Abstract
This study explores how the resin composition/structure affects the physicochemical properties of copolymers and their amorphous calcium phosphate (ACP)-filled composites. A series of photo-polymerizable binary and ternary matrices were formulated utilizing 2,2-bis[p-(2′-hydroxy-3′-methacryloxypropoxy)phenyl]propane, 2,2-bis[p-(2′-methacryloxypropoxy)phenyl]propane (EBPADMA) or a urethane dimethacrylate as base monomers, and triethylene glycol dimethacrylate or hexamethylene dimethacrylate (HmDMA) with or without 2-hydroxyethyl methacrylate (HEMA) as diluent monomer. Unfilled copolymers and composites filled with 40 % by mass zirconia-hybridized ACP were evaluated for biaxial flexure strength (BFS), degree of conversion (DC), mineral ion release, polymerization shrinkage (PS) and water sorption (WS). The average DC values were (82 to 94) % and (74 to 91) % for copolymers and composites, respectively. Unrelated to the resin composition, the PS values of composites were up to 8.4 vol. % and the BFS values of wet composite specimens were on average (51 ± 8) MPa. The maximum WS values attained in copolymers and composites reached 4.8 mass %. Inclusion of hydrophobic HmDMA monomer in the matrices significantly reduced the WS. The levels of Ca and PO4 released from all types of composites were significantly above the minimum necessary for the re-deposition of apatite to occur. Elevated Ca, and to a lesser extent PO4 release, was observed in HEMA-containing, ternary EBPADMA fromulations. Further resin reformulations may be needed to improve the PS of composite specimens. Poor dispersion of “as-synthesized“ ACP within the composite contributes to their inferior mechanical performance.
Keywords: amorphous calcium phosphate, biaxial flexure strength, composite, copolymer, degree of vinyl conversion, ion release, polymerization shrinkage, water sorption
INTRODUCTION
Calcium phosphate (CaP)-based restorative materials are appealing due to their biocompatibility. Amorphous calcium phosphate (ACP)-based materials capable of providing the extended supply of Ca and PO4 ions needed to reform damaged mineral structures are particularly attractive as bioactive, anti-demineralizing/remineralizing composites. When embedded in photo-polymerizable methacrylate matrices [1, 2] and exposed to an aqueous environment, ACP may promote re-deposition of thermodynamically stable, apatitic tooth mineral [3, 4]. A problem with dental composites of all types is their inability to resist cracking under masticatory stress due to their low strength and toughness. In the case of ACP composites the uncontrolled aggregation of ACP particles was identified as one of the main reasons for a poor interfacial interaction with dental resins which leads to mechanical instability for these materials [5] and inferior strength when compared to glass-reinforced composites. To overcome this shortcoming we have focused on developing strategies for improving the ACP filler/polymer matrix interfacial properties by better controlling the particle size distribution and surface properties of ACP fillers and/or by fine-tuning the resin [6–8].
In this study we report on the utility of the resin composition as a tool to control the physicochemical properties of the composites. The working hypothesis was that improved ion release could be achieved by substituting relatively flexible, low viscosity base monomer (ethoxylated bisphenol A dimethacrylate; EBPADMA) or a more flexible aliphatic base monomer (urethane dimethacrylate; UDMA) for rigid, aromatic base monomer (2,2-bis[p-(2′-hydroxy-3′-methacryloxypropoxy)phenyl]propane; Bis-GMA) in the matrix. Additionally, we wanted to assess the effect of introducing the hydrophobic diluent monomer (hexamethylene dimethacrylate; HmDMA) instead of the more hydrophilic triethylene glycol dimethacrylate (TEGDMA) in binary resin formulations as well as to evaluate the effect of including the very hydrophilic component, 2-hydroxyethyl methacrylate (HEMA) in ternary matrices. To test the above hypothesis, as-synthesized zirconia-ACP (Zr-ACP) filler was formulated into composites with a series of binary and ternary Bis-GMA-, EBPADMA and UDMA-based resins and evaluated for degree of vinyl conversion (DC), biaxial flexure strength (BFS), polymerization shrinkage (PS), water sorption (WS) and mineral ion release. In addition, the DC, BFS and WS of unfilled resins (copolymers) were determined to clarify the possible effect(s) of the filler on these parameters.
MATERIALS AND METHODS
Synthesis of Zirconia-hybridized ACP (Zr-ACP) Filler
Zr-ACP was synthesized as detailed earlier [2, 4]. It precipitated instantaneously in a closed system at 23 °C upon rapidly mixing equal volumes of a 800 mmol/L Ca(NO3)2 solution, a 536 mmol/L Na2HPO4 solution that contained a molar fraction of 2 % Na4P2O7 as a stabilizing component for ACP, and an appropriate volume of a 250 mmol/L ZrOCl2 solution (mole fraction of 10 % ZrOCl2 based on Ca reactant). The reaction pH varied between 8.6 and 9.0. The suspension was filtered, the solid phase washed subsequently with ice-cold ammoniated water and acetone, and then lyophilized. To avoid exposure to humidity, Zr-ACP was kept under vacuum (2.7 kPa) until utilized in composite formulations.
Formulation of the Resins
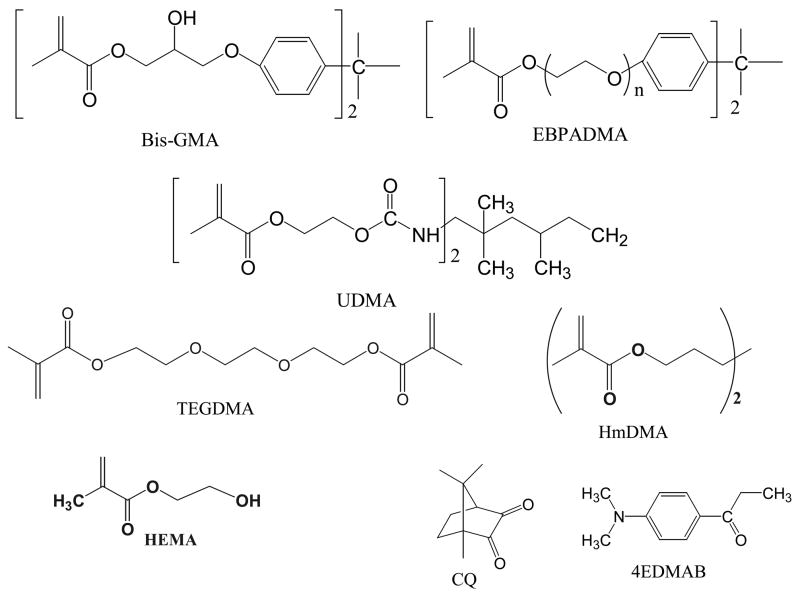
Two series of binary and ternary matrix resins were formulated from the commercially available monomers and the components of the photo-initiator system (Tables 1, 2; Fig. 1). The indicated acronyms are used throughout this manuscript. Resins were photo-activated by the inclusion of 0.20 % by mass camphorquinone and 0.80 % by mass ethyl-4-N, N-dimethylaminobenzoate as photo-oxidant and photo-reductant, respectively.
Table 1.
Base monomers, diluent monomers and the components of photo-initiator system employed in resin formulations.
| Component | Chemical nomenclature | Acronym |
|---|---|---|
| Base monomer | 2,2-bis[p-(2′-hydroxy-3′-methacryloxypropoxy)phenyl]propane
2,2-bis[p-(2′-methacryloxypropoxy)phenyl]propane urethane dimethacrylate |
Bis-GMA
EBPADMA UDMA |
| Diluent | 2-hydroxyethyl methacrylate | HEMA |
| monomer | hexamethylene dimethacrylate
triethylene glycol dimethacrylate |
HmDMA
TEGDMA |
| Photo-initiators | Camphorquinone
ethyl-4-N,N-dimethylaminobenzoate |
CQ
4EDMAB |
Table 2.
| Resin | Bis-GMA | EBPADMA | UDMA | TEGDMA | HmDMA | HEMA |
|---|---|---|---|---|---|---|
| BT
BHm BTH BHmH |
49.5
52.4 35.5 37.0 |
-
- - - |
-
- - - |
49.5
- 35.5 - |
-
46.6 - 32.8 |
-
- 28.0 29.2 |
| ET
EHm ETH EHmH |
-
- - - |
46.7
49.6 32.9 34.3 |
-
- - - |
52.3
- 36.9 - |
-
49.4 - 34.2 |
-
- 29.2 30.5 |
| UT
UHm UTH UHmH |
-
- - - |
-
- - - |
48.8
51.8 34.8 36.2 |
50.2
- 35.9 - |
-
47.2 - 33.3 |
-
- 28.3 29.5 |
Molar ratio base monomer (Bis-GMA, EBPADMA or UDMA): diluent monomer (TEGDMA or HmDMA) = 1.0 : 1.8 for binary mixtures. Molar ratio base monomer: diluent monomer: HEMA = 1: 1.8 : 3.1 for ternary mixtures.
Photoinitiator system consisted of 0.2 % by mass CQ and 0.8 % by mass 4EDMAB.
Fig. 1.
Chemical structure of the monomers and photo-curing agents utilized in the study.
Preparation of Composite and Copolymer Specimens
Composite pastes were made by mixing the appropriate resin (60 % by mass) and Zr-ACP filler (40 % by mass) using hand spatulation. The homogenized pastes were kept under a moderate vacuum (2.7 kPa) overnight to eliminate the air entrained during mixing. The pastes were molded into disks (14.9 mm to 15.3 mm in diameter and 1.31 mm to 1.53 mm in thickness) by filling the circular openings of flat Teflon molds, covering each side of the mold with a Mylar film plus a glass slide, and then clamping the assembly together with spring clips. The disks were photo-polymerized by irradiating sequentially each face of the mold assembly for 120 s with visible light (Triad 2000, Dentsply International, York, PA, USA). The copolymer disk specimens were prepared and irradiated in an identical manner.
Physicochemical Appraisal of ACP Filler, Copolymers and Composites
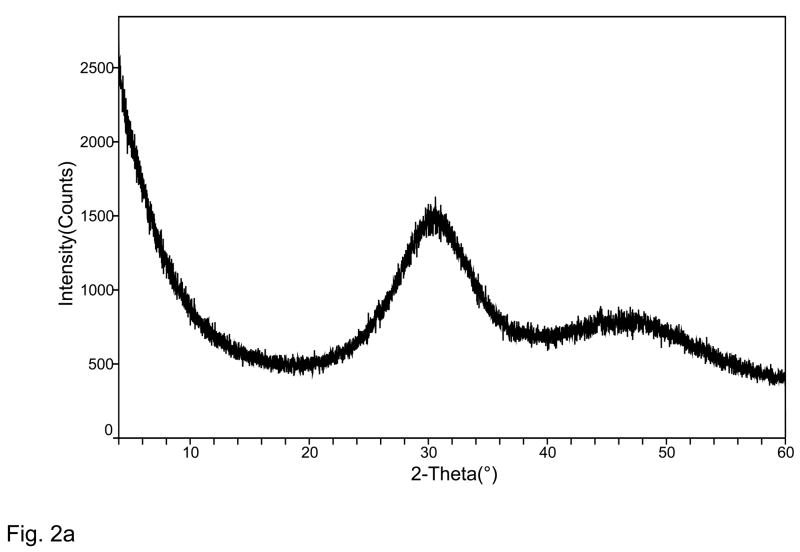
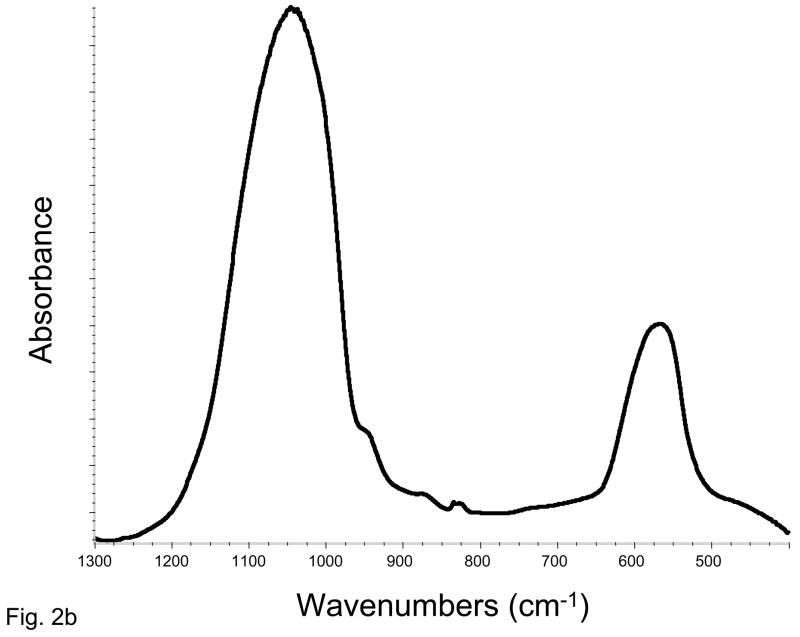
The amorphous state of the filler was verified by powder X-ray diffraction (XRD; Rigaku X-ray diffractometer) and Fourier-transform spectroscopy (FTIR; Nicolet Magna-IR FTIR System 550). XRD patterns were recorded from 4° to 60° 2θ with CuKα radiation (λ = 0.154 nm) at 40 kV and 40 mA. The samples were step-scanned in intervals of 0.010° 2θ at a scanning speed of 1.000 deg/min. The FTIR spectra (4000 cm−1 to 400 cm−1) were recorded using a KBr pellet technique (0.8 to 1.0 mg ACP/400 mg KBr).
A non-destructive near-FTIR spectroscopic technique (NIR) was utilized to determine the degree of conversion (DC) of the vinyl groups in the unfilled resins (copolymers) and their ACP-filled composites by monitoring the reduction in the =CH overtone absorption band at 6165 cm−1 for the methacrylate group [9]. NIR spectra were acquired before photo-cure and at 24 h post-cure by collecting 64 co-added scans at four wave-number resolution. Triplicate measurements were performed for each experimental group. Use of an internal reference was not required, provided that the thickness of uncured (monomer) and cured specimen (polymer) has been measured. DC was calculated from the decrease in integrated peak area/sample thickness values according to the expression:
| (1) |
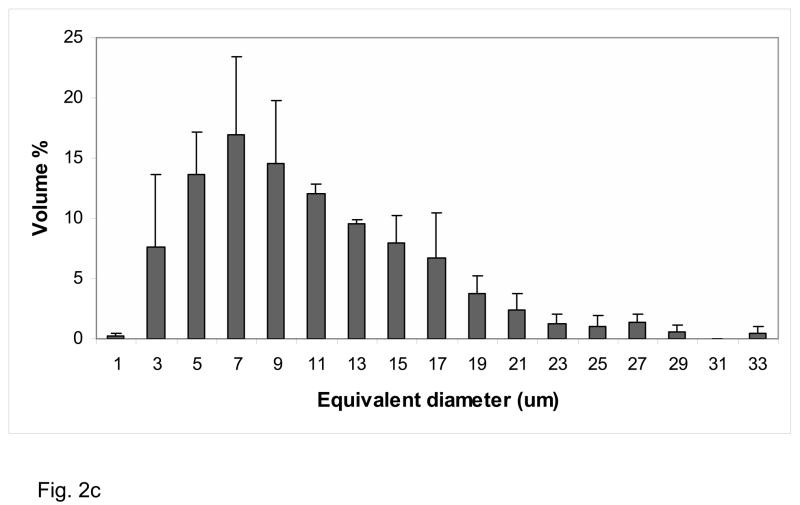
The particle size distribution (PSD) of Zr-ACP filler was measured using a laser light scattering particle size analyzer (Ankersmid CIS-100, Metropolitan Computing Corporation, East Hanover, NJ, USA). The ACP powder was dispersed in isopropanol and utrasonicated for 10 min at room temperature prior to the analysis (measurements were run in triplicate). From the PSD, the median particle size diameter (dm) of the sample was obtained and it was taken as an indicator of the aggregation of the ACP particulates (the higher the dm value, the more aggregated is the ACP). The PSD data were compared with morphological data obtained by scanning electron microscopy.
Surface morphology/topology of Zr-ACP, after the specimen was sputter-coated with gold, was determined by scanning electron microscopy (SEM) using a JSM-5400 instrument (JEOL Inc., Peabody, MA, USA).
The polymerization shrinkage (PS) of composite samples (n=9/group) was measured by a computer-controlled mercury dilatometer (PRC-ADAF, Gaithersburg, MD, USA). Composite pastes were cured using a standard 60 s/30 s exposure and data acquisition of 60 min + 30 min. The volumetric shrinkage of a specimen corrected for temperature fluctuations during the measurement was plotted as a function of time. The overall PS (% by volume) was calculated based on the known mass of the sample (50 mg to 100 mg) and its density. The latter was determined by means of the Archimedean displacement principle using an attachment to a microbalance.
The biaxial flexure strength (BFS) of copolymers and composites was determined by using a computer-controlled Universal Testing Machine (Instron 5500R, Instron Corp., Canton, MA, USA) operated by Testworks 4 software. The disk specimens were approximately 15 mm in diameter and 1.5 mm in thickness. The failure stress was calculated according to the following equation [10]:
| (2) |
where A = −[3/4π(X−Y)], X=(1+ν)ln(r1/rs)2 +[(1−ν)/2](r1/rs)2, Y = (1+ν)[1 + ln(rsc/rs)2], and where ν. = Poisson’s ratio, rl = radius of the piston applying the load at the surface of contact, rsc = radius of the support circle, rs = radius of disk specimen, L = applied load at failure, and t = thickness of disk specimen.
Water sorption (WS) profiles of copolymer and composite specimens were determined as follows. A minimum of 7 replicate disks in each experimental group were initially dried over anhydrous CaSO4 until a constant mass was achieved (± 0.1 mg). Specimens were then exposed to an air atmosphere of 75 % relative humidity (RH) at 37 °C by keeping them suspended over saturated aqueous NaCl slurry in closed systems. Gravimetric mass changes of dry-padded specimens were recorded at 5 d, 15 d and 30 d of exposure to this RH. The WS of any individual specimen at a given time interval (t), expressed as % by mass, was calculated using a simple equation:
| (3) |
where Wt represents the sample mass at the time t, and Wo is the initial mass of the dry sample.
Mineral ion release from composite disk specimens (n = 3/group) was examined at 37 °C, in a continuously stirred, buffered (pH=7.40) saline solution. Kinetic changes in the Ca and PO4 levels were determined by utilizing spectroscopic methods. Ca was determined as a complex with Arsenazo-III and PO4 was determined as a blue molybdato complex in an acidified ammonium molybdate solution [11, 12]. A Carey Model 219 spectrophotometer (Varian Analytical Instruments, Palo alto, CA, USA) was used at wavelengths of 650 nm and 882 nm for Ca and PO4, respectively. Ion release data were corrected for variations in the total area of the disk exposed to the immersion solution using the simple relation for a given surface area, A: normalized value = measured value X (500/A).
Experimental data were analyzed by ANOVA (α = 0.05). Significant differences between specific groups were determined by all pair-wise multiple comparisons (two-tail t-test; unequal variances). One standard deviation (SD) is given in this paper for comparative purposes as the estimated standard uncertainty of the measurements.
RESULTS
XRD pattern of the Zr-ACP filler utilized in the study (Fig. 2a) consisted of the two diffuse broad bands in 2θ (°) = (4 to 60) region. The corresponding FTIR spectrum (Fig. 2b) revealed two wide absorbance bands typical for non-crystalline phosphate: PO4 stretching and PO4 banding at (1200 to 900) cm−1 and (630 to 500) cm−1, respectively. The PSD data (Fig. 2c) disclosed heterogeneous size distribution ranging from submicron to approx. 40 μm with the median particle diameter being (8.6 ± 2.4) μm. A representative SEM image of the filler is shown in Fig. 2d.
Fig. 2.
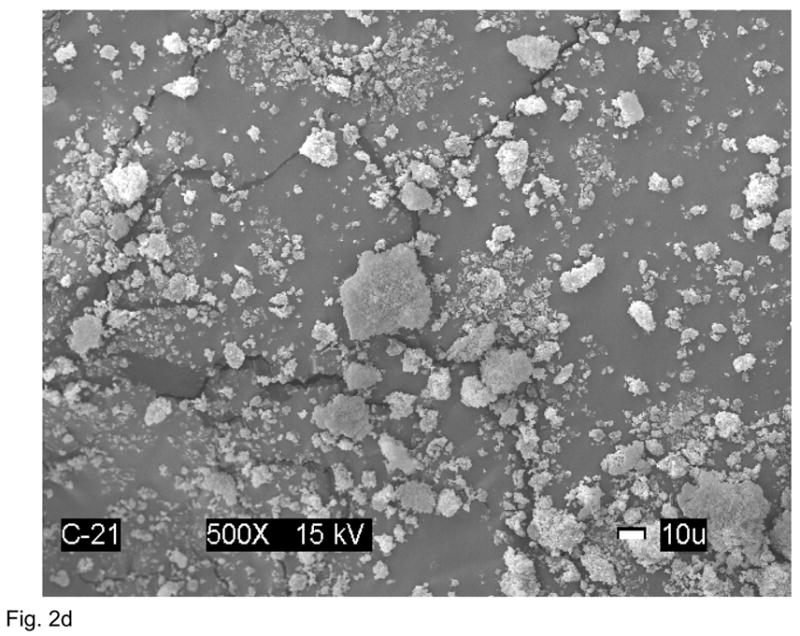
Physicochemical characteristics of Zr-ACP filler: Structural features (XRD and FTIR spectra; parts a and b, respectively), particle size distribution (PSD, part c) and the surface morphology (SEM image; part d).
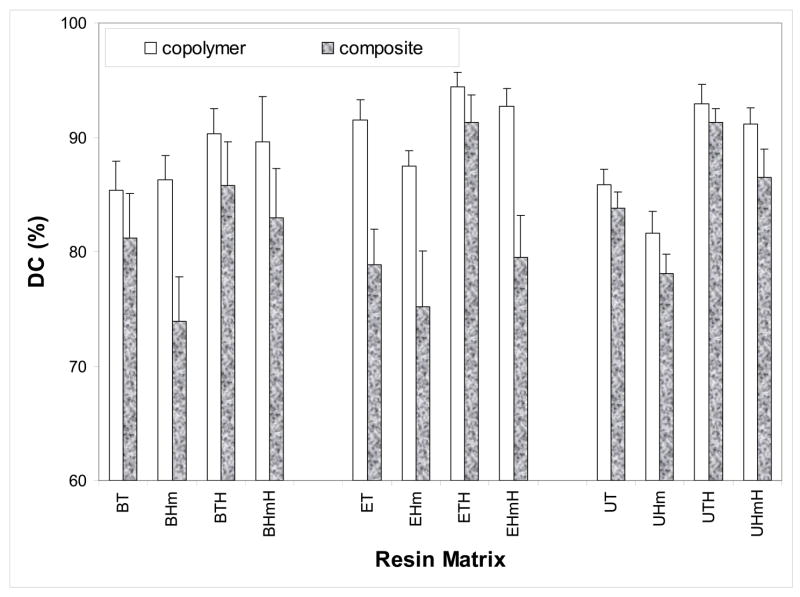
The NIR double bond conversion data are presented in Fig. 3. The 24 h post-cure DC attained in copolymer series ranged from 82 % to 94 % for the combined binary and ternary Bis-GMA-, EBPADMA- and UDMA-based matrices. The DC values in the corresponding composite series were between 74 % and 91 %. For each group of base monomers, in both copolymer and composite series, HEMA containing ternary formulations consistently achieved higher DC values than the non-HEMA, binary resins. On average, that increase in DC of ternary over binary resins was between 4 % and 8 % combined for copolymers and composites. The average DC of composites compared to the corresponding copolymers was lower (5 to 14) %, (3 to 14) % and (2 to 5) % for Bis-GMA, EBPADMA and UDMA matrices, respectively.
Fig. 3.
Degree of conversion (DVC; mean + standard deviation (SD; indicated by bars)) attained in copolymer and composite specimens at 24 h post-curing. The number of samples in each group n ≥ 7.
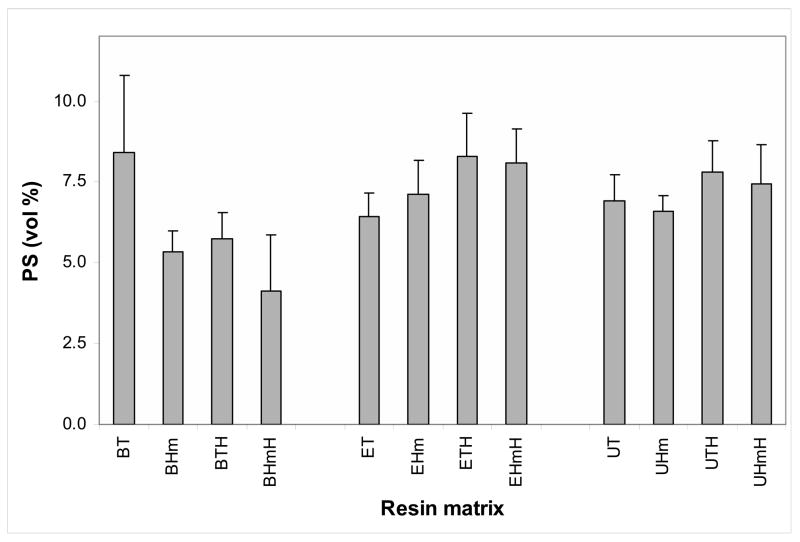
Results of PS measurements on the Zr-ACP composites in all three series of resins are illustrated in Fig. 4. The range of average PS values for the combined binary and ternary resin composites were (4.1 to 8.4) %, (6.4 to 8.3) % and (6.6 to 7.8) % for Bis-GMA, EBPADMA and UDMA matrices, respectively. No correlation with either the type of the base monomer or the type of diluent comonomer was established for the given resin formulations.
Fig. 4.
Polymerization shrinkage (PS; mean + SD) of Zr-ACP composites formulated with various binary and ternary matrices. The number of samples in each group n = 9.
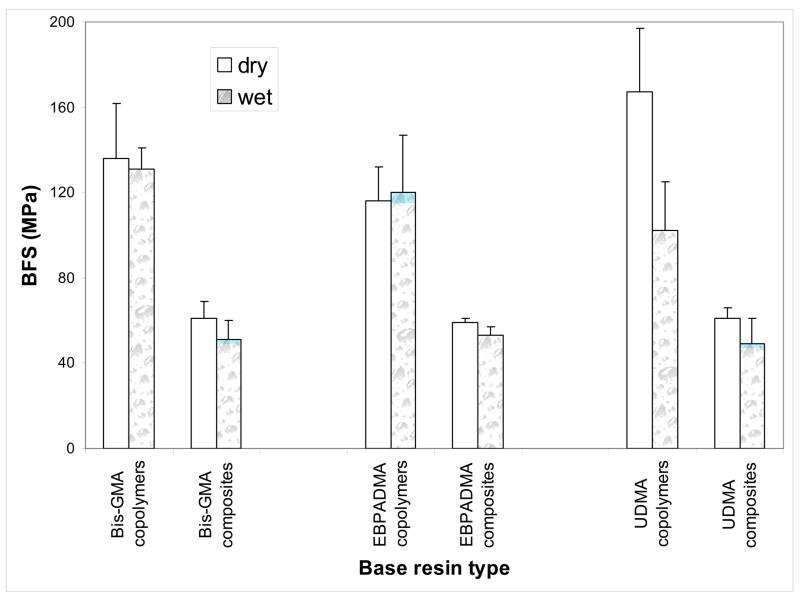
No apparent correlation existed between the resin composition and both dry and wet BFS values of copolymers and their corresponding composites. All-pair comparisons did not reveal significant differences within the Bis-GMA, EBPADMA and UDMA groups due to the resin compositional variations. Therefore, the BFS results for binary and ternary matrix materials were combined and compared as the average BFS values for Bis-GMA-, EBPADMA- and UDMA-based copolymers and composites (Fig. 5). Dry UDMA-based copolymers exhibited higher BFS ((167 ± 30) MPa) compared to dry Bis-GMA- and EBPADMA-based copolymers (on average (126 ± 22) MPa). However, the wet specimens in all three groups showed no significant differences (on average (118 ± 20) MPa). The BFS of dry composite specimens was on average (60 ± 5) MPa. The strength of all specimens was reduced to an average (51 ± 8) MPa after 2 weeks of soaking in saline.
Fig. 5.
Biaxial flexure strength (BFS; mean + SD) of dry and wet Bis-GMA, EBPADMA and UDMA copolymers and composites. The number of samples in each group n ≥ 14.
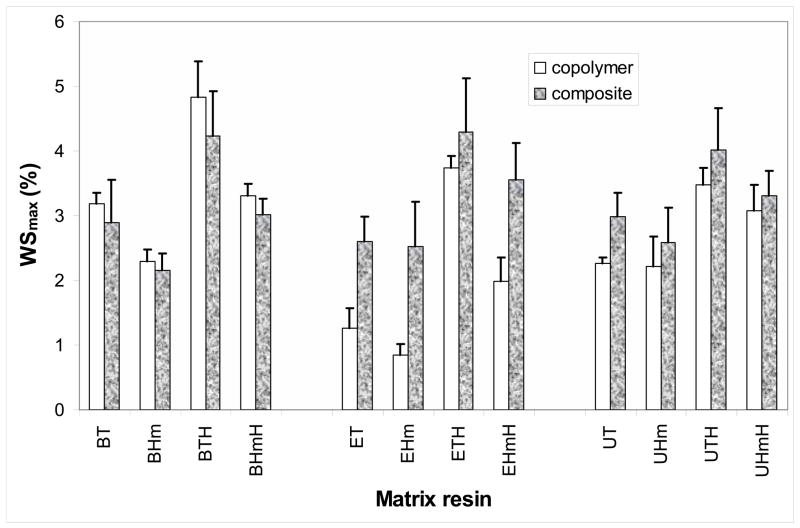
The maximum levels of WS (WSmax; mass %) following the 30 d of exposure to 75 % RH for copolymers and their composites are compared in Fig. 6. The WSmax values in copolymer series ranged from 0.9 % to 4.8 % for the combined binary and ternary Bis-GMA-, EBPADMA- and UDMA-based matrices. The WSmax values in the corresponding composite series were between 2.2 % and 4.3 %. HmDMA-containing copolymers and composites absorbed less water compared to copolymers and composites without HmDMA in the matrix. The reduction in the WS due to the inclusion of HmDMA was up to 47 % for copolymers and up to 29 % for composite specimens. Bis-GMA based composites absorbed (7 to 14) % less water than their corresponding copolymers. A reverse trend, however, was seen in EBPADMA and UDMA series: these composites absorbed (13 to 66) % and (7 to 24) % more water, respectively, than the corresponding copolymers.
Fig. 6.
Maximum water sorption, Wmax (mean + SD; in mass %), of binary and ternary copolymers and their corresponding composites after 30 d of exposure to 75 % relative humidity at room temperature. Number of specimens in each group n ≥ 4.
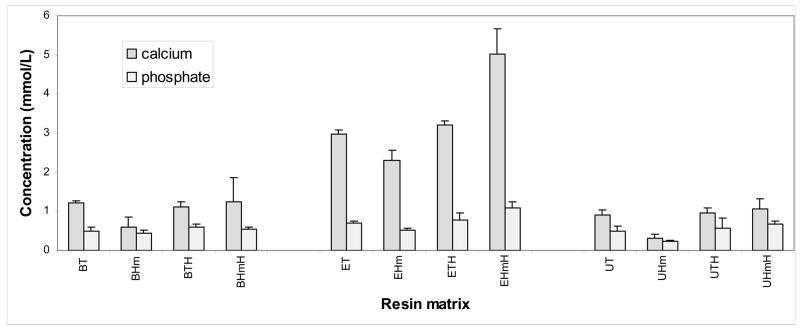
Concentrations of Ca and PO4 released from Zr-ACP filled Bis-GMA, EBPADMA and UDMA binary and ternary resin composites after 216 h of immersion in buffered saline are presented in Fig. 7. Ion release was moderately reduced in BHm and UHm matrices. In all other resin formulations of Bis-GMA and UDMA series, the levels of released ions were on average (1.1 ± 0.2) mmol/L for Ca, and (0.6 ± 0.1) mmol/L for PO4. Elevated ion release from EBPADMA matrices (on average (3.4 ± 0.5) mmol/L Ca and (0.8 ± 0.2) mmol/L PO4) was seemingly unaffected by the resin composition. Preferential Ca release from EBPADMA composites resulted in significantly higher Ca/PO4 ratios in their immersing solutions (4.3 ± 0.3) compared to Bis-GMA (2.1 ± 0.3) and UDMA (1.7 ± 0.2) systems.
Fig. 7.
Concentration of the mineral ions (mean + SD) attained after 216 h of immersion of composite specimens in buffered saline. The number of samples in each group n = 3.
DISCUSSION
Despite less than ideal performance with regard to critical properties such as PS, incomplete conversion (low DC) due to inhibition by oxygen on polymerization, stability in the oral milieu, resistance to wear and adhesion to dentin, conventional acrylic monomers and polymers still maintain their dominant position as dental polymeric materials for restorative, sealant, and adhesive applications. For example, the frequently utilized monomer systems based on Bis-GMA typically yields composites subject to loss of physicochemical and mechanical properties after prolonged exposure to the oral environment. To obtain Bis-GMA based dental resins with tractable viscosities, considerable amounts of lower molecular mass diluent monomers, such as TEGDMA, are routinely used in matrix formulations. However, the introduction of these type of diluent monomers into formulations may negatively affect the PS, and in Bis-GMA/TEGDMA or similar monomer mixtures, elevate water sorption of the resins. To overcome these deficiencies of conventional acrylic monomers, newly designed monomers, oligomers and polymers, both acrylic and non-acrylic are being synthesized/developed [13]. Dental material researchers still strive to develop resin system(s) that would achieve upon the polymerization both high DC and minimal PS. In this study we assessed the structure/property relationships of copolymers and their ACP composites utilizing as base monomers an ethoxylated Bisphenol A and an urethane dimethacrylate in addition to Bis-GMA. Besides, the more hydrophobic diluent monomer HmDMA was evaluated as a substitute for TEGDMA. Furthermore, the simplest methacrylate of the hydroxyl group-containing surface active monomers, HEMA, was introduced into ternary formulations as a potential adhesion-promoting diluent co-monomer.
The level or degree of vinyl conversion of a monomer or resin system is determined by a number of parameters, including chemical structure which affects viscosity and the glass transition temperature (Tg). For most monomer or resin systems Tg is well below room temperature. For example, Bis-GMA, a very viscous dimethacrylate quickly reaches the gel point on photo-polymerization at ambient temperatures resulting in a relatively low DC. The low conversion results from the retardation of the diffusion of monomer and other reactive species to the radical sites or pendant vinyl groups on the relatively immobilized polymer network structure. The slowdown in diffusion can be attributed to the rising Tg or viscous gel state of the growing polymer network. During photo-polymerization both the Tg and the viscosity of the growing polymer chain increase rapidly, especially as the gel point is reached. Both Tg and viscosity of the starting monomer/resin as well as Tg and viscosity changes that occur on polymerization are important factors affecting conversion.
The level of conversion of methacrylate double bonds attained during polymerization has been used to indirectly assess the potential leachability of unreacted monomeric species from bioactive ACP composites [1, 4]. Regardless of the type of base monomer utilized in this study, all copolymers, and to somewhat lesser extents their ACP composites, achieved high DCs (the lowest DC was 74 %) suggesting that these materials would likely cause only limited biocompatibility concern in clinical applications. Reportedly [14] at levels of up to 40 % by mass in Bis-GMA/TEGDMA copolymers, Bis-GMA governs the final DC via controlling the diffusion-controlled termination process. At higher levels, DC is expected to diminish due to the reduction in resin’s mobility. Surprisingly, in our experiments Bis-GMA/TEGDMA matrix containing approx. 50 % by mass Bis-GMA still attained very high DC (85 %). Under given conditions, all of our experimental formulations (copolymers and composites alike) exceeded the DC values typically obtained with Bis-GMA/TEGDMA copolymers ((55–75) % [14]). The higher DCs attained in ternary, HEMA-containing systems could be attributed to the high diffusivity of this monomer especially since the HEMA content in the resins was relatively high ((28 to 30) mass %). Addition of hydroxypropyl methacrylate, also a diluent monomethacrylate monomer structurally similar to HEMA, had similar effect on DC of Bis-GMA based resins [15].
UDMA, a less viscous and more flexible dimethacrylate with lower hydroxyl type hydrogen bonding interactions than Bis-GMA, was expected to exhibit enhanced DC relative to Bis-GMA monomer systems. We found no experimental evidence for this when the DCs of their copolymers were compared (Fig. 3). However, less reduction in DC going from copolymer to composite was seen in UDMA based formulations ((1.7 to 5.2) %) compared to BiS-GMA ((4.9 to 14.4) %) and EBPADMA matrices ((3.3 to 14.2) %). The observed effect may be attributed to the greater reactivity of the UDMA resin systems in the presence of ACP. Thus, the interaction of ACP with the UDMA resins does not appear to retard the polymerization process to the extent found with the Bis-GMA and EBAPDMA resins.
Since PS is directly correlated with DC [16] it is of no surprise that the composites based on the matrices that attained, on average, (82 ± 6) % double bond conversion, also showed relatively high contraction upon polymerization ((6.9 ± 1.3) % by volume). Undesirable consequence of such high PS would be the higher probability of formation of internal strains and gaps in the micro-structure of the composites and at the composite/tooth interface that would serve as potential sites for microleakage. The PS results appear not to be influenced by the resin matrix composition. ACP-filled (filler level 40 % by mass) experimental composites exhibited greater shrinkage than the commercial silica-filled (filler level usually (75 to 80) % by mass but much higher on than ACP on a volume basis) restorative composite materials (PS = (1.9 to 4.1) %; [17]). Their average PS values were close to the upper end of PS values for the commercial flowable composites and the lower end of PS values for adhesive resins (6.0 % and 6.7 %, respectively [18]). Intensified hydrogen bonding that may have occurred in HEMA-containing ternary matrices leading to the densification of polymerization [1] can partially explain high PS values obtained in those systems. However, for almost identical extent of shrinkage seen in binary matrices with all three base monomers we can provide no explanation. It certainly appears that from the PS standpoint our experimental resins would require compositional adjustments. Exploring beyond methacrylates and possibly employing ring-opening monomers in resin formulations [19, 20] may potentially lead to composites with improved PS without compromising their biocompatibility.
The BFS of dry and wet copolymer and composite specimens was unaffected by the composition of the resin matrix. However, ACP composites were drastically weaker than their corresponding copolymers under both dry and wet conditions. Highly agglomerated Zr-ACP particulates dispersed poorly throughout the matrix [5] regardless of the overall hydrophilicity/hydrophobicity (compositional makeup) of the resin. As a result of the uneven dispersion of ACP filler the filler/matrix interfaces become susceptible to random spatial changes for the duration of water sorption and the subsequent mineral ion release from composites. It is, therefore, the inability of such hetero-dispersed (coarse) ACP filler to closely interlock with any of the experimental resins that is responsible for the lowered strength of composite specimens. According to our latest studies performed with the more complex, four-component EBPADMA–based matrices [21] it appears that by making the ACP filler with finer particle size distribution (narrower distribution range and an order of magnitude smaller median particle diameter) a more intimate contact of ACP particles and the resin is achievable resulting in enhanced mechanical stability of the composite specimens upon soaking.
WS affects the mechanical stability of dental composites primarily via plasticization and disruption of the polymeric matrix [22]. The WS of ACP-based composites is additionally affected by the water-ACP interactions. The observed increase in WS of EBPADMA and UDMA composites vs their corresponding copolymers could be attributed to the unrestricted sorption of water by ACP agglomerates in those composites. It is, however, difficult to explain the opposite trend observed in Bis-GMA based formulations. One can only speculate that a lesser number of water sorption-prone subsurfaces/defects/voids, typical for coarse-ACP composites [5], existed in Bis-GMA resin composites.
Factors that determine the kinetics of mineral ion release from anti-demineralizing/remineralizing ACP composites include besides the nature of the polymer network structure, its permeability to water, internal pH and the rate of intra-composite conversion of ACP to thermodynamically stable apatite. Ion release data from the examined composites (Fig. 7) clearly show the accelerated Ca release, and to a lesser extent PO4 release, from EBPADMA matrices. The explanation for the observed effect could be that both binary and ternary EBPADMA polymers have lower cross-linking density compared to the analogous Bis-GMA and UDMA based polymers. Consequently, they form a more open network structure the result of which is the enhanced diffusion of ions into immersing milieu. As a product of such unrestricted ion release, the unexpectedly high Ca/PO4 ratios are seen in EBPADMA systems. Additionally, in ternary EBPADMA composites compared to the binary EBPADMA composites there was a clear upward trend in ion release with HEMA being introduced into resin formulation. It is believed that hydrophilic HEMA may increase internal mineral saturation levels by either allowing more water to be absorbed or simply enabling the filler to better access water that is already entrained. ACP filler itself contains on average 17 % by mass water of which approx. 2/3 is surface-bound [21].
CONCLUSIONS
The chemical structure and composition of the matrix phase of ACP-filled composites affected ion release, water sorption and degree of double bond conversion while having practically no effect on polymerization shrinkage and the composite’s mechanical stability upon aqueous immersion. Higher Ca and PO4 release was achieved in HEMA/EBPADMA-containing polymers. Including new types of methacrylates in resin formulation may be required to reduce the polymerization contraction of both copolymers and composites. Preliminary results with milled Zr-ACP fillers indicate that the mechanical performance of composites may be improved by better intra-composite dispersion of smaller sized ACP of narrower particle size distributions.
Acknowledgments
The authors thank the National Institute of Dental and Craniofacial Research for its support through a research grant R01 DE13169-07 and Esstech, Essington, PA, for generous contribution of the Bis-GMA, EBPADMA, UDMA, TEGDMA and HEMA monomers.
Footnotes
Certain commercial materials and equipment are identified in this work for adequate definition of the experimental procedures. In no instance does such identification imply recommendation or endorsement by ADAF or NIST, or that the material and the equipment identified is necessarily the best available for the purpose.
References
- 1.Skrtic D, Stansbury JW, Antonucci JM. Volumetric Contraction and Methacrylate Conversion in Photo-polymerized Amorphous Calcium Phosphate/methacrylate Composites. Biomaterials. 2003;24:2443–2449. doi: 10.1016/s0142-9612(02)00574-4. [DOI] [PubMed] [Google Scholar]
- 2.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physicochemical Evaluation of Bioactive Polymeric Composites Based on Hybrid Amorphous Calcium Phosphates. J Biomed Mat Res (Appl Biomater) 2000;53:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative Assessment of the Efficacy of Amorphous Calcium Phosphate/methacrylate Composites in Remineralizing Caries-like Lesions Artificially Produced in Bovine Enamel. J Dent Res. 1996;75(9):1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 4.Skrtic D, Antonucci JM, Eanes ED. Amorphous Calcium Phosphate-based Bioactive Polymeric Composites for Mineralized Tissue Regeneration. J Res Natl Inst Stands Technol. 2003;108(3):167–182. doi: 10.6028/jres.108.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skrtic D, Antonucci JM, Eanes ED, Eidelman N. Dental Composites Based on Hybrid and Surface-modified Amorphous Calcium Phosphates – A FTIR Microspectroscopic Study. Biomaterials. 2004;25:1141–1150. doi: 10.1016/j.biomaterials.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Antonucci JM, McDonough WG, Liu DW, Skrtic D. Effect of Polymer Matrices on Methacrylate Conversion and Mechanical Strength of Bioactive Composites Based on Amorphous Calcium Phosphate. Polymer Preprints. 2002;43(2):741–742. [Google Scholar]
- 7.Antonucci JM, McDonough WG, Liu DW, Skrtic D. Effect of Acidic Comonomers on Methacrylate Conversion and Mechanical Strength of Bioactive Composites Based on Amorphous Calcium Phosphate. Polymeric Materials: Science & Engineering. 2003;88:50–51. [Google Scholar]
- 8.Antonucci JM, Skrtic D. Matrix Resin Effects on Selected Physicochemical Properties of Amorphous Calcium Phosphate Composites. J Bioact Comp Polym. 2005;20(1):29–49. [Google Scholar]
- 9.Stansbury JW, Dickens S. Determination of Double Bond Conversion in Dental Resins by Near Infrared Spectroscopy. Dent Mater. 2001;17:71–79. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 10.ASTM F394-78 (re-appproved 1991). Standard Test Method for Biaxial Strength (Modulus of Rapture) of Ceramic Substrates.
- 11.Vogel GL, Chow LC, Brown WE. A Microanalytical Procedure for the Determination of Calcium, Phosphate and Fluoride in Enamel Biopsy Samples. Caries Res. 1983;17:23–31. doi: 10.1159/000260645. [DOI] [PubMed] [Google Scholar]
- 12.Murphy J, Riley JP. Single Solution Method for the Determination of Phosphate in Natural Waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- 13.Antonucci JM, Stansbury JW. Molecularly Designed Dental Polymers. In: Arshady R, editor. Desk Reference of Functional Polymers. Syntheses and Applications. American Chemical Society Publications; 1997. pp. 719–738. [Google Scholar]
- 14.Lovell LG, Stansbury JW, Syrpes DC, Bowman CN. Effects of Composition and Reactivity on the Reaction Kinetics of Dimethacrylate/dimethacrylate Copolymerizations. Macromolecules. 1999;32:3913–3921. [Google Scholar]
- 15.Venhoven BAM, DeGee AJ, Davidson CL. Polymerization Contraction and Conversion of Light-curing Bis-GMA-based Methacrylate Resins. Biomaterials. 1993;14(11):871–875. doi: 10.1016/0142-9612(93)90010-y. [DOI] [PubMed] [Google Scholar]
- 16.Silikas N, Eliades G, Watts DC. Light Intensity Effects on Resin Composite Degree of Conversion and Shrinkage Stress. Dent Mater. 2000;16:292–296. doi: 10.1016/s0109-5641(00)00020-8. [DOI] [PubMed] [Google Scholar]
- 17.Price RB, Rizkalla AS, Hall GC. Effect of Stepped Lifgt Exposure on the Volumetric Polymerization Shrinkage and Bulk Modulus of Dental Composites and an Unfilled Resin. Am J Dent. 2000;13:176–180. [PubMed] [Google Scholar]
- 18.Labella R, Lambrechts P, VanMeerbeek B, Vanherle G. Polymerization Shrinkage and Elasticity of Flowable Composites and Filled Adhesives. Dent Mater. 1999;15:128–137. doi: 10.1016/s0109-5641(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 19.Guggenberger R, Weinmann W. Exploring Beyond Methacrylates. Am J Dent. 2002;13:82D–84D. [PubMed] [Google Scholar]
- 20.Tilbrook DA, Clarke RL, Howle NE, Braden M. Photocurable Epoxy-polyol Matrices for Use in Dental Composites. Biomaterials. 2000;21:1743–1753. doi: 10.1016/s0142-9612(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Regnault WF, Antonucci JM, Skrtic D. Effect of Particle Size of an Amorphous Calcium Phosphate Filler on the Mechanical Strength and Ion release of Polymeric Composites. J Biomed Mater Res. 2005 doi: 10.1002/jbm.b.30561. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Fiero JL, Aleman JV. Sorption of Water by Epoxide Prepolymers. Macromolecules. 1982;15:1145–1149. [Google Scholar]