Summary
The authors determined the safety and efficacy of recombinant high-dose interleukin-2 administration in patients with brain metastases. This retrospective review included 1,069 patients with metastatic melanoma or renal cell carcinoma who received high-dose interleukin-2 alone or in combination with other immunotherapy or chemotherapy from July 1985–July 2000. All patients were evaluated for both toxicity and response. Only the first exposure to interleukin-2 was considered. Parameters evaluated among the groups included toxicity profiles, reasons for stopping treatment, number of interleukin-2 doses per cycle, and response to therapy. Three patient groups were compared. Group 1 (n = 27) comprised patients with previously treated brain metastases (surgery or radiation), group 2 (n = 37) comprised patients with untreated brain metastases, and group 3 (n = 1,005) comprised patients without brain metastases. For most comparisons between patients with brain metastases and those without, no significant differences were noted in toxicity profiles or reasons for stopping interleukin-2 therapy. Patients with previously treated brain metastases received fewer interleukin-2 doses per cycle (median, 6.5) than patients with previously untreated brain metastases (median, 7.5) or patients without brain metastases (median, 7.5). Patients with previously treated brain metastases demonstrated an 18.5% overall clinical response to interleukin-2 treatment. However, patients with evaluable (previously untreated) brain metastases had an overall 5.6% response rate, which was less than the 19.8% response rate of patients without brain metastases. Two of thirty-six patients with evaluable brain metastases demonstrated objective regression of intracranial and extracranial disease after receiving interleukin-2. Carefully selected patients with brain metastases can safely receive high-dose interleukin-2, and some can experience a response to treatment at intracranial and extracranial disease sites.
Keywords: Interleukin, 2, Melanoma, Brain metastases, Safety
Metastatic central nervous system lesions are a common complication of malignant disease and represent a significant cause of morbidity and mortality. Brain metastases are detected clinically in 8%–46% of patients with malignant melanoma (1). Brain metastases develop in 8% of patients with cutaneous melanoma as the first site of distant disease, with a subsequent median survival of 5 months that decreases to 1.4 months if extracranial metastatic disease is present (2). The incidence of brain metastases in patients with renal cell carcinoma is 10%–13% (3). Patients with brain metastases have generally been excluded from treatment with recombinant high-dose interleukin-2 (IL-2) because of perceived potential toxicities. However, the ability to use IL-2 would be desirable because its use has resulted in objective clinical tumor regression in 17% of patients with metastatic melanoma or renal cell carcinoma (4). The US Food and Drug Administration has approved high-dose IL-2 for the treatment of patients with metastatic renal cell carcinoma and metastatic melanoma.
High-dose IL-2 has been associated with various self-limited toxicities, and the use of IL-2 in patients with brain metastases has been avoided (5). The fear of increased intracranial pressure from edema caused by IL-2–induced capillary leak syndrome by treatment, and of the potential for hemorrhage into these lesions during IL-2–associated thrombocytopenia, led to the exclusion of patients with brain metastases from entry into clinical trials. Additionally, the use of IL-2 has been avoided in patients with central nervous system lesions because IL-2 may induce behavioral changes, confusion, lethargy, and (rarely) coma (5).
Anecdotal evidence supporting the use of IL-2 in carefully selected patients with brain metastases has been published. No apparent additional morbidity among this patient population has been reported, and evidence of tumor regression has been observed (6,7). The results of IL-2 therapy in the treatment of patients with metastatic melanoma and renal cell carcinoma have prompted interest in broadening the application of IL-2 therapy to include carefully selected patients with brain metastases. In this retrospective review, the safety and efficacy of IL-2 administration in patients with brain and widely metastatic disease was evaluated.
MATERIALS AND METHODS
This retrospective review included 1,069 patients with metastatic melanoma or renal cell carcinoma who received IL-2 at the Surgery Branch of the National Cancer Institute from July 1985–July 2000. All patients had measurable progressive metastatic melanoma or renal cell carcinoma, and had received no treatment of their disease for 1 month before enrollment into the clinical trial. Patients who were pregnant, steroid dependent, hepatitis B surface antigen positive, or antihuman immunodeficiency virus antibody positive were ineligible. Additionally, patients with severe respiratory, cardiovascular, or renal disease were ineligible for enrollment in clinical trials.
All patients received IL-2 by intravenous bolus infusion at a dose of 720,000 IU/kg (Cetus-Oncology Division, Chiron; Emeryville, CA, U.S.A.) alone or in combination with lymphokine-activated killer cells, tumor-infiltrating lymphocytes, chemotherapy agents, or vaccines (7–11). Doses of IL-2 were administered as tolerated by the patient to a maximum of 12–15 consecutive doses per treatment cycle. A treatment cycle was discontinued when moderate to severe toxicity was sustained and not readily reversed by supportive therapeutic interventions, or by patient refusal.
All patients were evaluated for toxicity and response. Toxicities sustained by patients receiving IL-2 were documented and recorded on a graded scale from 0–4, generally according to National Cancer Institute common toxicity criteria. Grade 0–2 represented absent or low toxicity, whereas moderate toxicity was scored as grade 3 and severe toxicity as grade 4. Only the first exposure to IL-2 was considered for entry into the study. Response to IL-2 therapy was assessed approximately 2 months after the start of each course of therapy. A complete response was defined as the disappearance of all measurable sites of disease. A partial response was defined as a 50% or greater decrease in the sum of the product of the longest perpendicular diameters of all lesions lasting 1 month, without an increase of any tumor or the appearance of any new tumor.
In most of the clinical protocols used, the presence of brain metastases was generally considered an exclusion criterion for administration of high-dose IL-2. However, carefully selected patients received treatment with IL-2. Patients with a limited number of brain metastases that were small, had little or no edema, or were effectively managed with surgery or radiation were considered eligible for IL-2–based therapy. All such patients that received high-dose IL-2 were included in this study and compared with patients without brain metastases. Patients were divided into three groups for the purposes of this analysis. Group 1 (n = 27) comprised patients with a history of brain metastases and in whom central nervous system disease was managed with surgery, stereotactic radiosurgery, whole-brain irradiation, or with a combination of these therapies before the initiation of IL-2 treatment. Group 2 (n = 37) comprised patients with untreated brain metastases documented by computed tomography or magnetic resonance imaging, and group 3 (n = 1,005) comprised patients without brain metastases. Three types of comparisons were made for each variable analyzed: patients with brain metastases previously treated versus patients without brain metastases; patients with brain metastases previously untreated versus patients without brain metastases; and patients with brain metastases (either previously treated or untreated, n = 64) versus patients without brain metastases.
Parameters evaluated among the patient groups included grade 3 and 4 toxicity profiles, reasons for stopping IL-2 therapy, doses of IL-2 per cycle, and response to IL-2–based therapy. Variables such as number of IL-2 doses and laboratory values (leukocyte, platelet, and eosinophil counts; hematocrit, serum sodium, blood urea nitrogen, creatinine, and bilirubin levels) were assessed using a Wilcoxon rank sum test for each of the three comparisons. Variables such as toxicity, reasons for stopping IL-2 administration, and response to treatment were assessed with Fisher’s exact test.
A large number of statistical comparisons were performed. Because the probability of declaring any individual association to be significant increases with the number of such comparisons, techniques exist for identifying exact thresholds to apply to p values when multiple comparisons are made. However, because comparisons were being made between different groups of patients and with varying numbers of parameters, potentially confusing multiple thresholds for significance were needed. In addition, in view of the large number of comparisons being performed, the thresholds needed under formal rules for multiple comparisons may have been so small as to overly restrict interpretation of findings. To allow consistent interpretation of findings and consider concerns regarding multiple comparisons, p ≦ 0.005 was chosen to indicate statistical significance, p > 0.005, ≦ 0.01 indicated a potentially significant difference, and p > 0.01, ≦ 0.05 indicated a trend.
RESULTS
This study evaluated 1,069 patients with metastatic melanoma or renal cell carcinoma and focused on 64 patients with brain metastases (37 patients had no previous therapy for brain disease). Among all patients, 65% were male, 35% were female, and 80% were between the ages of 30 and 60 years. Race was predominantly white (96%) and Eastern Cooperative Oncology Group performance was 0 or 1 in 86% of patients. Most patients were pretreated with surgery, chemotherapy, radiotherapy, hormonal therapy, immunotherapy, or a combination of therapies before receiving IL-2 treatment. No significant differences in gender, age, race, performance status, and prior therapy were observed among the groups compared. All 27 patients with brain metastases previously treated had metastatic melanoma, whereas 34 (92%) patients with untreated brain metastases had metastatic melanoma and 3 (8%) patients had metastatic renal cell carcinoma. Among patients with no brain metastases, 749 (70%) had metastatic melanoma and 320 (30%) had metastatic renal cell carcinoma. Although protocol therapies varied over the period examined as experimental combination regimens were developed, all patients among the groups compared received a similar IL-2 dosing regimen of 720,000 IU/kg intravenously every 8 hours. Interleukin-2 alone was received by 39% of all patients, 14% received IL-2 plus lymphokine-activated killer cells, 11% received IL-2 plus tumor-infiltrating lymphocytes or peripheral blood lymphocytes, 7% received IL-2 plus chemotherapy, and 29% received IL-2 plus vaccine.
Twenty-seven categories of grade 3 and 4 toxicity were evaluated. Only one potentially statistically significant difference was noted among patients with previously treated (n = 27) or untreated (n = 37) brain metastases, or all patients with brain metastases (n = 64) compared with patients without brain metastases (n = 1,005). Patients with previously treated brain metastases experienced less nausea and vomiting than patients without brain metastases (incidence of 4% versus 25%, respectively; p = 0.006). The frequency of neurologic toxicity from IL-2 (disorientation and alteration in level of consciousness) was similar in all groups (Table 1).
TABLE 1.
Incidence of grade 3 and 4 neurologic toxicities during the first course of IL-2 treatment
Twenty-nine reasons for stopping IL-2 based treatment were also compared. Using stringent criteria, only one statistically significant difference was noted when comparing the groups of patients with brain metastases (previously treated or untreated, or all brain metastases) with patients with no brain metastases (Table 2). Cutaneous toxicity was more frequent (p = 0.002) in patients with untreated brain metastases than in patients without brain metastases. There was a trend toward greater disorientation (p = 0.029) and depressed level of consciousness (p = 0.031) as reasons for stopping IL-2 therapy in patients with brain metastases previously treated when compared with patients without brain metastases. Interestingly, the incidence of grade 3 and 4 toxicity in these categories was similar among the patient groups.
TABLE 2.
Potentially significant differences in reasons for stopping IL-2 during the first course of IL-2 therapy*
| Frequency to toxicity
|
|||
|---|---|---|---|
| Reason for stopping IL-2 | Brain mets previously treated n = 27 (%) | Brain mets untreated n = 37 (%) | No brain mets n = 1005 (%) |
| Disorientation | 9 (33)† | 6 (16) | 158 (16) |
| Level of consciousness | 4 (15)‡ | 3 (8.1) | 43 (4.3) |
| Cutaneous | 0 | 3 (8.1)§ | 5 (0.5) |
Brain metastases (previously treated or untreated) versus no brain metastases.
p = 0.029 (trend toward significance).
p = 0.031 (trend toward significance).
p = 0.002 (significantly more than patients without brain metastases).
The number of IL-2 doses administered per cycle during the first course of treatment was evaluated. Patients with previously treated brain metastases received fewer doses (median, 6.5; range, 1–9.5) of IL-2 than patients without brain metastases (median, 7.5 doses; range, 1–18) (p = 0.013, not adjusted for multiple comparisons). Patients with untreated brain metastases received a similar number of IL-2 doses (median 7.5; range, 2–10) as patients without brain metastases.
The brain has previously been considered an immune-privileged site. Despite regression of extracranial metastases, brain metastases have been observed to develop or grow. In addition to evaluating the safety of IL-2 administration among patients with brain metastases, this study also evaluated the clinical responses to IL-2 among patients with previously treated and untreated brain metastases. In patients with previously treated brain metastases, the residual brain lesions were not considered evaluable sites. The objective response rate in these 27 patients was 18.5%, which is similar to the response rate of 19.8% in patients without brain metastases at the time of treatment (Table 3). However, only 2 (5.6%) of 36 patients with evaluable brain metastases exhibited an objective response (at intracranial and extracranial sites), which is fewer than patients without brain metastases(p = 0.031, not adjusted for multiple comparisons) (Figs. 1 and 2). Among the 34 nonresponding patients, none achieved a true partial response at extracranial sites. Thus, although patients can experience an objective response in the brain, it is less common than disease at other sites.
TABLE 3.
Overall clinical response to IL-2 treatment in evaluable patients
| Response | Brain metastases previously treated n = 27 (%) | Brain metastases untreated n = 36 (%) | No brain metastases n = 1003 (%) |
|---|---|---|---|
| CR | 0 | 1 (2.8) | 58 (5.8) |
| PR | 5 (18.5) | 1 (2.8) | 141 (14.1) |
| Total | 5 (18.5)* | 2 (5.6)† | 199 (19.8) |
| 7/63 (11.1) | |||
p = NS versus no brain metastases.
p = 0.031 by Fisher’s exact test (trend toward significance) versus no brain metastases.
FIG. 1.
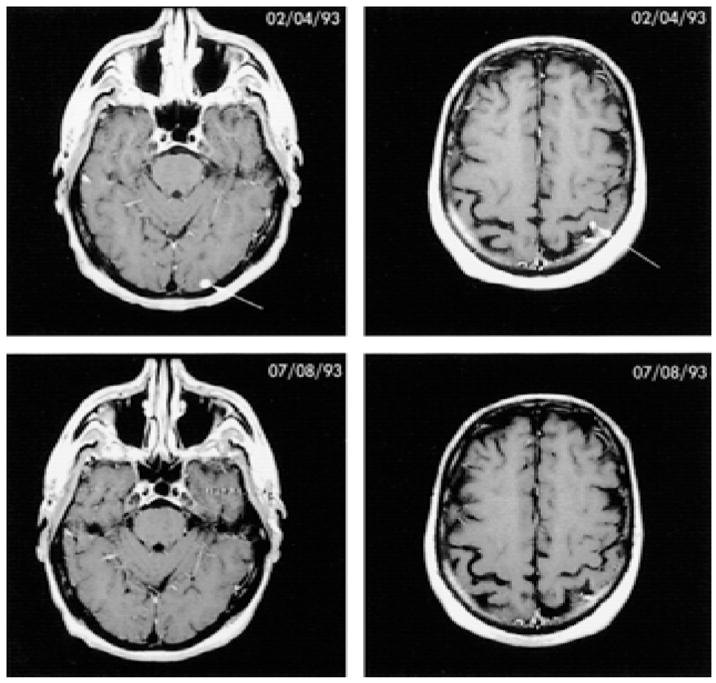
Complete response of two metastatic lesions in the brain after treatment with tumor-infiltrating lymphocytes plus high-dose interleukin-2. The patient remained free of disease for 38 months, after which time recurrent disease developed in the brain.
FIG. 2.
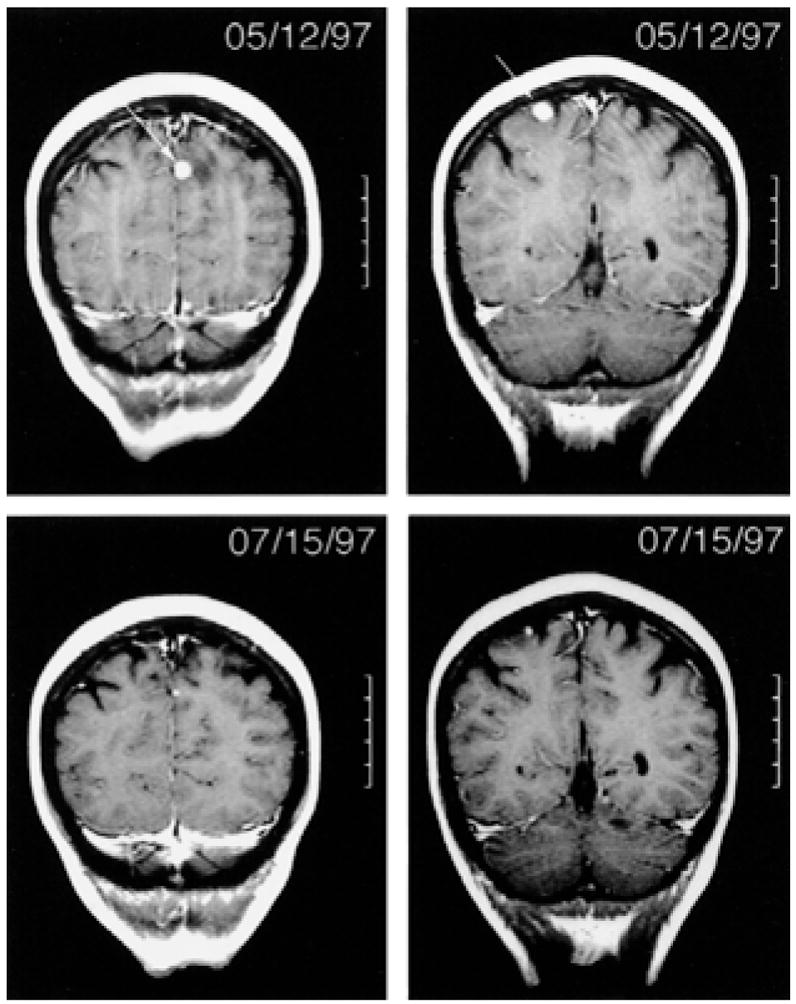
Partial response of four metastatic lesions (largest two shown) in the brain after treatment with vaccine and high-dose interleukin-2 (7). Progressive disease was noted in the brain 3 months later.
Overall, there was no significant difference in response rates between 63 patients with brain metastases (treated or untreated) and 1,003 patients without brain metastases (p = 0.10, Table 3). The characteristics of patients with brain metastases (treated and untreated) that achieved an overall clinical response to IL-2 are listed in Table 4. All patients with brain metastases (treated and untreated) sustaining a complete or partial response had a diagnosis of metastatic melanoma. The first site of the treatment failure was intracranial sites eventually progressed in five of the seven patients, and extracranial sites progressed in two patients; one patient is living with disease.
TABLE 4.
Characteristics of patients with brain metastases (all had a diagnosis of metastatic melanoma) achieving an overall clinical response to IL-2 based therapies
| Patient | Brain metastases previously treated | Evaluable sites of disease | Systematic treatment received | Overall response to IL-2 | Duration of response (months) | Initial site of treatment failure | Status |
|---|---|---|---|---|---|---|---|
| 1 | yes | SQ, lung | IL-2 + TIL | PR | 4 | brain | DOD |
| 2 | yes | SQ, lung | IL-2 + TIL | PR | 4 | brain, lung | DOD |
| 3 | yes | lung, liver, spleen | IL-2 alone | PR | 8 | brain | DOD |
| 4 | no* | SQ, paraspinous muscle | IL-2 + vaccine | PR | 12 | paraspinous muscle | DOD |
| 5 | yes | SQ, lymph node | IL-2 + vaccine | PR | 11 | SQ | AWD |
| 6 | no | brain, lung | IL-2 + TIL | CR | 38 | brain | DOD |
| 7 | no | lymph node, lung, liver, brain | IL-2 + vaccine | PR | 3 | brain | DOD |
SQ, subcutaneous; PR, partial response; CR, complete response; DOD, dead of disease; AWD, alive with disease.
Brain metastasis (5 mm in size) disappeared with vaccine and GM-CSF prior to IL-2.
DISCUSSION
The use of IL-2–based therapies in patients with metastatic melanoma and metastatic renal cell carcinoma has resulted in an objective cancer response rate of 17% (4). Recombinant high-dose IL-2 is associated with a broad range of well-described toxicities (5). Multiple comprehensive studies of renal, cardiopulmonary, and hematologic toxicities have led to the development of effective strategies for avoiding or effectively treating these toxicities, which are typically short lived. The current practice of high-dose IL-2 administration has resulted in diminished toxicities and emphasizes the safety of this agent when administered by experienced physicians (12). This review provides evidence supporting the safe administration of IL-2 to a carefully selected subset of patients with brain metastases.
Patients with a limited number of brain metastases that were small, had little or no edema, or were effectively treated with surgery or radiation were considered eligible for IL-2–based therapy. All such patients that received high-dose IL-2 were included in this study and were compared with patients without brain metastases. Patients with previously treated or untreated brain metastases experienced similar grade 3 and 4 toxicities as patients without brain metastases, and stopped IL-2 dosing for similar reasons. Specific measures of neurologic toxicity, orientation, and level of consciousness were similar among groups. However, there was a trend toward more frequently termination of IL-2 therapy due to disorientation and an altered level of consciousness in patients with previously treated brain metastases. This group received fewer doses of IL-2 per cycle (median, 6.5) than patients without brain metastases (median, 7.5), but interestingly achieved a similar overall clinical response rate (18.5% versus 19.8%, respectively). The subset of patients with evaluable brain metastases received the same median number of doses of IL-2 (7.5) as patients without brain metastases.
Of eight laboratory measurements evaluated before and during therapy, only five apparently clinically inconsequential differences were noted between groups (data not shown). A statistically significant lower baseline blood urea nitrogen and creatinine levels and peak creatinine level was noted among all patients with brain metastases, most likely because of a lower proportion of patients with a diagnosis of renal cell cancer. Patients with renal cell carcinoma have higher baseline and peak creatinine levels than patients with melanoma (13). A significantly higher increase in eosinophil count was noted among patients without brain metastases compared with all patients with brain metastases. Eosinophilia is a common finding after the administration of IL-2 (5). The difference in eosinophil counts and observed higher peak leukocyte counts in patients without brain metastases compared with patients with previously treated brain metastases is of unknown significance.
The brain has been considered by many to be an immune-privileged site, largely because of the observation that the brain could be a single site of failure in the setting of clinical remission at extracranial sites (14). The ability of intracranial disease to respond to immunotherapy has not been carefully studied. In fact, treatment of patients with brain metastases with high-dose IL-2 has largely been avoided in the past, primarily because of concerns regarding compounding the neurologic toxicity of IL-2 and the potential of intracranial bleeding during periods of thrombocytopenia. None of these concerns was observed in this review. In this study, 2 of 36 patients with previously untreated evaluable brain metastases did demonstrate objective regression of intracranial and extracranial disease while receiving IL-2–based therapies. However, response rates in this group were diminished compared with patients without brain metastases, possibly because of the small numbers of patients in this analysis. Among the 34 nonresponding patients with evaluable brain metastases, none achieved a partial response at extracranial sites of disease. Without discordant results of response in the brain and in extracranial sites, it is not possible to conclude that the brain is an immune-privileged site. The finding that the two patients who responded to IL-2 at extracranial sites also responded in the brain suggests that the brain may not be an immune-privileged site.
In the group of patients with previously treated brain metastases, the systemic response rate was similar to that of patients without brain metastases. Overall, patients with brain metastases (treated or untreated) experienced objective regression of cancer at extracranial sites of disease with similar frequency as patients without brain metastases. The median time to treatment failure among patients with brain metastases achieving a systemic response to IL-2 was 8 months, which is similar to patients without brain metastases (4). Durable complete responses were infrequent, occurring in only one patient and lasting only 38 months because of failure in the brain.
This study has shown that carefully selected patients with melanoma metastatic to the brain can safely receive high-dose IL-2 and experience a response to treatment. Patients with a limited number of brain metastases that are small and have little or no edema can be considered eligible for IL-2–based therapy. In addition, patients with brain metastases that have been effectively controlled with surgery, radiation, or both may be considered eligible for high-dose IL-2.
References
- 1.Lotze MT, Dallal RM, Kirkwood JM, Flickinger JC. Cutaneous melanoma. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 2012–69. [Google Scholar]
- 2.Balch CM, Soong S, Shaw HM, Urist MM, McCarthy WH. An analysis of prognostic factors in 8500 patients with cutaneous melanoma. In: Balch CM, Houghton AN, Milton GW, Sober AJ, Soong S, editors. Cutaneous Melanoma. Philadelphia, PA: J.B. Lippincott; 1992. pp. 165–87. [Google Scholar]
- 3.Jennings SB, Linehan WM. Renal, perirenal, and ureteral neoplasms. In: Gillenwater JY, Grayhack JT, Howards SS, Duckett JW, editors. Adult and Pediatric Urology. St. Louis, MO: Mosby; 1996. pp. 643–94. [Google Scholar]
- 4.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–19. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartzentruber DJ. Interleukin-2: Clinical applications. Principles of administration and management of side effects. In: Rosenberg SA, editor. Principles and Practice of the Biologic Therapy of Cancer. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 32–50. [Google Scholar]
- 6.Citterio G, Di Lucca G, Scaglietti U, Gilberti S, Baldini M, Rugarli C. Reduction of brain metastasis after immunotherapy with interleukin-2 for stage IV renal cell cancer. Acta Oncol. 1997;36:228–228. doi: 10.3109/02841869709109236. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA. 1994;271:907–13. [PubMed] [Google Scholar]
- 9.Rosenberg SA, Lotze MT, Yang JC, et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993;85:622–32. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor infiltrating lymphocytes and interleukin-2. J Natl Cancer Inst. 1994;86:1159–66. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, decarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol. 1999;17:968–75. doi: 10.1200/JCO.1999.17.3.968. [DOI] [PubMed] [Google Scholar]
- 12.Kammula US, White DE, Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer. 1998;83:797–805. [PubMed] [Google Scholar]
- 13.Guleria AS, Yang JC, Topalian SL, et al. Renal dysfunction associated with the administration of high-dose interleukin-2 in 199 consecutive patients with metastatic melanoma or renal cell carcinoma. J Clin Oncol. 1994;12:2714–22. doi: 10.1200/JCO.1994.12.12.2714. [DOI] [PubMed] [Google Scholar]
- 14.Hurst R, White DE, Heiss J, Lee DS, Rosenberg SA, Schwartzentruber DJ. Brain metastasis after immunotherapy in patients with metastatic melanoma or renal cell cancer: is craniotomy indicated? J Immunother. 1999;22:356–62. doi: 10.1097/00002371-199907000-00009. [DOI] [PubMed] [Google Scholar]


