Abstract
This review focuses on the emerging evidence that attenuation of the production of reactive oxygen species (ROS) and inhibition of inflammatory pathways play a central role in the anti-aging cardiovascular effects of caloric restriction (CR). Particular emphasis is placed on the potential role of the plasma membrane redox system in CR-induced pathways responsible for sensing oxidative stress and increasing cellular oxidative stress resistance. We propose that CR increases bioavailability of NO, decreases vascular ROS generation, activates the Nrf2/ARE pathway inducing ROS detoxification systems, exerts anti-inflammatory effects and, thereby, suppresses initiation/progression of vascular disease that accompany aging.
Historical perspective
Almost a century ago Moreschi and Rous published separately their observations on the impact of underfeeding laboratory animals on transplanted and induced tumors 1,2. Two decades later, McCay and colleagues first observed lifespan extension in laboratory rats maintained on a CR diet 3. Since then, CR has been studied intensively with consistent results showing its beneficial effects on longevity, age-associated diseases, attenuation of functional declines, and carcinogenesis across a broad variety of species and diet formulations 4–5. Despite these observations the precise mechanism(s) underlying the effects of CR protection and lifespan extension remain unknown. It is safe to say that, calorie restriction reduces metabolic rate and oxidative damage, improves markers of diabetes such as insulin sensitivity.
CR decreases the incidence of cardiovascular disease and has been shown to alter neuroendocrine and sympathetic nervous system in laboratory animals and some of these are replicating now in ongoing human studies. In particular, the National Institute on Aging through its program, CALERIE (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy, initiated in 2002) endeavors to fund clinical trials address the feasibility of using CR as therapeutical tool as well as its effects and mechanisms in disease prevention. CALERIE studies examine the delay of aging-related comorbidities, particularly those associated with metabolic rate and biomarkers of aging, studying those that predict age-related diseases such as cardiovascular disease and type 2 diabetes 6–13.
Oxidative stress, aging and the plasma membrane
Mitochondria are the main source of ATP production. During mitochondrial oxidative phosphorylation, reactive oxygen species [ROS] are produced. ROS are associated with damage to DNA, lipids and proteins 14–16. The pathology of aging and age-related diseases involves oxidative stress as an early stage in its development 17–19 as confirmed by a decrease in antioxidant defenses and an increase in oxidative damage 20, 21. Aging is also associated with changes in levels of antioxidant capacity and oxidative damage ostensibly leading to mitochondrial impairment. These changes have been coupled to increased oxidative damage to DNA 22–25, lipids 26, 27 and proteins 23, 28–30. Accumulation of mitochondrial DNA mutations, commonly identified in age-related diseases, induce impairments of mitochondrial complexes 31–33, including mitochondrial complex III activity in aged heart 34. Impaired mitochondrial function causes shortage of ATP supply, resulting in induction of further problems in biochemical pathways 31.
The free radical theory of aging 35, 36 has generated considerable interest regarding the search for possible biochemical bases of aging processes. Many past studies have shown that CR decreases production of reactive oxygen species (ROS) production thus minimizing oxidative damage 37, 38. These studies have lead collectively to the hypothesis that CR by reducing oxidative stress extends the lifespan. The mitochondrial 39 and plasma 40 membranes are sites of active and abundant ROS production and thus are at high risk of ROS damage. Therefore, it follows that a central mechanism for the actions of CR may involve membrane alterations that either reduce ROS production or resist oxidative damage.
It has been proposed that life span is inversely related to the degree of membrane phospholipid unsaturation 41, 42 and that elucidation of this relationship can provide insight on the mechanism for life span extension with CR43. Modulation of membrane susceptibility to peroxidation, however, may be too simplistic to explain aging processes since this hypothesis, for the most part, does not consider other membrane-associated processes. Such processes include changes in cellular signaling, leakage of protons (and other ions) 44, production of ROS 39, induction of apoptosis 45, and maintenance of antioxidant systems 46–49. Membrane-induced alterations in any of these processes could have major consequences that influence oxidative stress and life span.
CR Increases CoQ-Dependent Reductases in Plasma Membranes in vivo and in vitro
Coenzyme-Q (CoQ) contributes to stabilize plasma membrane, regenerates antioxidants such as ascorbate and α–tocopherol, and regulates the extracellulary-induced ceramide-dependent apoptosis pathway49, 50. NAD(P)H-dependent reductases act at the plasma membrane to regenerate CoQH2, contributing to maintain its antioxidant properties. As a whole, both CoQ and its reductases (Fig. 1) constitute a trans-plasma membrane antioxidant redox system responsible of the above described functions 51–53.
Figure 1.
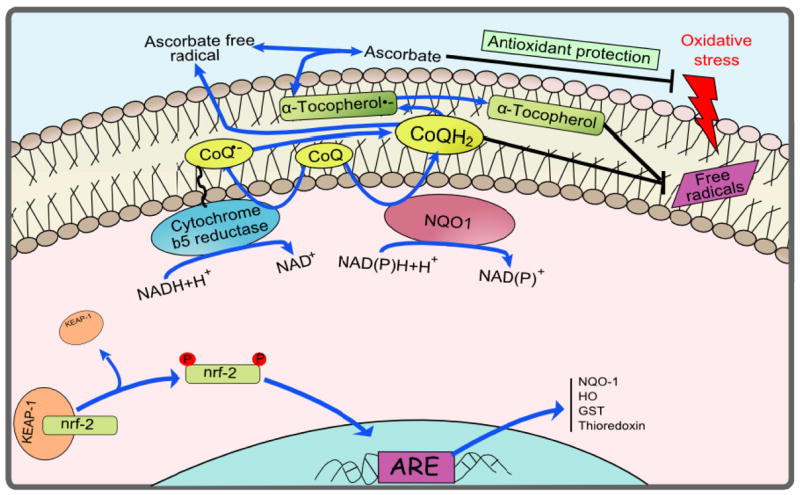
A diagram of the plasma membrane redox system. The redox cycle is shown in blue. CoQ, oxidized form of coenzyme Q; CoQ.−,semiquinone radical; CoQH2, reduced form of coenzyme Q; NQO1, NADH-quinone oxidoreductase. Modified from Hyun et al. (2006a).
The aforementioned antioxidants are maintained in their reduced forms at the plasma membrane by different CoQ-dependent reductases, NADH-dependent cytochrome b5-reductase 54 and NAD(P)H:Quinone-oxidoreductase-1 (NQO1) 55. Different dietary modifications can modulate these enzyme activities to protect the plasma membrane 56–58. Our previous work has shown that these two enzyme activities are increased in plasma membranes from rat and mouse tissues under long term CR compared to ad libitum conditions 46–48. Increases in the activities of these enzymes are due to enhanced concentration of these proteins at the plasma membrane 46, 47. Both enzyme activities are known to be present in the cardiovascular system 59–62 and we posit that they are regulated by CR in a similar manner. Data from our laboratories and others, provide support that the plasma membrane redox system is, at least in part, responsible of the maintenance of the antioxidant capacity during oxidative stress challenges induced by the diet and aging. The up-regulation of the plasma membrane redox system that occurs during CR decreases the levels of oxidative stress in aged membranes 46, 48, 63, 64. CR modifies composition of fatty acid in the plasma membrane, resulting in decreased oxidative damage including lipid peroxidation 65 66. More importantly, plasma membrane redox activities and also the content of CoQ, which decline with age, are enhanced by CR providing protection to phospholipids and preventing the lipid peroxidation reaction progression 46, 48, 63, 64.
The plasma membrane also contributes to the regulation of the cellular redox homeostasis through the maintenance of NAD(P)+/NAD(P)H ratio 67. This function is driven in cooperation with mitochondria, an interaction particularly observed in ρ° cells 68, 69 48. The ratio of pyridine nucleotides is considered an important regulator of yeast life span, as well as the establishment of respiration 70. The ratio of NAD+/NADH is also an important regulator of the deacetylase activity of Sir2, an enzyme involved in the regulation of life span in yeast. We and others have shown that expression of mammalian Sir2 (SIRT1) is induced under CR in laboratory animals and humans, as well as in cells in culture that are treated with serum from CR animals11, 47, 71–73. As we have indicated above, CR increases the activity of NAD(P)H-dependent reductases in the plasma membrane and CoQ, which likely contributes to the regulation of NAD(P)+/NAD(P)H ratio. Since NADH and NADPH are substrates for NAD(P)H oxidases, the availability of these electron donors also influences the generation of ROS by these enzymes38. There is increasing evidence for age-related up-regulation of NAD(P)H oxidases in the cardiovascular system74, 75, however, neither the role of CR-induced alterations in NAD(P)+/NAD(P)H ratio in modulation of NAD(P)H oxidase activity nor the role of the plasma membrane redox system in this process are well understood. Plasma membrane-associated redox system and mitochondria are the major source of ROS in cells, which are generated mainly when CoQ-dependent electron transport is disrupted 37, 76. Aging is associated with increased rates of stress-induced apoptosis in multiple organs77, including an increased rate of endothelial apoptosis75, 78. CR promotes the activation of stress response genes and attenuates the stress-induced apoptosis by inducing SIRT1 72, 79. Ceramide is a major signal molecule that mediates stress responses 80, and induces apoptosis through the activation of caspases 81. We have previously shown that CoQ within plasma membranes prevent the cytosolic accumulation of ceramide by inhibiting the neutral sphingomyelinase present in membranes 50, 82. It is conceivable that changes in CoQ concentration observed in liver plasma membrane induced by CR (see above) modulates the activity of neutral sphingomyelinase. We have studied this activity in plasma membrane-enriched fractions of rat liver and brain and observed that the activity of neutral sphingomyelinase decreases significantly after long-term CR 47, 46, 48
CR Induces SIRT1 Protein Levels In Vivo and In Vitro
SIRT1 is distributed in all mammalian tissues studied and modulates cellular and tissue homeostasis interacting with metabolic and stress response proteins and factors. Mounting evidence suggests that SIRT1 regulates energy metabolism, endocrine signaling and some stress responses 83. SIRT1 is also inducible by a broad variety of signals, in response to CR 79 or fasting 84, suggesting a broad role in mammalian physiology. It is becoming clear that sirtuins are regulated by stress and nutritional status in yeast, worms, flies and mammals 79, 85–87. Endocrine and energy metabolism pathways coordinate organismal development and physiology, and are intrinsic to pathologies such as cancer, neurodegeneration and diabetes. These systems respond to a variety of external signals, as diverse as environment, stress and nutrients. Sir2 regulates, in opposite ways, both replicative 88 and chronological life span in yeast 89. Extra copies of sirtuin genes extend the life spans of multicellular organisms such as worms, flies and fish 86, 90, 91]. In principle, understanding how these pathways respond to environmental and nutritional factors could enable us to better understanding to develop successful therapies.
SIRT1 regulates several transcription factors that regulate stress responses, energy metabolism and endocrine signaling, including peroxisome proliferator-activated receptor γ (PPARγ), PPARγ-coactivator 1α (PGC1-α), forkhead-box transcription factors (FOXOs), LXR and p5392–98. There is mounting data supporting that SIRT1 regulates energy metabolism, endocrine signaling and some stress responses 83, 99. The biological effects identified for sirtuins have fueled speculation that sirtuins modulate processes that affect longevity, age-related disease, diabetes and tumorigenesis 100.
CR animals and humans have significantly higher levels of SIRT1 protein in most tissues including brain, kidney, muscle, visceral fat pads, and liver 11, 79, 101. Up regulation of SIRT1 by CR is also observed in cultured cell models that recapitulate the key in vivo proliferative and phenotypic features of CR 72. Increasing the resistance of cells to apoptosis is beneficial if a cell is not critically damaged and is difficult to replace. However, this situation is clearly not always desirable if, for example, a cell is mutated or otherwise irreparably damaged. Under conditions of severe stress or pro-apoptotic signals such as TNF, SIRT1 can switch into a pro-apoptotic mode 79. A recent study by Alt et al. 102, 103 found that mouse embryonic cells lacking the SIRT1 gene continue to divide long after they should have senesced due to chronic cell stress, indicating that SIRT1 is able to suppress the proliferation of damaged cells. SIRT1 regulates several transcription factors that regulate stress responses, energy metabolism and endocrine signaling, including peroxisome proliferator-activated receptor γ (PPARγ) 97, PPARγ-coactivator 1α (PGC1-α) 98, forkhead-box transcription factors (FOXOs)92–96, LXR104 and p53. There is mounting data supporting that SIRT1 regulates energy metabolism, endocrine signaling and some stress responses83, 99. Recent reports associate SIRT1 with the regulation of apoptosis, senescence and proliferation 79, 105–107.
Vasoprotective effects of CR
CR was shown to attenuate atherogenesis in rodents108. The cardiovascular effects of CR observed so far are consistent with the view that CR may confer vasoprotection in humans, although the effects of CR on progression of atherosclerosis and plaque composition in elderly humans or aged primates109 are still not well documented. In general, CR may affect vascular health both by improving systemic risk factors for coronary artery disease (CAD) (e.g. plasma lipid and glucose levels, blood pressure) and by modulating cellular functions and gene expression in endothelial and smooth muscle cells that create a microenvironment in the vascular wall, which does not favor atherogenesis (e.g. attenuation of ROS production, anti-inflammatory effects).
Caloric restriction improves cardiovascular risk factor profile
Most current knowledge on the effects of CR on cardiovascular risk factors in humans emanates from studies in which obese individuals were treated with some form of relatively short-term dietary restriction to loose weight. High calorie diets and the resulting obesity are major risk factors for hypertension and coronary artery disease, In addition, weight loss has been associated with significant improvement in the cardiovascular risk factor profile in these individuals (including a decreased weight, body mass index, waist circumference, hip circumference, waist-to-hip ratio, total body fat, total cholesterol, serum triglyceride) 110, 111. CR exerts beneficial effects on risk factors of atherosclerosis in non-obese individuals as well. This effect has also been shown both in studies on the eight individuals (including Dr. Roy Walford, an early proponent of CR) sealed inside Biosphere 2 for two years, who had to restrict their calorie intake due to a technical problem112 and on 18 individuals who had been on voluntary CR for an average of 6 years6. Accordingly, CR in non-obese individuals elicits significant decreases in serum cholesterol, triglycerides, fasting glucose and fasting insulin levels as well as in systolic and diastolic blood pressure6, 10, 112. Studies of the effects of CR in rhesus monkeys have also shown reductions in serum triglyceride113, Lp(a) in males114 and fasting plasma glucose and insulin levels115, which likely contribute to the cardioprotective effect of CR. The available rodent data seem to corroborate this conclusion116–118.
CR increases bioavailability of NO and improves endothelial function
The direct effects of CR on vascular function and phenotype in aging are not well characterized. It is generally accepted that tonic release of NO from the endothelium exerts vasculoprotective and cardioprotective effects, such as maintenance of normal coronary blood flow, inhibition of platelet aggregation and inflammatory cell adhesion to endothelial cells and disruption of pro-inflammatory cytokine-induced signaling pathways. Abundant experimental and clinical data show that aging impairs endothelial NO production (recently reviewed elsewhere119, which has been suggested to play a role in atherogenesis. The severe impairment of NO bioavailability in aging also limits cardiac blood supply and alters myocardial O2 consumption and cardiac contractility120. Our recent data suggest that lifelong CR in rats prevents aging-induced endothelial dysfunction. Accordingly, CR elicited significant improvement of both agonist- and flow-induced, NO-mediated dilation of resistance arteries from the skeletal muscle of aged F344 rats (Fig. 2A–B), suggesting that CR increases bioavailability of NO. Available data also suggest that weight reduction with very low calorie diets improves flow-mediated vasodilation in obese individuals121, 122. It is yet to be determined whether CR can also improve endothelial function in non-obese aged monkeys109 and elderly humans independent of weight reduction.
Figure 2.
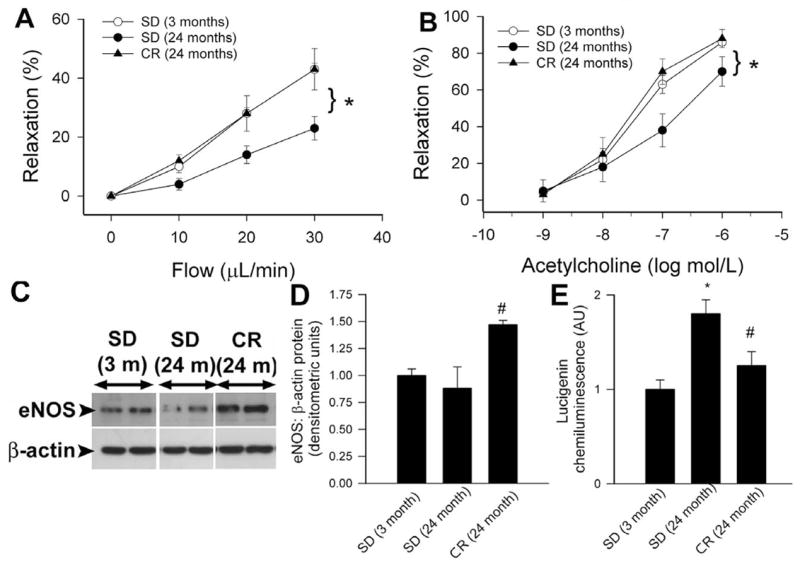
Dilations in response to step increases in intraluminal flow (Panel A) or administration of acetylcholine (Panel B) in isolated, cannulated, first order gracilis muscle arterioles (d: ~100 μm; pressurized to 80 mmHg74) of aged (24 month old) F344 rats fed a standard diet (SD) are impaired, as compared to young vessels. Lifelong caloric restriction (CR) preserved microvascular endothelial function. *P<0.05 vs. aged SD. Data are mean±S.D. (n=4 in each group). Panel C: Original Western blots showing that expression of eNOS is up-regulated in carotid arteries of aged CR rats. Bar graphs (Panel D) are summary densitometry data. #P<0.05 vs. SD. Panel E: Lucigenin chemiluminescence measurements revealed that age-related increases in O2.- production in the aorta of F344 rats are prevented by lifelong caloric restriction (CR). *P<0.05 vs. young, #P<0.05 vs. standard diet (SD)-fed rats.
The mechanisms by which CR increases bioavailability of NO improving endothelial function in aged rodents likely include up-regulation of eNOS (Fig. 2C–D). Although the upstream mediator(s) of the vascular effects of CR are not well understood, there is data suggesting that CR may regulate both eNOS activity and expression via activation of SIRT-1. An interesting study recently reported that SIRT1 and eNOS colocalize in endothelial cells, and SIRT1 deacetylates eNOS, stimulating eNOS activity and increasing endothelial nitric oxide123. Moreover, CR in mice leads to deacetylation of eNOS123, whereas SIRT1 overexpression or SIRT1 activators were shown to induce eNOS expression in endothelial cells124. Further studies are definitely needed to elucidate whether SIRT-1 activation results in increased NO bioavailability improving endothelial function in aged CR individuals.
CR may attenuate vascular inflammation in aging
Atherosclerotic vascular disease is now recognized as a chronic inflammatory disease125. There is abundant evidence showing that aging is associated with vascular inflammation promoting atherogenesis (reviewed recently elsewhere 119, 126, 127). For example, aging promotes endothelial activation, increasing the expression of adhesion molecules75, 124, 128, 129 and enhancing leukocyte adhesion to the endothelial cells124, 129, 130. Previous studies by this and other laboratories have shown that endothelial activation in aging is mediated, at least in part, by oxidative stress-induced increased NF-κB activation124, 129. In this regard it is important that CR seems to attenuate vascular NF-κB induction and endothelial activation in aged rats128, 129. CR also protected against the age-associated increase of JNK and P38 activities in aged rat aortas131. Moreover, CR similarly reversed the age-related increase of AP-1 DNA binding activity131. In aging a pro-inflammatory shift develops in the vascular cytokine expression profile (including up-regulation of TNFα, IL-1β and IL-6)74, 78, 132. Aging is also associated with increased plasma levels of inflammatory mediators (e.g. TNFα, IL-6 and CRP), both in humans and rodents7, 133, 134. In studies of CR in rats and mice, it was found that CR results in marked decreases in these inflammatory markers135, 136. The observation that CR in humans also seem to decrease serum CRP and TNFα137 provides preliminary evidence that CR may also reduce vascular inflammation in humans.
CR attenuates oxidative stress in the vasculature
Advanced age is associated with endothelial oxidative stress, which leads to functional inactivation of NO by high concentrations of O2.− resulting in an enhanced ONOO− formation74, 120, 138, 139. The role of increased oxidative and nitrosative stress in eliciting endothelial dysfunction and activation of proatherogenic inflammatory processes in aging has been recently reviewed119, 126. In 1996 Dr. Richard Weindruch’s group38 proposed that the anti-aging action of CR stems from the attenuation of the age-associated increase in oxidative stress140. Indeed, it has been amply demonstrated that CR decreases the age-associated accumulation of oxidatively damaged lipids, proteins, and nucleic acids in multiple organ systems, including the liver and skeletal muscle141–143. Our findings suggest that CR in aged rats significantly decreases vascular O2.− production (Fig. 2E). This data is in line with the findings that endothelial cells obtained from CR mice exhibit decreased O2.− and H2O2 production as compared with those obtained from mice fed ad libitum130. CR also significantly attenuates oxidative DNA damage144 and normalizes the tissue content of lipid peroxidation-derived aldehydes (HNE, MDA) in aortas of aged rats131. There are studies extant suggesting that reduction of oxidative stress in the arterial wall may contribute to the anti-atherogenic effect of CR in ApoE−/− mice108. In parenchymal tissues of experimental animals CR modulate the expression of various antioxidant enzymes, however, at present it is unclear whether this is the case in the vasculature as well. Previous studies have identified vascular NAD(P)H oxidases as an important source of ROS production in small coronary arteries, aorta and carotid arteries of aged rodents74, 75, 119. In addition, aging also increases mitochondrial ROS generation in the endothelial cells124. Future studies should elucidate how CR affects NAD(P)H oxidase activity/expression and mitochondrion-derived ROS generation145, 146 in the aged blood vessels.
There is data in the literature attributing some of the effects of CR to a decreased insulin-like signaling. Studies in Caenorhabditis elegans provided the first evidence that reduced insulin-like signaling may actually promote longevity in lower organisms. By now it is well established that insulin-like signals promote the phosphorylation and deactivation of DAF-16, a forkhead transcription factor, which is a key regulator of oxidative stress resistance and metabolism in C. elegans (reviewed in Ref.147). There is also evidence that loss of IGF-like signaling contributes to longevity response to CR in Drosophila148. The first evidence to support a role of insulin-like signals in regulation of mammalian longevity came from the observation that mice with hereditary dwarfism (Ames dwarf) have low circulating IGF-1, extended longevity and exhibit many symptoms of delayed aging149. However, the link between IGF signaling and vascular oxidative stress is likely complex. In Ames dwarf aortas endothelial ROS generation are more than in vessels of wild type mice (Ungvari, submitted 2008). Moreover, in cultured coronary arterial endothelial cells treatment with IGF significantly reduces cellular O2− and H2O2 production and ROS generation by mitochondria and up-regulates expression of antioxidant enzymes and eNOS (Ungvari, submitted 2008). These in vitro findings accord with the observations that in humans GH and IGF-I deficiency is associated with premature atherosclerosis and elevated cardiovascular disease mortality150. Recent evidence suggests that cardiovascular disease risk may also be elevated among apparently healthy individuals who have serum IGF-1 levels in the low-normal range151. There is also increasing evidence that IGF-1 may exert vasculoprotective effects in aging152, 153. By now it has been firmly established that IGF-1 protects myocardiocytes from apoptotic cell death154–156. Cardiac stem cells and early committed cells were also demonstrated to express IGF-1 receptors and secrete IGF-1157 and IGF-1 was shown to promote cardiac stem cell survival and proliferation157, 158. The findings that cardiac overexpression of IGF-1 significantly improved cardiomyocyte contractile function in old mice159 support the view that IGF-1 signaling plays protective role in the cardiovascular system and that loss of IGF-1 contributes to cardiac aging. Thus, low IGF-1 levels are less likely to be the cause of reduced ROS production and increased bioavailability of NO in the vasculature in CR.
Nrf2: a novel pathway for vasoprotection
Nrf2 (NF-E2-related factor 2) is a transcription factor that binds to the antioxidant response element (ARE) of target genes and increases the transcription of a variety of antioxidant proteins. Kelch-like ECH-associated protein-1 (Keap1) normally sequesters Nrf2 in the cytoplasm, but upon oxidation of cysteine residues Nrf2 dissociates from Keap1, translocates to the nucleus and binds to ARE sequences leading to transcriptional activation of phase II detoxifying genes (such as glutathione-S-transferase and NQO1 and antioxidant enzymes (such as glutathione reductase, glutathione peroxidase and catalase). In parenchymal tissues of the aged rat there is a significant decline in transcriptional activity of Nrf2, which causes age-related loss of glutathione synthesis160 likely promoting cellular oxidative stress. In a series of studies currently we are testing the hypothesis whether Nrf2 induction plays a role in attenuation of cellular oxidative stress in aged tissues. In this context our recent studies demonstrated that induction of Nrf2 is responsible for the anti-carcinogenic effects of CR, but is dispensable for increased insulin sensitivity. Accordingly, Nrf2 deficient mice developed tumors more readily in response to carcinogen exposure than did wild-type mice, and CR was ineffective in suppressing tumors in the Nrf2-deficient mice (Pearson K and de Cabo R, in press). The aforementioned Nrf2-dependent ROS detoxification systems are expressed in endothelial cells and previous studies have provided solid evidence that the ARE-mediated genes are regulated by atheroprotective laminar flow through a Nrf2-dependent mechanism103, 161–163. Also, induction of Nrf2 in cultured endothelial cells results in a marked increase in ARE-driven transcriptional activity and protected the cells from H2O2 -mediated cytotoxicity103. Nrf2 also suppresses TNFα-induced endothelial activation and inhibits monocyte adhesiveness to the endothelial cells103. Although presently it is unknown how aging affects Nrf2 transcriptional activity in the vascular endothelial and smooth muscle cell, we have strong evidence for an age-dependent decline in glutathione synthesis in aged rat aortas, which is prevented by CR (Csiszar A, Ungvari Z, Pinto J, unpublished data 2008). Further studies are evidently needed to test the hypothesis that the Nrf2/ARE pathway is induced in aged arteries, which acts as an endogenous atheroprotective system for antioxidant protection and suppression of redox-sensitive vascular inflammation.
Conclusions and perspectives
Oxidative stress plays an important role in the pathogenesis of CAD by mediating expression of inflammatory genes and eliciting oxidative modification of lipoprotein particles. CR seems to attenuate both vascular oxidative stress and exert anti-inflammatory effects in aged animals. We posit that CR activates the Nrf2/ARE pathway, which may serve as an endogenous antioxidant system within the vasculature increasing cellular oxidative stress tolerance. CR also increases bioavailability of anti-atherogenic NO and augments endothelial function. In addition, CR exerts beneficial effects on a range of systemic cardiovascular risk factors. There is a great deal of effort on dissecting the pathways that invoke CR benefits in order to develop pharmacological agents that would act as CR mimetics164–166. Several of the currently proposed CR mimetics are phytochemicals (resveratrol, quercetin and curcumin) that act, at least in part, through the activation of Nrf2 pathway 167 168–170. Importantly, newly identified CR mimetics, such as resveratrol, exert cardiovascular effects that are remarkably similar to those of CR. Accordingly, resveratrol increases vascular oxidative stress resistance171, up-regulate eNOS171, inhibit endothelial activation172 and vascular inflammatory gene expression171 and activates both SIRT1 and the Nrf2/ARE pathways providing a pharmacological alternative for CR for the prevention of CAD in the elderly.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging and grants from the American Heart Association (0430108N and 0435140N) and the NIH (HL077256 and HL43023 to ZU).
References
- 1.Moreschi C. Beziehungen zwischen Ernahrung und Tumorwachstum. Z fur Immunitatsforsch. 1909;2:661–675. [Google Scholar]
- 2.Rous P. The influence of diet on transplanted and spontaneous tumors. Journal of Experimental Medicine. 1914;20:433–451. doi: 10.1084/jem.20.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCay CCM, Crowell MMF, Maynard LLA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Journal of Nutrition. 1935;10:63–79. [PubMed] [Google Scholar]
- 4.Tannenbaum A. The initiation and growth of tumors. Introduction. Effects of underfeeding. American Journal of Cancer. 1940;38:335–350. [Google Scholar]
- 5.Kritchevsky D. Caloric restriction and experimental carcinogenesis. Hybridoma and hybridomics. 2002;21:147–151. doi: 10.1089/153685902317401753. [DOI] [PubMed] [Google Scholar]
- 6.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans 1. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilbronn LK, Clifton PM. C-reactive protein and coronary artery disease: influence of obesity, caloric restriction and weight loss. J Nutr Biochem. 2002;13:316–321. doi: 10.1016/s0955-2863(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 8.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS medicine. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. The American journal of clinical nutrition. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 10.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 11.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. The Journal of clinical endocrinology and metabolism. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. The American journal of clinical nutrition. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B. Free radicals, proteins and DNA: oxidative damage versus redox regulation. Biochem Soc Trans. 1996;24:1023–1027. doi: 10.1042/bst0241023. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 17.Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Barja G. Aging in vertebrates, and the effect of caloric restriction: a mitochondrial free radical production-DNA damage mechanism? Biol Rev Camb Philos Soc. 2004;79:235–251. doi: 10.1017/s1464793103006213. [DOI] [PubMed] [Google Scholar]
- 19.Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 20.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53 Suppl 3:S26–36. S36–28. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 21.Moreira PI, Honda K, Liu Q, Santos MS, Oliveira CR, Aliev G, Nunomura A, Zhu X, Smith MA, Perry G. Oxidative stress: the old enemy in Alzheimer’s disease pathophysiology. Curr Alzheimer Res. 2005;2:403–408. doi: 10.2174/156720505774330537. [DOI] [PubMed] [Google Scholar]
- 22.Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 23.Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 24.Fitzmaurice PS, Shaw IC, Kleiner HE, Miller RT, Monks TJ, Lau SS, Mitchell JD, Lynch PG. Evidence for DNA damage in amyotrophic lateral sclerosis. Muscle Nerve. 1996;19:797–798. [PubMed] [Google Scholar]
- 25.Warita H, Hayashi T, Murakami T, Manabe Y, Abe K. Oxidative damage to mitochondrial DNA in spinal motoneurons of transgenic ALS mice. Brain Res Mol Brain Res. 2001;89:147–152. doi: 10.1016/s0169-328x(01)00029-8. [DOI] [PubMed] [Google Scholar]
- 26.Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 27.Volchegorskii IA, Shemyakov SE, Turygin VV, Malinovskaya NV. The age dynamics of monoamine oxidase activity and levels of lipid peroxidation products in the human brain. Neurosci Behav Physiol. 2004;34:303–305. doi: 10.1023/b:neab.0000018736.84877.4f. [DOI] [PubMed] [Google Scholar]
- 28.Cakatay U, Telci A, Kayali R, Tekeli F, Akcay T, Sivas A. Relation of oxidative protein damage and nitrotyrosine levels in the aging rat brain. Exp Gerontol. 2001;36:221–229. doi: 10.1016/s0531-5565(00)00197-2. [DOI] [PubMed] [Google Scholar]
- 29.Shaw PJ, Ince PG, Falkous G, Mantle D. Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann Neurol. 1995;38:691–695. doi: 10.1002/ana.410380424. [DOI] [PubMed] [Google Scholar]
- 30.Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- 31.Schapira AH. Mitochondrial dysfunction in neurodegenerative disorders. Biochim Biophys Acta. 1998;1366:225–233. doi: 10.1016/s0005-2728(98)00115-7. [DOI] [PubMed] [Google Scholar]
- 32.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 34.Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda-Saito M, Turkaly PJ, Hoppel CL. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J Mol Cell Cardiol. 2001;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- 35.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 36.Droge W. Free radicals in the physiological control of cell function 1. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 37.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 38.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Donnell VB, Azzi A. High rates of extracellular superoxide generation by cultured human fibroblasts: involvement of a lipid-metabolizing enzyme. Biochem J. 1996;318:805–812. doi: 10.1042/bj3180805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pamplona R, Barja G, Portero-Otin M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? 2002;959:475–490. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 42.Hubert MF, Laroque P, Gillet JP, Keenan KP. The effects of diet, ad Libitum feeding, and moderate and severe dietary restriction on body weight, survival, clinical pathology parameters, and cause of death in control Sprague-Dawley rats. 2000;58:195–207. doi: 10.1093/toxsci/58.1.195. [DOI] [PubMed] [Google Scholar]
- 43.Yu BP, Lim BO, Sugano M. Dietary restriction downregulates free radical and lipid peroxide production: plausible mechanism for elongation of life span 2. 2002;48:257–264. doi: 10.3177/jnsv.48.257. [DOI] [PubMed] [Google Scholar]
- 44.Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. 2000;29:946–968. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 45.Mimeault M. New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett. 2002;530:9–16. doi: 10.1016/s0014-5793(02)03432-4. [DOI] [PubMed] [Google Scholar]
- 46.Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Cabo R, Cabello R, Rios M, Lopez-Lluch G, Ingram DK, Lane MA, Navas P. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver 5. Exp Gerontology. 2004;39:297–304. doi: 10.1016/j.exger.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Hyun DH, Hunt ND, Emerson SS, Hernandez JO, Mattson MP, de Cabo R. Up-regulation of plasma membrane-associated redox activities in neuronal cells lacking functional mitochondria. J Neurochem. 2007;100:1364–1374. doi: 10.1111/j.1471-4159.2006.04411.x. [DOI] [PubMed] [Google Scholar]
- 49.Navarro F, Navas P, Burgess JR, Bello RI, de Cabo R, Arroyo A, Villalba JM. Vitamin E and selenium deficiency induces expression of the ubiquinone-dependent antioxidant system at the plasma membrane 6. FASEB J. 1998;12:1665–1673. doi: 10.1096/fasebj.12.15.1665. [DOI] [PubMed] [Google Scholar]
- 50.Navas P, Fernandez-Ayala DM, Martin SF, Lopez-Lluch G, De Caboa R, Rodriguez-Aguilera JC, Villalba JM. Ceramide-dependent caspase 3 activation is prevented by coenzyme Q from plasma membrane in serum-deprived cells. Free Radic Res. 2002;36:369–374. doi: 10.1080/10715760290021207. [DOI] [PubMed] [Google Scholar]
- 51.Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 2007;7 Suppl:S34–40. doi: 10.1016/j.mito.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Villalba JM, Crane FL, Navas P. In: Plasma Membrane Redox System and their role in Biological Stress and Disease. Asard H, Berczi A, Caubergs RJ, editors. Vol. 1. Dordrecht: Kluwer; 1998. [Google Scholar]
- 53.Villalba JM, Navarro F, Cordoba F, Serrano A, Arroyo A, Crane FL, Navas P. Coenzyme Q reductase from liver plasma membrane: purification and role in trans-plasma-membrane electron transport. Proc Natl Acad Sci USA. 1995;92:4887–4891. doi: 10.1073/pnas.92.11.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villalba JM, Navarro F, Cordoba F, Serrano A, Arroyo A, Crane FL, Navas P. Coenzyme Q reductase from liver plasma membrane: purification and role in trans-plasma-membrane electron transport. 1995;92:4887–4891. doi: 10.1073/pnas.92.11.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beyer RE. The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and coenzyme Q 28. 1994;26:349–358. doi: 10.1007/BF00762775. [DOI] [PubMed] [Google Scholar]
- 56.Navarro F, Navas P, Burgess JR, Bello RI, de Cabo R, Arroyo A, Villalba JM. Vitamin E and selenium deficiency induces expression of the ubiquinone-dependent antioxidant system at the plasma membrane 6. 1998;12:1665–1673. doi: 10.1096/fasebj.12.15.1665. [DOI] [PubMed] [Google Scholar]
- 57.Mataix J, Manas M, Quiles J, Battino M, Cassinello M, Lopez-Frias M, Huertas JR. Coenzyme Q content depends upon oxidative stress and dietary fat unsaturation. Mol Aspects Med. 1997;18 Suppl:S129–135. doi: 10.1016/s0098-2997(97)00019-8. [DOI] [PubMed] [Google Scholar]
- 58.Gomez-Diaz C, Bello RI, Lopez-Lluch G, Forthoffer N, Navas P, Villalba JM. Antioxidant response induced by serum withdrawal protects HL-60 cells against inhibition of NAD(P)H:quinone oxidoreductase 1 2. 2003;18:219–228. doi: 10.1002/biof.5520180224. [DOI] [PubMed] [Google Scholar]
- 59.Merker MP, Audi SH, Bongard RD, Lindemer BJ, Krenz GS. Influence of pulmonary arterial endothelial cells on quinone redox status: effect of hyperoxia-induced NAD(P)H:quinone oxidoreductase 1. Am J Physiol Lung Cell Mol Physiol. 2006;290:L607–619. doi: 10.1152/ajplung.00302.2005. [DOI] [PubMed] [Google Scholar]
- 60.Merker MP, Audi SH, Lindemer BJ, Krenz GS, Bongard RD. Role of mitochondrial electron transport complex I in coenzyme Q1 reduction by intact pulmonary arterial endothelial cells and the effect of hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;293:L809–819. doi: 10.1152/ajplung.00448.2006. [DOI] [PubMed] [Google Scholar]
- 61.Merker MP, Bongard RD, Kettenhofen NJ, Okamoto Y, Dawson CA. Intracellular redox status affects transplasma membrane electron transport in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L36–L43. doi: 10.1152/ajplung.00283.2001. [DOI] [PubMed] [Google Scholar]
- 62.Wolin MS, Ahmad M, Gao Q, Gupte SA. Cytosolic NAD(P)H regulation of redox signaling and vascular oxygen sensing. Antioxid Redox Signal. 2007;9:671–678. doi: 10.1089/ars.2007.1559. [DOI] [PubMed] [Google Scholar]
- 63.De Cabo R, Cabello R, Rios M, Lopez-Lluch G, Ingram DK, Lane MA, Navas P. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol. 2004;39:297–304. doi: 10.1016/j.exger.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 64.López-Lluch G, Rios G, Lane MA, Navas P, de Cabo R. Mouse liver plasma membrane redox system activity is altered by aging and modulated by calorie restriction. AGE. 2005;27:153–160. doi: 10.1007/s11357-005-2726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng J, Mutcherson R, 2nd, Helfand SL. Calorie restriction delays lipid oxidative damage in Drosophila melanogaster. Aging Cell. 2005;4(4):209–216. doi: 10.1111/j.1474-9726.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 67.Merker MP, Bongard RD, Kettenhofen NJ, Okamoto Y, Dawson CA. Intracellular redox status affects transplasma membrane electron transport in pulmonary arterial endothelial cells. 2002;282:L36–L43. doi: 10.1152/ajplung.00283.2001. [DOI] [PubMed] [Google Scholar]
- 68.Larm JA, Vaillant F, Linnane AW, Lawen A. Up-regulation of the plasma membrane oxidoreductase as a prerequisite for the viability of human Namalwa rho 0 cells 3. 1994;269:30097–30100. [PubMed] [Google Scholar]
- 69.Gomez-Diaz C, Villalba JM, Perez-Vicente R, Crane FL, Navas P. Ascorbate stabilization is stimulated in rho(0)HL-60 cells by CoQ10 increase at the plasma membrane 17. 1997;234:79–81. doi: 10.1006/bbrc.1997.6582. [DOI] [PubMed] [Google Scholar]
- 70.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae 4. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 71.Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Cabo R, Furer-Galban S, Anson RM, Gilman C, Gorospe M, Lane MA. An in vitro model of caloric restriction. Exp Gerontol. 2003;38:631–639. doi: 10.1016/s0531-5565(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 73.Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis 1. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 74.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 75.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective Effects of Anti-Tumor Necrosis Factor-{alpha} Treatment in Aging. The American journal of pathology. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macho A, Calzado MA, Munoz-Blanco J, Gomez-Diaz C, Gajate C, Mollinedo F, Navas P, Munoz E. Selective induction of apoptosis by capsaicin in transformed cells: the role of reactive oxygen species and calcium. Cell Death Differ. 1999;6:155–165. doi: 10.1038/sj.cdd.4400465. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Chong E, Herman B. Age-associated increases in the activity of multiple caspases in Fisher 344 rat organs. Exp Gerontol. 2002;37:777–789. doi: 10.1016/s0531-5565(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 78.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 79.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 80.Hannun YA. Functions of ceramide in coordinating cellular responses to stress 7. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 81.Mizushima N, Koike R, Kohsaka H, Kushi Y, Handa S, Yagita H, Miyasaka N. Ceramide induces apoptosis via CPP32 activation. FEBS Lett. 1996;395:267–271. doi: 10.1016/0014-5793(96)01050-2. [DOI] [PubMed] [Google Scholar]
- 82.Martin SF, Gomez-Diaz C, Bello RI, Navas P, Villalba JM. Inhibition of neutral Mg2+-dependent sphingomyelinase by ubiquinol-mediated plasma membrane electron transport. Protoplasma. 2003;221:109–116. doi: 10.1007/s00709-002-0070-3. [DOI] [PubMed] [Google Scholar]
- 83.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 84.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 85.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae 4. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 86.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Oh SW, Deplancke B, Luo J, Walhout AJ, Tissenbaum HA. C. elegans 14-3-3 proteins regulate life span and interact with SIR-2.1 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:741–747. doi: 10.1016/j.mad.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 90.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 91.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 92.Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004;14:408–412. doi: 10.1016/j.tcb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 94.Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16:237–243. [PubMed] [Google Scholar]
- 95.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 96.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 97.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 99.Leibiger IB, Berggren PO. Sirt1: a metabolic master switch that modulates lifespan. Nat Med. 2006;12:34–36. 36. doi: 10.1038/nm0106-34. [DOI] [PubMed] [Google Scholar]
- 100.Yang T, Chan NY, Sauve AA. Syntheses of nicotinamide riboside and derivatives: effective agents for increasing nicotinamide adenine dinucleotide concentrations in Mammalian cells. J Med Chem. 2007;50:6458–6461. doi: 10.1021/jm701001c. [DOI] [PubMed] [Google Scholar]
- 101.Bartke A, Masternak MM, Al-Regaiey KA, Bonkowski MS. Effects of dietary restriction on the expression of insulin-signaling-related genes in long-lived mutant mice. Interdiscip Top Gerontol. 2007;35:69–82. doi: 10.1159/000096556. [DOI] [PubMed] [Google Scholar]
- 102.Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, Serrano L, Sternglanz R, Reinberg D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006;20:1256–1261. doi: 10.1101/gad.1412706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 104.Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27:708–715. doi: 10.1111/j.1478-3231.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 105.Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol. 2003;15:241–246. doi: 10.1016/s0955-0674(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 106.Chua KF, Mostoslavsky R, Lombard DB, Pang WW, Saito S, Franco S, Kaushal D, Cheng HL, Fischer MR, Stokes N, Murphy MM, Appella E, Alt FW. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell metabolism. 2005;2:67–76. doi: 10.1016/j.cmet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 107.Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. The EMBO journal. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo Z, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, Richardson A, Reddick R. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mechanisms of ageing and development. 2002;123:1121–1131. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 109.Cefalu WT, Wang ZQ, Bell-Farrow AD, Collins J, Morgan T, Wagner JD. Caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): metabolic, physiologic, and atherosclerotic measures from a 4-year intervention trial. J Gerontol A Biol Sci Med Sci. 2004;59:1007–1014. doi: 10.1093/gerona/59.10.b1007. [DOI] [PubMed] [Google Scholar]
- 110.Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, Kim SM, Lee SY, Ahn SM, Kim YK, Kim HJ, Kim DJ, Lee KW. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. 2007 doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 111.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. Jama. 2004;292:2482–2490. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 112.Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci U S A. 1992;89(23):11533–11537. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verdery RB, Ingram DK, Roth GS, Lane MA. Caloric restriction increases HDL2 levels in rhesus monkeys (Macaca mulatta) The American journal of physiology. 1997;273:E714–719. doi: 10.1152/ajpendo.1997.273.4.E714. [DOI] [PubMed] [Google Scholar]
- 114.Edwards IJ, Rudel LL, Terry JG, Kemnitz JW, Weindruch R, Zaccaro DJ, Cefalu WT. Caloric restriction lowers plasma lipoprotein (a) in male but not female rhesus monkeys. Experimental gerontology. 2001;36:1413–1418. doi: 10.1016/s0531-5565(01)00107-3. [DOI] [PubMed] [Google Scholar]
- 115.Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergman RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. The American journal of physiology. 1994;266:E540–547. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- 116.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 117.Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J Gerontol. 1985;40:671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- 118.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- 119.Csiszar A, Pacher P, Kaley G, Ungvari Z. Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol. 2005;3:285–291. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2003;285:H1015–1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- 121.Raitakari M, Ilvonen T, Ahotupa M, Lehtimaki T, Harmoinen A, Suominen P, Elo J, Hartiala J, Raitakari OT. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:124–128. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- 122.Sasaki S, Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, Matsuura H, Goto C, Oshima T, Chayama K. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. American journal of hypertension. 2002;15:302–309. doi: 10.1016/s0895-7061(01)02322-6. [DOI] [PubMed] [Google Scholar]
- 123.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 125.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 126.Ungvari Z, Csiszar A, Kaley G. Vascular Inflammation in Aging. Herz. 2004;29:733–740. doi: 10.1007/s00059-004-2625-x. [DOI] [PubMed] [Google Scholar]
- 127.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 128.Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction 1. 2004;18:320–322. doi: 10.1096/fj.03-0849fje. [DOI] [PubMed] [Google Scholar]
- 129.Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim MS, Kim YJ, Lee J, Yu BP, Chung HY. Upregulation of aortic adhesion molecules during aging. The journals of gerontology. 2006;61:232–244. doi: 10.1093/gerona/61.3.232. [DOI] [PubMed] [Google Scholar]
- 130.Yang H, Shi M, Story J, Richardson A, Guo Z. Food restriction attenuates age-related increase in the sensitivity of endothelial cells to oxidized lipids. J Gerontol A Biol Sci Med Sci. 2004;59:316–323. doi: 10.1093/gerona/59.4.b316. [DOI] [PubMed] [Google Scholar]
- 131.Castello L, Froio T, Cavallini G, Biasi F, Sapino A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto E. Calorie restriction protects against age-related rat aorta sclerosis. The FASEB journal. 2005;19:1863–1865. doi: 10.1096/fj.04-2864fje. [DOI] [PubMed] [Google Scholar]
- 132.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. The FASEB journal. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 133.Ershler WB, Sun WH, Binkley N, Gravenstein S, Volk MJ, Kamoske G, Klopp RG, Roecker EB, Daynes RA, Weindruch R. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–230. [PubMed] [Google Scholar]
- 134.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. The FASEB journal. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 135.Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 136.Kalani R, Judge S, Carter C, Pahor M, Leeuwenburgh C. Effects of caloric restriction and exercise on age-related, chronic inflammation assessed by C-reactive protein and interleukin-6. The journals of gerontology. 2006;61:211–217. doi: 10.1093/gerona/61.3.211. [DOI] [PubMed] [Google Scholar]
- 137.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 138.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 140.Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. American journal of physiology. 2005;289:E429–438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- 141.Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Harper ME, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Ageing, oxidative stress, and mitochondrial uncoupling. Acta Physiol Scand. 2004;182:321–331. doi: 10.1111/j.1365-201X.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 143.Sanz A, Gredilla R, Pamplona R, Portero-Otin M, Vara E, Tresguerres JA, Barja G. Effect of insulin and growth hormone on rat heart and liver oxidative stress in control and caloric restricted animals. Biogerontology. 2005;6:15–26. doi: 10.1007/s10522-004-7380-0. [DOI] [PubMed] [Google Scholar]
- 144.Guo ZM, Yang H, Hamilton ML, VanRemmen H, Richardson A. Effects of age and food restriction on oxidative DNA damage and antioxidant enzyme activities in the mouse aorta. Mechanisms of ageing and development. 2001;122:1771–1786. doi: 10.1016/s0047-6374(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 145.Minamiyama Y, Bito Y, Takemura S, Takahashi Y, Kodai S, Mizuguchi S, Nishikawa Y, Suehiro S, Okada S. Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. The Journal of pharmacology and experimental therapeutics. 2007;320:535–543. doi: 10.1124/jpet.106.110460. [DOI] [PubMed] [Google Scholar]
- 146.Judge S, Judge A, Grune T, Leeuwenburgh C. Short-term CR decreases cardiac mitochondrial oxidant production but increases carbonyl content. American journal of physiology. 2004;286:R254–259. doi: 10.1152/ajpregu.00502.2003. [DOI] [PubMed] [Google Scholar]
- 147.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 148.Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mechanisms of ageing and development. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 149.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 150.Elhadd TA, Abdu TA, Oxtoby J, Kennedy G, McLaren M, Neary R, Belch JJ, Clayton RN. Biochemical and biophysical markers of endothelial dysfunction in adults with hypopituitarism and severe GH deficiency. The Journal of clinical endocrinology and metabolism. 2001;86:4223–4232. doi: 10.1210/jcem.86.9.7813. [DOI] [PubMed] [Google Scholar]
- 151.Roubenoff R, Parise H, Payette HA, Abad LW, D’Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 152.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 153.Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer’s disease. The Journal of neuroscience. 2007;27:824–831. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leri A, Liu Y, Wang X, Kajstura J, Malhotra A, Meggs LG, Anversa P. Overexpression of insulin-like growth factor-1 attenuates the myocyte renin-angiotensin system in transgenic mice. Circulation research. 1999;84:752–762. doi: 10.1161/01.res.84.7.752. [DOI] [PubMed] [Google Scholar]
- 156.Li B, Setoguchi M, Wang X, Andreoli AM, Leri A, Malhotra A, Kajstura J, Anversa P. Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circulation research. 1999;84:1007–1019. doi: 10.1161/01.res.84.9.1007. [DOI] [PubMed] [Google Scholar]
- 157.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circulation research. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 158.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 159.Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Endocrinol Metab. 2007;292:H1398–1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 160.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circulation research. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 162.Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, Ishii T, Kodama T, Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free radical biology & medicine. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 163.Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. The Journal of biological chemistry. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 164.Ingram DK, Anson RM, de Cabo R, Mamczarz J, Zhu M, Mattison J, Lane MA, Roth GS. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 165.Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 166.Lane MA, Roth GS, Ingram DK. Caloric restriction mimetics: a novel approach for biogerontology. Methods Mol Biol. 2007;371:143–149. doi: 10.1007/978-1-59745-361-5_11. [DOI] [PubMed] [Google Scholar]
- 167.Hsieh TC, Lu X, Wang Z, Wu JM. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element ARE and is accompanied by nuclear translocation of transcription factor Nrf2. Medicinal chemistry (Shariqah, United Arab Emirates) 2006;2:275–285. doi: 10.2174/157340606776930709. [DOI] [PubMed] [Google Scholar]
- 168.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochemical and biophysical research communications. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 169.Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 170.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. American journal of physiology. 2007;292:H2417–2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 172.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: role of NF-{kappa}B inhibition. The American journal of physiology. 2006;291:H1694–1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]


