Abstract
Cognitive processes mediated by the hippocampus and cortex are influenced by estradiol (E2); however, the mechanisms by which E2 has these effects are not entirely clear. As such, studies were conducted to begin to address the role of actions at the β form of the intracellular estrogen receptor (ERβ) for E2’s cognitive effects in adult female mice. We investigated whether E2 improved performance of wildtype (WT) and ERβ knockout (βERKO) mice in tasks considered to be mediated by the cortex and hippocampus, the object recognition and object placement tasks. WT and βERKO mice were ovariectomized (ovx) and E2 (0.1 mg/kg), an ERβ selective ER modulator (SERM), diarylpropionitrile (DPN; 0.1 mg/kg), or oil vehicle was administered to mice following training in these tasks. We hypothesized that if E2 has mnemonic effects, in part, due to its actions at ERβ, then WT mice administered E2 or DPN would have improved performance compared to vehicle WT controls, which would not be different from βERKO mice administered vehicle, E2 or DPN. Alternatively, activation of ERα (with E2, which is a ligand for both ERα and ERβ) may produce opposing effects on cognition and/or the activation of ERα and ERβ vs. either receptor isoform alone may produce a different pattern of effects. Results obtained supported the hypothesis that ERβ activation is important for mnemonic effects. Ovx WT, but not βERKO, mice administered E2 or DPN had a greater percentage of time exploring a novel object in the object recognition task and a displaced object in the object placement task. Thus, actions at ERβ may be important for E2 or SERMs to enhance cognitive performance of female mice in the object recognition and placement tasks.
Keywords: estrogen, SERMs, allopregnanolone, hippocampus, cortex, learning, memory
1. Introduction
Steroid hormones, such as estradiol (E2), can have trophic effects in the central nervous system. However, results of recent clinical trials suggest that E2-based replacement therapies are not beneficial to all women (Almeida, Lautenschlager, Vasikaran, Leedman, Gelavis and Flicker, 2006; Espeland, Rapp, Shumaker, Brunner, Manson, Sherwin, Hsia, Margolis, Hogan, Wallace, Dailey, Freeman and Hays, 2004; LeBlanc, Janowsky, Chan and Nelson, 2001; Shumaker, Legault, Kuller, Rapp, Thal, Lane, Fillit, Stefanick, Hendrix, Lewis, Masaki and Coker, 2004; Yaffe, Sawaya, Lieberburg, and Grady, 1998). These initial reports included findings from women seventy years old and older, who had been post-menopausal for an average of 20 years. More recent re-analyses of these data suggest that E2-based therapies can confer some benefit to women when treatment begins closer to the onset of menopause (reviewed in Sherwin, 2007). Because of the clear clinical relevance, and the need for a greater understanding of E2’s effects for cognitive behavior, more research is needed in this area.
Because age, duration of E2 deprivation, and other factors may influence the effects of E2 on cognitive processes, it can be advantageous to investigate effects of E2 in animal models, where these factors can be controlled. The validity of using an animal model to investigate effects of E2 is supported by studies with older rodents. For instance, in studies using middle-age female rats and mice, spatial memory decline coincides with the transition to reproductive senescence, which is characterized by decline in ovarian function and E2 levels (Markowska, 1999; Frick, Burlingame, Arters and Berger-Sweeney, 2000). As well, the effects of long-term E2-replacement to enhance working memory are only observed when replacement begins at time of ovariectomy (ovx), rather than when initiated months post-ovx (Daniel, Hulst and Berbling, 2006). Comparisons of endogenous fluctuations in, and/or exogenous administration, of E2 to young rodent are another approach to utilize to investigate the effects of E2 for cognitive performance. Studies using this approach suggest that the capacity for E2 to alter cognitive processes appear to be E2 concentration- and/or task-dependent. During proestrus, or when proestrous-like levels of E2 are mimicked in ovx rodents, performance in tasks that involve hippocampus-mediated ‘place’ strategy are enhanced, perhaps due the favorable effects of high E2 on hippocampal function during consolidation (Frye and Rhodes, 2002; Korol and Kolo, 2002; Korol, Malin, Borden, Busby, and Couper-Leo, 2004; Rhodes and Frye, 2004) or increases in hippocampal dendritic spine density, synaptic proteins, or long-term potentiation (Brake, Alves, Dunlop, Lee, Bulloch, Allen, Greengard, and McEwen, 2001; Choi, Romeo, Brake, Bethea, Rosenwaks, and McEwen, 2003; Cordoba Montoya and Carrer, 1997; Day and Good, 2005; McEwen, Akama, Alves, Brake, Bulloch, Lee, Li, Yuen, and Milner, 2001; Warren, Humphreys, Juraska, and Greenough, 1995; Woolley and McEwen, 1992). Notably, these high, physiological E2 levels can impair performance in other hippocampally-mediated tasks with aversive components, which implies that some of E2’s effects may occur when E2 is present during training and testing and/or may be due to altering stress responses (e.g. Chesler and Juraska, 2000; Frye, 1995; Koch, 1998; Wood and Shors, 1998). As well, performance in tasks that are mediated by the striatum and/or dependent upon response learning is enhanced with lower levels of E2 (Daniel and Lee, 2004; Davis, Jacobson, Aliakbari, and Mizumori, 2005; Holmes, Wide, and Galea, 2002; Korol and Kolo, 2002; Quinlan, Graffe, Duncan, and Brake, 2006; Nofrey, Ben-Shahar, and Brake, 2007; Wide, Hanratty, Ting, and Galea, 2004. Given these differences in E2’s effects, the present study investigates the effects of post-training E2 regimen that produces proestrous-like E2 levels in young adult, ovx mice for performance in tasks mediated by the hippocampus and cortex (i.e. object placement and object recognition).
In addition to elucidating the nature and neural substrates of E2’s effects on cognitive performance, it is also important to ascertain the mechanisms by which E2 enhances cognitive performance. E2 receptors (ERs) have been localized to the hippocampus of both rats (Shughrue, Lane and Merchenthaler, 1997) and mice (Shughrue, Scrimo, Lane, Askew and Merchenthaler, 1997). Further, ER expression is reduced among middle-age female rats (Wilson, Rosewell, Kashon, Shughrue, Merchenthaler and Wise, 2002). E2 binds with a high affinity to ER isoforms, ERα and ERβ. Both ERα and ERβ are expressed in the hippocampus and cortex (Shughrue and Merchenthaler, 2001, Shughrue et al., 1997 and Shughrue et al., 1998), albeit ERβ expression may predominate in these regions. Although, E2 can have actions through both ERα and ERβ, whether actions at ERβ may underlie some cognitive effects of E2 are of interest. ERβ knockout mice (βERKOs) administered E2 had delayed acquisition of learning in the water maze compared to their wildtype (WT) counterparts (Rissman et al., 2002). Moreover, ERβ selective ER modulators (SERMS) or dietary phytoestrogens, which have selective actions at ERβ, enhance performance in hippocampal tasks, such as the water maze, radial arm maze, and inhibitory avoidance tasks (Lund, West, Tian, Bu, Simmons, Setchell, Adlercreutz, Lephart, 2001; Rhodes and Frye, 2006) and performance in tasks mediated by both the hippocampus and cortex (i.e. object recognition and object placement) in some, but not all, studies (Frye, Duffy, and Walf, 2007; Luine, Jacome and MacLusky, 2003; Walf, Rhodes, and Frye, 2006). Given these different patterns of effects with SERMs, it is important to further investigate the role of ERβ for E2’s effects on performance in hippocampally- and/or cortically-mediated tasks, such as the object placement and object recognition tasks. We hypothesized that if E2 has mnemonic effects, in part, due to actions at ERβ, then WT mice administered E2 or an ERβ SERM, diarylpropionitrile (DPN), would have improved performance in the object placement and object recognition tasks, compared to vehicle WT controls, which would not be different from βERKO mice administered vehicle, E2 or DPN. Alternative hypotheses about residual effects of ERα and/or ERβ, and the effects of the ERβ knockout and/or non-ER-mediated effects could be determined with the following comparisons. Residual activation of ERα for observed effects would be supported if there are differences between WT and βERKO mice administered E2 (which is a ligand for both ERα and ERβ). Residual activation of ERβ for observed effects would be supported if there are differences between WT mice administered E2 and DPN. Differences due to the knockout of ERβ alone could be accounted for by differences in WT and βERKO mice administered vehicle. Differences in effects of vehicle or DPN to βERKO mice would suggest non-ERβ-mediated effects of DPN.
2. Methods
All procedures were approved by the Institutional Animal Care and Use Committee of The University at Albany, and conformed to the guidelines established by The National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.1 Mouse Husbandry
Subjects were 8–10 weeks old, female mice (N=104). Mice were group-housed (4/5 per cage) in polycarbonate cages (45 × 24 × 21 cm) in a temperature-controlled room (21 ± 1 °C) in the Laboratory Animal Care Facility in The Life Sciences Research Building at The University at Albany. Mice were maintained on a 12/12-hour reversed light cycle (lights off at 8:00 am) with continuous access to Purina Rodent Chow and tap water in their home cages at all times.
2.2 Strain and Genotyping
WT (n=56) and homozygous βERKO (n=48) mice were raised on a C57BL/6 background and derived from breeder pairs obtained from Jackson Laboratories (Bar Harbor, ME). To determine the genotype of mice, DNA was isolated from tails and analyzed by PCR. PCR was conducted in the laboratory of Dr. Anne Messer at The Wadsworth Center by K. Manley, in the Molecular Core Facility at SUNY Albany, or in our laboratory. Briefly, DNA was denatured at 94°C for 3 min, followed by 35 cycles of amplification: 94°C for 30 secs, 60°C for 30 secs, 72°C for 30 secs and a final primer extension step at 72°C for 2 min. Specific primers used: ESR2-1, which lies upstream of insertion site in exon 2 (5’-GTTGTGCCAGCCCTGTTACT-3’), ESR2-1, which lies downstream of the insertion site in exon 2 (5’-TCACAGGACCAGACACCGTA-3’), and ESR2-3, a neo gene-specific primer (5’-GCAGCCTCTGTTCCACATACAC-3’). Primers were obtained from Integrated DNA Technologies (Coralville, IL). Bands of approximately 106 and 160 base pairs were amplified for WT and βERKO mice, respectively. For this study, only WT and homozygous βERKO mice were included.
2.3 Screening procedure
Before inclusion in the study, the general health of mice and their normative responses to external stimuli were evaluated (Crawley, Chen, Puri, Washburn, Sullivan, Hill, Young, Nadler, Moy, Young, Caldwell, and Young, 2007). Observers, who were blind to the genotypes of mice, performed these evaluations so that potential differences in these measures could be ruled out as contributors to genotypic differences in behavioral responses to E2 or DPN in the present study. Measures included: the general appearance of mice (clean fur, whiskers, posture, gait, muscle tone) and normative behavior (fur grooming, nest-building with Nestlet cotton squares provided in home cage, huddling with cage mates, ability to cage climb, paw withdrawal when gently pulled) and reflexes (blinking to cotton swab placed close to eyes, ear twitch when cotton swab is gently placed on ear). No differences were noted in WT or βERKO mice for these measures and all mice were included in the study.
2.4. Ovariectomy
Approximately 7 to 10 days prior to behavioral testing, mice were ovariectomized to remove the primary endogenous source of steroid hormones. Briefly, mice were anesthetized using sodium pentobarbital (80 mg/kg, IP, or to effect). An incision in the skin and then abdominal wall was made away from the midline at the level of the pelvis. The ovary, oviduct, and top of the fallopian tubes were ligated and removed. The abdominal wall was sutured. The skin was closed with surgical adhesive and wound clips. After recovery from anesthesia, mice were returned to group-housing in their home cages.
2.5. Hormone treatment
After ovariectomy, mice were randomly-assigned to receive subcutaneous (SC) injections of vehicle (vegetable oil), 17β-estradiol (E2; 0.1 mg/kg; Sigma Chemical Co., St. Louis, MO), or an ERβ-selective SERM, DPN (0.1 mg/kg; Meyers et al., 2001; Tocris Chemical Co., Ellisville, MO) immediately after the completion of training in each task. E2 and DPN were dissolved in vegetable oil. Dosing for both was based upon pilot studies and published reports (Frye et al., 2007; Walf et al., 2006; 2007), which indicated that this regimen of E2 produced proestrous-like levels of E2 in plasma (Edwards, 1970; Frye & Vongher, 1999) and in brain (see Table 1). Mice were randomly assigned to groups to be administered E2 (WT, n = 18; βERKO, n=19), DPN (WT, n = 18; βERKO, n=26) or vehicle (WT, n = 22; βERKO, n=27).
Table 1.
Mean (± sem) prefrontal cortex concentrations of estradiol (E2), progesterone (P4) and 3α,5β-THP of ovariectomized wildtype (WT) or estrogen receptor β knockout (βERKO) mice administered subcutaneous vehicle (far left), 17β-E2 (middle), and DPN (far right).
| CONDITION | ||||||
|---|---|---|---|---|---|---|
| vehicle | 17β-E2 | DPN | ||||
| WT | βERKO | WT | βERKO | WT | βERKO | |
| n= | 17 | 15 | 15 | 12 | 23 | 17 |
| E2 (pg/g) | 1.5 ± 0.4 | 1.4 ± 0.3 | *9.2 ± 1.5 | *10.6 ± 2.0 | - | - |
| P4 (ng/g) | 2.9 ± 0.5 | 3.5 ± 0.6 | 4.4 ± 0.9 | 2.5 ± 0.9 | 4.6 ± 0.6 | 6.0 ± 1.0 |
| 3α,5α-THP (ng/g) | 3.1 ± 0.6 | 4.0 ± 0.7 | 5.4 ± 0.8 | 3.2 ± 1.0 | 3.8 ± 0.8 | 4.8 ± 1.1 |
indicates a significant (P ≤ 0.05) effect of E2 or DPN, which is attributable to higher levels vs. vehicle.
2.6. Habituation/Behavioral testing
To habituate mice to handling, mice were picked up for 5 min/day for 5 consecutive days and exposed to a novel stimuli/situation on days 1–4 (new clean cage, weighing scale, novel open field testing chamber (which was utilized in subsequent behavioral tasks that are described below), SC injection of oil vehicle; as per Frye, Sumida, Dudek, Harney, Lydon, O'Malley, Pfaff, Rhodes, 2006). This procedure occurred one week prior to the start of behavioral testing. The mice were housed in a room with a 12:12 reversed light/dark cycle (lights off at 07:00). All training and testing was performed in the core behavioral testing facility, which is adjacent to the animal housing area. Mice were transported in their home cages on a cart to the behavioral testing facility. Each mouse was individually housed prior to training (which occurred between 0900–1100 hours) and was returned to their home cage after testing (between 1300 and 1500). Approximately 7–10 days after ovariectomy, mice were tested in the object recognition or object placement task using a 4-hr delay. Seven to 10 days later, mice were tested in whichever task (object recognition or object placement) that they had not been tested in previously. Hormone injections were given at the completion of training, as described below. The majority of experimental mice were tested once in each task. A few mice were tested in either task twice. Analyses of data from the few mice that were tested in the same task on more than one occasion did not reveal test decay effects, as has been reported (Frye and Walf, 2008; Luine et al., 2003). Whether mice were tested initially in the object recognition or object placement task was randomized and counterbalanced across groups.
2.6.1. Object recognition
The object recognition task, a test of non-spatial reference memory, was conducted in an open field (46 × 57 × 30 cm), constructed of white laminate and located in a quiet room under dim lighting. This same open field was utilized in the habituation procedure described above. Small, plastic, washable toys that have distinct circular (oranges, lemons, apples) or cone-like shapes (buoys, bottles) were utilized as target stimuli. A digital camera was mounted on the ceiling above the testing chamber and connected to a computer with a video-tracking system that objectively monitored and quantified animals’ movements (Any-maze- Stoelting, Inc., Wood Dale, IL). Testing, which takes advantage of the natural affinity of mice for novelty, was conducted as described previously (Walf et al., 2006). Briefly, during training, each mouse was placed in the open field box for 180 secs and allowed to explore two identical objects (placed approximately 15 cm from the northeast and northwest walls). After 180 secs, each mouse was removed from the box, immediately injected with vehicle or E2 or DPN and returned to its individual holding cage. Mice were tested in the choice phase 4 hrs later. One familiar object (identical to that which was used in training) and one novel object were placed in the same location of the box as was used during training. Whether the novel object was placed in the northeast or northwest location was counterbalanced within and between groups. The time spent exploring each object was recorded during both the training and testing phases using Anymaze video tracking system. Object exploration was scored when the mouse was sniffing, climbing on, or touching the object and was a body’s length or less away from the object (i.e. less than 3 cm) while facing the object and/or oriented towards it. Four hours later, the time mice spent exploring the objects were recorded during testing. An increased percentage of time spent exploring the displaced object compared to the total amount of time spent exploring both objects during testing (duration spent with novel object/(duration spent with novel object + duration spent with familiar object) × 100) was considered an index of enhanced performance in this task. Chance levels of performance are when mice spend 50% of the total exploration time investigating each object.
2.6.2. Object placement
The object placement task, a test of spatial reference memory, was conducted as per Frye et al., 2007, using methods similar to that described above. Mice were trained in the open field box for 180 secs and allowed to explore two identical objects (placed approximately 15 cm from the northeast and northwest walls). After 180 secs, each mouse was removed from the box, immediately injected with vehicle, E2, or DPN and returned to its individual holding cage. Mice were tested 4 hrs later. The same objects used in training were employed but one was placed in the same location of the box as was used during training and the other was displaced to a novel location (southeast or southwest). Whether the northeast or northwest object was displaced was counterbalanced within and between groups. An increased percentage of time spent exploring the displaced object compared to the total amount of time spent exploring both objects during testing (duration spent with displaced object/(duration spent with displaced object + duration spent with non-displaced object) × 100) was considered an index of enhanced performance in this task. Chance levels of responding are considered when mice spend 50% of the total exploration time investigating each object.
2.7. Tissue Collection/Dissection
One week after the completion of object recognition and placement testing, some mice that were initially assigned to receive vehicle, E2, or DPN were re-injected their assigned condition. One hr later, mice were cervically-dislocated, decapitated, and brains were rapidly dissected from the skull and placed on dry ice. This time frame was used because E2 has to be present within 1 hour of training for post-training estradiol to enhance memory (Frye et al., 2007; Packard and Teather, 1997; Walf et al., 2006). Whole brains were then stored at −80°C until immediately prior to radioimmunoassay. The cortex and hippocampus were dissected out from whole brain on ice immediately before radioimmunoassay (as per Frye and Walf, 2008).
2.8. Radioimmunoassay of Steroid Hormones
To address the effects of hormone treatments and possible genotypic differences in E2 or progestin biosynthesis, E2, progesterone (P4), and its metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP), were measured in cortex and hippocampus, as described below, using previously reported methods (Frye and Bayon, 1999). Radioactive probes utilized, E2 (NET-317, 51.3 Ci/mmol), P4 (NET-208: specific activity = 47.5 Ci/mmol), and 3α,5α-THP (NET-1047: specific activity = 65.0 Ci/mmol), were purchased from Perkin Elmer (Boston, MA).
E2, P4, and 3α,5α-THP were extracted from brain tissues that had been processed with a glass/glass homogenizer in 50% MeOH;1% acetic acid. Brain tissues were centrifuged at 3,000 × g, followed by chomatographic separation of the supernatant using Sepak-cartridges equilibrated with 50% MeOH:1% acetic acid. Steroids were eluted with increasing concentrations of MeOH (i.e. 50% MeOH followed by 100% MeOH). Solvents were evaporated to dryness in a Savant. Immediately before radioimmunoassay set-up, samples were reconstituted in 150 µl phosphate assay buffer.
The E2 antibody (E#244, Dr. G.D. Niswender, Colorado State University, Fort Collins, CO), which typically binds between 40% and 60% of [3H] E2, was used in a 1:40,000 dilution and bound 54% in the present study. Because of concerns about cross-reactivity between E2 and DPN, only effects of E2 and vehicle administration are reported here. The P4 antibody (P#337), obtained from Dr. G.D. Niswender (Colorado State University), when used in a 1:30,000 dilution typically binds between 30% and 50% of [3H]P4, and bound 48% in the present study. The 3α,5α-THP antibody (#921412-5), obtained from Dr. Robert Purdy (Veterans Medical Affairs, La Jolla, CA), when used in a 1:5000 dilution binds between 40–60% of [3H] 3α,5α-THP and bound 47% in the present study.
The range of the standard curves, prepared in duplicate, was 0–1000 pg for E2, and 0–8000 pg for P4 and 3α,5α-THP. Standards were added to assay buffer followed by addition of the appropriate antibody (described above) and 3H steroid. Total assay volumes were 750 µl for E2 and P4, and 950 µl for 3α,5α-THP. All assays were incubated overnight at 4°C.
Separation of bound and free steroid was accomplished by rapidly adding dextran-coated charcoal to assay tubes. Following 20 minute incubation with charcoal, samples were centrifuged at 3000 g for 20 minutes and the supernatant was decanted into a glass scintillation vial with 5 ml scintillation cocktail (Scintiverse BD). Sample tube concentrations were calculated using the logit-log method of Rodbard and Hutt (1974), interpolation of the standards, and correction for recovery with Assay Zap. The inter- and intra-assay reliability co-efficients were: 0.05 and 0.06 for E2, 0.08 and 0.10 for P4, and 0.09 and 0.10 for 3α,5α-THP.
2.9. Data analyses
Two-way analysis of variance (ANOVA) was utilized with hormone (E2, DPN, and/or vehicle) and genotype (WT, βERKO) as independent variables using Statview and/or SuperANOVA statistical software. Post-hoc analyses were run, as appropriate, to determine group differences. The α level for statistical significance was 0.05.
3. Results
3.1 Hormone Measures
E2 administration similarly increased concentrations of E2 in cortex and hippocampus of WT and βERKO mice. Compared to vehicle, E2 administration significantly increased E2, but neither P4, nor 3α,5α-THP, levels in the prefrontal cortex (F2,93 = 16.60, P < 0.01; Table 1). E2 and DPN administration similarly increased concentrations of 3α,5α-THP in the hippocampus of WT and βERKO mice. E2 administration significantly increased E2 (F2,93 = 24.16, P < 0.01; Table 2) and 3α,5α-THP (F2,93 = 2.94, P < 0.05; Table 2), and DPN increased 3α,5α-THP levels in the hippocampus compared to vehicle administration
Table 2.
Mean (± sem) hippocampal concentrations of estradiol (E2), progesterone (P4) and 3α,5α-THP of ovariectomized wildtype (WT) or estrogen receptor β knockout (βERKO) mice administered subcutaneous vehicle (far left), 17β-E2 (middle), and DPN (far right).
| CONDITION | ||||||
|---|---|---|---|---|---|---|
| vehicle | 17β-E2 | DPN | ||||
| WT | βERKO | WT | βERKO | WT | βERKO | |
| n= | 17 | 15 | 15 | 12 | 23 | 17 |
| E2 (pg/g) | 1.4 ± 0.4 | 1.5 ± 0.5 | *9.3 ± 1.1 | *11.9 ± 1.9 | - | - |
| P4 (ng/g) | 3.9 ± 0.8 | 2.0 ± 0.5 | 5.2 ± 0.8 | 5.5 ± 1.1 | 5.8 ± 0.8 | 8.3 ± 0.9 |
| 3α,5α-THP (ng/g) | 3.9 ± 1.0 | 4.1 ± 1.1 | *8.6 ± 1.9 | *6.9 ± 2.2 | *8.9 ± 1.6 | *4.6 ± 1.1 |
indicates a significant (P ≤ 0.05) effect of E2 or DPN, which is attributable to higher levels vs. vehicle.
3.1 Behavioral Measures
3.1.1 Object Recognition
There were no statistical differences between groups in the time spent exploring the objects on the training trials. All mice investigated both objects during training (mean ± sem in secs): WT + vehicle (left: 9.0 ± 3.0, right: 6.7 ± 2.5), βERKO + vehicle (left: 16.4 ± 7.6, right: 6.3 ± 2.4), WT + E2 (left: 8.5 ± 4.8, right: 14.4 ± 7.4), βERKO + E2 (left: 7.9 ± 3.4, right: 9.0 ± 3.2), WT + DPN (left: 5.8 ± 3.1, right: 4.4 ± 1.6), βERKO + DPN (left: 13.7 ± 6.7, right: 6.3 ± 2.7).
Administration of E2 or DPN to WT, but not βERKO, mice significantly increased the percentage of time spent investigating the novel object, over that of vehicle-administered control mice, during the test phase (Figure 1). Hormone and genotype condition interacted (F2,124 = 3.79, P< 0.03) to influence the percentage of time spent investigating the novel object (vs. the familiar object as a function of the total time exploring both objects during testing). There were also main effects of genotype (F1,124 = 24.19, P< 0.01) and hormone condition (F2,124 = 5.44, P< 0.01) during testing. The time mice spent with each object (mean ± sem in seconds) is as follows: WT + vehicle (novel: 10.1 ± 4.0, familiar: 8.0 ± 3.0), βERKO + vehicle (novel: 6.6 ± 2.4, familiar: 9.5 ± 4.0), WT + E2 (novel: 12.8 ± 5.6, familiar: 7.9 ± 3.9), βERKO + E2 (novel: 11.0 ± 4.1, familiar: 10.3 ± 4.2), WT + DPN (novel: 4.8 ± 1.0, familiar: 2.3 ± 0.3), βERKO + DPN (novel: 5.3 ± 1.7, familiar: 8.6 ± 3.3).
Figure 1.
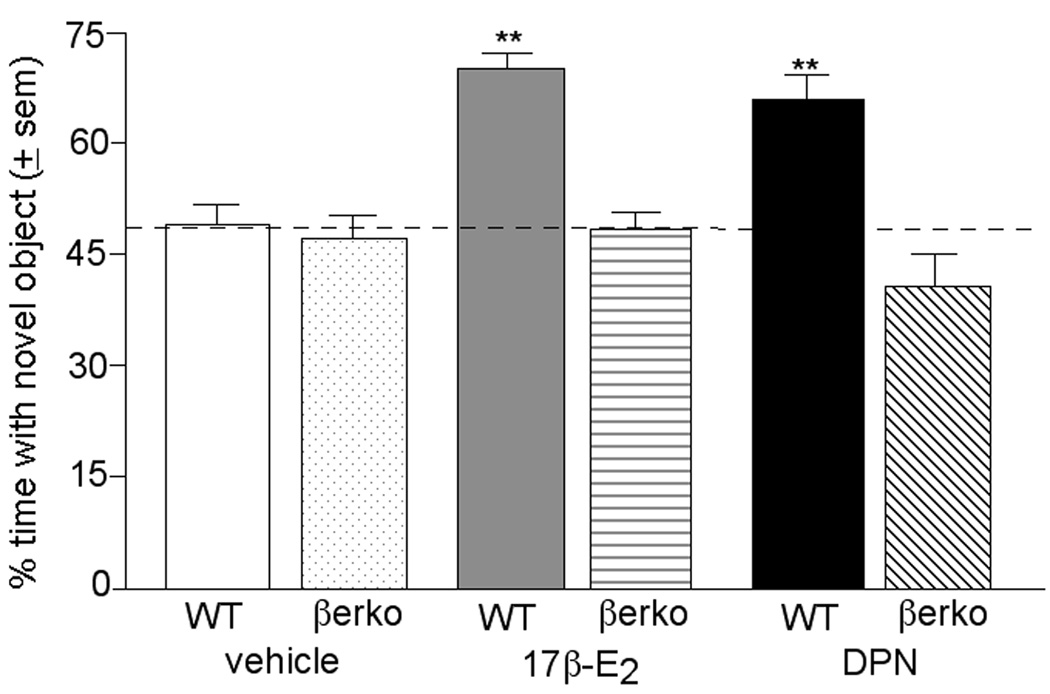
Mean (± sem) percentage of time spent exploring the novel object by vehicle-administered WT or estrogen receptor β knockout (βERKO) mice, 17β-E2-administered WT or βERKO mice, or DPN-administered WT or βERKO mice. ** indicates a significant (P ≤ 0.05) interaction between genotype and hormone condition. Chance levels of responding are considered when mice spend 50% of the total exploration time investigating each object, as indicated by dashed line.
3.1.2 Object Placement
There were no statistical differences between groups in the time spent exploring the locations during the training trial. All mice investigated both objects during training (mean ± sem in seconds): WT + vehicle (left: 3.3 ± 0.4, right: 4.3 ± 0.8), βERKO + vehicle (left: 3.6 ± 0.5, right: 2.9 ± 0.4), WT + E2 (left: 2.9 ± 0.6, right: 3.3 ± 0.7), βERKO + E2 (left: 2.8 ± 0.5, right: 3.0 ± 0.8), WT + DPN (left: 2.2 ± 0.6, right: 2.5 ± 0.5), βERKO + DPN (left: 2.2 ± 0.3, right: 2.2 ± 0.3).
Administration of E2 or DPN to WT, but not βERKO, mice significantly increased the percentage of time spent investigating the displaced object (vs. the non-displaced object as a function of the total time exploring both objects during testing) over that of vehicle administered control mice (Figure 2). During the test period, there were main effects of genotype (F1,124 = 16.15, P< 0.01) and hormone condition (F2,124 = 4.12, P< 0.0.2), and an interaction between these variables to influence the percentage of time spent investigating the displaced object (F2,124 = 3.06, P< 0.05). The time mice spent with each object (mean ± sem in seconds) during testing is as follows: WT + vehicle (displaced: 3.8 ± 0.5, non-displaced: 4.9 ± 0.8), βERKO + vehicle (displaced: 3.0 ± 0.4, non-displaced: 5.0 ± 0.6), WT + E2 (displaced: 3.4 ± 0.4, non-displaced: 2.5 ± 0.5), βΒERKO + E2 (displaced: 4.0 ± 0.9, non-displaced: 5.7 ± 1.0), WT + DPN (displaced: 2.7 ± 0.4, non-displaced: 1.8 ± 0.3), βERKO + DPN (displaced: 2.7 ± 0.3, non-displaced: 2.6 ± 0.4).
Figure 2.
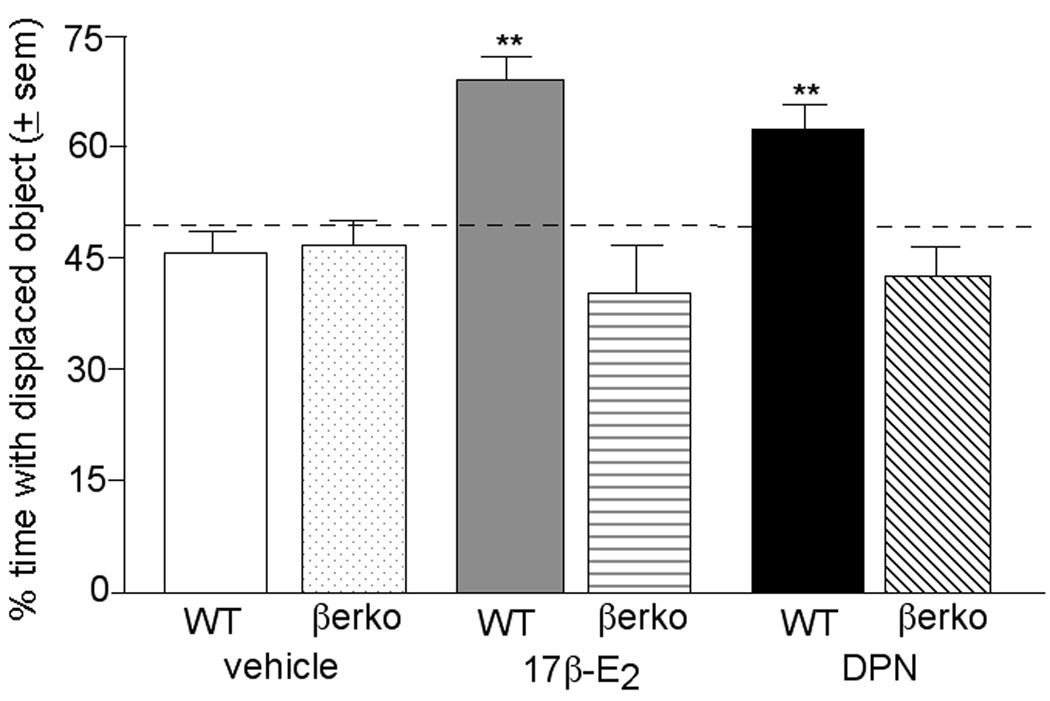
Mean (± sem) percentage of time spent exploring the displaced object by vehicle-administered WT or estrogen receptor β knockout (βERKO) mice, 17β-E2-administered WT or βERKO mice, or DPN-administered WT or βERKO mice. ** indicates a significant (P ≤ 0.05) interaction between genotype and hormone condition. Chance levels of responding are considered when mice spend 50% of the total exploration time investigating each object, as indicated by dashed line.
4. Discussion
Our hypothesis that actions at ERβ may be important for cognitive performance in hippocampally- and cortically-mediated cognitive tasks was supported. Administration of E2, or the ERβ selective SERM, DPN, significantly increased the percentage of time spent investigating the novel or displaced object over that of vehicle-administered WT mice. A similar pattern of effects for E2 and DPN were not seen in βERKO mice, despite E2 and DPN administration producing similar increases in 3α,5α-THP levels in the hippocampus. This implies that actions at ERβ may underlie the effects of E2 and/or DPN (and perhaps 3α,5α-THP) to enhance performance in these tasks.
The present findings that post-training E2 (0.1 mg/kg, SC) enhanced performance in the object recognition task confirm and extend previous research utilizing this behavioral assay. In the object recognition task, pre-training or post-training E2 (0.2 mg/kg) improved performance of young adult, ovx mice when they were tested 48-hrs later (Gresack and Frick, 2004, 2006b). Post-training injection of E2 also improved object recognition memory of young adult, ovx rats (Luine et al., 2003; Walf et al., 2006). Notably, these effects of E2 are observed with different E2 regimen, rodent species, and training/testing paradigms. Regarding the latter, in some object recognition protocols, mice are trained until they reach a set total investigation time, which ensures consistent duration of exposure to the objects across individuals during training (Gresack and Frick, 2004, 2006b). Given this longer time spent in contact with training stimuli, it is then possible to examine and demonstrate effects of E2, 24 to 48 hours later, when E2 levels are on the decline and may be less of a concern for effects on performance during the test phase. The protocol that we employ provides rodents the opportunity to investigate target stimuli for a fixed training phase duration (180 secs), which typically involves a much shorter duration of exposure to the stimuli during training than do these other approaches. Mice are then tested four hours later. This delay is utilized based upon previous findings from our laboratory demonstrating that this interval is sufficient to reveal effects of endogenous and/or post-training administered steroids (Frye et al., 2007; Walf et al., 2006; 2007). A shorter interval between training and testing produces E2 levels that are similarly elevated during consolidation and testing. However, the latter factor limits our interpretation of E2 to effects on cognitive performance, rather than learning/memory. In the present study, there were apparent differences in the time mice spent exploring the objects during training in the object recognition and object placement tasks, which would not be expected because order of exposure to these tasks was counter-balanced. Despite mice having these differences in time spent with objects during training before treatment, mice in the same treatment groups spent comparable duration with the objects during testing. To take into potential differences between groups in total time spent exploring both objects during testing (which were not observed in the present study), data during testing are analyzed as a function of time spent with the target object (i.e. novel object, displaced object) compared to both objects. Indeed, similar enhancements in performance were observed in both tasks following E2 or DPN administration to WT mice.
The object recognition and placement tasks were selected for use in this experiment because they provide multiple indices of hippocampal function and each task taps into different types of memory encoded by the hippocampus. The object placement task requires intact hippocampal function (Ennaceur et al., 1997). There is some controversy on the neural substrates for object recognition (Mumby, 2001), but performance in this task may involve both the hippocampus and the prefrontal cortex (Baker and Kim, 2002, Clark, Zola and Squire, 2000). E2 and DPN to WT mice similarly improved performance in both tasks in the present study. Considering the present findings that post-training E2 enhanced performance in both the object recognition and object placement tasks, in conjunction with previous findings that E2 enhances CA1 dendritic spine density (Frick et al., 2004, Woolley and McEwen, 1992, 1993), long-term potentiation (Foy et al., 1999, Warren et al., 1995), and neurogenesis in the dorsal hippocampus (Tanapat et al., 1999), effects of E2 on hippocampal structure and/or function may underlie some of the behavioral changes observed in the present experiment.
A lack of effect in βERKO mice may indicate that non-mnemonic processes may have interfered with their performance in this task (i.e. aberrant brain ontogeny or developmental differences or other compensatory processes associated with their mutation). Although βERKO mice can show increased anxiety and poorer cognitive function (Krezel, Dupont, Krust, Chambon, and Chapman, 2001; Rissman et al., 2002; Walf and Frye, 2006), we found that WT and βERKO mice similarly explored the novel stimuli utilized in both of these tasks. Given these effects, we do not expect that differences in motivation and/or neophobia underlies effects on performance in either the object recognition or object placement tasks utilized in this study. Thus, the lack of beneficial cognitive effects of E2 among βERKO mice in the object recognition and object placement task did not appear to be associated with baseline deficits.
Because of concerns regarding interpretation of studies relying exclusively on knockout mice, we also utilized the ERβ selective agonist, DPN, to test the hypothesis that ERβ is involved in object recognition and/or object placement performance. Post-training administration of DPN had effects similar to E2 to enhance performance in these tasks among ovx WT, but not βERKO, mice. Systemic administration of DPN has effects within 30 mins that are maintained for up to 12 hours to increase expression of nuclear ER (Lund et al., 2005). Furthermore, other studies have demonstrated that DPN can enhance performance in object recognition tasks of ovx rats (Walf et al., 2006). An important question is how ERβ may mediate some of the effects of E2 on performance. One possibility is that some of the effects of ERβ may be secondary to actions at ERα. Indeed, we (Frye et al., 2007) and others (Luine et al., 2003) have demonstrated that propyl-pyrazole-triol (PPT), which binds with 400-fold greater affinity for ERα and demonstrates minimal binding to ERβ (Stauffer, Coletta, Tedesaco, Nishiguchi, Carlson, Sun, Katzenellenbogen, Katzenellenbogen, 2000), can also enhance performance in the object placement task compared to vehicle or DPN. Similarly, post-training administration of E2 or coumestrol (a SERM with greater affinity for ERβ than ERα, but one that is not as specific for ERβ as DPN) enhanced performance in the inhibitory avoidance task of ovx rats, unlike DPN or vehicle post-training (Rhodes and Frye, 2006). Indeed, co-administration of DPN with 17α-E2 (which has greater affinity for ERα than ERβ) improved performance in this task (Rhodes and Frye, 2006). That there may be interactions between ERα and ERβ need to be investigated further (Toran-Allerand, 2004). In the present study, differences between WT mice administered E2 and DPN in either task were not observed, suggesting that residual effects of ERα were not likely. Furthermore, residual effects of ERβ were not supported as there was little evidence for differences between WT and βERKO mice administered E2. Future studies comparing effects of E2, ERβ- and ERα- SERMs may reveal the interactions between these isoforms for the functional effects observed.
There are a number of non-ER actions that should be considered. First, rapid, non-ER mediated effects of E2 have been observed (Mhyre and Dorsa, 2006; Sheldahl et al., 2007; Deecher, Swiggard, Frail, and O'Connor, 2003). Second, findings from in vivo and in vitro models have suggested the intriguing possibility is that actions of E2 at nuclear ER and via rapid membrane actions may integrate for their functional effects (Vasudevan et al., 2005) and this may be extended to effects on learning and memory processes. Third, the effects of 3α,5α-THP, which has trophic actions, neuroprotective effects in models of neural injury, and can enhance cognitive performance (Ciriza et al., 2004; Frye et al., 2007; Garcia-Estrada et al., 1993; He et al., 2004; Rhodes et al., 2004; Vongher & Frye, 1999; Walf et al., 2006), should also be considered. Indeed, E2 increases activity of metabolism enzymes necessary for 3α,5α-THP synthesis and can increase 3α,5α-THP levels in the hippocampus (Cheng & Karavolas, 1973; Frye & Rhodes, 2005; Vongher & Frye, 1999). The present data, that E2 and DPN increased hippocampal (but not cortical) levels of 3α,5α-THP and improved performance in tasks mediated by the hippocampus, substantiate further investigation of the role of 3α,5α-THP in some of the mnemonic effects observed in E2 and/or SERM-administered rodents. Thus, there are many possible ER and non-ER mechanisms that may underlie the mnemonic effects of E2.
In studies using aging rats, there is evidence of different underlying mechanisms for cognitive processes. For example, ER binding in the hippocampus declines among middle-aged female rats (Wilson, Rosewell, Kashon, Shughrue, Merchenthaler and Wise, 2002), yet E2 increases dentate spine density in aged rats (Miranda, Williams and Einstein, 1999), synaptophysin levels in middle-aged (Fernandez and Frick, 2004) and aged mice (Frick et al., 2002), and nerve growth factor levels in middle-aged mice (Fernandez and Frick, 2004). These findings imply that some cognitive effects of E2 in younger and aged individuals may involve different mechanisms. Given the potential beneficial role of E2 on cognition and the direct relevance for the aging population, the question of how E2 has actions for cognition will be the subject of much ongoing investigation by our lab and others.
Acknowledgments
This research was supported in part by grants from the National Science Foundation (IBN03-16083), National Institute of Mental Health (MH0676980), and Department of Defense (BC051001). The assistance of Kevin Manley in genotyping is appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: Effect on mood, cognition and quality of life. Neurobiology of Aging. 2006;27:141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning and Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Hormones and Behavior. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144:4734–7738. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. Journal of Neuroendocrinology. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. Journal of Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba Montoya DA, Carrer HF. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Research. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiology of Learning and Memory. 2004;82:142–149. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiology of Learning and Memory. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Day M, Good M. Ovariectomy-induced disruption of long-term synaptic depression in the hippocampal CA1 region in vivo is attenuated with chronic estrogen replacement. Neurobiology of Learning and Memory. 2005;83:13–21. doi: 10.1016/j.nlm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Deecher DC, Swiggard P, Frail DE, O'Connor LT. Characterization of a membrane-associated estrogen receptor in a rat hypothalamic cell line (D12) Endocrine. 2003;22:211–223. doi: 10.1385/ENDO:22:3:211. [DOI] [PubMed] [Google Scholar]
- Edwards DA. Induction of estrus in female mice: Estrogen-progesterone interactions. Hormones and Behavior. 1970;1:299–304. [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. Journal of the American Medical Association. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behavioral Neuroscience. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RE, Berger TW. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. Journal of Neurophysiology. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety, and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. European Journal of Neuroscience. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiology & Behavior. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. Journal of Neuroendocrinology. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiology of Learning and Memory. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O'Malley BW, Pfaff DW, Rhodes ME. Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology. 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Research. 2002;956:285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Research. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. Progesterone has rapid and membrane effects in the facilitation of female mouse sexual behavior. Brain Research. 1999;815:259–269. doi: 10.1016/s0006-8993(98)01132-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Effects of progesterone administration and APPswe+PSEN1<DELTA>e9 mutation for cognitive performance of mid-aged mice. Neurobiology of Learning and Memory. 2008;89:7–26. doi: 10.1016/j.nlm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Estrada J, Del rio JA, Luquin S, Soriano E, Garcia-Sequra LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain research. 1993;628:271–278. doi: 10.1016/0006-8993(93)90964-o. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Research. 2006a;1115:135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacology of Biochemistry and Behavior. 2006b;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restorative Neurology and Neuroscience. 2004;22:19–31. [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behavioral Neuroscience. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiology of Behavior. 1998;64:625–628. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behavioral Neuroscience. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and Behavior. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Janowsky J, Chan BKS, Nelson HD. Hormone replacement therapy and cognition. Systematic review and meta-analysis. Journal of the American Medical Association. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Lund TD, West TW, Tian LY, Bu LH, Simmons DL, Setchell KD, Adlercreutz H, Lephart ED. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neuroscience. 2001;2:20. doi: 10.1186/1471-2202-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory:Relevance to alterations in the estrous cycle. Journal of Neuroscience. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proceedings of the National Academy of Science U S A. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. Journal of Medicinal Chemistry. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor α and estrogen receptor β in neurons and glia. Journal of Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. Journal of Neuroscience. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behavioral Brain Research. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Nofrey BS, Ben-Shahar OM, Brake WG. Estrogen abolishes latent inhibition in ovariectomized female rats. Brain and Cognition. 2007 doi: 10.1016/j.bandc.2007.06.003. (in press) [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997;8(14):3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Quinlan MG, Graffe N, Duncan A, Brake WG. Postpubertal but not prepubertal rats show sex differences in latent inhibition. Society for Neuroscience Abstracts. 2006 [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass MLS, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. The Women's Health Initiative Memory Study: A randomized controlled trial. Journal of the American Medical Association. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. 47. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic-enhancing effects in the inhibitory avoidance task. Pharmacology, Biochemistry, and Behaviour. 2004;78:551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERβ-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiology of Learning and Memory. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, McCormick CM, Frye CA. 3α,5α-THP mediates progestins’ effects to protect against adrenalectomy-induced cell death in the dentate gyrus of female and male rats. Pharmacology, Biochemistry, and Behaviour. 2004;78:505–512. doi: 10.1016/j.pbb.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor β gene impairs spatial learning in mice. Proceedings of the National Academy of Science U S A. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D, Hutt DM. Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. In: International Atomic Energy Agency, editor. Symposium on Radioimmunoassay and Related Procedures in Medicine. New York: Uniput; 1974. pp. 209–233. [Google Scholar]
- Sheldahl LC, Marriott LK, Bryant DM, Shapiro RA, Dorsa DM. Neuroprotective effects of estrogen and selective estrogen receptor modulators begin at the plasma membrane. Minerva Endocrinology. 2007;32:87–94. [PubMed] [Google Scholar]
- Sherwin BB. The clinical relevance of the relationship between estrogen and cognition in women. The Journal of Steroid Biochemistry and Molecular Biology. 2007;106:151–156. doi: 10.1016/j.jsbmb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. Journal of the American Medical Association. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-β mRNA in forebrain regions of the estrogen receptor-α nockoutmouse. Endocrinology. 1997;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-β mRNA and estrogen receptor-α immunoreactivity in neurons of the rat forebrain. Endocrinolog. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. Journal of Comparative Neurology. 2001;436:64–81. [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. Journal of Medicinal Chemistry. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. Journal of Neuroscience. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff DW. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacology, Biochemistry, and Behaviour. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Research. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Frye CA. Adult female wildtype, but not estrogen receptor β knockout, mice have decreased depression-like behavior during proestrus and following administration of estradiol or diarylpropionitrile. Journal of Psychopharmacology. 2007 doi: 10.1177/0269881108089598. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiology of Learning and Memory. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behavioral Brain Research. 2004;155:45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) gene expression in specific regions of the rat brain. Mechanisms of Ageing and Development. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Science U S A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal of Neuroscience. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. Journal of Comparative Neurology. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: Effects on cognitive function and dementia. Journal of the American Medical Association. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]


