Abstract
Since trivalent chromium (Cr3+) enhances glucose metabolism, interest in the use of Cr3+as a therapy for type 2 diabetes has grown in the mainstream medical community. Moreover, accumulating evidence suggests that Cr3+ may also benefit cardiovascular disease (CVD) and atypical depression. We have found that cholesterol, a lipid implicated in both CVD and neurodegenerative disorders, also influences cellular glucose uptake. A recent study in our laboratory shows that exposure of 3T3-L1 adipocytes to chromium picolinate (CrPic, 10 nM) induces a loss of plasma membrane cholesterol. Concomitantly, accumulation of intracellularly sequestered glucose transporter GLUT4 at the plasma membrane was dependent on the CrPic-induced cholesterol loss. Since CrPic supplementation has the greatest benefit on glucose metabolism in hyperglycemic insulin-resistant individuals, we asked here if the CrPic effect on cells was glucose-dependent. We found that GLUT4 redistribution in cells treated with CrPic occurs only in cells cultured under high glucose (25 mM) conditions that resemble the diabetic-state, and not in cells cultured under non-diabetic (5.5 mM glucose) conditions. Examination of the effect of CrPic on proteins involved in cholesterol homeostasis revealed that the activity of sterol regulatory element-binding protein (SREBP), a membrane-bound transcription factor ultimately responsible for controlling cellular cholesterol balance, was upregulated by CrPic. In addition, ABCA1, a major player in mediating cholesterol efflux was decreased, consistent with SREBP transcriptional repression of the ABCA1 gene. Although the exact mechanism of Cr3+-induced cholesterol loss remains to be determined, these cellular responses highlight a novel and significant effect of chromium on cholesterol homeostasis. Furthermore, these findings provide an important clue to our understanding of how chromium supplementation might benefit hypercholesterolemia-associated disorders.
Keywords: Cholesterol, Chromium picolinate, GLUT4
1. Introduction
The case for trivalent chromium (Cr3+) supplementation in the management of type 2 diabetes and other insulin-resistant conditions began in 1957 with the discovery that a substance in brewer’s yeast prevented an age-related decline of glucose tolerance in rats. Shortly after, Cr3+ was identified as the active substance [1]. In 1977, Cr3+ was declared an essential nutrient when significant elevations in blood glucose were first observed in a hospitalized patient receiving total parenteral nutrition devoid of Cr3+ [2,3]. Blood glucose levels normalized after the addition of Cr3+ to her diet. More recently, studies have revealed that Cr3+ may also be beneficial in cardiovascular disease [4] and atypical depression [5]. These findings beget the question if Cr3+ affects a cellular process mutually involved in the regulation of cardiovascular, cognitive and metabolic functions.
A role of cholesterol in the pathogenesis of cardiovascular disease is well recognized and an appreciation for this lipid in other abnormalities such as neurode-generative disorders is emerging [6–8]. Interestingly, we recently found that the content of cholesterol in the plasma membrane of 3T3-L1 adipocytes influences the well-characterized regulation of glucose transport in these cells by insulin [9]. Under normal conditions, glucose homeostasis occurs by the ability of insulin to stimulate the storage and metabolism of glucose, helping to return elevated plasma glucose levels toward normal. It is in muscle and adipose tissues where insulin accelerates the removal of excess circulatory glucose by regulating the subcellular trafficking of the glucose transporter GLUT4. In the basal state, GLUT4 cycles continuously between the plasma membrane and one or more intracellular compartments, with the vast majority of the transporter residing within the cell interior [10–12]. Activation of the insulin receptor triggers a large increase in the rate of GLUT4 vesicle exocytosis and a smaller but important decrease in the rate of internalization by endocytosis [13–16]. The overall insulin-dependent shift in the cellular dynamics of GLUT4 vesicle trafficking results in a net increase of GLUT4 protein levels at the plasma membrane, where these transporters facilitate cellular glucose uptake. Recent work from our group concerning the role of cholesterol in this process show that its removal from the plasma membrane is associated with plasma membrane GLUT4 accumulation [9]. This finding and the observation by Evans and Bowman [17] documenting that Cr3+ in the picolinate salt form (CrPic) increases membrane fluidity, prompted us to test if CrPic action was cholesterol-dependent. We found that that the content of cholesterol in the plasma membrane fell in cells exposed to CrPic and that loading exogenous cholesterol back into the plasma membrane blocked the action of CrPic [18].
Membrane physiology as a basis for the cellular effects of CrPic on glucose transport is compelling on several levels. First, in most studies conducted in non-diabetic subjects with normal insulin sensitivity and glucose tolerance, Cr3+ supplements have only modest or no effects on insulin or glucose levels. In contrast, nearly all studies report that Cr3+ supplements have positive effects in lowering elevated blood glucose, insulin or lipid levels in subjects with insulin resistance and type 2 diabetes [19]. Interestingly, unfavorable plasma membrane phospholipid changes correlating with impaired insulin sensitivity are a characteristic of erythrocytes obtained from obese individuals. In particular, Younsi et al. [20] recently found that erythrocyte membranes from insulin-resistant overweight women had a significantly higher content of cholesterol than that present in erythrocyte membranes from insulin-sensitive lean women.
Second, in direct support of our postulate, moderate increases in plasma membrane fluidity have been documented to increase glucose transport [21–23]. Studies show that basal glucose transport is not fully active in fat cells and that it can be increased further by augmenting membrane fluidity [21]. Consistent with membrane fluidity influencing insulin responsiveness, insulin-stimulated glucose transport decreased when fluidity diminished [22].
Third, recent data suggest that the anti-diabetic drug metformin enhances insulin action by increasing membrane fluidity [24,25]. As we have observed following CrPic treatment [18], metformin treatment has been reported to increase GLUT4 translocation [26–31]. The relative enhancing effect of metformin was higher in cells incubated in 25 mM glucose rather than in 5 mM glucose, consistent with its selective action in hyperglycemic conditions in vivo [30,32]. As reported for metformin [33–36], the effects of CrPic are not attributed to increased expression of GLUT4 protein but rather its translocation and/or activation state [18,37–39]. Combined, these results predict that during the hyperglycemic state Cr3+ may functionally enhance insulin action by targeting the plasma membrane.
Finally, in addition to the employment of Cr3+ supplementation to improve glucose tolerance, recent clinical trials suggest that Cr3+ may have potential as an antide-pressant agent [5] and in lowering certain risk factors (total serum cholesterol, LDL cholesterol and serum triglycerides) for cardiovascular disease [40,41]. Evidence has shown elevated total serum cholesterol to be unfavorable for cardiovascular health. Interestingly, a recent study also suggests that elevated serum cholesterol at midlife may increase the risk of dementia and Alzheimer disease. Given the potential importance of Cr3+ in cholesterol-related disorders, we sought to test if the relative enhancing effect of CrPic on the glucose transport system was higher in cells incubated in 25 mM glucose rather than in 5.5 mM glucose, consistent with its selective action in hyperglycemic conditions in vivo. Studies also examined proteins involved in cellular cholesterol homeostasis in response to CrPic. We examined SREBP, a sterol regulating transcription factor that localizes to the ER/Golgi membranes in its immature form. When the cell is in need of cholesterol, cleavage of immature ER/Golgi-localized SREBP results in the release of the mature form of SREBP from the ER/Golgi membrane and accumulation of this active transcription factor in the nucleus where it binds sterol response elements controlling the expression of proteins regulating cholesterol homeostasis. For example, SREBP represses the expression of ABCA1, an ATP-binding cassette that mediates the cellular efflux of excess cholesterol. The subsequent report provides an account of these studies supporting a novel action of CrPic on cellular cholesterol homeostasis.
2. Material and methods
2.1. Antibodies, plasmids, and reagents
Polyclonal rabbit SREBP-1 antibody and horseradish peroxidase (HRP)-conjugated goat anti-rabbit and mouse antibodies were obtained from Santa Cruz (Santa Cruz, CA). Polyclonal rabbit ABCA1 antibody was purchased from Novus Biologicals (Little, CO). Monoclonal mouse GLUT4 antibody was purchased from Biogenesis (Kingston, NH). Near-infrared IR-dye 800- and 700-conjugated anti-mouse IgG secondary antibodies were purchased from Rockland (Gilbertsville, PA). Amplex Red Cholesterol Assay Kit was purchased from Molecular Probes (Eugene, OR). Dulbecco’s modified MEM was from Invitrogen (Grand Island, NY). FBS and FCS were obtained from Hyclone Laboratories Inc. (Logan, UT). Chromium picolinate (CrPic) was from Tokyo Kasei Kogyo Co. (Tokyo, Japan). All other chemicals were from Sigma (St. Louis, MO).
2.2. Cell culture and treatments
Murine 3T3-L1 preadipocytes were purchased from American Type Culture Collection (Manassas, VA). Murine 3T3-L1 preadipocytes were cultured in DMEM containing 25 mM glucose and 10% calf serum at 37 °C in an 8% CO2 atmosphere. Confluent cultures were induced to differentiate into adipocytes as previously described [9]. Adipocytes were then cultured in DMEM/10% fetal bovine serum growth medium containing either 5.5 or 25 mM glucose for 48 h before the experimental treatment. All studies were performed on adipocytes, which were between 8 and 12 days after differentiation. The cells were left untreated or treated with CrPic (10 nM) in serum free DMEM medium for 16 h. The preparation of methyl-β-cyclodextrin:cholesterol (βCD:cholesterol) complex was performed as we have previously described [9]. The use of βCD for manipulating cellular cholesterol content has been described [42] and previously demonstrated not to cause adverse effects on membrane and cellular function [9,18]. In the cholesterol replenishment experiments, cells were incubated with this solution βCD:cholesterol for 30 min following the CrPic treatment.
2.3. Plasma membrane sheet assay
Preparation of plasma membrane sheets from the adipocytes was performed as previously described [9]. After the isolation of plasma membrane sheets, these purified membranes were used for immunofluorescence. The sheets were fixed for 20 min at 25 °C in a solution containing 2% paraformaldehyde, 70 mM KCl, 30 mM HEPES, pH 7.5, 5 mM MgCl2 and 3 mM EGTA. The sheets were then blocked in 5% milk for 60 min at 25 °C and incubated overnight at 4 °C with a 1:1000 dilution of monoclonal GLUT4 antibody, followed by incubation with a 1:100 dilution of IR-dye 800-conjugated anti-mouse IgG secondary antibody for 60 min at 25 °C. The amount of glucose transporter on the plasma membrane was quantitated by digital image processing as described previously [43,44].
2.4. Subcellular fractionation
Adipocyte subcellular membrane fractions were obtained using the differential centrifugation method previously described [45] with slight modification. Briefly, control and insulin-stimulated 3T3-L1 adipocytes were washed and resuspended in HES buffer (20 mM HEPES, pH 7.4, 1 mM EDTA and 255 mM sucrose containing 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin, 10 μg/ml aprotinin and 5 μg/ml leupeptin). Cell lysates were prepared by shearing the cells through a 22-gauge needle 10 times. Lysates were then centrifuged at 19,000 × g for 20 min at 4 °C. The intracellular membrane pellet was obtained by centrifugation of the resulting supernatant at 180,000 × g for 75 min at 4 °C. The plasma membrane pellet was obtained by resuspending the pellet from the initial 19,000 × g centrifugation in HES buffer followed by layering onto a 1.12 M sucrose cushion for centrifugation at 100,000 × g for 60 min. The plasma membrane layer was removed from the sucrose cushion and centrifuged at 40,000 × g for 20 min. All pelleted fractions were resuspended in a detergent-containing lysis buffer and assayed for soluble protein content.
2.5. Preparation of nuclear extracts
Nuclear extracts were collected as previously described [46]. Briefly, 3T3-L1 adipocytes were washed with PBS and 2 ml of hypotonic buffer (10 mM HEPES, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT containing 20 mM leupeptin, 2 mM pepstatin, 2 mM aprotinin and 0.5 M PMSF). Cell lysates were prepared by shearing the cells through a 22-gauge needle 5 times. Prepared lysates were centrifuged at 800 g for 10 min at 4 °C. The pellet was then carefully dried and resuspended in 0.5 ml of buffer C (10 mM HEPES, pH 7.4, 0.42 M NaCl, 25% glycerol (v/v), 1.5 mM MgCl2, 0.5 mM EDTA containing 20 mM leupeptin, 2 mM pepstatin, 2 mM aprotinin and 0.5 M PMSF). Nuclei were then visualized by staining with 0.2% trypan blue. Proteins were allowed to swell out of the nuclei by vortexing for 15 s every 10 min for 40 min. The mixture was then centrifuged at full speed in a microcentrifuge at 4 °C for 15 min. Supernatant was collected and assayed for soluble protein content.
2.6. Electrophoresis and immunoblotting
Plasma membrane, intracellular membrane, and nuclear extract fractions were resolved on 7.5% (ABCA1, SREBP-1) or 10% (GLUT4) SDS-polyacrylamide gels. The resolved proteins were transferred to nitrocellulose (GLUT4) or PVDF (ABCA1, SREBP-1) membranes and immunoblotted with polyclonal rabbit ABCA1 or SREBP-1 antibodies, or monoclonal GLUT4 antibody. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit and anti-mouse antibodies were then used and immunoblots were subjected to ECL detection.
2.7. Cholesterol analyses
Plasma membrane pellets were resuspended in 0.2 ml of HES buffer, and cholesterol content was determined by using an enzymatic, fluorometric kit (Cat No. A12216: Amplex Red Cholesterol Assay Kit) purchased from Molecular Probes (Eugene, OR) for the quantitative determination of total cholesterol. Briefly, 0.15 ml of the resuspended plasma membrane pellet was vigorously mixed with 3 ml of chloroform–methanol (2:1 v/v) extraction solution for 10 min. The mixture was then centrifuged (3000 rpm, 10 min), and 1.0 ml of the supernatant was added to a glass tube and evaporated via a 100 °C water bath. The residue was reconstituted with 0.08 ml of an isopropanol-Triton X-100 solution (9:1 v/v), and 0.05 ml of cholesterol-containing sample was mixed with 0.05 ml of Amplex Red reagent/HRP/cholesterol oxidase/cholesterol esterase solution. After incubation for 30 min at 37 °C, absorbance was measured at 600 nm.
2.8. Statistical analysis
All values are presented as means ± S.E. Analysis of variance was used to determine differences among groups. Where a significant difference was indicated, the Tukey’s test was used to determine significant differences between groups. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Insulin mimetic activity of CrPic is cholesterol-dependent
Recently we reported that both CrPic and CrCl3 at concentrations as low as 10 nM stimulates the mobilization of GLUT4 to the plasma membrane of 3T3-L1 adipocytes [18]. A novel observation was that this redistribution of GLUT4 was dependent on a Cr3+-induced loss of plasma membrane cholesterol. Although these data are consistent with Cr3+ exerting a positive effect on the glucose transport system and lowering elevated blood glucose in type 2 diabetic individuals, a confounding issue is that non-diabetic individuals do not experience a change in blood glucose following CrPic treatment. Since the standard medium used to culture 3T3-L1 adipocytes contains 25 mM glucose, a concentration of glucose akin to that in a poorly controlled diabetic individual, we tested if the cellular response to Cr3+ treatment would be diminished in cells cultured in a normal level of glucose (5.5 mM).
Consistent with an enhancing effect of CrPic on GLUT4 translocation in cells cultured in 25 mM glucose, plasma membrane fractions from these cells displayed an increase in GLUT4 compared with plasma membrane fractions prepared from untreated cells (Fig. 1A, compare lanes 1 and 2). Interestingly, the same treatment parameters did not increase the basal-state plasma membrane level of GLUT4 in cells cultured in medium containing 5.5 mM glucose (Fig. 1A, compare lanes 3 and 4). With our previous observations that CrPic action requires changes in plasma membrane cholesterol, we next compared the plasma membrane cholesterol levels in the high glucose/CrPic-sensitive and low glucose/CrPic-resistant cells.
Fig. 1.
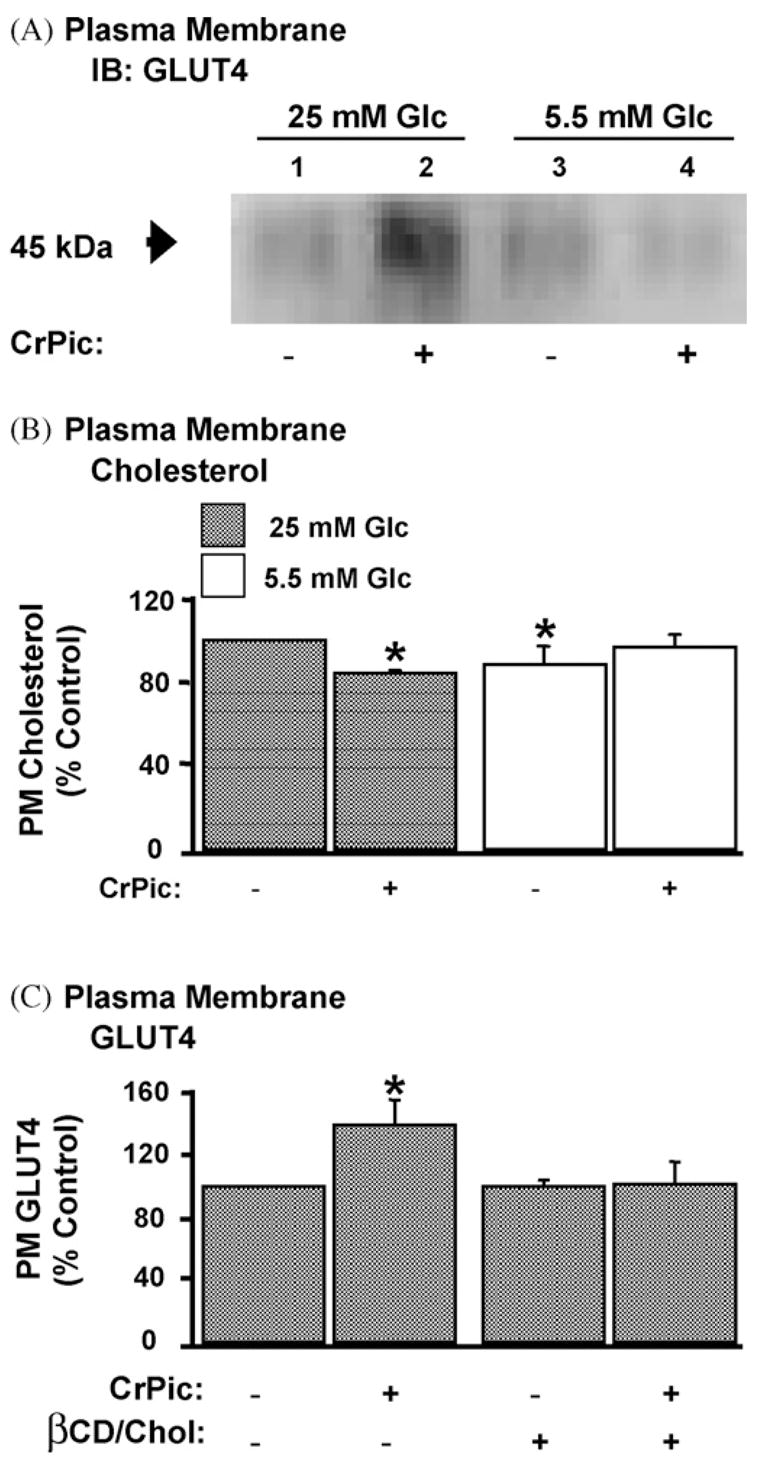
GLUT4 translocation and cholesterol loss stimulated by CrPic in cells cultured in high, but not low, glucose containing medium. 3T3-L1 adipocytes were left untreated or treated with 10 nM CrPic in serum-free DMEM containing 25 mM glucose (Glc) or 5.5 mM Glc for 16 h. (A) GLUT4 immunoblot (IB) and (B) cholesterol content of plasma membrane (PM) fractions prepared by ultracentrifugation as described in Section 2. (C) Quantitated GLUT4 content in PM fragments prepared by sonication as described in Section 2 from cells left untreated or treated with 10 nM CrPic in serum-free DMEM containing 25 mM Glc for 16 h. (A) Representative IB from three independent experiments and (B and C) results, expressed as percent of 25 mM glucose control, representing the mean (±S.E., *P < 0.05) from 3 to 4 independent experiments.
Fig. 1B shows that plasma membrane fractions prepared from cells cultured in 25 mM glucose were more cholesterol enriched by 13% (P < 0.05) than membranes prepared from cells cultured in 5.5 mM glucose, and CrPic selectively lowered this excess cholesterol only in the “high glucose” cells by 18% (P < 0.05). Notably, cells cultured in 5.5 mM glucose contain a similar level of plasma membrane cholesterol to those cultured in 25 mM glucose/10 nM CrPic, and treatment of the low-glucose cultured cells tended to increase plasma membrane cholesterol, but this was not statistically significant. Therefore, we next confirmed if addition of exogenous cholesterol in the culture medium prevented CrPic action. As previously reported [9,18], this experimental manipulation that replenished the reduced cholesterol effectively prevented the translocation of GLUT4 to the plasma membrane elicited by CrPic (Fig. 1C).
3.2. CrPic induces a reciprocal response in SREBP and ABCA1
In order to confirm the CrPic-induced change in cellular cholesterol we next studied whether the activity of SREBP-1, a predominantly expressed SREBP in 3T3-L1 adipocytes that can stimulate all SREBP-responsive genes including those involved in cholesterol homeostasis, was affected in cells treated with CrPic. In contrast to our initial thoughts that CrPic may decrease plasma membrane cholesterol by repressing the cleavage of the ER/Golgi-localized 125 kDa precursor SREBP-1, CrPic exposure was associated with a clear loss of this precursor from the intracellular membrane fraction (Fig. 2A). At the same time, immunoblot analyses of nuclear extracts revealed an increased abundance of the 68 kDa mature SREBP-1 (Fig. 2B).
Fig. 2.
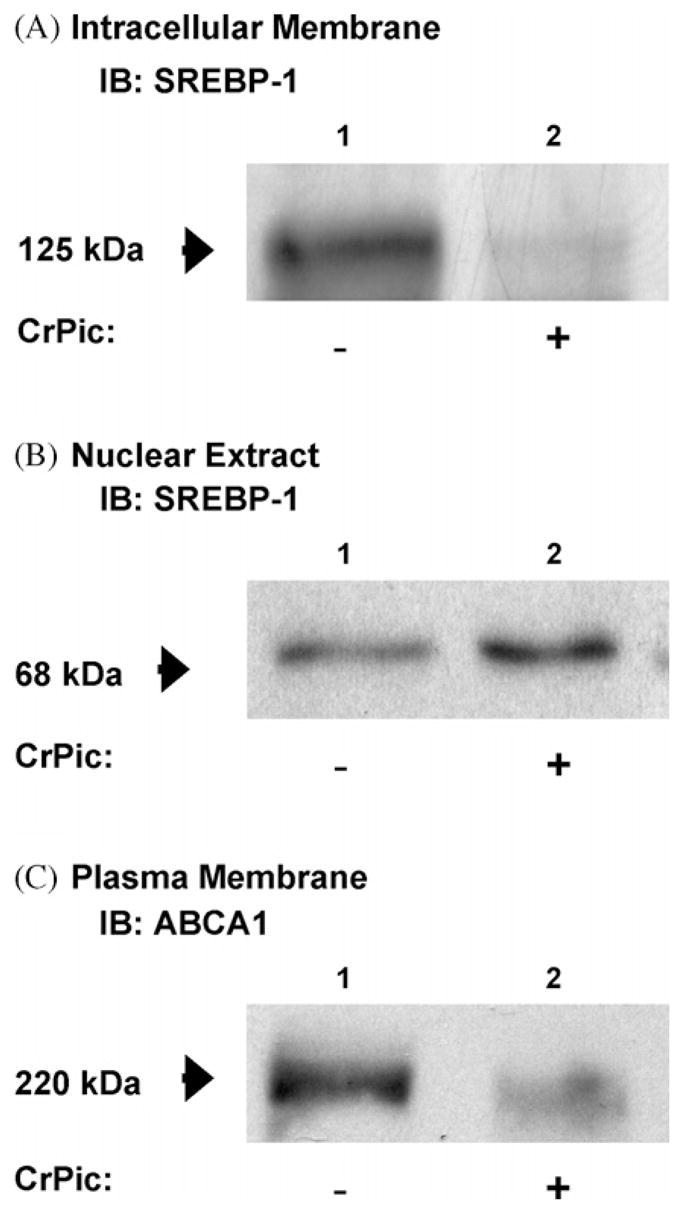
SREBP-1 and ABCA1 protein response in cells exposed to CrPic. Plasma membrane, intracellular membrane and nuclear extracts were prepared as described in Section 2 from 3T3-L1 adipocytes treated with 10 nM CrPic in serum-free DMEM containing 25 mM Glc for 16 h. IBs for (A) SREBP-1 in the intracellular membrane fraction, (B) SREBP-1 in the nuclear extract fraction and (C) ABCA1 in the plasma membrane fraction.
In parallel, we tested if the increased nuclear localization of SREBP-1 induced the predicted shutdown of expression of ABCA1, a major player in mediating the cellular efflux of cholesterol. As shown in Fig. 2C, plasma membrane fractions prepared from cells treated with CrPic displayed a decrease in the basal-state plasma membrane level of ABCA1.
4. Discussion
Thirty-eight years after the landmark discovery of insulin, Cr3+ was proposed to be a separate factor that is necessary for the maintenance of normal glucose tolerance. Unlike the intensive research in pursuit of understanding the molecular mechanisms of insulin signaling and resistance to its biological action associated most significantly with obesity and type 2 diabetes, the molecular basis of Cr3+ action has been intermittently studied over the years. Recent data is consistent with the theory that Cr3+ supplements, especially CrPic, enhance the metabolic action of insulin and lower certain risk factors for cardiovascular disease. For example, several randomized, placebo-controlled, double blind clinical trials, found supplementation with CrPic (200–1000 μg Cr/day) decreased total cholesterol and/or low-density lipoprotein (LDL) cholesterol [40,41,47]. These data, together with recent findings showing the negative impact cholesterol has on cellular glucose uptake [9,18] and neuronal function [6–8], two processes positively affected by Cr3+ [5,38], prompted us to ask if Cr3+ influenced cellular cholesterol homeostasis.
Recently, we reported that CrCl3 and CrPic treatments mobilized the glucose transporter, GLUT4, to the plasma membrane in 3T3-L1 adipocytes [18]. Concomitant with an increase in GLUT4 at the plasma membrane, insulin-stimulated glucose transport was enhanced. We also observed that Cr3+ action did not result from effects on known mediators of insulin action such as the insulin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase and Akt. Consistent with a reported effect of CrPic on increasing membrane fluidity [17], we found CrPic treatment decreased plasma membrane cholesterol. Exogenous cholesterol add-back to the plasma membrane prevented the beneficial effect of CrPic on both GLUT4 mobilization and insulin-stimulated glucose transport. An exciting prediction from these findings is that Cr3+ supplementation may lower blood glucose by altering the plasma membrane composition of cholesterol in fat and muscle cells. However, since Cr3+ supplementation has the greatest benefit in overweight, insulin-resistant individuals, a presumption also has to be that cells from these individuals possess cholesterol-laden plasma membranes. In contrast, the plasma membrane cholesterol content of cells from non-diabetic subjects with normal insulin sensitivity and glucose tolerance would be lower and not be affected by Cr3+. In line with this reasoning, chronic hyperglycemia has been shown to increase cholesterol ester accumulation concomitantly with reduced insulin-stimulated glucose uptake in cultured human skeletal muscle cells [48]. The findings presented in this report are consistent with hyperglycemia promoting lipid metabolism and show for the first time that plasma membrane cholesterol content is increased. Moreover, the beneficial cholesterol-dependent mobilization of GLUT4 to the plasma membrane occurred in cells cultured in high glucose, but not in cells cultured in low glucose.
We think that it is of particular interest that a SREBP response accompanied CrPic action. In addition, to confirming that cells exposed to CrPic undergo a change in membrane cholesterol levels, the observation that SREBP is activated and ABCA1 protein levels are reduced following 16 h of CrPic treatment suggests that CrPic-induced cholesterol loss occurs at an earlier time point. Preliminary qualitative assessment of CrPic-induced GLUT4 accumulation at the plasma membrane appears to show elevated levels of this transporter at the plasma membrane following 4 h of CrPic exposure. Data providing a glimpse of CrPic action on plasma membrane cholesterol levels, GLUT4 trafficking, and amounts/activities of proteins involved in cholesterol synthesis over time will be very informative. Studies with this objective are currently underway in our laboratory. Given the nature of the parameters of interest, careful planning and experimental optimization to allow for same sample analyses is critically important. Findings from these ongoing Cr3+ studies are anticipated to shed new insight into the mechanism of action of this transition metal.
Interestingly, it is very possible that the interaction of heavy metals with cellular membranes may contribute to, at least partially, the toxicity associated with these metals [49]. For example, cell membranes are dynamic, fluid structures, and most of their molecules are able to move in the plane of the membrane. Fluidity is the quality of ease of movement and represents the reciprocal value of membrane viscosity. Fluid properties of biological membranes are essential for numerous cell functions. Even slight changes in membrane fluidity may cause aberrant function and pathological processes. Several findings suggest that trace elements, e.g., iron, copper, zinc, selenium, chromium, cadmium, mercury and lead may influence membrane fluidity. In particular, hexavalent chromium (Cr6+), which is well recognized to pose an environmental health risk, has been shown to increase membrane fluidity [50]. Consistent with this membrane change having an insulin-like action on glucose transport, hexavalent chromium significantly stimulates glucose uptake into rat adipocytes [39]. Taken together with the data presented in this report, the extraordinary complexity of understanding chromium biology and toxicology certainly may involve an underappreciated cast of membrane lipids, including cholesterol.
5. Conclusion
These results are significant, because they provide the first novel insight into how Cr3+ supplementation may selectively target the growing numbers of insulin-resistant individuals in this country. In addition, this work highlights the elusive importance of membrane lipids in the biological activities and toxicological consequences of heavy metals. Although our direct research focus is on understanding how Cr3+ plays a role in normal carbohydrate metabolism, it is very noteworthy that Cr3+ supplementation may have beneficial effects beyond diabetes to cardiovascular disease and atypical depression. In closing, given the enormous public health cost of these and other cholesterol-associated diseases, the prospect of being able to use Cr3+ – a relatively low-cost dietary supplement – as a therapy to correct cholesterol-dependent abnormalities merits further study.
Acknowledgments
This work was supported in part by a National Center for Complementary and Alternative Medicine Grant No. R01-AT001846 (JSE) and an American Diabetes Research Grant No. 7-05-RA-37 (JSE).
References
- 1.Mertz W. Chromium research from a distance: from 1959 to 1980. J Am Coll Nutr. 1998;17:544–547. doi: 10.1080/07315724.1998.10718801. [DOI] [PubMed] [Google Scholar]
- 2.Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, Bruce-Robertson A. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am J Clin Nutr. 1977;30:531–538. doi: 10.1093/ajcn/30.4.531. [DOI] [PubMed] [Google Scholar]
- 3.I.o.M. Food and Nutrition Board, Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc, 2001.
- 4.Rajpathak S, Rimm EB, Li T, Morris JS, Stampfer MJ, Willett WC, Hu FB. Lower toenail chromium in men with diabetes and cardiovascular disease compared with healthy men. Diabetes Care. 2004;27:2211–2216. doi: 10.2337/diacare.27.9.2211. [DOI] [PubMed] [Google Scholar]
- 5.Davidson JR, Abraham K, Connor KM, McLeod MN. Effectiveness of chromium in atypical depression: a placebo-controlled trial. Biol Psychiat. 2003;53:261–264. doi: 10.1016/s0006-3223(02)01500-7. [DOI] [PubMed] [Google Scholar]
- 6.Michikawa M. Neurodegenerative disorders and cholesterol. Curr Alzheimer Res. 2004;1:271–275. doi: 10.2174/1567205043331983. [DOI] [PubMed] [Google Scholar]
- 7.Lukiw WJ, Pappolla M, Pelaez RP, Bazan NG. Alzheimer’s disease—a dysfunction in cholesterol and lipid metabolism. Cell Mol Neurobiol. 2005;25:475–483. doi: 10.1007/s10571-005-4010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sjogren M, Blennow K. The link between cholesterol and Alzheimer’s disease. World J Biol Psychiat. 2005;6:85–97. doi: 10.1080/15622970510029795. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, Leffler BJ, Weeks LK, Chen G, Bouchard CM, Strawbridge AB, Elmendorf JS. Sphingomyelinase activates GLUT4 translocation via a cholesterol-dependent mechanism. Am J Physiol Cell Physiol. 2004;286:C317–C329. doi: 10.1152/ajpcell.00073.2003. [DOI] [PubMed] [Google Scholar]
- 10.Kandror KV, Pilch PF. The insulin-like growth factor II/mannose 6-phosphate receptor utilizes the same membrane compartments as GLUT4 for insulin-dependent trafficking to and from the rat adipocyte cell surface. J Biol Chem. 1996;271:21703–21708. doi: 10.1074/jbc.271.36.21703. [DOI] [PubMed] [Google Scholar]
- 11.Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- 12.Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 13.Satoh S, Nishimura H, Clark AE, Kozka IJ, Vannucci SJ, Simpson IA, Quon MJ, Cushman SW, Holman GD. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J Biol Chem. 1993;268:17820–17829. [PubMed] [Google Scholar]
- 14.Jhun BH, Rampal AL, Liu H, Lachaal M, Jung CY. Effects of insulin on steady state kinetics of GLUT4 subcellular distribution in rat adipocytes. Evidence of constitutive GLUT4 recycling. J Biol Chem. 1992;267:17710–17715. [PubMed] [Google Scholar]
- 15.Yang J, Holman GD. Comparison of GLUT4 and GLUT1 sub-cellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J Biol Chem. 1993;268:4600–4603. [PubMed] [Google Scholar]
- 16.Czech MP, Buxton JM. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J Biol Chem. 1993;268:9187–9190. [PubMed] [Google Scholar]
- 17.Evans GW, Bowman TD. Chromium picolinate increases membrane fluidity and rate of insulin internalization. J Inorg Biochem. 1992;46:243–250. doi: 10.1016/0162-0134(92)80034-s. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Liu P, Pattar GR, Tackett L, Bhonagiri P, Strawbridge AB, Elmendorf JS. Chromium activates GLUT4 trafficking and enhances insulin-stimulated glucose transport in 3T3-L1 adipocytes via a cholesterol-dependent mechansim. Mol Endocrinol. 2006;20:857–870. doi: 10.1210/me.2005-0255. [DOI] [PubMed] [Google Scholar]
- 19.Althuis MD, Jordan NE, Ludington EA, Wittes JT. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am J Clin Nutr. 2002;76:148–155. doi: 10.1093/ajcn/76.1.148. [DOI] [PubMed] [Google Scholar]
- 20.Younsi M, Quilliot D, Al-Makdissy N, Delbachian I, Drouin P, Donner M, Ziegler O. Erythrocyte membrane phospholipid composition is related to hyperinsulinemia in obese non-diabetic women: effects of weight loss. Metabolism. 2002;51:1261–1268. doi: 10.1053/meta.2002.35184. [DOI] [PubMed] [Google Scholar]
- 21.Czech MP. Insulin action and the regulation of hexose transport. Diabetes. 1980;29:399–409. doi: 10.2337/diab.29.5.399. [DOI] [PubMed] [Google Scholar]
- 22.Pilch PF, Thompson PA, Czech MP. Coordinate modulation of D-glucose transport activity and bilayer fluidity in plasma membranes derived from control and insulin-treated adipocytes. Proc Natl Acad Sci USA. 1980;77:915–918. doi: 10.1073/pnas.77.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitesell RR, Regen DM, Beth AH, Pelletier DK, Abumrad NA. Activation energy of the slowest step in the glucose carrier cycle: break at 23 °C and correlation with membrane lipid fluidity. Biochemistry. 1989;28:5618–5625. doi: 10.1021/bi00439a042. [DOI] [PubMed] [Google Scholar]
- 24.Wiernsperger NF. Membrane physiology as a basis for the cellular effects of metformin in insulin resistance and diabetes. Diabetes Metab. 1999;25:110–127. [PubMed] [Google Scholar]
- 25.Muller S, Denet S, Candiloros H, Barrois R, Wiernsperger N, Donner M, Drouin P. Action of metformin on erythrocyte membrane fluidity in vitro and in vivo. Eur J Pharmacol. 1997;337:103–110. doi: 10.1016/s0014-2999(97)01287-9. [DOI] [PubMed] [Google Scholar]
- 26.Matthaei S, Reibold JP, Hamann A, Benecke H, Haring HU, Greten H, Klein HH. In vivo metformin treatment ameliorates insulin resistance: evidence for potentiation of insulin-induced translocation and increased functional activity of glucose transporters in obese (fa/fa) Zucker rat adipocytes. Endocrinology. 1993;133:304–311. doi: 10.1210/endo.133.1.8391425. [DOI] [PubMed] [Google Scholar]
- 27.Kozka IJ, Holman GD. Metformin blocks downregulation of cell surface GLUT4 caused by chronic insulin treatment of rat adipocytes. Diabetes. 1993;42:1159–1165. doi: 10.2337/diab.42.8.1159. [DOI] [PubMed] [Google Scholar]
- 28.Pryor PR, Liu SC, Clark AE, Yang J, Holman GD, Tosh D. Chronic insulin effects on insulin signalling and GLUT4 endocytosis are reversed by metformin. Biochem J. 2000;348:83–91. [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer Y, Thomas J, Rosen P, Kammermeier H. Action of metformin on glucose transport and glucose transporter GLUT1 and GLUT4 in heart muscle cells from healthy and diabetic rats. Endocrinology. 1995;136:412–420. doi: 10.1210/endo.136.2.7835271. [DOI] [PubMed] [Google Scholar]
- 30.Sarabia V, Lam L, Burdett E, Leiter LA, Klip A. Glucose transport in human skeletal muscle cells in culture. Stimulation by insulin and metformin. J Clin Invest. 1992;90:1386–1395. doi: 10.1172/JCI116005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hundal HS, Ramlal T, Reyes R, Leiter LA, Klip A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology. 1992;131:1165–1173. doi: 10.1210/endo.131.3.1505458. [DOI] [PubMed] [Google Scholar]
- 32.Klip A, Guma A, Ramlal T, Bilan PJ, Lam L, Leiter LA. Stimulation of hexose transport by metformin in L6 muscle cells in culture. Endocrinology. 1992;130:2535–2544. doi: 10.1210/endo.130.5.1572281. [DOI] [PubMed] [Google Scholar]
- 33.Ciaraldi TP, Kong AP, Chu NV, Kim DD, Baxi S, Lovis-cach M, Plodkowski R, Reitz R, Caulfield M, Mudaliar S, Henry RR. Regulation of glucose transport and insulin signaling by troglitazone or metformin in adipose tissue of type 2 diabetic subjects. Diabetes. 2002;51:30–36. doi: 10.2337/diabetes.51.1.30. [DOI] [PubMed] [Google Scholar]
- 34.Thomas CR, Turner SL, Jefferson WH, Bailey CJ. Prevention of dexamethasone-induced insulin resistance by metformin. Biochem Pharmacol. 1998;56:1145–1150. doi: 10.1016/s0006-2952(98)00151-8. [DOI] [PubMed] [Google Scholar]
- 35.Handberg A, Kayser L, Hoyer PE, Voldstedlund M, Hansen HP, Vinten J. Metformin ameliorates diabetes but does not normalize the decreased GLUT4 content in skeletal muscle of obese (fa/fa) Zucker rats. Diabetologia. 1993;36:481–486. doi: 10.1007/BF02743261. [DOI] [PubMed] [Google Scholar]
- 36.Rouru J, Koulu M, Peltonen J, Santti E, Hanninen V, Pesonen U, Huupponen R. Effects of metformin treatment on glucose transporter proteins in subcellular fractions of skeletal muscle in (fa/fa) Zucker rats. Br J Pharmacol. 1995;115:1182–1187. doi: 10.1111/j.1476-5381.1995.tb15022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris B, Gray T, MacNeil S. Evidence for chromium acting as an essential trace element in insulin-dependent glucose uptake in cultured mouse myotubes. J Endocrinol. 1995;144:135–141. doi: 10.1677/joe.0.1440135. [DOI] [PubMed] [Google Scholar]
- 38.Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle GLUT4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J Nutr. 2002;132:1107–1114. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- 39.Goto Y, Kida K. Insulin-like action of chromate on glucose transport in isolated rat adipocytes. Jpn J Pharmacol. 1995;67:365–368. doi: 10.1254/jjp.67.365. [DOI] [PubMed] [Google Scholar]
- 40.Rabinovitz H, Friedensohn A, Leibovitz A, Gabay G, Rocas C, Habot B. Effect of chromium supplementation on blood glucose and lipid levels in type 2 diabetes mellitus elderly patients. Int J Vitam Nutr Res. 2004;74:178–182. doi: 10.1024/0300-9831.74.3.178. [DOI] [PubMed] [Google Scholar]
- 41.Press RI, Geller J, Evans GW. The effect of chromium picolinate on serum cholesterol and apolipoprotein fractions in human subjects. West J Med. 1990;152:41–45. [PMC free article] [PubMed] [Google Scholar]
- 42.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 43.Hausdorff SF, Frangioni JV, Birnbaum MJ. Role of p21ras in insulin-stimulated glucose transport in 3T3-L1 adipocytes. J Biol Chem. 1994;269:21391–21394. [PubMed] [Google Scholar]
- 44.Strawbridge AB, Elmendorf JS. Phosphatidylinositol 4,5-bisphosphate reverses endothelin-1-induced insulin resistance via an actin-dependent mechanism. Diabetes. 2005;54:1698–1705. doi: 10.2337/diabetes.54.6.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elmendorf JS. Fractionation analysis of the subcellular distribution of GLUT4 in 3T3-L1 adipocytes. Meth Mol Med. 2003;83:105–111. doi: 10.1385/1-59259-377-1:105. [DOI] [PubMed] [Google Scholar]
- 46.Dugail I. Transfection of adipocytes and preparation of nuclear extracts. Meth Mol Biol. 2001;155:141–146. doi: 10.1385/1-59259-231-7:141. [DOI] [PubMed] [Google Scholar]
- 47.Anderson RA, Cheng N, Bryden NA, Polansky MM, Chi J, Feng J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 48.Aas V, Kase ET, Solberg R, Jensen J, Rustan AC. Chronic hyperglycaemia promotes lipogenesis and triacylglycerol accumulation in human skeletal muscle cells. Diabetologia. 2004;47:1452–1461. doi: 10.1007/s00125-004-1465-9. [DOI] [PubMed] [Google Scholar]
- 49.Garcia JJ, Martinez-Ballarin E, Millan-Plano S, Allue JL, Albendea C, Fuentes L, Escanero JF. Effects of trace elements on membrane fluidity. J Trace Elem Med Biol. 2005;19:19–22. doi: 10.1016/j.jtemb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Belagyi J, Pas M, Raspor P, Pesti M, Pali T. Effect of hexavalent chromium on eukaryotic plasma membrane studied by EPR spectroscopy. Biochim Biophys Acta. 1999;1421:175–182. doi: 10.1016/s0005-2736(99)00129-7. [DOI] [PubMed] [Google Scholar]


