Abstract
The maintenance of wakefulness test (MWT) is a daytime polysomnographic procedure which quantifies wake tendency by measuring the ability to remain awake during soporific circumstances. We present normative data based on 64 healthy subjects (27 males and 37 females) who adhered to uniform MWT procedural conditions including polysomnographic montage, illuminance level, seating position, room temperature, meal timing, and subject instructions. When allowed a maximum trial duration of 40 min, subjects’ mean sleep latency to the first epoch of sustained sleep was 35.2 ± 7.9 min. The lower normal limit, defined as two standard deviations below the mean, was 19.4 min. Calculation of data on the basis of a maximum trial duration of 20 min and sleep latency to the first appearance of brief sleep (a microsleep episode or one epoch of any stage of sleep) yielded a mean sleep latency of 18.1 ± 3.6 min and a lower normal limit of 10.9 min. Sleep latency scores were significantly higher than those previously reported in patients with disorders of excessive somnolence. Therefore, the MWT appears to be a useful procedure in differentiating groups with normal daytime wake tendency from those with impaired wake tendency and in identifying individuals with pathologic inability to remain awake under soporific circumstances.
Keywords: Maintenance of wakefulness test, MWT, Excessive daytime somnolence, Sleepiness, Alertness, Polysomnography, Narcolepsy
1. Introduction
The maintenance of wakefulness test (MWT) is a polysomnographic procedure for the evaluation of daytime somnolence/wakefulness. It assesses an individual’s ability to remain awake while resisting the pressure to fall asleep during soporific circumstances (Mitler et al., 1982a; Mitler et al., 1982b), a process to which we will refer to as ‘wake tendency’. It is used clinically in disorders associated with excessive somnolence such as narcolepsy and sleep apnea syndrome (Browman et al., 1983, 1986; Poceta et al., 1992; Sangal et al., 1992a; Sangal and Sangal, 1996). It has also been utilized to examine treatment efficacy (Sangal et al., 1992b; Fry et al., 1986; Mitler et al., 1990, 1986). Despite its extensive clinical use, however, there are no published reports of systematically obtained normative data. The goal of this investigation was to gather such data.
2. Methods and materials
The sleep disorders centers affiliated with the institutions listed above collaborated in this multi-center study. The design called for 40 males and 40 females with ages ranging between 30 and 70 years (10 males and 10 females in each decade), who did not have complaints regarding sleep or alertness, who were free of major medical and psychiatric conditions and drug use disorders, and who were not taking CNS-active medications. An equal number of potential subjects from each age and gender category were allocated to each center. Subjects were recruited over the course of one year by each center on the basis of posted advertisements and word of mouth and were offered monetary compensation for their participation. Those who met screening criteria and who gave informed consent underwent a medical and psychiatric interview, physical examination, and mental status examination. Participating subjects denied major past or current medical and psychiatric conditions, symptoms compatible with premenstrual syndrome, habitual snoring, and symptoms of other sleep disorders. All smokers and all habitual nappers (napping more than once per week) were excluded. All subjects were night sleepers, accustomed to getting out of bed by 08:30 h at the latest. Subjects took no CNS-active medications for at least 2 weeks prior to testing. For the 5 days preceding the MWT, subjects limited alcohol consumption to one drink per day and caffeine consumption to 2 drinks per day and consumed no caffeine after noon. No caffeine or alcohol were allowed on the night and day of testing. All candidates who met initial inclusion criteria kept a sleep log for 2 weeks prior to testing to insure sleep regularity, defined by a latest out of bed time of 08:30 h, and lack of napping at a frequency of greater than once per week. Subjects then underwent urine drug screens for psychoactive substances which yielded negative results in all entered subjects. They also underwent nocturnal polysomnography with a montage including C3/A2, O1/A2, bilateral electrooculography (EOG), submental electromyogram (EMG), airflow through mouth and nares, respiratory effort, electrocardiogram (EKG), bilateral anterior tibialis EMG, and oxyhemoglobin saturation (SaO2). Recording began within 30 min of the subject’s usual bedtime, and total recording time was equal to the usual time in bed and ended by 08:30 h. For subjects between 30 and 59 years, polysomnographic exclusion criteria included a respiratory disturbance index greater than 5, lowest oxyhemoglobin saturation less than 85%, periodic limb movement arousal index greater than 5, or total sleep time less than 210 min (3.5 h). The frequency of sleep-related respiratory disturbances and periodic limb movements is known to increase with age (Bliwise, 1993). Therefore, we varied polysomnographic exclusion criteria for subjects between 60 and 69 years, in the following way: Respiratory disturbance index greater than 10, lowest oxyhemoglobin saturation less than 85%, periodic limb movement arousal index greater than 10, or total sleep time less than 210 min. Nocturnal polysomnographic and MWT recordings were scored for sleep architectural parameters according to standard criteria (Rechtshaffen and Kales, 1968).
On the next day, subjects were given four 40 min MWT trials at 2 h intervals with the first trial beginning at 1000. All trials were performed in the same bedroom using a simplified montage (C3/A2, O1/A2, EMG, EOG). Bedrooms were insulated from external light and equipped with a dim light (7.5 W night light positioned behind the subject’s head just out of the field of vision, 1 foot off the floor, and 3 feet lateral to the bed). At the subject’s eye level, illuminance was 0.10–0.13 lux, established by a photometer positioned at the corneal level. During each MWT trial subjects sat up in bed with their backs and heads supported by a bedrest cushion (Chintz floor cushion type 30K; Strouds, Orange, CA) such that the neck was not uncomfortably flexed/extended during any sleep that might have occurred. Their backs were at an angle of 45–90 degrees with respect to the bed and legs were straight out with some flexing at the knees to maximize comfort. Ambient temperature was recorded at the beginning of each trial and kept as close to 22°C (72°F) as possible. Breakfast was provided at least 1 h prior to the first MWT trial, and lunch immediately after the termination of the noon trial. Prior to each trial, subjects were instructed as follows: ‘Please sit still and remain awake for as long as possible. Look ahead of you, and do not look directly at the light.’ Subjects were not allowed to maintain wakefulness by using extraordinary measures such as slapping the face or singing. Recordings were monitored by a trained technologist. Each trial was terminated either at the first onset of sleep (sleep onset) or, if sleep onset was not achieved, after a maximum in-bed duration of 40 min. The protocol called for the exclusion of subjects if they were inadvertently allowed to sleep more than 10 min during the first 3 trials. Inspection of records did not, however, reveal such errors. A subject’s participation ended at termination of the fourth trial.
As noted above, the termination of each trial was contingent upon the achievement of sleep onset. Erroneous termination of a trial prior to the onset of true sleep would have invalidated data. To avoid such a technical error, we selected a clearly defined and indisputable definition of sleep onset for the purposes of trial termination, i.e. the first occurrence of sustained sleep defined as 3 consecutive 30 s epochs of stage 1 or any single 30 s epoch of another sleep stage (II, III, IV, or REM). Nevertheless, we noted that previous MWT studies with patient samples had utilized recording and scoring protocols with varied definitions of sleep onset and trial durations (Mitler et al., 1982a; Browman et al., 1983; Browman et al., 1986; Poceta et al., 1992; Sangal et al., 1992a). In order to compare our data with data of these studies we calculated sleep latency scores on the basis of their criteria as well, i.e. sleep onset defined as the first epoch of any sleep stage and the first occurrence of 10 s of continuous sleep (sleep). However, since these two definitions yielded equivalent sleep latency data (P > 0.05), we did not distinguish between them in the presentation of the data. Similarly, although we conducted the study with a trial duration of 40 min, we also calculated our data on the basis of 20 min trial durations. In summary, we calculated our results utilizing the following four protocols:
-
SUSMWT40
40 min MWT trials with sleep onset defined as 3 continuous epochs of stage 1 sleep or any single epoch of another sleep stage
-
MWT40
40 min MWT trials with sleep onset defined as the first appearance of sleep, whether 10 s of sleep or the first epoch of sleep
-
SUSMWT20
20 min MWT trials with sleep onset defined as 3 continuous epochs of stage 1 sleep or any single epoch of another sleep stage
-
MWT20
20 min MWT trials with sleep onset defined as the first appearance of sleep, whether 10 s of sleep or the first epoch of sleep
Polysomnographic recordings of sleep onsets were photocopied and distributed to other sites to insure agreement of scoring criteria. Unless otherwise noted, in all comparisons we utilized T-tests for differences between means and Pearson correlational methods for relationships between variables.
3. Results
Sixty-four subjects (27 males and 37 females) successfully completed the protocol within the 1 year recruitment period. However, the goal of 80 entered subjects was not met because of the failure of some potential subjects to meet entrance criteria prior to, and following, polysomnography. As is evident in Table 1, which summarizes the distribution of subjects within each age group and gender category, most gaps in subject requirements fell in the older age groups. These subjects were excluded due to sleep-related respiratory disturbances, periodic limb movements, medications, and medical conditions. Table 2 presents demographic and nocturnal variables for the entire sample. Subjects did not meet polysomnographic criteria for major sleep disorders as set forth by our entrance requirements. Review of polyosmnographic records also did not reveal evidence of other, unanticipated, sleep disorders. Variability between centers was noted for both SUSMWT40 mean sleep latency and nocturnal total sleep time (both ANOVA Fs > 2.75; df = 5,58, P < 0.025). However, these differences could not be ascribed to procedural issues inasmuch as scrutiny did not reveal protocol violations or systematic differences in subject entry, study recording, or record scoring procedures.
Table 1.
Distribution of subjects by age and gender
| Age Group | Female | Male | Total |
|---|---|---|---|
| 30–39 | 10 | 8 | 18 |
| 40–49 | 10 | 10 | 20 |
| 50–59 | 10 | 5 | 15 |
| 60–69 | 7 | 4 | 11 |
| Total | 37 | 27 | 64 |
Table 2.
Demographic and Nocturnal Variables
| Variable | Mean | SD |
|---|---|---|
| Age | 47.8 | 11.4 |
| Body mass index | 24.8 | 3.9 |
| Sleep latency | 10.9 | 11.9 |
| Total sleep time | 416.7 | 63.1 |
| Stage 1 | 36.0 | 17.3 |
| Stage 2 | 230.5 | 58.7 |
| Stage 3 | 32.21 | 14.92 |
| Stage 4 | 36.5 | 31.5 |
| REM | 80.3 | 25.5 |
| REM latency | 106.6 | 54.2 |
| Respiratory disturbance index | 1.96 | 2.92 |
| Average SaO2 nadir | 91.41 | 4.72 |
| PLM arousal index | 1.32 | 3.18 |
Polysomnographic variables are in minutes and refer to nocturnal sleep. The respiratory disturbance index refers to the number of apneas and hypopneas per hour of sleep. Abbreviations: PLM, periodic limb movement; SaO2, oxyhemoglobin saturation.
Table 3 presents averages of sleep latency scores for individual trials and across all four trials for our main (SUSMWT40) protocol. As is evident, the use of 40 min MWT trials produced a truncated distribution as a result of a high proportion of mean latency scores approximating 40 min. Thus, most information was conveyed by the minima and lowest percentiles. The mean sleep latency across all trials was 35.2 ± 7.9 min. 38 (59%) of subjects did not fall asleep during any of the MWT trials (Fig. 1). A total 256 MWT trials were administered. In all but 52 (20%) of the trials, wakefulness was maintained for 40 min. Had the trials been terminated at 20 min (SUSMWT20), the mean sleep latency across all trials would have been 18.7 ± 2.6 min. 44 (69%) of subjects would have failed to meet sleep onset criteria on all trials, and wakefulness would have been maintained in all but 36 (14%) trials.
Table 3.
Individual and average MWT sleep latency scores for the SUSMWT40 protocol
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | Mean | |
|---|---|---|---|---|---|
| Mean | 36.3 | 34.0 | 34.2 | 36.5 | 35.2 |
| SD | 9.2 | 12.0 | 11.5 | 8.8 | 7.9 |
| Minimum | 3.0 | 4.5 | 3.5 | 1.2 | 7.1 |
| Maximum | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| 10th pertl | 18.2 | 9.6 | 11.5 | 25.4 | 21.7 |
| 25th pertl | 40.0 | 40.0 | 40.0 | 40.0 | 32.8 |
| 50th pertl | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| 75th pertl | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| 90th pertl | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
Sleep latency scores are in minutes. SD, standard deviation; pertl, percentile.
Fig. 1.
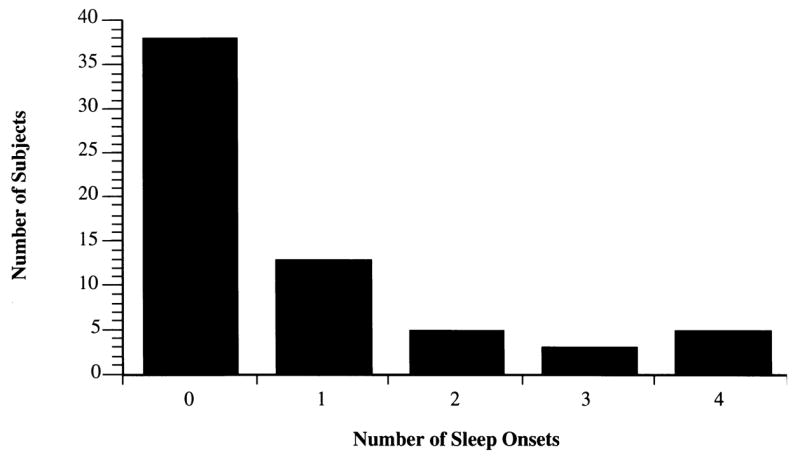
Distribution of sleep onsets prior to trial termination for the SUSMWT40 Protocol.
Data based on our main and extrapolated protocols are displayed in boxplot form in Fig. 2. Each box represents the middle 50% of the sample, i.e. the interquartile range, or the distance between the 25th and 75th sample percentiles. The tails represent outliers, as noted in the legend. The truncated distribution of the data is apparent in all four boxplots as the middle 50% of the distribution clusters close to the trial termination limit. Nevertheless, the SUSMWT40 protocol’s mean sleep latency score is higher and skewness greater than the MWT40, probably attributable to the stricter definition of sleep onset. Utilizing 20 min trial durations yields highly truncated plots regardless of the definition of sleep onset.
Fig. 2.
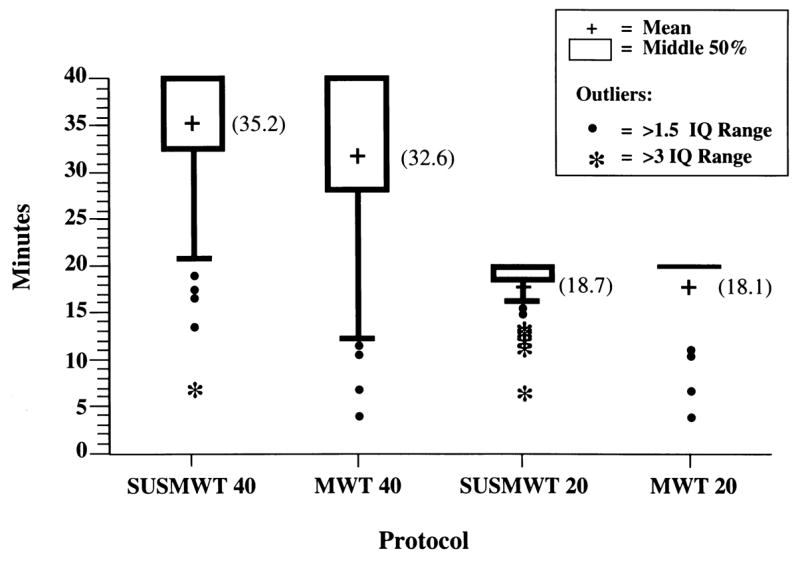
Distribution of mean MWT sleep latency scores for various protocols For definitions of protocols, please see text. Abbreviations: IQ, interquartile, i.e. the distance between the 25th and 75th sample percentiles, represented by the box. Therefore, each box encompasses the middle 50% of the distribution. The vertical lines extend each box to a distance of 1.5 interquartile ranges from the box. Black circles encompass scores falling within three interquartile ranges and asterisks denote extreme outliers falling outside this range.
Direct comparisons of out present data to those of previously published clinical studies appear in Table 4. Regardless of the definition of sleep latency or the maximum trial duration, mean MWT sleep latency scores from the present, normative, study were significantly higher than scores obtained with clinical samples, and did not differ from scores of previously reported normal controls.
Table 4.
Comparison of MWT normative with clinical data
| Study | Sample | N | Sleep onset criteria | Trial duration | Protocol | Mean sleep latency | P-valuea |
|---|---|---|---|---|---|---|---|
| Doghramji et al. (this work) | Normals | 64 | A | 40 | SUSMWT40 | 35.2 ± 7.9 | |
| Doghramji et al. (this work) | A | 20 | SUSMWT20 | 18.7 ± 2.6 | |||
| Doghramji et al. (this work) | Bb | 40 | MWT40 | 32.6 ± 9.9 | |||
| Doghramji et al. (this work) | Bb | 20 | MWT20 | 18.1 ± 3.6 | |||
| Poceta et al., 1992 (5) | OSA | 322 | A | 40 | SUSMWT40 | 25.9 ± 11.8 | <0.0001 |
| Sangal et al., 1992a (6) | Excessive daytime sleepiness | 258 | B | 20c | MWT20c | 15.9 ± 5.0 | <0.001 |
| Sangal et al., 1992a (6) | Excessive daytime sleepiness | 258 | B | 40 | MWT40 | 26.5 ± 12.4 | <0.001 |
| Browman et al., 1986 (4) | Narcolepsy | 11 | A | 20 | SUSMWT20 | 10.7 ± 5.3 | <0.001 |
| Browman et al., 1986 (4) | Normal controls | 11 | A | 20 | SUSMWT20 | 19.0 ± 1.5 | >0.05 |
| Browman et al., 1983 (3) | Narcolepsy | 12 | A | 20 | SUSMWT20 | 11.0 ± 5.6 | <0.001 |
| Browman et al., 1983 (3) | OSA | 12 | A | 20 | SUSMWT20 | 11.0 ± 4.8 | <0.001 |
| Browman et al., 1983 (3) | Normal controls | 10 | A | 20 | SUSMWT20 | 18.3 ± 4.0 | >0.05 |
| Mitler et al., 1982a (1) | Narcolepsy | 10 | A | 20 | SUSMWT20 | 9.9 ± 6.1 | <0.001 |
| Mitler et al., 1982a (1) | Normal controls | 8 | A | 20 | SUSMWT20 | 17.9 ± 4.4 | >0.05 |
For definitions of protocols, please see text. Trial durations are in minutes and mean sleep latency scores in minutes ± SD.
(A) Three 30-s epochs of stage 1 or one epoch of any other sleep stage.
(B) One 30-s epoch of any sleep stage.
Compared with corresponding measure of sleep latency in this work.
Or 10 s of sleep.
Data based on a 40-min protocol were recalculated for the 20-min protocol.
With respect to the effect of age, there was a significant, negative, correlation between age and nocturnal total sleep time (R = −0.38, P < 0.01), i.e. younger subjects tended to sleep longer. There was also a weak, but statistically significant, positive correlation between age and mean MWT40 sleep latency (Table 5), i.e. older subjects tended to maintain wakefulness longer on MWT40 trials, yet this relationship was not evident with any of the other protocols. Note that there was no relationship between either nocturnal sleep latency or nocturnal total sleep time and MWT sleep latency for any of the protocols. Indeed, the individuals with the two lowest nocturnal sleep times maintained wakefulness on all SUSMWT40 trials. However, sleep efficiency was inversely related to MWT sleep latency for all protocols except SUTMWT20. Table 6 summarizes selected variables as a function of gender. Males did not differ from females in terms of major nocturnal sleep parameters or performance for our main (SUSMWT40) protocol.
Table 5.
Relationship of selected variables with mean MWT sleep latency scores utilizing various protocols
| Correlation coefficients (R)
|
||||||
|---|---|---|---|---|---|---|
| Mean | SD | SUSMWT40 | MWT40 | SUSMWT20 | MWT20 | |
| Age | 47.7 | 11.4 | 0.28 | 0.29* | 0.24 | 0.24 |
| Noc SL | 10.9 | 11.9 | 0.16 | 0.20 | 0.16 | 0.19 |
| Noc TST | 416.7 | 63.1 | −0.08 | −0.14 | −0.07 | −0.11 |
| SE | 0.86 | 0.09 | −0.26* | −0.31* | −0.25 | −0.27* |
Noc SL, nocturnal sleep latency; Noc TST, nocturnal total sleep time; SE, sleep efficiency (total sleep time divided by the time in bed, expressed as a percentage). For definitions of protocols, please see text.
P < 0.05.
Table 6.
Selected variables as a function of gender
| Noc SL | Noc TST | Noc REM | Mean SUSMWT40 sleep latency | No. SUSMWT40 failures | |
|---|---|---|---|---|---|
| Males | 7.8 ± 7 | 422.1 ± 51 | 83.7 ± 24 | 36.2 ± 6 | 0.63 ± 0.9 |
| Females | 13.2 ± 14 | 412.7 ± 71 | 77.8 ± 27 | 34.6 ± 9 | 0.95 ± 1.0 |
Values are mean ± SD. Noc SL, nocturnal sleep latency; Noc TST, nocturnal total sleep time; Noc REM, minutes of nocturnal REM sleep; No. SUSMWT40 failures, number of trials meeting criteria for sleep onset prior to trial termination. Males did not differ from females with respect to any of the variables listed (P>0.05).
4. Discussion
This is the first systematic study of MWT performance in normal individuals. Whereas previous MWT studies had utilized variable experimental conditions, the present study provides uniform procedural conditions which were stringently adhered to. These conditions included polysomnographic montage, illuminance level, seating position, room temperature, meal timing, and patient instructions. The availability of such uniform guidelines offers the possibility of making meaningful comparisons between the results of future studies. Additionally, whereas the study was performed utilizing set definitions of sleep onset and trial duration, results were also calculated in accordance with other criteria to allow for comparison of our data with those of previously reported clinical investigations.
For these normal subjects, adherence to our primary protocol of a 40 min trial duration and a definition of sleep onset as 3 continuous epochs of stage 1 sleep or any single epoch of another sleep stage (SUSMWT40) yields a mean sleep latency of 35.2 ± 7.9 min. A 20 min trial duration (SUSMWT20) yields a mean sleep latency of 18.7 ± 2.6 min. The distribution of sleep latencies is truncated for both protocols, with a ceiling effect in over 75% of 40 min trials and in about 85% of 20 min trials. Making the definition of sleep onset more lenient (i.e. the first appearance of sleep, whether 10 s of sleep or the first epoch of sleep) diminishes the magnitude of the ceiling effect and yields a mean sleep latency of 32.6 ± 9.9 min for the 40 min trial duration (MWT40), and of 18.1 ± 3.6 min for the 20 min trial duration (MWT20).
The MWT has been extensively utilized to quantify changes following therapeutic maneuvers in disorders of excessive somnolence (Sangal et al., 1992b; Fry et al., 1986; Mitler et al., 1990, 1986). Our results also indicate, however, that the MWT is useful in differentiating groups with normal daytime alertness from those with impaired ability; regardless of the protocol utilized, our normal group exhibited scores that were significantly higher than those of previously studied sleepy patients. An additional clinical application of the MWT may be its ability to assess whether a given individual is ‘normal’ in terms of wake tendency during soporific daytime tasks. Such an application would necessitate cutoff points for normality. A statistical criterion commonly utilized for this purpose is the equivalent of two standard deviations (SD) from the mean (American Electroencephalographic Society, 1994). Lower limits for normality as assessed by the mean minus 2 SDs are displayed in Table 7. Although actual limits for normality differ with each protocol, the proportion of subjects scoring lower than these limits is less than 10% for all protocols. Enhancing the level of certainty to the 5% level diminishes the sleep latency by approximately 1 min for the MWT protocols and by approximately 2 min for the SUSMWT protocols. In applying the 2 SD definition of normality to previous, clinical, samples, we note that of the 258 patients with subjective sleepiness reported by Sangal and colleagues (Sangal et al., 1992a), 19% had mean sleep latency less than 10.9 min on the MWT20, and the same number had mean sleep latency less than 12.9 min on the MWT40. Thus, 19% of these patients were ‘too sleepy’ by these criteria. For either the MWT 20 or the MWT 40, sleep latency scores for the 258 patients were significantly lower than those of normal controls (P < 0.001). Finally, we note that for the 20 min protocols, normal cutoff scores are close to the corresponding score of 10 min for a similar daytime polysomnographic test, the multiple sleep latency test (MSLT; Carskadon, 1986), which also utilizes a 20 min maximum trial duration. However, although the normal MWT scores are based on a statistical definition of normality, the normal MSLT cutoff of 10 min is consensus-based.
Table 7.
Lower limits for normality as assessed by two standard deviations lower than the mean for various MWT protocols
| Protocol | Lower limit (mean minus 2 SD in minutes) | % Subjects scoring less than lower limit |
|---|---|---|
| SUSMWT40 | 19.4 | 8 |
| MWT40 | 12.9 | 9 |
| SUSMWT20 | 13.5 | 6 |
| MWT20 | 10.9 | 8 |
For definitions of protocols, please see text. Abbreviations: SD, standard deviation.
We applied various definitions of sleep onset and trial duration in calculating sleep latency scores. The question arises as to which of these protocols should be utilized in each individual case. This determination may be based on the specific clinical need and the nature of practical constraints. For example, if the sample being tested is likely to have subjects with a low level of sleepiness, maximizing the test’s sensitivity in detecting sleep onset and maximizing the duration of each trial may minimize the potential for a ceiling effect and, in turn, allow for more meaningful comparison among subjects tested. In this case, therefore, utilizing the 40 min trial duration and the first appearance of any epoch of sleep as the definition of sleep onset (MWT40) may be optimal. The same protocol may be best suited for assessing treatment response. However, if diagnostic accuracy is critical, as in medicolegal settings or if test results are to be utilized to establish the presence or absence of an illness, the more stringent definition of sleep onset (3 epochs of stage 1 or one epoch of any other sleep stage) may be preferred. Practical, economic, limitations may favor the use of the 20 min duration. Table 5 shows that the latency to sleep on the MWT40 is significantly related to age. The latency to sleep on either 20 min MWT, however, is not. This suggests that the ability to stay awake for the first 20 min is not related to age. However, as the time required to stay awake increases to 40 min, age becomes a factor, with increasing age being associated with ability to stay awake longer. This would argue for using the MWT20 in routine clinical settings in determining pathological inability to stay awake, so as not to compound the test with the effects of age.
The MWT and MSLT are objective tests and considered, therefore, to be preferable to the recently introduced subjective scale, the Epworth Sleepiness Scale (ESS; Johns, 1994), for the assessment of daytime somnolence. However, the former are more costly. One method of diminishing cost for the MWT is conducting fewer trials. To examine the feasibility of conducting the MWT with two trials instead of four, we analyzed MWT20 results using the first two trials, MWT20(1_2), and the last two trials, MWT20(3_4). We also analyzed the SUSMWT20 using the first two trials, SUSMWT20(1_2), and the last two trials, SUSMWT20 (3_4). Table 8 shows correlation coefficients for the relationship of the abbreviated (2 trial) protocols with the complete (4 trial) protocols. The highly significant correlation coefficients for the relationship of MWT20 with MWT20 (1_2) and MWT20(3_4) (0.92 and 0.93, respectively) imply that an insignificant amount of variance is lost by using 2 trials instead of 4 trials when the latency to sleep is measured to the first onset of any stage of sleep. In contrast, the correlation coefficients for the relationship of SUSMWT20 with SUSMWT20(1_2) and SUSMWT20 (3_4) are lower (0.81 for both), suggesting that a greater amount of the variance in the data is lost by using 2 trials instead of 4 trials when the latency to sleep is measured to 3 continuous epochs of stage 1 or any epoch of stage 2, 3, 4 or REM. Examination of the scatter plot for MWT20 vs. MWT20 (1_2) reveals that only two of the 64 subjects who are MWT20-abnormal (sleepy), i.e. having scores less that 2SDs from the mean, fall within the normal range for the MWT20(1_2). Of the 258 subjectively sleepy patients reported by Sangal et al. (1992a), nine patients were sleepy on the MWT20(1_2) and not on the MWT20. Ten patients were sleepy on the MWT20 but not the MWT20 (1_2). Therefore, we would suggest that resource utilization and cost of the MWT can be reduced substantially by using 2 trials instead of 4 trials. However, the latency to sleep must then be measured to the first onset of any stage of sleep.
Table 8.
Relationship of complete and abbreviated 20-min MWT protocol results
| Protocol | Correlation coefficients (R)
|
|||
|---|---|---|---|---|
| Mean | SD | MWT20 | SUSMWT20 | |
| MWT20 | 18.1 | 3.6 | 1.00* | 0.95* |
| MWT20 (1_2) | 18.1 | 3.8 | 0.92* | 0.83* |
| MWT20 (3_4) | 18.0 | 4.1 | 0.93* | 0.88* |
| SUSMWT20 | 18.7 | 2.6 | 0.95* | 1.00* |
| SUSMWT20 (1_2) | 18.6 | 3.2 | 0.83* | 0.81* |
| SUSMWT20 (3_4) | 18.8 | 3.3 | 0.72* | 0.81* |
For definitions of protocols, please see text. SD, standard deviation.
P < 0.001.
As noted above, despite significant variability among study sites in SUSMWT40 sleep latency and nocturnal total sleep time, we uncovered no systematic procedural differences or protocol violations. Therefore, we cannot question the validity of our data on the basis of these differences. We cannot account for these differences other than to speculate that they may reflect true differences in sleep habits of different metropolitan areas. Further research, appropriately designed and with a sufficient number of subjects will be required to address this possibility. Additionally, no consistent relationship was discovered between either nocturnal sleep latency or nocturnal total sleep time and MWT sleep latency for any of the MWT protocols. Although manipulations of sleep such as nocturnal sleep restriction and deprivation have been shown to be associated with changes in MWT (Carskadon, 1986) and MSLT (Carskadon and Dement, 1987) results, our study examined subjects who naturally varied with respect to their habitual total sleep times. Therefore, the lack of a relationship between nocturnal total sleep time and MWT sleep latency for the sample as a whole may reflect the natural variability of sleep needs.
Another intriguing finding is the ability of older individuals to maintain wakefulness for a longer period of time during the MWT40 and their lack of impairment in ability to maintain wakefulness on all other protocols, despite their lower total sleep time during the prior night. Any conclusions regarding this finding must be considered in light of the relatively low number of elderly individuals in our sample; 11 subjects (17%) fell into the 60–69 age range. Nevertheless, to our knowledge, this is the first such reported finding as assessed by the MWT. If such a finding is replicated, it may suggest that the elderly have a diminished need for sleep. MSLT studies have shown discrepant results in the elderly (for a review, see Bliwise, 1993). Some of these discrepancies may be due to methodological inconsistencies such as failure to exclude medical, psychiatric, and sleep disorders. Indeed, the severity of respiratory sleep disturbance in the elderly has been reported to be related to the severity of daytime sleepiness as assessed by the MSLT (Valencia-Flores et al., 1993). In contrast, our sample was carefully screened for these conditions.
To date, the most widely utilized polysomnographic procedure for these and other clinical purposes has been the MSLT (Standards of Practice Committee, American Sleep Disorders Association, 1992). Although the MWT and the MSLT have many procedural similarities, a critical difference between the two lies in the instructions to the subject. MSLT subjects are instructed to not resist the urge to fall asleep while lying down in a dark room. Therefore, the MSLT measures the ability of subjects to fall asleep, while the MWT measures the ability of an individual to remain awake, under soporific conditions. That the two tests assess such separate functions is supported by the lack of a consistent relationship between the two when applied to the same patient groups (Browman et al., 1986; Sangal et al., 1992a). Therefore, each may have a unique set of clinical applications. For example, the MWT has been shown to be more sensitive than the MSLT in detecting effects of treatment (Sangal et al., 1992b) and manipulations of the prior night’s sleep quality and quantity (Sugerman and Walsh, 1989) on daytime alertness. It also stands to reason that the MWT may by more accurate in assessing the risk of falling asleep unintentionally during soporific activities where individuals are attempting to stay awake, such as driving and reading. However, no such comparisons between the two tests have been performed, to our knowledge, in such activities of daily life. Clearly greater studies are needed to explore the reasons for the discordance between the two tests and the relative utility of each. We anticipate that the availability of uniform procedures and normative data for the MWT will make such future studies possible and will lead to greater clinical experience with the MWT.
In conclusion, we have provided a methodology for the performance of the MWT as well as normative data based on that methodology. Our data indicate that MWT sleep latency scores have a truncated distribution and that altering basic parameters such as the definition of sleep onset and the trial termination limit changes the shape of the distribution. Nevertheless, all mean MWT sleep latencies so derived were significantly higher than those obtained by previous studies utilizing patient samples. Based on a definition of normality of 2 SDs lower than the mean, we also determined that normal lower cutoff points for the SUSMWT40, MWT40, SUSMWT20, and MWT20 protocols were 19.4, 12.9, 13.5, and 10.9 min, respectively. Therefore, in addition to its value in quantifying therapeutic effects in disorders of excessive somnolence, the MWT appears to be useful in differentiating groups with normal ability to remain awake from those with impaired ability and in assessing whether an individual is pathologically impaired in the ability to remain awake in soporific situations.
5. Consensus statement
Following the initial reviews of this paper, the authors were made aware of the need for a consensus statement regarding the optimal recording and scoring guidelines for routine clinical use of the MWT. As we have indicated above, the determination of which protocol to use in each case should, optimally, be based on the specific clinical objectives and the nature of practical constraints. Nevertheless, we also appreciate the need for uniformity in methodology so that clinical results can be meaningfully compared to one another and to a normative cutoff value. In this context, we favor the MWT20 protocol for the following reasons: First, the 20 min protocols are more cost-effective than the 40 min protocols and have, therefore, a practical advantage. Second, the 20 min protocols are not affected by age in a consistent manner, whereas the MWT40 protocol is. Third, of the two 20 min protocols, the MWT20, in which sleep latency is measured to the first onset of any stage of sleep, is more conducive to the use of a less costly 2 trial protocol than the SUSMWT20, in which latency is measured to the first epoch of sustained sleep; less variance is lost by using 2 trials intead of 4 trials in the former protocol than in the latter. In summary, our recommendations are as follows:
-
Lighting
The room should be maximally insulated from external light. The light source should be positioned slightly behind the subject’s head such that it is just out of his field of vision, and should deliver an illuminance of 0.10–0.13 lux at the corneal level (in our study a 7.5 W night light was utilized, 1 ft. off the floor, and 3 ft. laterally removed from the subject’s head).
-
Seating position
Sitting in bed, back and head supported by a bedrest such that neck is not uncomfortably flexed/extended during sleep.
-
Room temperature
As close to 22°C (72°F) as possible. Temperature should be recorded at the beginning of each trial.
-
Meals
A light breakfast at least 1 h prior to the first nap, and a light lunch immediately after the termination of the noon nap.
-
Instructions to patients
‘Please sit still and remain awake for as long as possible. Look directly ahead of you, and do not look directly at the light.’ Patients should be disallowed from using extraordinary measures such as slapping the face or singing.
-
Monitoring montage
C3/A2, O1/A2, EMG, EOG
-
Sleep onset
The first occurrence of one epoch of any stage of sleep.
-
Trials
Should be performed at 2 h intervals, the first beginning at 1000 h.
-
Trial termination
At sleep onset or (b) after 20 min in bed if sleep onset not achieved.
-
Scoring
Sleep latency is defined as the time from trial onset to the first epoch of any sleep stage.
-
Data to be recorded
Sleep latency, total sleep time, total wake time, stages of sleep achieved for each trial.
-
Interpretation
Impairment in wake tendency exists if the mean sleep latency is less than 11 min.
Acknowledgments
We gratefully acknowledge the technical assistance of William Breuninger, R.PSG.T., Joanne Saccomandi, Tina DeFinis, and Patricia Zwiffelhoffer. We are also indebted to the reviewers of this manuscript who provided us with the useful term ‘wake tendency’ in describing what the MWT measures.
References
- American Electroencephalographic Society. Guidelines in electroencephalography evoked potentials and polysomnography. J Clin Neurophysiol. 1994;11:1–147. [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Browman CP, Gujavarty KG, Sampson MG, Mitler MM. REM sleep episodes during the maintenance of wakefulness test in patients with sleep apnea and narcolepsy. Sleep. 1983;6:23–28. doi: 10.1093/sleep/6.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman CP, Gujavarty KS, Yolles S, Mitler MM. Forty-eight-hour polysomnographic evaluation of narcolepsy. Sleep. 1986;9:183–188. doi: 10.1093/sleep/9.1.183. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Daytime sleepiness: Quantification of a behavioral state. Neurosci Biobehav Rev. 1987;11:307–317. doi: 10.1016/s0149-7634(87)80016-7. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- Fry JM, Pressman MR, DiPhillipo MA, Frost-Paulus M. Treatment of narcolepsy with codeine. Sleep. 1986;9:269–274. doi: 10.1093/sleep/9.1.269. [DOI] [PubMed] [Google Scholar]
- Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness scale. Sleep. 1994;17:703–710. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Gujavarty KS, Browman CP. Maintenance of wakefulness test: a polysomnographic technique for evaluating treatment efficacy in patients with excessive somnolence. Electroenceph clin Neurophysiol. 1982a;53:658–661. doi: 10.1016/0013-4694(82)90142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler MM, Gujavarty KS, Sampson MG, Browman CP. Multiple daytime nap approaches to evaluating the sleepy patient. Sleep. 1982b;5:S119–S127. doi: 10.1093/sleep/5.s2.s119. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Shafor R, Hajdukovich R, Timms RM, Browman CP. Treatment of narcolepsy: objective studies on methylphenidate, pemoline, and protriptyline. Sleep. 1986;9:260–264. doi: 10.1093/sleep/9.1.260. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Hajdukovic RM, Erman M, Koziol JA. Narcolepsy. J Clin Neurophysiol. 1990;7:93–118. doi: 10.1097/00004691-199001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poceta JS, Timms RM, Jeong D, Ho S, Erman M, Mitler MM. Maintenance of wakefulness test in obstructive sleep apnea syndrome. Chest. 1992;101:893–897. doi: 10.1378/chest.101.4.893. [DOI] [PubMed] [Google Scholar]
- Rechtshaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- Sangal RB, Sangal JM. Measurement of P300 and sleep characteristics in patients with hypersomnia: do P300 latencies, P300 amplitudes and multiple sleep latency and maintenance of wakefulness tests measure different factors? Clin Electroenceph. 1996 doi: 10.1177/155005949702800311. in press. [DOI] [PubMed] [Google Scholar]
- Sangal RB, Thomas L, Mitler MM. Maintenance of wakefulness test and multiple sleep latency test. Measurement of different abilities in patients with sleep disorders. Chest. 1992a;101:898–902. doi: 10.1378/chest.101.4.898. [DOI] [PubMed] [Google Scholar]
- Sangal RB, Thomas L, Mitler MM. Disorders of excessive sleepiness: treatment improves ability to stay awake but does not reduce sleepiness. Chest. 1992b;102:699–703. doi: 10.1378/chest.102.3.699. [DOI] [PubMed] [Google Scholar]
- Standards of Practice Committee of the American Sleep Disorders Association. The clinical use of the multiple sleep latency test. Sleep. 1992;15:268–276. doi: 10.1093/sleep/15.3.268. [DOI] [PubMed] [Google Scholar]
- Sugerman JL, Walsh JK. Physiological sleep tendency and ability to maintain alertness at night. Sleep. 1989;12:106–112. doi: 10.1093/sleep/12.2.106. [DOI] [PubMed] [Google Scholar]
- Valencia-Flores M, Ma Campos R, Mendez J, Haro R, Schenkel E, Bliwise D, Guilleminault C. Multiple sleep latency test (MSLT) and sleep apnea in aged women. Sleep. 1993;16:114–117. doi: 10.1093/sleep/16.2.114. [DOI] [PubMed] [Google Scholar]


