Abstract
Temporal correlates of the brain circuit underlying reward processing in healthy adults remain unclear. The current study investigated the P3 and contingent negative variation (CNV) as putative reward-related temporal markers. The effect of sustained monetary reward on these event-related potentials and on behavior was assessed using a warned reaction-time paradigm in 16 young healthy subjects. Monetary reward (0, 1 and 45 cents) varied across blocks of trials. While the CNV was unaffected by money, P3 amplitude was significantly larger for 45 than the 1 and 0 cent conditions. This effect corresponded to the monotonically positive subjective ratings of interest and excitement on the task (45>1>0). These findings suggest a difference between the P3 and CNV; the P3 is sensitive to the sustained effect of relative reward value while the CNV does not vary with reward magnitude.
DESCRIPTOR TERMS: ERP, REACTION TIME, MONETARY REWARD, P3, CNV
Introduction
Reward processing is complex and involves the contribution from multiple interacting brain regions. Numerous functional neuroimaging studies in humans have helped to spatially define this neural mesocorticolimbic dopaminergic reward circuitry that encompasses the ventromedial prefrontal cortex, orbitofrontal cortex, anterior cingulate, nucleus accumbens, midbrain (e.g., substantia nigra and ventral tegmentum), amygdala, and the hippocampus (for review, see (Goldstein & Volkow, 2002; Kelley & Berridge, 2002)). Further, neuroimaging studies have contributed to the functional dissociation of these regions based on their specific roles in reward processing (e.g., expectancy and probability, outcome and magnitude, valence) (Breiter, Aharon, Kahneman, Dale, & Shizgal, 2001; Elliott, Friston, & Dolan, 2000; Knutson, Taylor, Kaufman, Peterson, & Glover, 2005). However, current functional neuroimaging studies lack the temporal resolution to provide the precise chronological delineation of such reward-related activity. This can be attained through the use of event-related potentials (ERPs). Surprisingly, relatively few studies have employed ERPs to investigate intact reward processing; therefore, its temporal correlates remain to be determined.
One well-studied ERP component that seems to play a role in reward processing is the P3 (or P300), a positive wave usually peaking between 300 and 600 msec post-stimulus with largest amplitude at centro-parietal scalp sites (Sutton, Braren, Zubin, & John, 1965). The major factors affecting P3 amplitude include stimulus probability and task relevance (Squires, Donchin, Herning, & McCarthy, 1977). Stimuli with high emotional value, informative feedback stimuli, and target stimuli also elicit larger P3s than stimuli that do not have these properties (Johnson, 1988; Picton, 1992; Pritchard, 1981). We therefore expected the P3 to be elicited by monetary feedback manipulation; indeed the P3’s involvement in monetary reward, and specifically in marking reward’s magnitude, has been previously documented (Begleiter, Porjesz, Chou, & Aunon, 1983; Homberg, Grunewald, & Grunewald-Zuberbier, 1981; Otten, Gaillard, & Wientjes, 1995; Ramsey & Finn, 1997; Yeung & Sanfey, 2004). For example, Ramsey and Finn (1997) used a visual discrimination task where subjects were instructed to respond to target stimuli in a neutral condition (no monetary incentive) vs. an incentive condition (monetary gain of 50 cents for correct responses and loss of 50 cents for incorrect responses). Greater amplitude and shorter latency of the P3 was reported in the incentive condition as compared to the neutral condition. In a recent study, Yeung & Sanfey (2004) revealed a double dissociation between P3 and feedback negativity (a negative component occurring 200 to 300 ms after a feedback stimulus) such that reward magnitude (small: 6–11 cents vs. large: 32–40 cents) was reflected by the P3, but not by feedback negativity, and reward valence (win or loss) was reflected by feedback negativity only.
Unlike most previous studies, the current investigation was designed to induce sustained anticipation of graded monetary reward. This design allowed for comparisons between different amounts of money, which could highlight the role of the P3 in processing of relative reward and not only in processing reward’s absolute value (reward vs. no reward). We were interested in inspecting sustained (blocked) and not event-related (rapidly alternating) anticipation of reward, because of our interest in the examination of relative reward processing in a real-world context, where emotional/motivational information is more likely to occur in a sustained fashion over several minutes, rather than alternating rapidly with information of a different emotional tenor (Compton, et al., 2003). Our interest in sustained reward was further guided by the prospect of future studies utilizing functional magnetic resonance imaging (fMRI), where signal-to-noise ratio is higher in blocked vs. event-related designs (for a direct comparison see (Mechelli, Price, Henson, & Friston, 2003)), a concern that is particularly relevant in studies of clinical populations (e.g., with psychopathology that affects reward processing such as drug addiction).
While there have been several studies investigating the role of P3 in reward processing, less attention has been directed to the CNV (contingent negative variation), a slow component typically elicited in Go/No-go paradigms and associated with expectancy in the human brain (Walter, Cooper, Aldridge, McCallum, & Winter, 1964). In warned S1–S2 Go/No-go paradigms, the CNV develops early in response to the warning stimulus (S1), having a frontal distribution (the orienting, “O”, wave); its later part develops immediately preceding the target stimulus (S2), having a centroparietal distribution (late expectancy, “E”, wave). We were particularly interested in this later CNV component, which is anticipatory in nature, further related to motor response preparation or the readiness potential of the motor potential complex (Rohrbaugh, et al., 1986) and to motivation (Cant & Bickford, 1967).
However, although preparation to and anticipation of reward are core mechanisms in reward processing (Knutson, Fong, Adams, Varner, & Hommer, 2001; Volkow, et al., 2003), this slow ERP component has not been frequently targeted in the study of reward processing, and conflicting results abound to date. Thus, while some studies point to a role of the CNV in reward processing (Boyd, Boyd, & Brown, 1979; Pierson, Ragot, Ripoche, & Lesevre, 1987), other studies suggest otherwise (Lumsden, Howard, & Fenton, 1986; Sobotka, Davidson, & Senulis, 1992). For example, Pierson and colleagues (1987) conditioned subjects to associate one tone with monetary gain (reward) and another tone with loss of money (punishment); a third tone was not associated with reward or punishment (neutral stimulus). Following the conditioning phase, they found that CNV amplitude differed between rewarding conditioned stimuli and neutral or punishing conditioned stimuli. Other evidence supporting the relationship between the CNV and reward comes from the animal literature; Boyd and colleagues (1979) examined the CNV in the squirrel monkey and found that it varied as a function of reward (but not consistently in the same direction across all animals). Conversely, Sobotka and colleagues (1992) manipulated reward and punishment contingencies such that subjects were instructed whether they could potentially win or lose money (25¢) on each trial. To win money (on reward trials) or avoid losing money (on punishment trials), subjects had to press or release a button faster than a specified response time, while responding slower resulted in either no monetary gain or loss of money (respectively). While the CNV was larger for trials on which subjects had faster response times, it did not vary with reward or punishment.
The purpose of this study was to investigate cognitive ERPs, especially the P3 and CNV, evoked by warning (S1) and target (S2) stimuli in a Go/No-go paradigm and their modulation by magnitude of sustained monetary reward (high, low, and none as baseline) in the intact brain. Event-related potential variations were interpreted in conjunction with behavioral measures (including reaction time, accuracy, and self-reported interest and excitement ratings) in 16 healthy young subjects. While we hypothesized the P3 to be modulated by reward magnitude (high > low > none), our analyses of the CNV were more exploratory in nature.
Materials and Methods
Participants
Subjects were 16 college students (age: M = 21.56, SD = 1.9; education: M = 14.9, SD = 2.05; sex: 56 percent female; race: 56 percent Caucasian; handedness: 88 percent right) free of neurological disorder by self-report. Subjects were given a basic monetary fee ($5) for participating and the opportunity to earn additional money ($20) based on the ERP task performance. All procedures were undertaken in accordance with the Stony Brook University Committee on Research Involving Human Subjects (Institutional Review Board).
Task
A blocked design (sustained activation) was selected over an event-related paradigm (rapid alternation) as most appropriate for examining the continuous electrophysiological activation induced by predictable and constant monetary reward. Prior behavioral studies have demonstrated that emotional/motivational stimuli become more potent when they are grouped together into blocks, rather than when they are intermixed with neutral trials (Holle, Neely, & Heimberg, 1997); see also (Compton, Heller, Banich, Palmieri, & Miller, 2000; Dalgleish, 1995).
The overall design included three sequences/blocks each consisting of three monetary reward conditions: 0¢, 1¢, 45¢ (Figure 1, top and middle); each condition was comprised of 12 “Go” or “No-go” trials (Figure 1, bottom). The monetary conditions were pseudo-randomized and separated by a 30 sec fixation period. At each experimental condition onset, a screen displaying the monetary reward condition (0¢, 1¢, 45¢) was presented (visual angle equal to 8.64°) for 5000 msec (this screen, which preceded each experimental condition, is not depicted in Figure 1). The “Go” and “No-go” stimuli (total of 108) were pseudo-randomized across all trials (six of each within a block, no more than three of same type). The task was the same for all subjects. Stimuli presented during a brief training session were the same as those presented during the experimental conditions.
Figure 1.
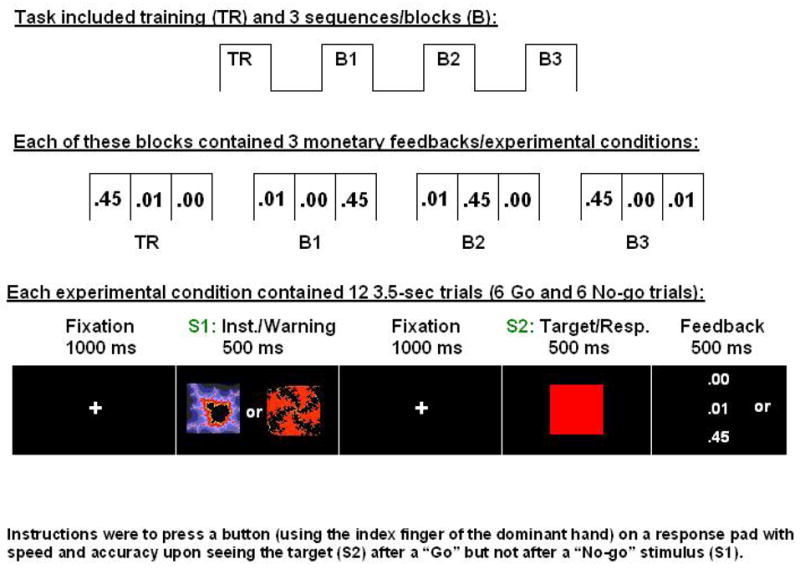
Experimental paradigm for the monetary incentive task. Overall design and experimental conditions are depicted at the top; at each condition onset (conditions were separated by 30 sec), a 5 sec screen (not depicted) displayed the monetary reward (0¢, 1¢, 45¢). Together with the feedback delivered at the end of each trial, this 5 sec screen (similar in appearance to the feedback screen) guaranteed the subjects were continuously aware of the reward contingencies.
Two distinct abstract (fractal) images (“Image 1” and “Image 2”; adapted from (Thut, et al., 1997)) served as the “Go” and “No-go” warning stimuli (S1) (Figure 1, bottom). The designation of the images as “Go” or “No-go” was counterbalanced across subjects such that the first eight subjects received Image 1 as the “Go” stimulus and the other eight subjects received Image 2 as the “Go” stimulus. The two stimuli (visual angle equal to 15.97°) were of 500 msec in duration, following a blank screen for 1000 msec. A target stimulus (S2) in the form of a red square (visual angle equal to 15.97°) was also of 500 msec duration, following another black screen for 1000 msec, which allowed for a very long preparation time before response was due. This long preparation time allowed for the creation of the full CNV (slow) component, and was used also to minimize errors for later studies comparing healthy individuals to subjects with cognitive impairments. A response window of 500 msec overlapped with the full presentation of S2. A fixation point remained in the center of the screen for the duration of each 3500 msec trial. All text was in a ROM 2 font.
The subject was instructed to press a button (using the index finger of the dominant hand) on a response pad with speed and accuracy upon seeing S2 after a “Go” stimulus and to not press the button upon seeing S2 after a “No-go” stimulus. Incorrect responses were trials where subjects pressed the button instead of refraining from responding (errors of commission); incorrect non-responses were trials where subjects did not press the button instead of pressing it (errors of omission). Feedback (the amount of money earned for correct responses/non-responses was: $0.00, $0.01, $0.45; for incorrect responses/non-responses: $0.00) was presented (visual angle equal to 3.75°) immediately after the offset of S2 for 500 msec. Thus, subjects were aware of the reward contingencies at the start of each block and after every trial.
Number of repetitions and overall block length was determined based on the prospective fMRI studies (ideal block length in fMRI is 20–40 sec) and pilot runs indicating this was optimal also for subjects’ sustained engagement in the task. Because our goal was to examine responses to the receipt of real money, the selection of number of trials was further guided by the monetary amount available to pay each volunteer ($20). Within this range, we chose the reward conditions that would allow the largest difference between the highest and lowest rewards and that would also incorporate a baseline non-reward condition ($0.00). We further chose the comparison of the $0.01 and $0.00 conditions because we were specifically interested in assessing the behavioral and brain sensitivity to very small levels of reward (indeed to the smallest monetary amount available).
Experimental Procedure
Participants were fitted with electrodes and positioned in a reclining chair in a sound-attenuating room. An LCD panel was placed 61 centimeters from the subject’s face. Instructions were provided and followed by a short training session, where no money could be earned. At the end of the experiment, subjects were told how much they earned and were mailed a check for that amount plus the basic fee for participating.
Electrophysiological Recording
Continuous recordings of the electroencephalogram (EEG, Neuroscan Inc., sterling USA) and electrooculogram (EOG) were obtained in all experimental conditions. Participants were fitted with a cap containing 64 silver-silver chloride electrodes, positioned according to a modified version of the international 10/20 System (American Electroencephalographic Society, 1991). All recordings were performed using a fronto-central electrode as ground, and electronically linked mastoid electrodes as reference. Electrodes were placed above and below the left eye to record vertical eye movements. The EEG was digitized at a rate of 500 Hz and amplified with a gain of 250, and a bandpass of 0 to 70 Hz. The amplifiers were calibrated prior to each recording. Electrode impedances were at or below 10 kilo-ohms for all electrodes used in the analysis.
Behavioral Measures
Reaction time and number of errors were recorded during all task trials and conditions. Upon completion of the task, participants were asked to rate their level of interest (Scale 1, ranged 0: boring to 7: interesting) and excitement (Scale 2, ranged 0: dull to 7: exciting) for each of the monetary conditions they completed.
Analysis
Event-Related Potentials
The digitized, continuous EEG was transformed using a DC offset algorithm and was divided into epochs extending from 200 ms before the onset of S1 to 2000 msec after . A linear detrend algorithm was applied to the epoched EEG and after baseline correction, epochs were inspected and those containing amplitudes greater than 75 μV or less than −75 μV were rejected to eliminate EOG and movement artifacts. After rejections, there was a minimum of 10 epochs per averaged waveform. Separate averages were composed (across sequences) for “Go” and “No-go” stimuli separately for the three money conditions (0¢, 1¢ and 45¢) for a total of six waveforms per subject. Grand average waveforms, across subjects, were composed for display purposes.
Five ERP components were identified (ordered by temporal appearance): N1 and P2 to the warning stimulus, P3 to the warning stimulus (P3-W), CNV, and P3 to the target stimulus (P3-T). Two time windows were selected for statistical analysis of the CNV: window 1 (600 to 800 ms after the onset of S1) and window 2 (1300 to 1500 ms after the onset of S1). Window 1 contained the early portion of the CNV (CNV1 or the “O” wave), while window 2 contained the late portion of the CNV (CNV2 or the “E” wave). The mean amplitude was calculated for each time window. The peak amplitudes and latencies of the P3-W (within the interval of 250 to 450 ms) and P3-T (within the interval of 1798–1998 ms) components were measured for all conditions. All grand averages were visually inspected and their scalp distributions evaluated for consistency with the literature. Then, an automated peak detection option in Neuroscan selected the largest peak for each component. To statistically analyze the scalp distribution of the amplitudes of these components, a 3 × 3 array of nine electrodes was selected: FC3, FCZ, FC4, CP3, CPZ, CP4, PO3, POZ and P04.
For each amplitude dependent measure (a total of six), a repeated-measures 2 × 3 × 3 × 3 ANOVA was conducted: Trial (Go, No-go), Money (0¢, 1¢, 45¢), ACP (anteroposterior scalp location: anterior, central, posterior) and LCR (lateral scalp location: left, central, right). For each of the latency dependent measures (a total of four: using the CPZ derivation for P3 and the FCZ derivation for N1 and P2 because the respective peak amplitudes were prominent at these sites), a repeated-measures 2 × 3 ANOVA was conducted: Trial (Go, No-go) and Money (0¢, 1¢, 45¢). To examine whether the first eight subjects (for whom Image 1 served as the “Go” image) differed from second eight subjects (for whom Image 2 served as the “Go” image), each of these ANOVAs was repeated with Image (Image 1, Image 2) as a between-subjects factor.
In cases where the assumption of Sphericity was not met (as tested by Mauchly’s Test of Sphericity), the Greenhouse-Geisser correction was applied. Contrasts were used to examine simple main and interaction effects. Significant effects were followed with LSD (least significant difference) tests or with multiple, paired t tests employing the Bonferroni correction in cases where sphericity was violated (Stevens, 1992).
Behavior: Reaction Time, Number of Errors, Post Task Rating Scale
Reaction time (msec) and number of errors were averaged across all subjects for each run (block) and each monetary condition. They were analyzed using a repeated-measures 3 × 3 ANOVA: Money (0¢, 1¢, 45¢) and Run (1, 2, 3). The subjective ratings of the post-task scale were analyzed using a repeated-measures 3 × 2 ANOVA: Money (0¢, 1¢, 45¢) and Scale (interest, excitement). Significant effects were then followed by paired samples t-tests. To protect against Type I error, a significance level of .01 was used for all behavior and ERP analyses.
Results
Event-Related Potentials
The Image serving as the “Go” image, which differed between the first and second eight subjects, did not significantly affect the ERP results, and was therefore removed from all further analyses.
Peak Amplitudes
N1. There was a significant main effect of ACP, F (1.29,19.3) = 10.76, p < .01 (partial eta squared = .42). Pairwise comparisons showed significantly smaller peak amplitudes at the posterior than anterior (p = .003) or central (p=.004) sites. There were no other significant main effects or interactions.
P2. There was a significant main effect of ACP, F (1.19,17.80) = 10.77, p < .01 (partial eta squared = .42). Pairwise comparisons showed significantly smaller peak amplitudes at the posterior than anterior (p = .006) or central (p = .0001) sites. There were no other significant main effects or interactions.
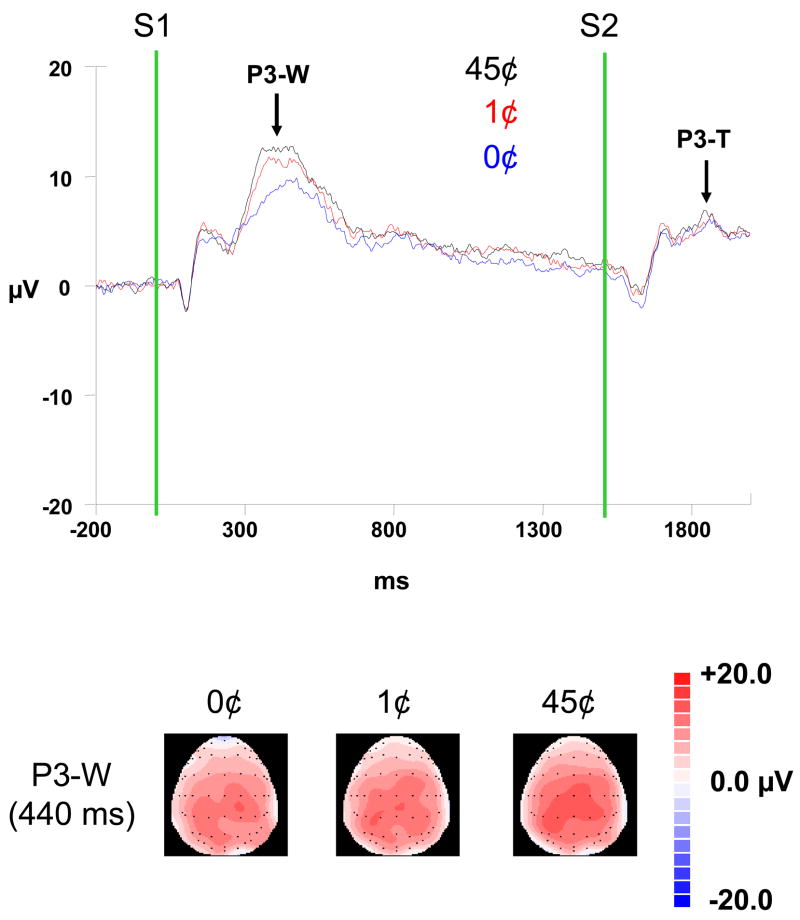
P3 (Figure 2). A main effect of Money was significant for the P3-W, F (2,30) = 10.08, p < .0001 (partial eta squared = .40). The P3-W was significantly larger in amplitude in the 45¢ condition than the 0¢ and 1¢ conditions (p = .002 for both), while the difference between the 0¢ and 1¢ conditions did not reach significant (p = .12). Note that the comparison of these waveforms was undertaken at the latency of the highest peak in the 45¢ condition, and at this latency, the difference between the 45¢ and 1¢ conditions (1.563) was indeed greater than the difference between the 0¢ and 1¢ conditions (1.003). There were no other significant main or interaction effects for the P3-W.
Figure 2.
(top) Grand average waveforms (N = 16) for three levels of monetary reward (0-cent, 1-cent, 45-cent) at the CZ electrode site; (bottom) 64-channel brain maps showing scalp topography of the P3-W at 440 ms.
For the P3-T, the main effect of Money approached significance, F (2,14) = 5.08, p = .02 (partial eta squared = .42). Further, nominal significance level was established for the main effects of Trial, F(1,15) = 26.00, p < .0001 (partial eta squared = .63) and ACP, F (1.15,17.21) = 7.83, p = .01 (partial eta squared = .34), and for their interaction, F (1.3,19.55) = 13.50, p = .001 (partial eta squared = .47). Larger differences were found between Go and No-go stimuli at the anterior as compared to the central (p = .001) or posterior (p = .001) sites. However, the overall peak amplitude was significantly larger over the central than posterior sites (p < .0001), which suggests a similar scalp distribution as P3-W.
Component Latency
There were no significant differences in latency for the N1, P2, P3-W and P3-T components as a function of Trial or Money.
CNV Mean Amplitude
There was no main effect of Money or ACP for the early or late portions of the CNV. For CNV1, although main effect of Trial was not significant, there was a two-way interaction between Trial and ACP, F(1.31,19.58) = 8.52, p < .01 (partial eta squared = .36) such that there were larger amplitudes to Go than No-go stimuli, and this difference was more prominent at anterior than central (p < .0001) or posterior (p = .01) sites. There was a main effect of Trial for CNV2, which was larger to Go than No-go stimuli, F(1,15) = 21.92, p < .0001 (partial eta squared = .59).
Behavior
Reaction Time and Number of Errors
There were no significant main or interaction effects of Money or Run for both reaction time and the number of errors, indicating that performance on the task was equivalent across all task conditions and blocks and differences in ERP measures could not be accounted for by task difficulty (Table 1).
Table 1.
Means and SEM of all behavioral and ERP components
| 0 | 1 | 45 | |
|---|---|---|---|
| Reaction time (msec) | 236.6 (8.1) | 235.7 (10.0) | 227.3 (8.9) |
| Number of errors | .52 (.14) | .31 (.10) | .17 (.06) |
| [minimum – maximum] | [0 – 1.3] | [0 – 1.0] | [0 – 0.7] |
| Post-task rating scale 1* | 3.19 (.51) | 4.19 (.38) | 5.19 (.37) |
| Post-task rating scale 2* | 3.13 (.48) | 4.10 (.32) | 5.56 (.34) |
| N1: amplitude/latency | −2.63 (.80)/100.58 (4.20) | −2.09 (.63)/99.63 (4.61) | −2.34 (.72)/103.50 (5.36) |
| P2: amplitude/latency | 4.99 (.65)/173.00 (5.84) | 6.34 (.72)/184.31 (7.19) | 6.39 (.79)/177.31 (8.14) |
| P3-W: amplitude/latency | 9.18 (.90)/399.56 (11.19) | 10.19 (.73)/401.50 (8.52) | 11.75 (.85)/395.06 (7.16) |
| P3-T: amplitude/latency | 5.97 (.63)/1880.25 (9.67) | 5.91 (.64)/1888.88 (9.36) | 6.96 (.59)/1884.63 (11.50) |
| CNV 1 (O): amplitude | 2.71 (.62) | 2.63 (.80) | 2.69 (.85) |
| CNV 2 (E): amplitude | .76 (.39) | .66 (.44) | .92 (.49) |
Note. Upon completion of the task, participants were asked to rate their level of interest (Scale 1, ranged 0: boring to 7: interesting) and excitement (Scale 2, ranged 0: dull to 7: exciting) for each of the monetary conditions they completed.
Post-Task Rating Scale
There was a significant main effect of Money on the post-task rating scale, F(1.11,16.71)=16.39, p<.01 (partial eta squared = .52) such that participants’ interest and excitement ratings were highest for the 45¢ condition and lowest for the 0¢ condition (Table 1 and Figure 3). The ratings for the 1¢ condition were significantly different from both the 45¢ (p = .00 1) and 0¢ (p = .003) conditions (Table 1). The image serving as the “Go” image, did not significantly affect the behavioral results.
Figure 3.
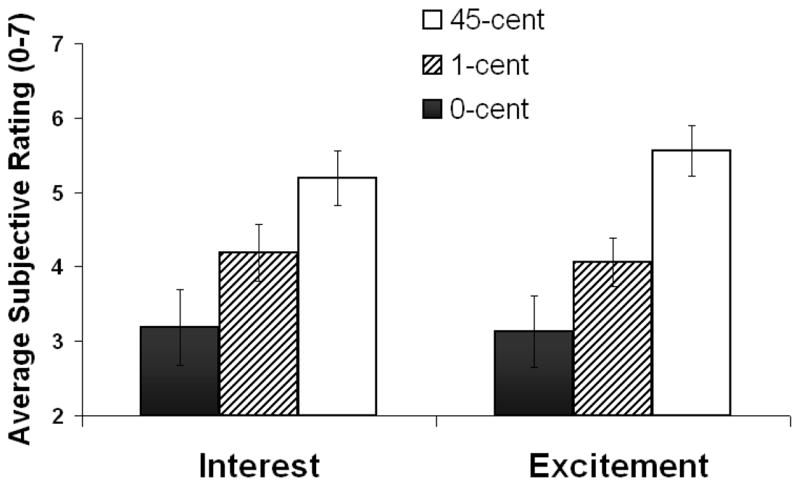
Average subjective ratings (N=16) on the Interest scale and the Excitement scale for three monetary reward conditions (0-cent, 1-cent, 45-cent). Error bars represent standard error of the mean.
Discussion
The purpose of this study was to investigate the relationship between behavior, cognitive ERPs, and reward processing in young healthy adults. As predicted, the amplitude of the P3 component to S1 showed a graded response to monetary reward, such that it was highest for the larger reward and lowest to the lowest reward (Figure 2). This P3 effect was accompanied by differential subjective responses such that interest and excitement ratings were highest for the 45¢ condition and lowest for the 0¢ condition (45¢>1¢>0¢) (Figure 3). In contrast, the CNV amplitude did not vary with monetary reward.
Sensitivity of the P3 amplitude to reward magnitude is consistent with previous reports (Begleiter, Porjesz, Chou, & Aunon, 1983; Homberg, Grunewald, & Grunewald-Zuberbier, 1981; Otten, Gaillard, & Wientjes, 1995; Ramsey & Finn, 1997; Yeung & Sanfey, 2004). However, the precise mechanism underlying this effect is not yet well understood. The P3 has been associated with motivation (Carrillo-de-la-Pena & Cadaveira, 2000), attention (Squires & Ollo, 1999) and arousal (Polich & Kok, 1995) which are in turn associated with reward processing in healthy individuals (Ressler, 2004). Similarly, patients with major depressive disorder (Bruder, et al., 1995), schizophrenia (Javitt, Doneshka, Grochowski, & Ritter, 1995), alcoholism (Porjesz, Begleiter, Bihari, & Kissin, 1987) or family history of alcoholism (Ramsey & Finn, 1997), disorders affecting reward processing but also motivation, attention, and arousal, show decreased P3 amplitude to cognitive and emotional targets. Note that although interrelated, reward processing, motivation, attention, and arousal may represent distinct neuronal processes.
Because we did not include autonomic measures in the current study, our results cannot elucidate the relationships between arousal and the P3. Regarding attention, it is unlikely that large fluctuations accompanied the three reward conditions because there were no significant monetary differences in task performance (speed or accuracy). Although this null result may have been influenced by statistical power (and increasing trial or sample size may have revealed higher speed or accuracy as a function of larger monetary reward), our current results point to the role of motivation as a possible modulating factor in the P3’s role in reward processing, as reflected by the graded ratings on the subjective interest and excitement scales (45¢>1¢>0¢) (Figure 3). Note that although a parallel graded response was visually discernible in the P3 waveforms (Figure 2), it was not statistically significant (45¢>1¢=0¢), possibly due to low statistical power or the restricted distinctiveness between the low and no reward conditions (see (Comerchero & Polich, 1998) and (Katayama & Polich, 1998) for a positive relationship between P3 amplitude and stimulus distinctiveness). To overcome this limitation, future studies could compare additional or more disparate reward conditions (e.g., $2 vs. $1 vs. 10¢).
Future studies may investigate other target components that could also be specific to reward. For example, the error- or feedback-related negativity which is elicited by error commission and presentation of feedback stimuli indicating incorrect performance, has recently been shown to be associated with the value of outcome (Hajcak, Moser, Holroyd, & Simons, 2006; Holroyd, Larsen, & Cohen, 2004). This component was not pertinent in the current investigation where error rate was minimal (mean <1 per condition) and outcome prediction was maximal. Indeed, our a priori goal was to study processing of reward that is predictable and we therefore used a blocked and not an event-related design. Such guaranteed response-reward association may have elicited a distinct type of reward motivation different than motivation elicited by conditions in which the outcome is more uncertain. This distinction possibly exemplifies the differences between consummatory vs. anticipatory reward processing, respectively, which differ in their neural representations (Knutson, Fong, Adams, Varner, & Hommer, 2001; Bjork, et al., 2004). Because the monetary main effect was observed for the P3-W (which may be considered anticipatory) and it also approached significance for the P3-T (which may be considered consummatory), the exact motivational processes elicited by the current task remain to be delineated, possibly with a task that directly contrasts these different reward mechanisms. In the current study, trial type affected the CNV (go>no-go), as possibly related to differences between the behavioral activation (go) and inhibition (no-go) systems (see Jeffrey Gray’s BIS/BAS systems - Fowles, 1994). However, CNV amplitude did not vary with monetary reward. This finding is in agreement with another study that utilized a similar S1–S2 rewarded task (Sobotka, Davidson, & Senulis, 1992). It is further possible that other individual variables, particularly those related to approach behavior, may have influenced these results. For example, in comparison to neutral stimuli, reward-associated stimuli elicited larger CNV amplitude in hedonic individuals as compared to anhedonic individuals (Pierson, Ragot, Ripoche, & Lesevre, 1987) suggesting that the trait ability to experience pleasure or positive emotionality may modulate the CNV’s response to reward and reward-conditioned stimuli. It is also possible that factors related to the task design have influenced these null findings; it would thus be of particular interest to inspect the late CNV waveform in a task that delivers reward in an event-related (i.e., unexpected) rather than a blocked (i.e., predictable) fashion.
Limitations of this study include the assessment of subjects’ interest and excitement at the end of the testing (i.e., retrospective assessments) and not at the end of each reward condition, possibly increasing the effect of demand characteristics on this assessment and predisposing it to other extraneous influences (e.g., expectancy, working memory). Future studies need to replicate these results using “online” assessments that may better reflect the actual emotions experienced during the experiment. Further, we did not perform both a blocked and an event-related task within the same subjects; this important comparison awaits future studies.
In summary, we showed a difference between the P3 and CNV ERP components with regard to reward processing in healthy young individuals. While P3 amplitude was sensitive to graded sustained monetary reward, the early and late CNV components were not. These results present the next challenge of studying clinical populations with known reward processing deficits (e.g., drug addicted individuals) and of synchronizing the temporal (ERP) with the anatomical (e.g., functional neuroimaging) aspects of human neural processing of reward.
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (DA06891-06; 1K23 DA15517-01; and T32 DA07316-01A1); Laboratory Directed Research and Development from U.S. Department of Energy (OBER); National Institute on Alcohol Abuse and Alcoholism (AA/ODO9481-04); NARSAD Young Investigator Award and Stony Brook/Brookhaven National Laboratory seed grant (79/1025459); and General Clinical Research Center (5-MO1-RR-10710).
References
- American Electroencephalographic Society guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1991;8(2):200–2. [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Chou CL, Aunon JI. P3 and stimulus incentive value. Psychophysiology. 1983;20(1):95–101. doi: 10.1111/j.1469-8986.1983.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd ES, Boyd EH, Brown LE. Observations on the M-wave and the CNV in the squirrel monkey. Electroencephalogr Clin Neurophysiol. 1979;46(3):320–36. doi: 10.1016/0013-4694(79)90206-2. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30(2):619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, Quitkin FM. Brain event-related potentials to complex tones in depressed patients: relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32(4):373–81. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Cant BR, Bickford RG. The effect of motivation on the contingent negative variation (CNV) Electroencephalogr Clin Neurophysiol. 1967;23(6):594. [PubMed] [Google Scholar]
- Carrillo-de-la-Pena MT, Cadaveira F. The effect of motivational instructions on P300 amplitude. Neurophysiol Clin. 2000;30(4):232–9. doi: 10.1016/s0987-7053(00)00220-3. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a, perceptual distinctiveness, and stimulus modality. Brain Res Cogn Brain Res. 1998;7(1):41–8. doi: 10.1016/s0926-6410(98)00009-3. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, Scalf PE, Webb A, Heller W. Paying attention to emotion: an fMRI investigation of cognitive and emotional stroop tasks. Cogn Affect Behav Neurosci. 2003;3(2):81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Heller W, Banich MT, Palmieri PA, Miller GA. Responding to threat: hemispheric asymmetries and interhemispheric division of input. Neuropsychology. 2000;14(2):254–64. doi: 10.1037//0894-4105.14.2.254. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. Performance on the emotional stroop task in groups of anxious, expert, and control subjects: a comparison of computer and card presentation formats. Cognition and Emotion. 1995;9:341–362. [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20(16):6159–65. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC. A motivational theory of psychopathology. Nebr Symp Motiv. 1994;41:181–238. [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol. 2006;71(2):148–54. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Holle C, Neely JH, Heimberg RG. The effects of blocked versus random presentation and semantic relatedness of stimulus words on response to a modified Stroop task among social phobics. Cognitive Therapy and Research. 1997;21:681–697. [Google Scholar]
- Holroyd CB, Larsen JT, Cohen JD. Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology. 2004;41(2):245–53. doi: 10.1111/j.1469-8986.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Homberg V, Grunewald G, Grunewald-Zuberbier E. The variation of p300 amplitude in a money-winning paradigm in children. Psychophysiology. 1981;18(3):258–62. doi: 10.1111/j.1469-8986.1981.tb03030.x. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52(7):550–8. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr Scalp-recorded P300 activity in patients following unilateral temporal lobectomy. Brain. 1988;111(Pt 6):1517–29. doi: 10.1093/brain/111.6.1517. [DOI] [PubMed] [Google Scholar]
- Katayama J, Polich J. Stimulus context determines P3a and P3b. Psychophysiology. 1998;35(1):23–33. [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25(19):4806–12. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J, Howard RC, Fenton GW. The contingent negative variation (CNV) to fear-related stimuli in acquisition and extinction. Int J Psychophysiol. 1986;3(4):253–61. doi: 10.1016/0167-8760(86)90034-6. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Henson RN, Friston KJ. Estimating efficiency a priori: a comparison of blocked and randomized designs. Neuroimage. 2003;18(3):798–805. doi: 10.1016/s1053-8119(02)00040-x. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Gaillard AW, Wientjes CJ. The relation between event-related brain potential, heart rate, and blood pressure responses in an S1–S2 paradigm. Biol Psychol. 1995;39(2–3):81–102. doi: 10.1016/0301-0511(94)00969-5. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9(4):456–79. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Pierson A, Ragot R, Ripoche A, Lesevre N. Electrophysiological changes elicited by auditory stimuli given a positive or negative value: a study comparing anhedonic with hedonic subjects. Int J Psychophysiol. 1987;5(2):107–23. doi: 10.1016/0167-8760(87)90015-8. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41(2):103–46. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol. 1987;4(4):283–7. doi: 10.1016/0741-8329(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychol Bull. 1981;89(3):506–40. [PubMed] [Google Scholar]
- Ramsey SE, Finn PR. P300 from men with a family history of alcoholism under different incentive conditions. J Stud Alcohol. 1997;58(6):606–16. doi: 10.15288/jsa.1997.58.606. [DOI] [PubMed] [Google Scholar]
- Ressler N. Rewards and punishments, goal-directed behavior and consciousness. Neurosci Biobehav Rev. 2004;28(1):27–39. doi: 10.1016/j.neubiorev.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh JW, McCallum WC, Gaillard AW, Simons RF, Birbaumer N, Papakostopoulos D. ERPs associated with preparatory and movement-related processes. A review. Electroencephalogr Clin Neurophysiol Suppl. 1986;38:189–229. [PubMed] [Google Scholar]
- Sobotka SS, Davidson RJ, Senulis JA. Anterior brain electrical asymmetries in response to reward and punishment. Electroencephalogr Clin Neurophysiol. 1992;83(4):236–47. doi: 10.1016/0013-4694(92)90117-z. [DOI] [PubMed] [Google Scholar]
- Squires KC, Donchin E, Herning RI, McCarthy G. On the influence of task relevance and stimulus probability on event-related-potential components. Electroencephalography and Clinical Neurophysiology. 1977;42(1):1–14. doi: 10.1016/0013-4694(77)90146-8. [DOI] [PubMed] [Google Scholar]
- Squires NK, Ollo C. Comparison of endogenous event-related potentials in attend and non-attend conditions: latency changes with normal aging. Clinical Neurophysiology. 1999;110:564–574. doi: 10.1016/s1388-2457(99)00003-6. [DOI] [PubMed] [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. 2. Lawrence Erlbaum, Associates; NewJersey: 1992. [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150(700):1187–8. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL. Activation of the human brain by monetary reward. Neuroreport. 1997;8(5):1225–8. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation Enhances the Regional Brain Metabolic and the Reinforcing Effects of Stimulants in Cocaine Abusers. J Neurosci. 2003;23(36):11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent Negative Variation: An Electric Sign of Sensorimotor Association and Expectancy in the Human Brain. Nature. 1964;203:380–4. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. J Neurosci. 2004;24(28):6258–64. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]