Abstract
Purpose
To achieve a better understanding of the involvement of phospholipases in the inflammation and wound-healing processes in human corneal epithelial cells (HCECs), expression of phospholipase A2s (PLA2s) and phospholipase Cs (PLCs) was examined in the human corneal epithelium.
Methods
Specific primers were designed for RT-PCR amplification of the known secreted (s)PLA2, cytosolic (c)PLA2, and PLC mRNAs. Corresponding PCR products were cloned and the DNA sequenced. Immunofluorescence of flatmounted corneal sections and Western blot analyses were used to detect the PLA2s and PLCs expressed by HCECs.
Results
The mRNAs for the following phospholipases were detected by RT-PCR in the HCECs: sPLA2GIII, -GX, and -GXIIA; cPLA2α and -γ; PLCβ1, -β2, -β3, -β4, -γ1, -γ2, -δ1, -δ3, -δ4, and -ε. Immunofluorescence analyses conducted on corneal epithelium cryosections and Western blot on freshly isolated HCECs demonstrated the presence of sPLA2GIII, -GX, and -GXIIA; cPLA2α and -γ; and PLCβ2, -β3, -γ1, -γ2, and -δ3.
Conclusions
Many phospholipase isoforms are expressed by HCECs and may play a major role in signal transduction (PLCs) as well as in the release of precursors of potent mediators of inflammation, such as leukotrienes and prostaglandins (PLA2s). Moreover, the sPLA2s expressed by the corneal epithelium could be involved in the normal antibacterial activity in the tears and in wound healing.
Corneal wound healing is a complex process that can be seriously impaired by inflammation. Indeed, keratitis or corneal inflammation often result in an opacification of the cornea that can usually be successfully treated but may also lead to the loss of vision or of the eye itself.1 Phospholipases are essential for cell proliferation, differentiation, transformation, apoptosis,2 and synthesis of potent precursors of mediators of inflammation.3 Some phospholipases also exhibit antibacterial properties.4,5 Phospholipases can therefore be considered major components in corneal wound healing and inflammation processes.6,7
Phospholipases hydrolyze membrane phospholipids to induce or transmit signals within the cell.8 There are different families of phospholipases, among which the phospholipase A2s (PLA2s) and phospholipase Cs (PLCs) families are physiologically essential. The PLA2 family is composed of two major subfamilies: secreted (s)PLA2 and cytosolic (c)PLA2, group (G)IV. In mammals, many groups of sPLA2s (GIB, GIIA, GIIC, GIID, GIIE, GIIF, GIII, GV, GX, GXIIA, and GXIIB)9–12 and four types of cPLA2s (α, β, γ, and δ; GIVA–D)11,13,14 hydrolyze phospholipids to generate a free fatty acid and a lysophospholipid, both of which can act as lipid signaling molecules. Frequently, the free fatty acid produced is arachidonic acid (AA).15 AA is the precursor of many potent mediators of inflammation, such as thromboxanes, prostaglandins, and leukotrienes. AA metabolites are, among others, responsible for ocular inflammation.16,17 sPLA2s and cPLA2s are therefore essential enzymes in the management of corneal inflammation. Furthermore, seven sPLA2s demonstrate potent antibacterial properties in vitro against Gram-positive bacteria.4,5 sPLA2GIIA accounts, at least in part, for the antibacterial properties of the tears18 and is secreted by both the lacrimal glands and canals.19,20 The corneal epithelium may also be responsible for part of the antibacterial properties of the tears, if it secretes sPLA2s.
There are 12 known mammalian isoforms of PLC divided into five different types: PLCβ,2,21,22 -γ,2,21,22 -δ,2,21–23 -ε,24,25 and -ζ.26,27 Some of these isoforms also undergo alternative splicing. PLCs are associated with the plasma membrane (β, γ, δ, and ε),2,25 the cytosol (β, γ, δ, ε, and ζ),2,25–27 or the nucleus (β and γ).2 After activation by the cell receptors,2 PLCs then preferentially hydrolyze phosphatidylinositol 4,5-bishosphate from the membrane to produce two potent second messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG).2 IP3 induces the release of intracellular calcium from the endoplasmic reticulum reserves, and the main cellular targets for DAG belong to the protein kinase C (PKC) family.2 The increase in intracellular calcium activates a very intricate signaling pathway in ocular tissues.28 It also activates cPLA2s through its translocation to the membrane.29,30
Phospholipases play a major role in signal transduction (PLCs) and in the release of potent precursors of mediators of inflammation (PLA2s and PLCs). These enzymes thus play central roles in the corneal epithelium, with particular reference to wound healing as well as to the antibacterial properties of the tears. However, no information is available on the expression of phospholipases by the human corneal epithelium. In the present study, the identity of the different PLA2s and PLCs expressed by human corneal epithelial cells (HCECs) was thus determined at the protein and transcript level by using immunofluorescence of tissue sections, Western blot analyses, and RT-PCR.
Materials and Methods
This study was conducted in accordance with our institution’s guidelines and the Declaration of Helsinki. The protocols were also approved by the institution’s Committee for the Protection of Human Subjects.
Isolation of Human Corneal Epithelium
Fresh corneas were isolated from 62 human donor eyes (age range, 39–88 years; average, 69) within 24 hours after death through the Banque d’Yeux Nationale (Sainte-Foy, Québec, Canada). Briefly, corneas were dissected according to the procedure described by Gipson and Grill.31 Epithelial sheets were then transferred to either a reagent for subsequent total RNA extraction (TriReagent; Sigma-Aldrich, St. Louis, MO), or PBS (150 mM NaCl, 9.1 mM Na2HPO4, and 1.7 mM NaH2PO4 [pH 7.4]) containing 1% (vol/vol) protease inhibitor cocktail (Sigma-Aldrich) for subsequent protein extraction.
Reverse Transcription–Polymerase Chain Reaction
Total RNA was extracted and pooled from human corneal epithelial tissues freshly isolated from 16 human eyes (age range, 48–84 years; average, 66) using extraction reagent (TriReagent; Sigma-Aldrich), as described previously.32,33 Reverse transcription of total RNA was performed with a reverse transcriptase kit (SuperScript II RNase H−; Invitrogen, Burlington, Ontario, Canada) according to the manufacturer’s instructions. cDNA sequences of sPLA2s, cPLA2s, and PLCs were amplified by PCR on a thermocycler (Techgene; Techne, Princeton, NJ). The primers (Table 1) were provided by the Service de Synthèse d’ADN from the Centre de Recherche du CHUL; Sainte-Foy, Québec, Canada) using a high-throughput DNA synthesizer (model 3900; Applied Biosystems, Foster City, CA). The amplification reactions consisted of 35 three-step cycles of denaturation for 50 seconds at 95°C, annealing for 40 seconds at 55°C, and elongation for 60 seconds at 72°C. The PCR products were then separated by electrophoresis on 1% (wt/vol) agarose gels and photographed (Gel-Doc 2000; Bio-Rad, Hercules, CA).
Table 1.
Sequences of the Forward and Reverse Primers Used for the PCR Amplification of Phospholipase Isoforms in Human Corneal Epithelium
| Phospholipase Isoform |
Forward Primer | Reverse Primer | Expected PCR Product Size (bp) | GenBank Accession Number |
|---|---|---|---|---|
| sPLA2GIB | TGGTCATCTCAGTTCTTTTC | TCAACTCTGACAATACTTCT | 482 | NM_000928 |
| sPLA2GIIA | AGCCACCAAGGAGGAGCAGG | CAGCACTGGGTGGAAGGTTT | 309 | NM_000300 |
| sPLA2GIID | GCCAGCATCTGCCTCCACT | AGGAACAGGGTAGAGGGTGA | 499 | NM_012400 |
| sPLA2GIIE | TGCTCCTTGTGCACCTC | CAGCTTGTTGGGATAATGGG | 437 | NM_014589 |
| sPLA2GIIF | AGAAGTTCTTCACCGT | TTGGTGACTGCAGGTGACCT | 480 | NM_022819 |
| sPLA2GIII* | TGCCTACAGAATCAGCACGA | TTGAGCAGCTGGAACTCGAT | 500 | AF_220490 |
| sPLA2GV | TGGATACCAATGTTCCGAC | CCTAGGAGCAGAGGATGTTG | 549 | NM_000929 |
| sPLA2GX | CGCCTATATGAAATATGGT | CAAGGTAGTCAGTCACACTTG | 327 | NM_003561 |
| sPLA2GXII | AAGGAGGCGTGGATATGGAGCT | ATCTGTCACTAGCTGTCGGCAT | 723 | NM_030821 |
| cPLA2α | GAGCTGAAAAAGGATCCTGACT | TGTCCCTAGAGTTTCATCCA | 440 | M68874 |
| cPLA2β† | CTTCATGATGCCAGCTGAGCGCCGCC | CCCGGCCATCAGTGGGGCCTGCGC | 2730 | AF121908 |
| cPLA2γ | CCACAGGCATCTATGTTGAA | ATTACTCTCTGACCGACTTC | 1020 | XM_055864 |
| cPLA2δ | AATTATGGAGAGCCTGTCACCTGGG | TATATCAGGTCTGTGCCCATGGAGG | 2457 | AB090876 |
| PLCβ1 | TTGGATGTGGGGAACATCGG | AAATAGTGAGAAAGGGGCTG | 690 | XM_045636 |
| PLCβ2 | GAGCAACTAGATTTCTGGAG | TCACCGGAATCTTCCCTTCA | 620 | BC009009 |
| PLCβ3 | AGCGGTTCCTGAACAAGCTG | AGGTCTTGAAGGCAGTCTCG | 550 | NM_000932 |
| PLCβ4 | CCCAGTGGAAAGAATGATGA | TTCCATCCCAGCAGTCAAGT | 500 | L41349 |
| PLCγ1 | GCTGCCTGCGGATGGGCTGT | GTACCACTCTTTGCTCTCGT | 924 | NM_002660 |
| PLCγ2 | GCTGCCTGCGCATGGGCTGT | GTACCACGGCTTGGACTCGT | 882 | BC_011772 |
| PLCδ1 | TGTCGCTACTCAAGTGAGTC | ATGGAGCCTGAGTGGTGGAT | 458 | BC050382 |
| PLCδ2 | CAGGGTAGGTTACCTATTAA | ATGTTCGTAACAAAACCTAC | 369 | N/A‡ |
| PLCδ3 | AGCCATGCTGTGCGGCCGCT | AGACGGTCGTTGTTGGAGTG | 716 | NM_133373 |
| PLCδ4 | CAGCTCACAGACACAGGAAA | GCTGGTGACAACATCCACCA | 468 | NM_032726 |
| PLCε§ | CAGCAGAAGGTAATGGCT | TCCCTTGGGCTTTGGGAAAT | 700 |
NM_016341
NM_015184 |
| PLCζ1 | GAGGGTATGCCAATTACACTT | GGCTGTTTTATTGCGATGCA | 447 | NM_033123 |
Cloning and DNA Sequencing
The bands containing the PCR products were excised, purified (Ultra-free-DA columns; Millipore, Bedford, MA), and inserted into a cloning vector (pGEM-T Easy; Promega, Madison, WI), according to the manufacturer’s instructions. The mixture was transformed into competent Escherichia coli DH5α bacterial cells (Invitrogen). The transformed cells were then plated and grown to a stationary phase and plasmid purification was performed (QIAprep Spin Miniprep kit; Qiagen, Mississauga, Ontario, Canada). DNA sequencing of positive clones was performed by the Service d’Analyse et de Synthèse d’Acides Nucléiques at Université Laval (Sainte-Foy), using T7 sequencing primers.
Indirect Immunofluorescence of Corneal Cryosections
Tissue biopsies (corneas) were obtained from the eyes of a 52-year-old human donor. Corneas were embedded in optimal cutting temperature (OCT) compound (Tissue-Tek; Bayers Canada, Etobicoke, Ontario, Canada), frozen in liquid nitrogen, and stored at −80°C until use. Indirect immunofluorescence assays were performed on acetone-fixed, 5-μm-thick cryosections, as previously reported.34 Sections were incubated with primary antibodies (Table 2) diluted 1:50. The large-scale production of the sPLA2GIII, -GX, or -GXIIA antibodies has been reported,35 except for the sPLA2GIII antiserum, which was prepared in rabbits, as described for the other anti-sPLA2s antisera,35 by using as an antigen the group III sPLA2 domain of sPLA2GIII. A secondary antibody was then added (Table 2). Cell nuclei were also labeled with Hoechst 33258 reagent (Sigma-Aldrich) after immunofluorescence staining. Cryosections were then observed under an epifluorescence microscope (Optiphot; Nikon, Tokyo, Japan) and photographed with a numeric charge-coupled device (CCD) camera (Sensys; Roper Scientific, Trenton, NJ). Negligible background was observed in control experiments in which primary antibodies were omitted. The expression of the PLCδ4 protein could not be tested by immunologic techniques, because specific antibodies for this phospholipase are not yet commercially available.
Table 2.
Primary and Secondary Antibodies
| Antibodies | Category | Source |
|---|---|---|
| Primary antibodies | ||
| sPLA2GIII (MG-12-02-02-1) | Rabbit polyclonal | Michael H. Gelb |
| sPLA2GX | Rabbit polyclonal | Michael H. Gelb |
| sPLA2GXIIA (MG-2-17-01-1) | Rabbit polyclonal | Michael H. Gelb |
| cPLA2α (MF-145) | Rabbit polyclonal | Merck Frost Center for Therapeutic Research, Montreal, QC, Canada |
| cPLA2γ | Rabbit polyclonal | Christina C. Leslie, National Jewish Medical and Research, Denver, CO |
| PLCβ1 (G-12) | Rabbit polyclonal | Santa Cruz Biotechnology, Santa Cruz, CA |
| PLCβ2 (Q-15) | Rabbit polyclonal | Santa Cruz Biotechnology |
| PLCβ3 (C-20) | Rabbit polyclonal | Santa Cruz Biotechnology |
| PLCβ4 (C-18) | Rabbit polyclonal | Santa Cruz Biotechnology |
| PLCγ1 (E-12) | Rabbit polyclonal | Santa Cruz Biotechnology |
| PLCγ2 (Q-20) | Rabbit polyclonal | Santa Cruz Biotechnology |
| PLCδ1 (C-20) | Goat polyclonal | Santa Cruz Biotechnology |
| PLCδ3 (2A11-D10-D8) | Mouse monoclonal | Steve Roffler, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan |
| PLCε | Goat polyclonal | Santa Cruz Biotechnology |
| Secondary antibodies (IF) | ||
| Anti-rabbit | Goat-TRITC | Chemicon, Temecula, CA |
| Anti-goat | Rabbit-TRITC | Jackson IRL, West Grove, PA |
| Anti-mouse | Goat-Alexa 594 | Molecular Probes, Eugene, OR |
| Secondary antibodies (Western blot) | ||
| Anti-rabbit | Donkey-HRP | Amersham Biosciences, Baie d’Urfé, Québec, Canada |
| Anti-goat | Bovine-HRP | Santa Cruz Biotechnology |
| Anti-mouse | Sheep-HRP | Amersham Biosciences, Baie d’Urfé, Québec, Canada |
IF, immunofluorescence; TRITC, tetramethylrhodamine isothiocyanate; HRP, horseradish peroxidase.
Protein Extraction from HCECs
Freshly isolated human corneal epithelial tissues from 60 eyes (age range, 39–88 years; average, 70) were pooled and lysed in RIPA buffer (150 mM NaCl, 9.1 mM Na2HPO4, 1.7 mM NaH2PO4 [pH 7.4], 1% [vol/vol] NP40, 0.5% [vol/vol] sodium deoxycholate, 0.1% [vol/vol] SDS, 100 mM NaVO3, and 1% [vol/vol] protease inhibitor cocktail) and then sonicated for 30 seconds. The homogeneous cell lysate was incubated on ice and then centrifuged. Protein concentration was measured using a bicinchoninic acid (BCA) assay kit (Pierce, Rockford, IL). The supernatant was kept at −80°C until use.
SDS-PAGE and Western Blot Analyses
Proteins from the human corneal epithelium (60 μg) and from each positive control (50 μg) were separated on a 6% (PLCs), 8% (cPLA2s and PLCδs), or 15% (sPLA2s) (wt/vol) polyacrylamide gel. Prestained broad-range protein molecular weight standards (MBI Fermentas, Burlington, Ontario, Canada) were used for calibration. The proteins from the gel were transferred onto a polyvinylidene difluoride membrane (Amersham Biosciences) for sPLA2s, or onto a nitrocellulose membrane (Bio-Rad) for cPLA2s and PLCs. The membranes were blocked, incubated with primary antibodies (Table 2), washed, and incubated with secondary antibodies (Table 2). The membranes were washed and then soaked in tris-buffered saline (TBS). These immunoconjugates were detected with the chemiluminescent substrate (SuperSignal West Pico; Pierce), and blots were visualized (Fluor-S Max System; Bio-Rad) except for PLCδ3, with which an autoradiographic film was used to reduce background.
Results
PLA2 and PLC mRNAs Expression by HCECs
RT-PCR experiments revealed the expression of mRNAs for sPLA2GIII, -GX, and -GXIIA (Fig. 1A) in the corneal epithelium, with an apparent size of 500, 327, and 723 bp, respectively, as was expected with the primers used (Table 1). As shown in Figure 1B, cPLA2α and -γ mRNA transcripts were expressed by HCECs, with a respective apparent size of 440 and 1020 bp (Table 1). Many different mRNA transcripts of PLCs were present in HCECs, including PLCβ1, -β2, -β3, -β4, -γ1, -γ2, -δ1, -δ3, -δ4, and -ε(Fig. 1C) with apparent sizes of 690, 620, 550, 500, 924, 882, 458, 716, 468 and 700 bp, respectively, as expected with the primers used (Table 1). sPLA2GIB, -GIIA, -GIID, -GIIE, and -GIIF (Fig. 1A); cPLA2β (Fig. 1B) and -δ(data not shown); and PLCδ2 and -ζ1 (data not shown) mRNA transcripts were not amplified in the corneal epithelium. After cloning and sequencing of the PCR products, identities of the different phospholipases were confirmed by comparison with the known phospholipase sequences using BLASTn (www.ncbi.nlm.nih.gov/BLAST). Additional bands were observed for some of the PLCs—namely, β1, β3, γ1, γ2, and ε(Fig. 1C). Moreover, faint bands were also observed for sPLA2IID and -IIF (Fig. 1A). These bands were excised from the agarose gel, cloned, sequenced, and found to correspond to nonspecific PCR products.
Figure 1.
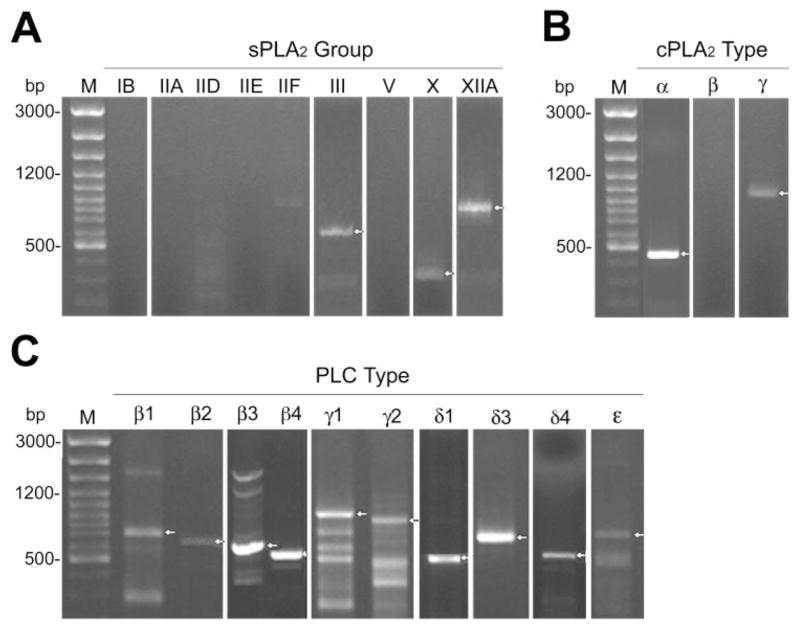
Identification of (A) sPLA2, (B) cPLA2, and (C) PLC transcripts in HCECs by PCR amplification. Lane M: 100-bp ladder. Arrows: position of each positive phospholipase band.
PLA2 and PLC Protein Expression by the Corneal Epithelium
As shown in Figure 2, indirect immunofluorescence analyses conducted on corneal cryosections revealed the presence of and sPLA2GIII, -GX, and -GXIIA (Fig. 2A); cPLA2α and -γ (Fig. 2B); and PLCβ2, -β3, -γ1, and -γ2 proteins (Fig. 2C). PLCβ1, -β4, -δ1, -δ3, and -ε proteins were not detected in corneal epithelium (data not shown). It can be seen in the micrographs in Figure 2 that all phospholipases were present in the cytoplasm of HCECs. sPLA-GIII and -GXIIA, cPLA2α and -γ, and PLCγ1 were also present in the nucleus. Whereas PLCβ3 was present uniformly in the cytoplasm of the cells throughout the epithelium, it is also markedly present in the basal region of the basal cells. sPLA2GIII and -GXIIA and cPLA2α and -γ were markedly present in the apical region of the cornea. It is interesting to note that among all PLA2s and PLCs tested, many are also expressed by stromal fibroblasts, particularly PLCγ2. To further validate these data, Western blot analyses were conducted on crude protein extracts obtained from the corneal epithelium of donor eyes. As shown in Figure 3A, protein bands with apparent molecular masses of 55, 20, and 21 kDa corresponding to sPLA2GIII, -GX, and -GXIIA, respectively, were detected in the human corneal epithelium. Protein bands with apparent molecular masses of 85 and 60 kDa corresponding respectively to cPLA2α and -γ, were detected in the human corneal epithelium (Fig. 3B). Similarly, protein bands with apparent molecular masses of 140, 150, 155, 120, and 85 kDa, corresponding respectively to PLCβ2, -β3, -γ1, -γ2, and -δ3 (Fig. 3C) were detected in the human corneal epithelium. The disagreement between the indirect immunofluorescence and Western blot analyses for PLCδ3 can be explained by the presence of a very low expression of PLCδ3 in this tissue, since a 1-hour exposure of the membrane was necessary to observe the PLCδ3 band at 85 kDa. The molecular masses of these bands are in good agreement with those obtained with the positive controls provided by the manufacturer (Fig. 3C), except for PLCγ2, which was 20 kDa lower than the positive control. This latter result could be explained by N-terminal proteolysis (epitope mapping at the C terminus), as no splicing has been reported for the PLCγ2 transcript. No protein was detected with PLCβ1, -β4, -δ1, and -ε antibodies (data not shown), in contrast with the data reported by Islam and Akhtar,36 who observed the presence of PLCβ1 in their cultures of rabbit corneal epithelium. This can be explained either by differences in the expression of PLCs in human and rabbit corneal epithelium or by the treatment of their corneal epithelium with epidermal growth factor (EGF). These data thus suggest that only sPLA2GIII, -GX, and -GXIIA; cPLA2α and -γ; and PLCβ2, -β3, -γ1, -γ2, and -δ3 are expressed by the human corneal epithelium. Additional bands were observed for three of these phospho, lipases: sPLA2GXIIA, cPLA2α and PLCγ2. Indeed, four bands of high molecular mass were detected for sPLA2GXIIA which likely correspond to different levels of aggregation of this enzyme. In addition, five and two bands of low molecular weight were detected respectively for cPLA2α and PLCγ2 which may correspond to protein degradation.
Figure 2.
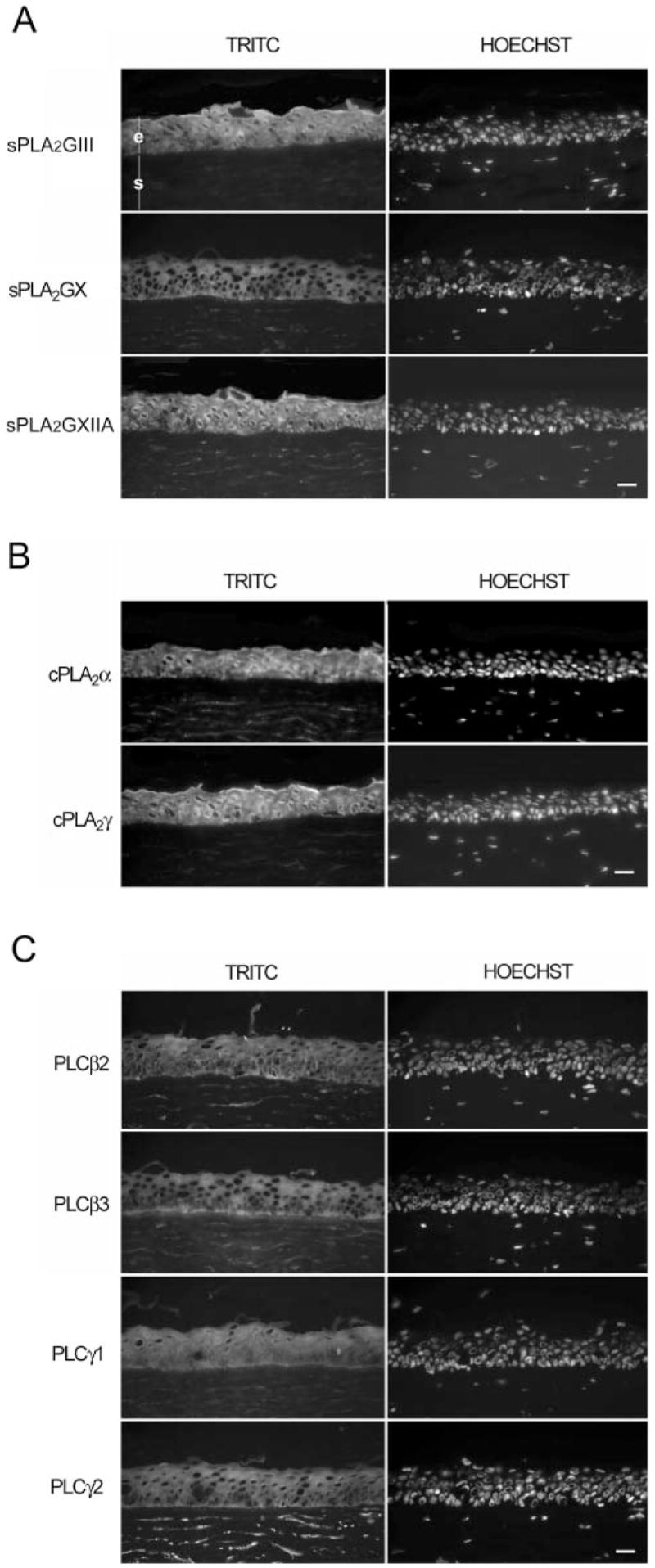
Immunofluorescence analysis of phospholipases expressed by the human cornea. Immunolocalization of all phospholipases was performed using the (A) sPLA2, (B) cPLA2, and (C) PLC antibodies shown, and further revealed with a secondary antibody labeled with TRITC or Alexa 594 (Table 2). Nuclei were counter-stained with Hoechst 33258 reagent. Negligible background was observed in control experiments, in which primary antibodies were omitted (data not shown). The corneal epithelium (e) and stroma (s) are indicated in (A). Scale bar, 100 μm.
Figure 3.
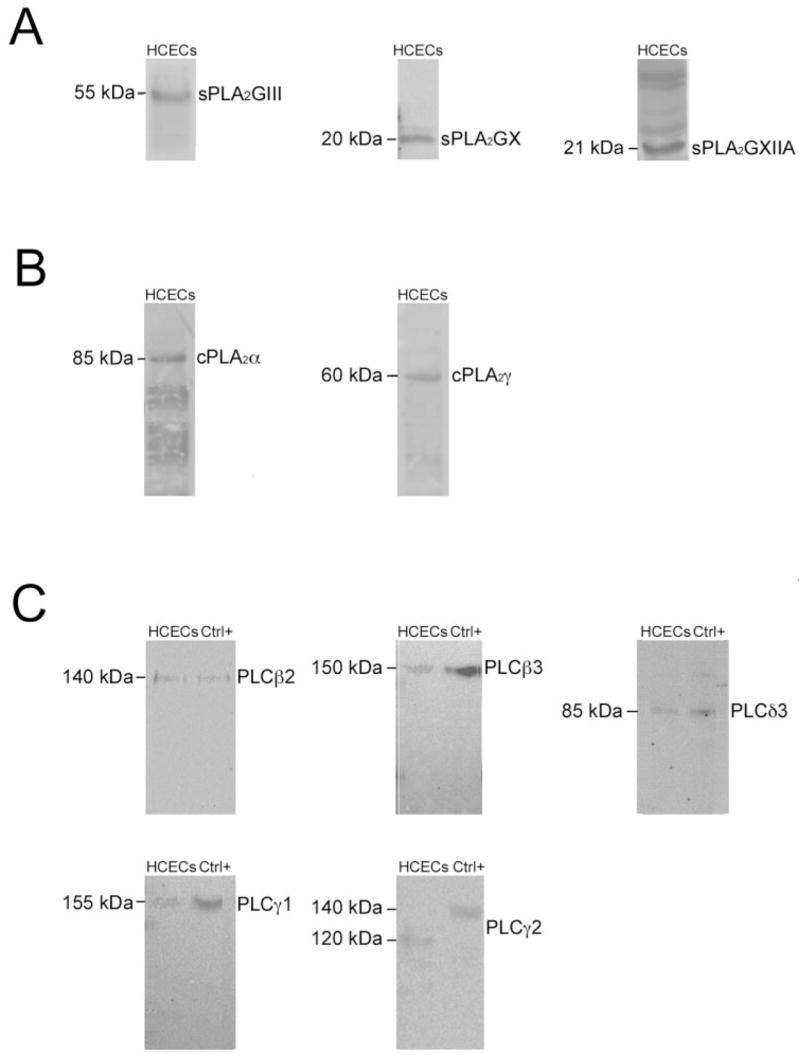
Western blot analysis of phospholipases in crude extracts from HCECs. Western blot analyses were performed using the (A) sPLA2, (B) cPLA2, and (C) PLC antibodies shown (Table 2). Left: molecular mass of the expected proteins. Ctrl+, positive controls RAW 264.7 for PLCs β2 and δ3, A-431 for PLCs β3 and γ1, and MCF7 for PLCγ2.
Discussion
No information was available on the expression of phospholipases by the human corneal epithelium. In fact, most of the studies on the expression of ocular phospholipases and their regulation were conducted using rabbit eyes.8,17,19,36 It is important to investigate the expression of phospholipases in the human eye because of the limitations of extrapolating from animal models. This is especially true of data derived from the rabbit eye,17 which has a much higher rate of AA metabolism than either the human or bovine eye.37 In the present study, the identity and localization of the different PLA2s and PLCs expressed by the human corneal epithelium were thus determined.
The antibacterial properties of sPLA2s are well recognized and may be the result of their catalytic action.4,5 The sPLA2GIIA present in tears 5 originates from lacrimal ducts and glands19,20 and accounts in part for the antibacterial properties of the tear film. However, sPLA2GIIA is not expressed by the corneal epithelium, which thus does not contribute to the production of this enzyme in the tear film. In vitro, sPLA2GIIA showed the strongest bactericidal activity against Gram-positive bacteria, followed by sPLA2GX, -GV, and -GXIIA.4 Only sPLA2GXIIA demonstrated a detectable bactericidal activity against the Gram-negative bacteria Escherichia coli.4 We demonstrated an even distribution of sPLA2GX proteins in the cytosol of HCECs and a cytosolic/nuclear distribution of sPLA2GIII and -GXIIA. It would be of interest to determine whether sPLA2GIII, -GX, and -GXIIA proteins can indeed be found in tears and then contribute to the antibacterial properties of the tear film. If this were indeed the case, then sPLA2s activity in tears would originate from lacrimal ducts and glands as well as HCECs, providing even more antibacterial protection to help maintain the sterility of a wound or at least fight against infection after corneal injury.
cPLA2α mRNA is ubiquitously expressed in most adult human tissues,38,39 whereas cPLA2γ mRNA is selectively expressed in some tissues.38,40,41 In this study, we demonstrated the expression of these two proteins in the cytosol and nucleus of corneal epithelial cells. cPLA2α has attracted special interest, because it is the only one of numerous PLA2s that selectively release AA over other fatty acids.42,43 cPLA2α initiates the immediate AA release.3 Its expression is elevated in some tissues in response to pathologic stimuli.44–47 cPLA2γ properties and regulation are less well known. Asai et al.48 demonstrated that cPLA2γ remains bound to membranes due to its lipid anchor at its C terminus.41 The immunofluorescence analyses of corneal tissue sections conducted in the present study demonstrated that this protein was markedly present on the cell membrane of HCECs. By using a cPLA2α inhibitor, Kang et al.49 have determined that EGF induces the production of prostaglandins through the activation of this cPLA2 in rabbit corneal epithelial cells. Given that the EGF level increases during corneal wound healing,50–52 the cPLA2α expressed by HCECs may be involved in corneal wound healing. Moreover, it has been proposed that increased expression of a cPLA2 in the corneal epithelium takes place after a platelet-activating factor (PAF) stimulus.53,54 Given that PAF is well known to mediate inflammatory and immune responses, these data suggest that cPLA2α-expressed by the corneal epithelium may be involved in these processes.
Because of their ability to induce numerous effects after hydrolysis of phospholipids,2 PLCs are believed to be important cell-signaling components during wound healing and inflammation processes. A wide variety of mRNAs coding for PLC isoforms were identified in HCECs (PLCβ1, -β2, -β3, -β4, -γ1, -γ2, -δ1, -δ3, -δ4, and -ε). However, in normal HCECs, only part of these mRNA are translated into proteins (PLCβ2, -β3, -γ1, -γ2, and -δ3). The other ones may then be expressed in pathologic conditions or after a proper stimulation by a growth factor as observed for PLCγ1 in EGF in cultures of rabbit corneal epithelium.36 Moreover, it is interesting to point out the variation in the localization of PLC proteins within the HCECs. PLCβ2, -β3, -γ1, and -γ2 proteins are all present throughout the cytoplasm of HCECs; PLCβ2 was more concentrated at the cell membrane, whereas PLCβ3 was very much present on the basal side of the basal cell layer of the corneal epithelium. PLCγ1 protein was also present in the nucleus of basal and intermediate cells, whereas PLCγ2 was the only phospholipase strongly detected in stromal fibroblasts among all those examined. This difference in cellular localization reinforces the hypothesis that the different PLC isoforms may play several critical functions within these cells in normal and most probably under pathologic conditions. In this regard, it has been shown that EGF stimulates the expression of PLCγ1.36,49,55 This PLC may be involved in corneal wound healing, given the involvement of EGF in this process.50–52 Moreover, by using a specific PLC inhibitor, Huang et al.56 have shown that the production of bradykinin, which is released during the inflammatory response of the cornea, leads to the activation of a PLC in canine cultured corneal epithelial cells.
The respective function played by each phospholipase (PLA2 and PLC) must be determined, to achieve a better understanding of their involvement in inflammation and wound healing of the corneal epithelium. It is clear that phospholipases play important roles in ocular physiology and pathophysiology and that modulation of their synthesis, sites of action, and inactivation comprise important pharmacological targets for the management of ocular disorders. Such knowledge could lead to new studies to determine which phospholipases represent good therapeutic targets in the establishment of a specific treatment for inflammatory disorders.57
Acknowledgments
The authors thank the Banque d’Yeux Nationale for providing the human eyes, Marc-André Laurin for the sPLA2 primer design, Sara-Edith Penney for PCR amplification of cPLA2δ, and Christina C. Leslie and Steve Roffler for providing the antibody against cPLA2γ and PLCδ3, respectively.
Supported by the Natural Sciences and Engineering Research Council of Canada, and Grant HL36235 from the National Institutes of Health (MHG). CS and SLG are both Chercheur Boursier National from the Fonds de la Recherche en Santé du Québec (FRSQ). SL and PC are recipients of studentships from the FRSQ. SL and SC hold studentships from the Canadian Institute of Health Research.
Footnotes
Disclosure: S. Landreville, None; S. Coulombe, None; P. Carrier, None; M.H. Gelb, None; S.L. Guérin, None; C. Salesse, None
References
- 1.Sharma S. Keratitis. Biosci Rep. 2001;21:419–444. doi: 10.1023/a:1017939725776. [DOI] [PubMed] [Google Scholar]
- 2.Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 3.Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Crit Rev Immunol. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- 4.Koduri RS, Gronroos JO, Laine VJ, et al. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277:5849–5857. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- 5.Qu XD, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aho VV, Nevalainen TJ, Saari KM. Group IIA phospholipase A2 content of tears in patients with keratoconjunctivitis sicca. Graefes Arch Clin Exp Ophthalmol. 2002;240:521–523. doi: 10.1007/s00417-002-0477-8. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood) 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 8.Bazan HE, King WD, Rossowska M. Metabolism of phosphoinositides and inositol polyphosphates in rabbit corneal epithelium. Curr Eye Res. 1985;4:793–801. doi: 10.3109/02713688509020036. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M, Masuda S, Shimbara S, et al. Cellular arachidonate-releasing function of novel classes of secretory phospholipase A2s (groups III and XII) J Biol Chem. 2003;278:10657–10667. doi: 10.1074/jbc.M211325200. [DOI] [PubMed] [Google Scholar]
- 10.Rouault M, Bollinger JG, Lazdunski M, Gelb MH, Lambeau G. Novel mammalian group XII secreted phospholipase A2 lacking enzymatic activity. Biochemistry. 2003;42:11494–11503. doi: 10.1021/bi0349930. [DOI] [PubMed] [Google Scholar]
- 11.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 12.Valentin E, Ghomashchi F, Gelb MH, Lazdunski M, Lambeau G. Novel human secreted phospholipase A2 with homology to the group III bee venom enzyme. J Biol Chem. 2000;275:7492–7496. doi: 10.1074/jbc.275.11.7492. [DOI] [PubMed] [Google Scholar]
- 13.Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase A2. Biochim Biophys Acta. 2000;1488:124–138. doi: 10.1016/s1388-1981(00)00115-3. [DOI] [PubMed] [Google Scholar]
- 14.Chiba H, Michibata H, Wakimoto K, et al. Cloning of a gene for a novel epithelial-specific cytosolic phospholipase A2, cPLA2delta, induced in psoriatic skin. J Biol Chem. 2004;279:12890–12897. doi: 10.1074/jbc.M305801200. [DOI] [PubMed] [Google Scholar]
- 15.Balsinde J, Winstead MV, Dennis EA. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/s0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 16.Catania LJ. Inflammation and the cornea. Optom Clin. 1995;4:1–18. [PubMed] [Google Scholar]
- 17.Bhattacherjee P. The role of arachidonate metabolites in ocular inflammation. Prog Clin Biol Res. 1989;312:211–227. [PubMed] [Google Scholar]
- 18.Saari KM, Aho V, Paavilainen V, Nevalainen TJ. Group II PLA2 content of tears in normal subjects. Invest Ophthalmol Vis Sci. 2001;42:318–320. [PubMed] [Google Scholar]
- 19.Aho HJ, Saari KM, Kallajoki M, Nevalainen TJ. Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Invest Ophthalmol Vis Sci. 1996;37:1826–1832. [PubMed] [Google Scholar]
- 20.Nevalainen TJ, Aho HJ, Peuravuori H. Secretion of group II phospholipase A2 by lacrimal glands. Invest Ophthalmol Vis Sci. 1994;35:417–421. [PubMed] [Google Scholar]
- 21.Williams RL. Mammalian phosphoinositide-specific phospholipase C. Biochim Biophys Acta. 1999;1441:255–267. doi: 10.1016/s1388-1981(99)00150-x. [DOI] [PubMed] [Google Scholar]
- 22.Katan M. Families of phosphoinositide-specific phospholipase C: structure and function. Biochim Biophys Acta. 1998;1436:5–17. doi: 10.1016/s0005-2760(98)00125-8. [DOI] [PubMed] [Google Scholar]
- 23.Meldrum E, Katan M, Parker P. A novel inositol-phospholipid-specific phospholipase C: rapid purification and characterization. Eur J Biochem. 1989;182:673–677. doi: 10.1111/j.1432-1033.1989.tb14878.x. [DOI] [PubMed] [Google Scholar]
- 24.Lopez I, Mak EC, Ding J, Hamm HE, Lomasney JW. A novel bifunctional phospholipase C that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. J Biol Chem. 2001;276:2758–2765. doi: 10.1074/jbc.M008119200. [DOI] [PubMed] [Google Scholar]
- 25.Song C, Hu CD, Masago M, et al. Regulation of a novel human phospholipase C, PLCepsilon, through membrane targeting by Ras. J Biol Chem. 2001;276:2752–2757. doi: 10.1074/jbc.M008324200. [DOI] [PubMed] [Google Scholar]
- 26.Cox LJ, Larman MG, Saunders CM, et al. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- 27.Saunders CM, Larman MG, Parrington J, et al. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 28.Duncan G, Collison DJ. Calcium signalling in ocular tissues: functional activity of G-protein and tyrosine-kinase coupled receptors. Exp Eye Res. 2002;75:377–389. [PubMed] [Google Scholar]
- 29.Clark JD, Schievella AR, Nalefski EA, Lin LL. Cytosolic phospholipase A2. J Lipid Mediat Cell Signal. 1995;12:83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima S. Protein kinase C alpha (PKC alpha): regulation and biological function. J Biochem (Tokyo) 2002;132:669–675. doi: 10.1093/oxfordjournals.jbchem.a003272. [DOI] [PubMed] [Google Scholar]
- 31.Gipson IK, Grill SM. A technique for obtaining sheets of intact rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1982;23:269–273. [PubMed] [Google Scholar]
- 32.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–534. 536–537. [PubMed] [Google Scholar]
- 33.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 34.Laplante AF, Germain L, Auger FA, Moulin V. Mechanisms of wound reepithelialization: hints from a tissue-engineered reconstructed skin to long-standing questions. FASEB J. 2001;15:2377–2389. doi: 10.1096/fj.01-0250com. [DOI] [PubMed] [Google Scholar]
- 35.Degousee N, Ghomashchi F, Stefanski E, et al. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J Biol Chem. 2002;277:5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- 36.Islam M, Akhtar RA. Epidermal growth factor stimulates phospholipase C gamma1 in cultured rabbit corneal epithelial cells. Exp Eye Res. 2000;70:261–269. doi: 10.1006/exer.1999.0783. [DOI] [PubMed] [Google Scholar]
- 37.Kulkarni PS. Arachidonic acid metabolism in human and bovine retina. J Ocul Pharmacol. 1991;7:135–139. doi: 10.1089/jop.1991.7.135. [DOI] [PubMed] [Google Scholar]
- 38.Pickard RT, Strifler BA, Kramer RM, Sharp JD. Molecular cloning of two new human paralogs of 85-kDa cytosolic phospholipase A2. J Biol Chem. 1999;274:8823–8831. doi: 10.1074/jbc.274.13.8823. [DOI] [PubMed] [Google Scholar]
- 39.Sharp JD, White DL. Cytosolic PLA2: mRNA levels and potential for transcriptional regulation. J Lipid Mediat. 1993;8:183–189. [PubMed] [Google Scholar]
- 40.Song C, Chang XJ, Bean KM, et al. Molecular characterization of cytosolic phospholipase A2-beta. J Biol Chem. 1999;274:17063–17067. doi: 10.1074/jbc.274.24.17063. [DOI] [PubMed] [Google Scholar]
- 41.Underwood KW, Song C, Kriz RW, et al. A novel calcium-independent phospholipase A2, cPLA2-gamma, that is prenylated and contains homology to cPLA2. J Biol Chem. 1998;273:21926–21932. doi: 10.1074/jbc.273.34.21926. [DOI] [PubMed] [Google Scholar]
- 42.Hanel AM, Gelb MH. Multiple enzymatic activities of the human cytosolic 85-kDa phospholipase A2: hydrolytic reactions and acyl transfer to glycerol. Biochemistry. 1995;34:7807–7818. doi: 10.1021/bi00024a004. [DOI] [PubMed] [Google Scholar]
- 43.Clark JD, Lin LL, Kriz RW, et al. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda T, Kim DK, Chin MR, Hales CA, Bonventre JV. Increased group IV cytosolic phospholipase A2 activity in lungs of sheep after smoke inhalation injury. Am J Physiol. 1999;277:L533–L542. doi: 10.1152/ajplung.1999.277.3.L533. [DOI] [PubMed] [Google Scholar]
- 45.Stephenson D, Rash K, Smalstig B, et al. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neuro-degeneration. Glia. 1999;27:110–128. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 46.Kol S, Ruutiainen-Altman K, Ben-Shlomo I, et al. The rat ovarian phospholipase A2 system: gene expression, cellular localization, activity characterization, and interleukin-1 dependence. Endocrinology. 1997;138:322–331. doi: 10.1210/endo.138.1.4826. [DOI] [PubMed] [Google Scholar]
- 47.Mangat H, Peterson LN, Burns KD. Hypercalcemia stimulates expression of intrarenal phospholipase A2 and prostaglandin H synthase-2 in rats: role of angiotensin II AT1 receptors. J Clin Invest. 1997;100:1941–1950. doi: 10.1172/JCI119725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asai K, Hirabayashi T, Houjou T, et al. Human group IVC phospholipase A2 (cPLA2gamma): roles in the membrane remodeling and activation induced by oxidative stress. J Biol Chem. 2003;278:8809–8814. doi: 10.1074/jbc.M212117200. [DOI] [PubMed] [Google Scholar]
- 49.Kang SS, Li T, Xu D, Reinach PS, Lu L. Inhibitory effect of PGE2 on EGF-induced MAP kinase activity and rabbit corneal epithelial proliferation. Invest Ophthalmol Vis Sci. 2000;41:2164–2169. [PubMed] [Google Scholar]
- 50.Wilson SE, Chen L, Mohan RR, Liang Q, Liu J. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. 1999;68:377–397. doi: 10.1006/exer.1998.0603. [DOI] [PubMed] [Google Scholar]
- 51.Sheardown H, Cheng YL. Tear EGF concentration following corneal epithelial wound creation. J Ocul Pharmacol Ther. 1996;12:239–243. doi: 10.1089/jop.1996.12.239. [DOI] [PubMed] [Google Scholar]
- 52.Sheardown H, Wedge C, Chou L, et al. Continuous epidermal growth factor delivery in corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1993;34:3593–3600. [PubMed] [Google Scholar]
- 53.Ma X, Bazan HE. Platelet-activating factor (PAF) enhances apoptosis induced by ultraviolet radiation in corneal epithelial cells through cytochrome c-caspase activation. Curr Eye Res. 2001;23:326–335. doi: 10.1076/ceyr.23.5.326.5445. [DOI] [PubMed] [Google Scholar]
- 54.Bazan HE, Varner L. A mitogen-activated protein kinase (MAP-kinase) cascade is stimulated by platelet activating factor (PAF) in corneal epithelium. Curr Eye Res. 1997;16:372–379. doi: 10.1076/ceyr.16.4.372.10699. [DOI] [PubMed] [Google Scholar]
- 55.Islam M, Akhtar RA. Upregulation of phospholipase Cgamma1 activity during EGF-induced proliferation of corneal epithelial cells: effect of phosphoinositide-3 kinase. Invest Ophthalmol Vis Sci. 2001;42:1472–1478. [PubMed] [Google Scholar]
- 56.Huang SC, Chien C, Hsiao L, et al. Mechanisms of bradykinin-mediated Ca2+ signalling in canine cultured corneal epithelial cells. Cell Signal. 2001;13:565–574. doi: 10.1016/s0898-6568(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 57.Yedgar S, Lichtenberg D, Schnitzer E. Inhibition of phospholipase A2 as a therapeutic target. Biochim Biophys Acta. 2000;1488:182–187. doi: 10.1016/s1388-1981(00)00120-7. [DOI] [PubMed] [Google Scholar]


