Neurosci. 18, 223–253 [DOI] [PubMed] [Google Scholar]
- 15.Brewer, G. J. ( 1997) Isolation and culture of adult rat hippocampal neurons. J. Neurosci. Methods 71, 143–155 [DOI] [PubMed] [Google Scholar]
- 16.Pereira, D. B., and Chao, M. V. ( 2007) The tyrosine kinase Fyn determines the localization of TrkB receptors in lipid rafts. J. Neurosci. 27, 4859–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. ( 1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 18.Zhang, G., Spellman, D. S., Skolnik, E. Y., and Neubert, T. A. ( 2006) Quantitative phosphotyrosine proteomics of EphB2 signaling by stable isotope labeling with amino acids in cell culture (SILAC). J. Proteome Res. 5, 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raiborg, C., Bache, K. G., Mehlum, A., Stang, E., and Stenmark, H. ( 2001) Hrs recruits clathrin to early endosomes. EMBO J. 20, 5008–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bache, K. G., Raiborg, C., Mehlum, A., Madshus, I. H., and Stenmark, H. ( 2002) Phosphorylation of Hrs downstream of the epidermal growth factor receptor. Eur. J. Biochem. 269, 3881–3887 [DOI] [PubMed] [Google Scholar]
- 21.Hunter, T. ( 2000) Signaling—2000 and beyond. Cell 100, 113–127 [DOI] [PubMed] [Google Scholar]
- 22.Tan, P. B., and Kim, S. K. ( 1999) Signaling specificity: the RTK/RAS/MAP kinase pathway in metazoans. Trends Genet. 15, 145–149 [DOI] [PubMed] [Google Scholar]
- 23.Sak, K., and Illes, P. ( 2005) Neuronal and glial cell lines as model systems for studying P2Y receptor pharmacology. Neurochem. Int. 47, 401–412 [DOI] [PubMed] [Google Scholar]
- 24.Pratt, J. M., Petty, J., Riba-Garcia, I., Robertson, D. H., Gaskell, S. J., Oliver, S. G., and Beynon, R. J. ( 2002) Dynamics of protein turnover, a missing dimension in proteomics. Mol. Cell. Proteomics 1, 579–591 [DOI] [PubMed] [Google Scholar]
- 25.Doherty, M. K., Whitehead, C., McCormack, H., Gaskell, S. J., and Beynon, R. J. ( 2005) Proteome dynamics in complex organisms: using stable isotopes to monitor individual protein turnover rates. Proteomics 5, 522–533 [DOI] [PubMed] [Google Scholar]
- 26.Huang, E. J., and Reichardt, L. F. ( 2003) Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki, S., Mizutani, M., Suzuki, K., Yamada, M., Kojima, M., Hatanaka, H., and Koizumi, S. ( 2002) Brain-derived neurotrophic factor promotes interaction of the Nck2 adaptor protein with the TrkB tyrosine kinase receptor. Biochem. Biophys. Res. Commun. 294, 1087–1092 [DOI] [PubMed] [Google Scholar]
- 28.Rajagopal, R., and Chao, M. V. ( 2006) A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Mol. Cell. Neurosci. 33, 36–46 [DOI] [PubMed] [Google Scholar]
- 29.Clague, M. J. ( 1998) Molecular aspects of the endocytic pathway. Biochem. J. 336, 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorkin, A., and Von Zastrow, M. ( 2002) Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 3, 600–614 [DOI] [PubMed] [Google Scholar]
- 31.Clague, M. J., and Urbe, S. ( 2001) The interface of receptor trafficking and signalling. J. Cell Sci. 114, 3075–3081 [DOI] [PubMed] [Google Scholar]
- 32.Chen, Z. Y., Ieraci, A., Tanowitz, M., and Lee, F. S. ( 2005) A novel endocytic recycling signal distinguishes biological responses of Trk neurotrophin receptors. Mol. Biol. Cell 16, 5761–5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clague, M. J., and Urbe, S. ( 2003) Hrs function: viruses provide the clue. Trends Cell Biol. 13, 603–606 [DOI] [PubMed] [Google Scholar]
- 34.Arevalo, J. C., Waite, J., Rajagopal, R., Beyna, M., Chen, Z. Y., Lee, F. S., and Chao, M. V. ( 2006) Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron 50, 549–559 [DOI] [PubMed] [Google Scholar]
- 35.Komada, M., and Kitamura, N. ( 1995) Growth factor-induced tyrosine phosphorylation of Hrs, a novel 115-kilodalton protein with a structurally conserved putative zinc finger domain. Mol. Cell. Biol. 15, 6213–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katzmann, D. J., Odorizzi, G., and Emr, S. D. ( 2002) Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3, 893–905 [DOI] [PubMed] [Google Scholar]
- 37.Asao, H., Sasaki, Y., Arita, T., Tanaka, N., Endo, K., Kasai, H., Takeshita, T., Endo, Y., Fujita, T., and Sugamura, K. ( 1997) Hrs is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine-induced cell growth. J. Biol. Chem. 272, 32785–32791 [DOI] [PubMed] [Google Scholar]
- 38.Row, P. E., Clague, M. J., and Urbe, S. ( 2005) Growth factors induce differential phosphorylation profiles of the Hrs-STAM complex: a common node in signalling networks with signal-specific properties. Biochem. J. 389, 629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urbe, S., Mills, I. G., Stenmark, H., Kitamura, N., and Clague, M. J. ( 2000) Endosomal localization and receptor dynamics determine tyrosine phosphorylation of hepatocyte growth factor-regulated tyrosine kinase substrate. Mol. Cell. Biol. 20, 7685–7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammond, D. E., Carter, S., McCullough, J., Urbe, S., Vande Woude, G., and Clague, M. J. ( 2003) Endosomal dynamics of Met determine signaling output. Mol. Biol. Cell 14, 1346–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanyaloglu, A. C., McCullagh, E., and von Zastrow, M. ( 2005) Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 24, 2265–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasdemir, B., Bunnett, N. W., and Cottrell, G. S. ( 2007) Hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) mediates post-endocytic trafficking of protease-activated receptor 2 and calcitonin receptor-like receptor. J. Biol. Chem. 282, 29646–29657 [DOI] [PubMed] [Google Scholar]
- 43.Hislop, J. N., Marley, A., and Von Zastrow, M. ( 2004) Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J. Biol. Chem. 279, 22522–22531 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.