Abstract
The mesolimbic dopaminergic system, originating from the ventral tegmental area (VTA) is implicated in the rewarding properties of ethanol. VTA dopaminergic neurons are under the tonic control of GABAergic innervations. Application of GABAergic agents changes ethanol consumption. However, it is unclear how acute ethanol modulates GABAergic inputs to dopaminergic neurons in the VTA. This report describes ethanol at clinically relevant concentrations (10–40 mM) dually modulates inhibitory postsynaptic currents (IPSCs). IPSCs were mediated by GABAA receptors and were recorded from VTA dopaminergic neurons in acute midbrain slices of rats. Acute application of ethanol reduced the amplitude and increased the paired pulse ratio of evoked IPSCs. Ethanol lowered the frequency but not the amplitude of spontaneous IPSCs. Nevertheless, ethanol had no effect on miniature IPSCs recorded in the presence of tetrodotoxin. These data indicate that ethanol inhibits GABAergic synaptic transmission to dopaminergic neurons by presynaptic mechanisms, and that ethanol inhibition depends on the firing of GABAergic neurons. Application of CGP 52432, a GABAB receptor antagonist did not change ethanol inhibition of IPSCs. Tyr-D-Ala- Gly-N-Me-Phe-Gly-ol enkephalin (DAMGO), a µ-opioid receptor agonist, conversely, silenced VTA GABAergic neurons and inhibited IPSCs. Of note, in the presence of saturating concentration of DAMGO (3 µM), ethanol potentiated the remaining IPSCs. Thus, ethanol dually modulates GABAergic transmission to dopaminergic neurons in the VTA. Ethanol modulation depends on the activity of VTA GABAergic neurons, which were inhibited by the activation of µ-opioid receptors. This dual modulation of GABAergic transmission by ethanol may be an important mechanism underlying alcohol addiction.
Keywords: mesolimbic system, drug addiction, postsynaptic current
INTRODUCTION
The mesolimbic dopaminergic (DA) system, originating from the ventral tegmental area (VTA) is implicated in the rewarding effects of ethanol (Imperato and Di Chiara, 1986, Ikemoto et al., 1997a, Nowak et al., 1998). Ethanol facilitates the firing of VTA DA neurons both in vivo (Imperato and Di Chiara, 1986) and in vitro (Brodie et al., 1990, Di Chiara and North, 1992, Brodie et al., 1999, Ye et al., 2006, Xiao et al., 2007).
VTA DA neurons are under tonic control of GABAergic inputs, which are from medium spiny neurons of the nucleus accumbens (NAcc) (Waddington and Cross, 1978, Kalivas et al., 1993) and the ventral pallidum (VP) (Kalivas et al., 1993), but the primarily inhibitory regulation is from collaterals of VTA GABAergic neurons (Johnson and North, 1992a). Blockade of GABAergic innervation enhances the activity of VTA DA neurons (Johnson and North, 1992a, Johnson and North, 1992b, Ye et al., 2004, Xiao et al., 2007), and causes burst firing in VTA DA neurons (Kitai et al., 1999). Previous in vivo studies on rodents demonstrated that VTA GABAergic neurons control ethanol consumption (Nowak et al., 1998, Gallegos et al., 1999, Koob, 2004, Stobbs et al., 2004, Besheer et al., 2006). In a recent study in midbrain slices, we found that ethanol-induced excitation of VTA DA neuron is significantly attenuated by an antagonist of GABAA receptors (Xiao et al., 2007). This result implicates that ethanol can excite VTA DA neurons indirectly, through inhibition of VTA GABAergic neurons, in addition to its direct excitatory action (Brodie et al., 1990, Brodie et al., 1999).
The µ-opioid receptors (MORs) in VTA are mostly expressed in VTA GABAergic neurons (Mansour et al., 1995, Steffensen et al., 1998, Garzon and Pickel, 2001). Activation of MORs hyperpolarizes and inhibits VTA GABAergic neurons (Di Chiara and North, 1992, Johnson and North, 1992a, Margolis et al., 2003). Several lines of evidence indicate that MORs are involved in ethanol-induced excitation of VTA DA neurons: (1) ethanol enhances the release of β-endorphin in several brain regions, which activates MORs (Stein, 1993, Herz, 1997, Marinelli et al., 2004); (2) Naloxone, an opioid receptor antagonist, greatly attenuates ethanol-induced inhibition of VTA GABAergic neurons (Xiao et al., 2007); (3) Both naloxone and MOR agonist attenuated the effect of ethanol on VTA DA neurons (Xiao et al., 2007).
Numerous evidence supports that potentiation of GABAergic synaptic transmission is of great importance for the behavioral and cognitive effects of ethanol (Mihic, 1999, Siggins et al., 2005, Weiner and Valenzuela, 2006). However, previous in vitro studies of ethanol on inhibitory postsynaptic currents (IPSCs) in several brain regions generated controversial results (Siggins et al., 2005, Weiner and Valenzuela, 2006). Moreover, the effects of ethanol on GABAergic synaptic transmission to VTA DA neurons are unknown. In the present study, we asked whether and how ethanol affects GABAergic IPSCs in DA neurons in VTA. We found that ethanol increases IPSCs when it was applied alone. However, ethanol enhanced the remaining IPSCs when VTA GABAergic neurons were fully depressed by a MOR agonist.
EXPERIMETNAL PROCEDURES
All experiments were performed in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey. The experiments were performed on Sprague-Dawley rats aged 14 to 28 postnatal (P) days.
Slice preparation
The midbrain slices were prepared as described previously (Ye et al., 2006). Animals were anesthetized and then sacrificed by decapitation. The brain was removed and a midbrain block (containing the VTA) was isolated. It was glued to the cutting stage of a VF-200 slicer (Precisionary Instruments Inc., Greenville, NC). While the brain was kept in ice-cold glycerol-based artificial cerebrospinal fluid (GACSF) – containing (in mM) 252 glycerol, 1.6 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 18 NaHCO3, and 11 glucose, and oxygenated with 95%O2/5%CO2 -- 250–300 µm thick slices were cut in the coronal plane (Ye et al., 2006). The slices (two per animal) were allowed to recover for at least 1 hr in a holding chamber in regular ACSF, which has the same composition as GACSF, except that glycerol was replaced with 126 mM NaCl.
Electrophysiological recording in midbrain slices
Cells in midbrain slices were visualized with an upright microscope (E600FN, Nikon, Tokyo) and near-infrared illumination. Electrical signals were obtained in whole-cell patch clamp technique with MultiClamp 700A amplifiers (Molecular Devices Co., Union City, CA, USA), a Digidata 1320A A/D converter (Molecular Devices Co.) and pCLAMP 9.2 software (Molecular Devices Co.). Data were filtered at 2 kHz and sampled at 5 kHz.
When filled with internal solutions, the patch electrodes had a resistance of 3 – 5 MΩ. Whole-cell currents were recorded with a CsF-based pipette solution containing (in mM): 135 CsF, 5 CsCl, 5 EGTA, 0.5 CaCl2, 10 HEPES, 2 Mg-ATP, and 0.1 GTP. Whole-cell voltages were recorded with a K-gluconate-based pipette solution. This solution has the some composition as CsF-based solution except that CsF was replaced with 135 mM K-gluconate, and CsCl with 5 mM KCl. The pH of pipette solutions was adjusted to 7.2 with tris-base, and the osmolarity to 280 – 300 mOsm with sucrose. A single slice was transferred into the 0.4 ml recording chamber where it was stabilized by a platinum ring. Throughout the experiments, the bath was continually perfused with ACSF (1.5 – 2.0 ml/min).
All IPSCs were recorded at a holding potential of 0 mV. To evoke monosynaptic IPSCs (eIPSCs), a glass stimulating electrode, filled with ACSF, was placed 50 – 100 µm away from the recorded VTA neuron. Electrical stimuli (100 – 200 µs in duration) were applied once every 20 s. An input/output curve was obtained near the start of recording, and the stimulation was set at 20 – 30% of maximum, an intensity that evoked stable responses with no failures. Paired eIPSCs were elicited with identical double stimuli at an interval of 50 ms. Most recordings were performed at 32 °C.
Unless indicated, all recordings were obtained from putative DA neurons identified by their pharmacological and physiological properties. Specifically, spontaneous firing of VTA neurons was first recorded with the loose-patch cell attached configuration. The depression of spontaneous firing by 0.2 µM quinpirole (QP), and facilitation by 1 µM DAMGO (probably owing to DAMGO-induced disinhibition) are characteristic features of VTA DA neurons (Johnson et al., 1992; Johnson and North, 1992; Margolis et al., 2003). Quinpirole is a dopamine D2/D3 receptor agonist. DAMGO is a µ-opioid receptor agonist. Further suction changed the recording to the whole-cell configuration. A prominent inward current (Ih) activated by hyperpolarizing voltage steps (between −60 and −160 mV) or a corresponding voltage-sag in response to a hyperpolarizing current pulse (−100 pA) (Lacey et al., 1989) confirmed the identity of putative DA neurons. The identification of VTA DA neuron by the responses to QP and the expression of Ih has been challenged (Margolis et al., 2006). However, we believe that in the population of neurons reported in this study (except the GABA neuron shown in Fig. 4A), (1) there were no GABAergic neurons because VTA GABAergic neurons have no prominent Ih (Jones and Kauer, 1999, Ibanez-Sandoval et al., 2006, Lee and Tepper, 2007) and they are inhibited by µ-opioid receptor agonists (Margolis et al., 2003), and (2) all the recorded VTA neurons were probably dopaminergic because in VTA, DA neurons are uniquely excited by DAMGO, via a mechanism of disinhibition (Margolis et al., 2003, Xiao et al., 2007).
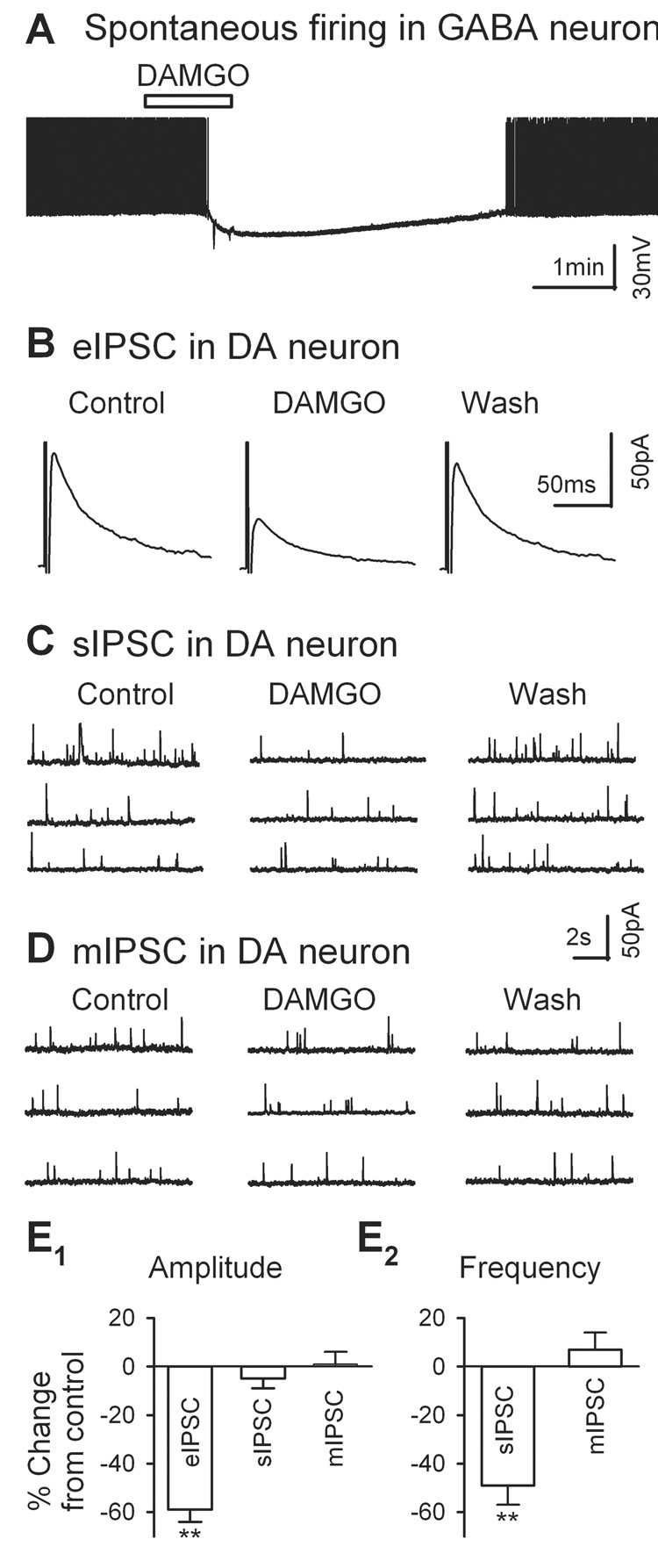
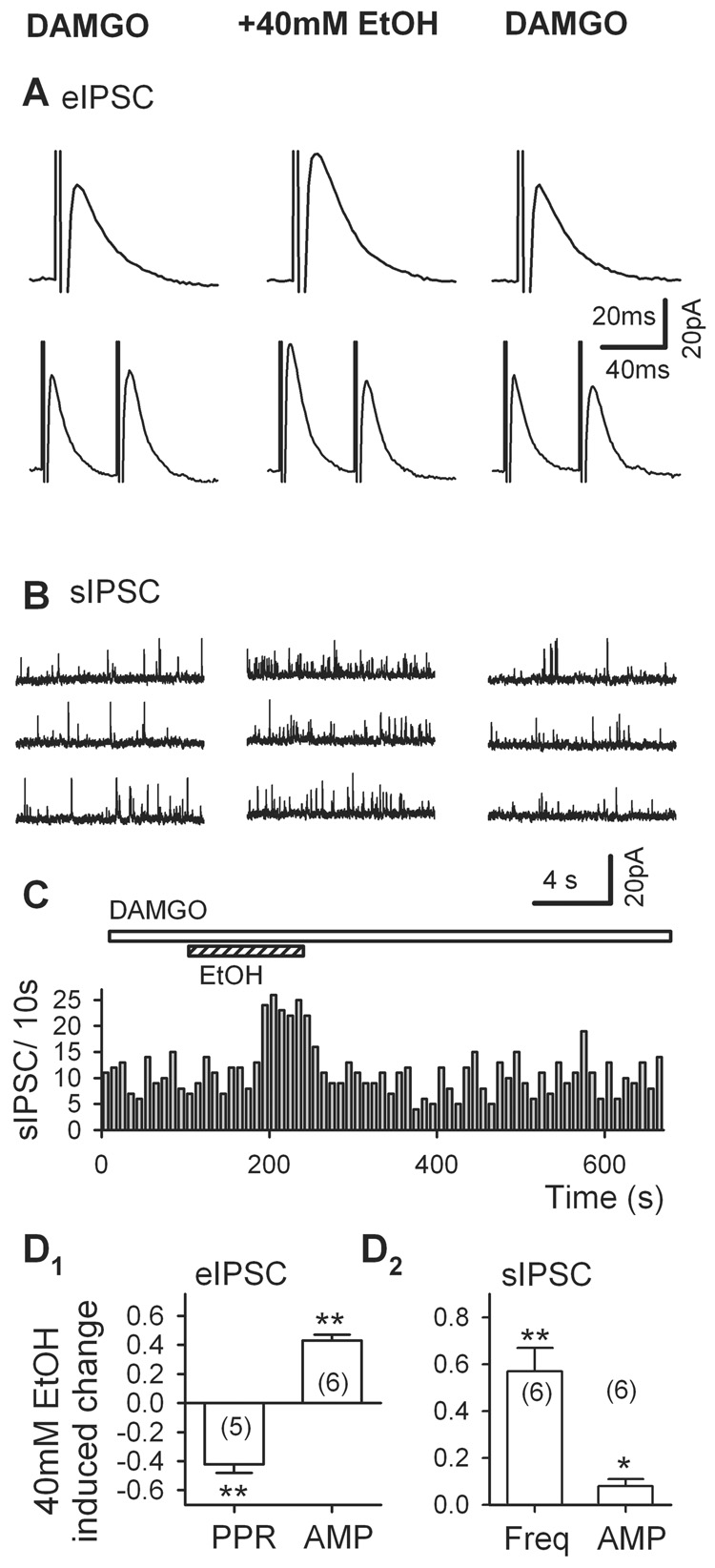
Fig. 4.
DAMGO (µ-opioid receptor agonist) inhibits IPSCs in VTA DA neurons. A, 3 µM DAMGO hyperpolarized and silenced the spontaneous firings of a putative GABAergic neuron in the VTA in a midbrain slice. B, 3 µM DAMGO suppressed a large portion of eIPSCs, recorded from a putative DA neurons in the VTA. C, DAMGO (3 µM) prominently inhibits sIPSC frequency. D, DAMGO (3 µM) did not change mIPSC frequency. E1–2, Summary of the effect of DAMGO on the amplitude (E1) of eIPSCs, sIPSCs, and mIPSCs and the frequency (E2) of sIPSC and mIPSC. Histograms show mean % changes (± SEM). ** p < 0.01, paired t test for DAMGO application vs. pre-DAMGO control.
Chemicals and applications
Most of the chemicals, including bicuculline, Tyr-D-Ala-Gly-N-Me-Phe-Glyol enkephalin (DAMGO), tetrodotoxin (TTX), DL-2-amino-5-phosphono-valeric acid (APV), 6,7-dinitroquinoxaline-2, 3-dione (DNQX), and (−)-Quinpirole hydrochloride (QP) were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). 3-[[(3, 4-dichlorophenol) methyl] amino] propyl] diethoxymethyl) phosphinic acid (CGP 52432) was from TOCRIS Bioscience (Ellisville, MO, USA). Ethanol (95% v/v, prepared from grain) was from Pharmco (Brookfield, CT, USA) and stored in glass bottles. Chemicals were added in known concentrations to the superfusate.
Data analysis
Spontaneous discharges and inhibitory postsynaptic currents (sIPSCs) were counted and analyzed with Clampfit 9.2 (Molecular Devices Co). For each experimental condition, we averaged the amplitudes of evoked IPSCs or paired pulse ratio (PPR = IPSC2 / IPSC1) in 10 – 20 traces. IPSC1 and IPSC2 are the IPSCs in response to the first and second stimulus of the paired pulses, respectively. In the figures, we showed averaged single or paired eIPSCs. Spontaneous IPSCs or miniature IPSCs (mIPSCs) were screened automatically (5 pA amplitude threshold), checked visually, and accepted or rejected according to their rise and decay times. Cumulative probability plots of the incidence of various inter-event intervals and amplitudes (for 100 – 1500 sIPSCs), recorded under different conditions from the same neuron, were compared with the Kolmogorov-Smirnov (K – S) test. For other plots, data obtained over a 1 – 2 min period at the peak of a drug response were normalized to the average values of the frequency and amplitude of sIPSCs or mIPSCs during the initial control period (4 – 5 min). Data were expressed as means (± SEM). The statistical significance of drug effects was assessed by a paired two-tailed t test on normalized data. Values of p < 0.05 were considered significant.
RESULTS
Ethanol depresses GABAA-receptor-mediated inhibitory postsynaptic currents (IPSCs) of VTA DA neurons
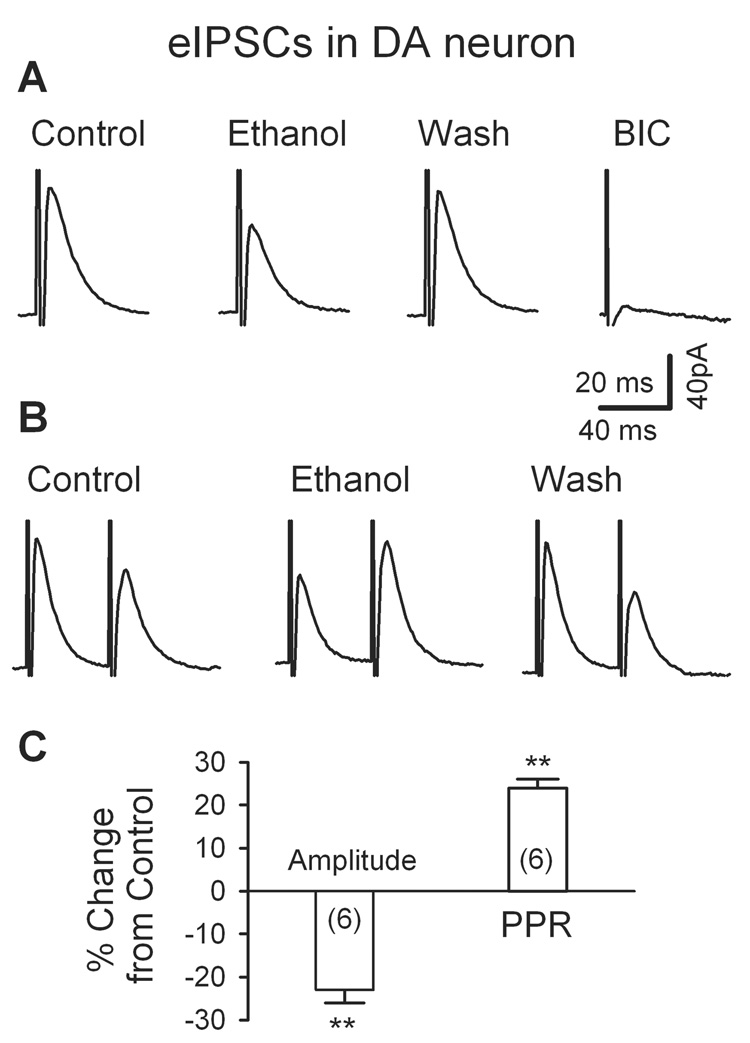
Monosynaptic IPSCs (eIPSCs) were evoked in the presence of APV (50 µM) and DNQX (20 µM) at a holding potential of 0 mV; their suppression by bicuculline (BIC, 10 µM; Fig. 1A) confirmed that they were mediated by GABAA receptors. As illustrated in Fig. 1A and C, ethanol (40 mM) significantly and reversibly diminished the peak amplitude of eIPSCs (by 23 ± 3%, n = 6, p < 0.001). To determine whether ethanol inhibits GABAergic synaptic transmission via a pre- or a post-synaptic mechanism, we recorded eIPSCs in response to a pair of stimuli separated by an interval of 50 ms and calculated paired pulse ratio (PPR = IPSC2/IPSC1) (Fig. 1B). It is well documented that if a modulator modifies PPR, it probably acts through a presynaptic mechanism (Debanne et al., 1996). An inhibition of transmitter release would depress the current (PSC1) in response to the first stimulus, while relatively enhance the current (PSC2) elicited by the second stimulus, thus increase PPR. As illustrated in Fig. 1B, 40 mM ethanol reduced the amplitude of the IPSC1, but potentiated the amplitude of IPSC2, which led to an increase of 24 ± 2% in the PPR (from 0.94 ± 0.08 to 1.17 ± 0.11, n = 6, p < 0.01; Fig. 1C). These results indicate that the effect of ethanol on eIPSCs involves a presynaptic mechanism.
Fig. 1.
Ethanol depresses evoked monosynaptic IPSCs (eIPSCs) in putative DA neurons in midbrain slices. A, IPSCs evoked by stimulation within the VTA was reduced by 40 mM ethanol and virtually abolished by 10 µM bicuculline (BIC). Data are averages of 10 traces. B, 40 mM ethanol significantly reduced the first, but enhanced the second of a pair of IPSCs evoked by paired stimuli (at 50 ms interval), thus increasing the paired pulse ratio (PPR). C, Summary of the effects of ethanol on the amplitude and the PPR of eIPSCs. For all figures, numbers in brackets are numbers of recorded neurons. ** p < 0.01, paired t test for ethanol application vs. pre-ethanol control. All IPSCs were recorded from putative DA neurons at a VH of 0 mV with CsF- containing pipette solution, in the presence of APV (50 µM) and DNQX (20 µM).
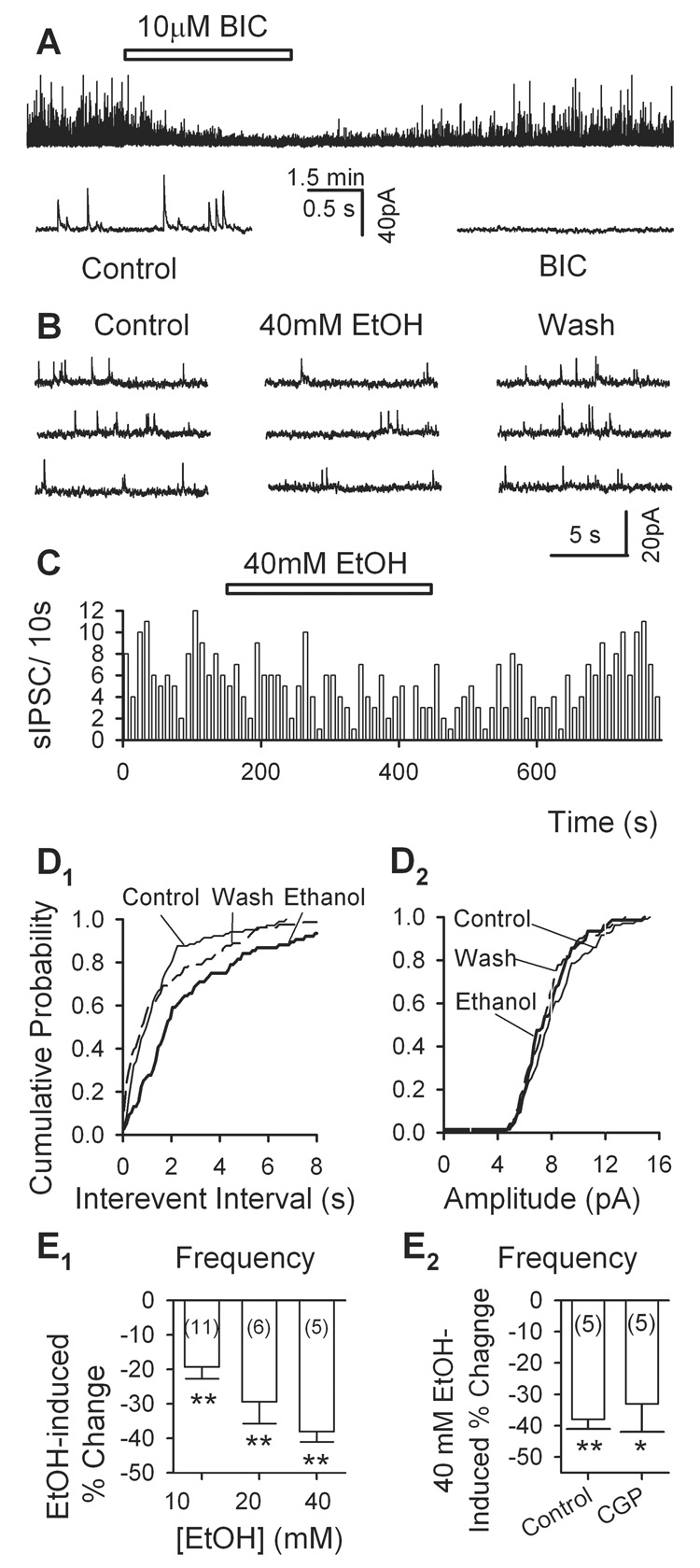
A second set of observations also favored the presynaptic action of ethanol. All the spontaneous IPSCs (sIPSCs) recorded in these experiments – in the presence of APV (50 µM) and DNQX (20 µM) at a holding potential of 0 mV--were eliminated by bicuculline (10 µM) and therefore mediated by GABAA receptors (Fig. 2A). Ethanol (40 mM) reversibly lowered the frequency of sIPSCs (Fig. 2B, C). This is confirmed by a significant rightward shift of the cumulative probability plot of inter-event intervals (K-S test, p < 0.001, Fig. 2D1). In 5 DA neurons, 40 mM ethanol decreased sIPSC frequency by 38 ± 3% (n = 5, p < 0.001; Fig. 2D2). However, 40 mM ethanol did not change sIPSC amplitude (by 1 ± 3%, n = 5, p > 0.05). As shown in Fig. 2E1, 10 and 20 mM ethanol also significantly reduced sIPSC frequency by 19.3 ± 3.4% (n = 11, p <0.01) and 29.4 ± 6.3% (n = 6, p < 0.01), respectively. These data indicate that like VTA GABAergic neurons (Xiao et al., 2007), GABAergic terminals which make synapses onto VTA DA neurons are very sensitive to ethanol.
Fig. 2.
Ethanol decreases the frequency of spontaneous IPSCs (sIPSCs) recorded in VTA DA neurons. A, sIPSCs were abolished by 10 µM bicuculline (BIC). B, 40 mM ethanol reduced the frequency of sIPSCs. C, time course of ethanol suppression of sIPSC frequency in one cell. D, cumulative probability plots confirm that ethanol sharply reduced sIPSC frequency (D1) but not the amplitude (D2). E1–2, Summary of the dose-response relationship of ethanol inhibition of sIPSC frequency (E1) and the effect of 40 mM ethanol on sIPSC frequency in the absence (Control) and presence (CGP) of 1 µM CGP 52432 (E2). * p < 0.05, ** p < 0.01, paired t test for ethanol application vs. pre-ethanol control.
Presynaptic GABAB receptors did not contribute to the effect of ethanol on sIPSCs
Most GABAergic synapses have presynaptic GABAB receptors which autoregulate GABA release (Misgeld et al., 1995). In hippocampal slices, ethanol enhances GABA release and facilitates the function of presynaptic GABAB receptors, which in turn inhibits GABA release (Ariwodola and Weiner, 2004). It is possible that, in analogue to what happened in the hippocampus, ethanol may enhance presynaptic GABAB receptor function, which leads to the inhibition of GABA release onto VTA DA neurons. We therefore compared the effects of ethanol on sIPSCs in VTA DA neurons in the absence and the presence of CGP 52432 (CGP, 1 µM), a GABAB receptor antagonist. In the presence of CGP 52432, 40 mM ethanol significantly lowered sIPSC frequency by 33 ± 9% (n = 5, p = 0.02, Fig. 2E2), which is comparable to its effect on sIPSCs in the absence of CGP52432 (38 ± 3%, n = 5, Fig. 2E2) (p = 0.30, t-test). This result suggests that GABAB receptors did not play a major role in ethanol inhibition of sIPSCs in VTA DA neurons.
Ethanol does not alter miniature IPSCs (mIPSCs) of VTA DA neurons
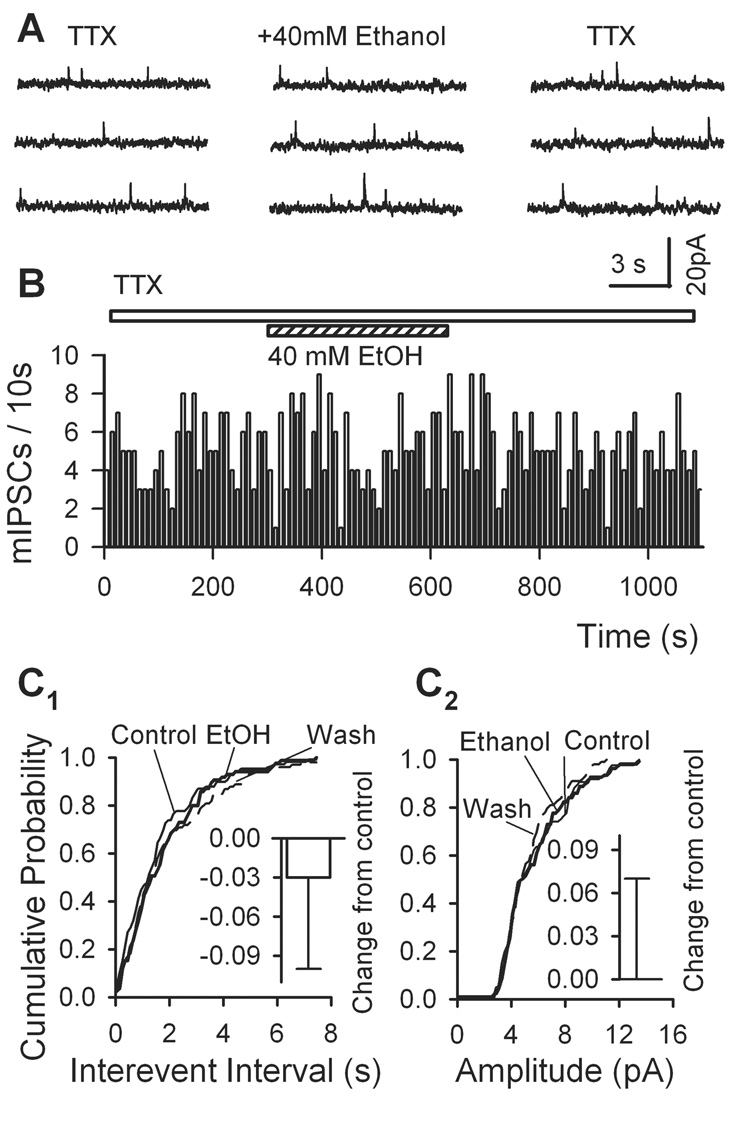
We next tested the effects of ethanol on the spontaneous IPSCs when action potentials were blocked by tetrodotoxin (TTX). TTX (1 µM) suppressed sIPSC frequency by 45 ± 2% (from 1.80 ± 0.60 Hz to 1.02 ± 0.35 Hz, n = 7, p = 0.00002) without altering their mean amplitude (15.2 ± 1.7 pA vs. 16.4 ± 1.5 pA, n = 7, p = 0.37) (not illustrated). It indicates that a considerable portion of sIPSCs are not action potential-dependent, which is so-called mIPSCs. In contrast to its effect on sIPSCs, 40 mM ethanol did not change the frequency and the amplitude of mIPSCs (Fig. 3A, B). This was confirmed by typical cumulative probability plots of intervals between two successive mIPSCs (Fig. 3C1) and amplitude of mIPSCs (Fig. 3C2). In 9 neurons, 40 mM ethanol altered neither frequency nor amplitude of mIPSCs (frequency: by −3 ± 7%, n = 9, p > 0.05; amplitude: by 0 ± 7%, n = 9, p > 0.05; Fig. 3C1, C2). These results indicate that the action of ethanol is linked to TTX-sensitive sodium channels.
Fig. 3.
Ethanol has no effect on mIPSCs in VTA DA neurons. mIPSCs were recorded in the presence of 1 µM tetrodotoxin (TTX). Typical traces (A) and time course (B) showed that ethanol had no effect on mIPSC frequency. Ethanol did not change the cumulative probability of inter-event intervals (C1) and amplitude (C2) for mIPSCs. Insets show mean % changes (± SEM, n = 9 neurons) induced by 40 mM ethanol.
DAMGO inhibits GABAergic IPSCs of VTA DA neurons
Numerous studies demonstrate the presence of MORs in the VTA (Mansour et al., 1994, Mansour et al., 1995, Garzon and Pickel, 2001). In the VTA, majority of MORs express on the somata and dendrites of non-dopaminergic neurons, and only 8% of them locate on axonal terminals, which formed inhibitory synapses (Dilts and Kalivas, 1989, Garzon and Pickel, 2001). It suggests that the effect of MORs on inhibitory inputs to VTA DA neurons might be largely mediated by VTA GABAergic neurons. As illustrated in Fig. 4A, bath perfusion of 3 µM DAMGO, a MOR agonist, hyperpolarized the membrane potential and blocked the ongoing discharge of a putative VTA GABAergic neuron in a midbrain slice, in keeping with previous studies (Johnson and North, 1992a, Margolis et al., 2003).
DAMGO (3 µM) also strongly depressed eIPSCs (Fig. 4B) recorded from DA neurons in brain slices by 59 ± 5% (from 88 ± 15 pA in control to 34 ± 6 pA in DAMGO, n = 11, p = 0.001, Fig. 4E1). As illustrated in Fig. 4C, DAMGO (3 µM) reversibly inhibited sIPSCs. In 7 cells, DAMGO (3 µM) significantly decreased sIPSC frequency by 49 ± 8% (0.43 ± 0.09 Hz in DAMGO vs. 1.09 ± 0.37 Hz in control, n = 7, p = 0.001, Fig. 4E2). By contrast, DAMGO (3 µM) did not alter the amplitude of sIPSCs, which remained at 95 ± 4% of control (in control, 21.3 ± 2.6 pA; in DAMGO, 19.8 ± 2.7 pA, n = 7, p = 0.25; Fig. 4E1). The DAMGO-induced inhibition was reversible: eIPSCs and sIPSCs recovered to control levels after washout of DAMGO.
Next, we tested whether DAMGO (3 µM) inhibits GABAergic synaptic transmission through silencing of VTA GABAergic neurons or / and direct hyperpolarization of GABAergic terminals. As illustrated in Fig. 4D, unlike sIPSCs, mIPSCs were not altered by DAMGO. In the presence of 3 µM DAMGO, mIPSC frequency was 107 ± 7% of control (in control, 0.42 ± 0.09 Hz; in DAMGO, 0.44 ± 0.09 Hz; n = 6, p = 0.2, Fig. 4E2), and mIPSC amplitude was 102 ± 5% of control (in control, 19.0 ± 3.2 pA; in DAMGO, 19.2 ± 3.2 pA, n = 7, p = 0.77, Fig. 4E1). These results suggest that DAMGO inhibits GABAergic IPSCs probably by silencing the VTA GABAergic neurons.
Ethanol enhanced evoked and spontaneous IPSC in the presence of DAMGO
Acute ethanol increases the release of β-endorphin, an endogenous ligand for MORs in the rat brain (Stein, 1993, Herz, 1997, Marinelli et al., 2004). Our recent study supports the postulation that ethanol might inhibit the activity of VTA GABAergic neurons through the activation of postsynaptic MORs following its enhancement of β-endorphin release (Xiao et al., 2007). Therefore, the inhibition of GABAergic IPSCs by ethanol might also be mediated by the increase of β-endorphin release and activation of postsynaptic MORs on VTA GABAergic neurons. To further test this hypothesis, we examined the effect of ethanol on the remaining evoked and spontaneous IPSC in the presence of DAMGO (3 µM). As illustrated in Fig. 5, in the presence of DAMGO (3 µM), 40 mM ethanol prominently increased the amplitude of evoked IPSC by 43 ± 4% (from 44 ± 13 pA in DAMGO to 64 ± 19 pA in DAMGO + ethanol, n = 6, p = 0.013, Fig. 5D, Ampl), and decreased the PPR by 42 ± 6% (from 1.61 ± 0.28 in DAMGO to 0.92 ± 0.16 in DAMGO + ethanol, n = 5, p = 0.008, Fig. 5D, PPR). In the presence of 3 µM DAMGO, 40 mM ethanol prominently increased sIPSC frequency by 57 ± 10% (n = 6, p = 0.006, Fig. 5E, Freq), whereas the amplitude of sIPSCs in 40 mM ethanol and DAMGO was 108 ± 3% (n = 6, p = 0.02, Fig. 5E, Ampl) of that in DAMGO alone. Note that although the effect of ethanol on sIPSC amplitude (<10%) is statistically significant, it is probably not significant in physiological function; moreover, this effect is much weaker than its effect on sIPSC frequency (57%). These results indicate that when the activity of VTA GABAergic neuron was suppressed, ethanol enhanced GABA transmission primarily via presynaptic mechanisms.
Fig. 5.
Ethanol facilitates IPSCs in the presence of DAMGO. All IPSCs were recorded from putative DA neurons in the VTA in slices in the presence of 3 µM DAMGO. A, 40 mM ethanol enhances eIPSC amplitude (upper traces), and decreases paired pulse ratio (PPR, lower traces). B, typical traces (B) and time course (C) showed that 40 mM ethanol increased the frequency of sIPSCs. D, Summary of aforementioned effects of ethanol on eIPSC (D1) and sIPSC (D2). ** p < 0.01, paired t test for ethanol application vs. pre-ethanol control.
DISCUSSION
Ethanol, at clinically relevant concentrations (10–40 mM) strongly suppressed action potential-dependent GABAergic synaptic transmission onto VTA DA neurons in acute midbrain slices of rats. DAMGO, a MOR agonist mimicked ethanol-induced inhibition of GABAergic transmission. Due to the fact that MORs are mostly expressed on the soma and dendritic area of GABAergic neurons in VTA, this result suggests that ethanol inhibition is probably resulted from suppression of GABAergic neurons in VTA. In addition, ethanol facilitated GABAergic transmission in the presence of DAMGO, indicating that ethanol enhances GABAergic transmission from sources other than the GABAergic neurons in VTA. The results of this study provide strong evidence that the GABAergic transmission in VTA is among the major targets of ethanol.
Ethanol suppresses GABA-mediated IPSCs in VTA DA neurons
The GABAergic synapse plays pivotal roles in mediating many behavioral consequences of acute and chronic ethanol exposure (Koob, 2004, Follesa et al., 2006). Ethanol effects on GABAergic transmission have been intensively studied for decades. Ethanol alters GABAergic synaptic transmission including the postsynaptic GABAA receptors (Mihic, 1999, Follesa et al., 2006, Wallner et al., 2006, Glykys et al., 2007) and the presynaptic machinery of GABA release in several brain regions, such as cerebellum (Carta et al., 2004), amygdala (Roberto et al., 2003, Nie et al., 2004, Zhu and Lovinger, 2006), and hippocampus (Ariwodola and Weiner, 2004, Li et al., 2006).
The mesolimbic dopaminergic system originating from VTA is implicated in the processes of reward (Ikemoto et al., 1997a, Ikemoto et al., 1997b). There are plenty of GABAergic terminals synapsing onto the soma or dendrites of VTA DA neurons (Waddington and Cross, 1978, Johnson and North, 1992a, Kalivas et al., 1993). These GABAergic inputs regulate the activity of VTA DA neurons and consequently alcohol consumption. Indeed, previous in vivo studies have demonstrated that microinjection of GABAA antagonist into the VTA increases the excitability of VTA DA neurons and the extracellular dopamine levels in NAcc, and animals will self-administer GABAA antagonist into VTA (Gessa et al., 1985, Ikemoto et al., 1997a, Ikemoto et al., 1997b), and this reduces ethanol intake (Nowak et al., 1998), probably because of their similar effects on NAcc dopamine level (Imperato and Di Chiara, 1986). Therefore, GABAergic transmission in the VTA might be a crucial target of ethanol. Note that although the GABAergic neurons that inhibit DA neurons in VTA were originally suggested as interneurons (Johnson and North, 1992b), later studies in substantial nigra (Tepper et al., 1995) and in VTA (Steffensen et al., 1998) identified these GABAergic neurons are either predominantly or exclusively projection neurons. Therefore, the VTA GABAergic neurons described in the current study are probably projection neurons.
In keeping with previous studies (Melis et al., 2002, Ye et al., 2004), we readily recorded bicuculline sensitive IPSCs from VTA DA neurons. Ethanol sharply lowered the frequency but not the amplitude of sIPSCs, the frequency or the amplitude of mIPSCs. Hence, the reduced sIPSC frequency can be ascribed mainly to the suppression of GABA neuronal firing (Xiao et al., 2007). We did not detect any significant effects of ethanol (≤ 40 mM) on the amplitude of sIPSCs and mIPSCs. This result is in agreement with previous studies which show that most synaptic GABAA receptor are insensitive to ethanol (Wallner et al., 2006).
On the other hand, ethanol reduced the amplitude of eIPSCs and increased their PPR, which suggests a depression of GABA release from the terminals. Yet ethanol had no effect on mIPSCs. Perhaps ethanol affects only some synaptic vesicles, in keeping with a recent report that different pools of vesicles generate mPSPs and ePSPs (Sara et al., 2005). In any case, these results indicate that ethanol acts presynaptically to lower the efficacy of GABAergic inhibition of DA neurons. This is consistent with previous reports that ethanol inhibits VTA GABAergic neurons in vivo (Gallegos et al., 1999, Stobbs et al., 2004) and in vitro (Xiao et al., 2007).
In hippocampus, presynaptic GABAB receptors modulate ethanol sensitivity of GABAergic synaptic activity (Wu et al., 2005), and limit ethanol facilitation of GABAergic synaptic transmission by ethanol (Ariwodola and Weiner, 2004). In amygdala, presynaptic GABAB receptors cause tolerance to ethanol in GABAergic terminals (Zhu and Lovinger, 2006). 1 µM CGP52432 did not prevent ethanol inhibition of sIPSCs in the VTA. Note that 1 µM of CGP52432 is 12 times greater than its EC50 for blocking GABAB receptors (Lanza et al., 1993). Moreover, CGP52432 is an effective antagonist of baclofen (a GABAB agonist) (Morl et al., 2003). Therefore, it is clear that ethanol inhibited GABA release in the VTA independently of GABAB receptors.
In our whole-cell recordings, on the other hand, only GABAA–mediated sIPSCs and eIPSCs were recorded because post-synaptic K+-mediated GABAB events are suppressed by high internal Cs+ (Ling and Benardo, 1994). Note that a previous study has shown that GABAB responses did not occur spontaneously in VTA DA neurons and there were distinct afferents mediating the GABAA and GABAB response (Sugita et al., 1992). Still, GABAB receptor agonist was reported to attenuate ethanol intake in ethanol dependent rats (Walker and Koob, 2007). It is noteworthy that the baseline level of GABA release is elevated during ethanol dependence (Roberto et al., 2004), and GABAB receptor agonist exerts stronger inhibition of GABA release in these animals, in comparison with naïve ones.
The role of µ-opioid receptors in ethanol inhibition of GABAergic IPSCs in VTA DA neurons
Accumulating evidence implicates the endogenous opioid system in the development and maintenance of alcoholism (Oswald and Wand, 2004). Both µ- and δ-opioid receptors are important in ethanol consumption, because their selective antagonists and agonists respectively decrease or increase ethanol intake (Herz, 1997). In the current study, we observed that ethanol and DAMGO, a MOR agonist have similar effects on GABAergic transmission to VTA DA neurons. First, both ethanol and DAMGO inhibit VTA GABAergic neurons (Johnson and North, 1992a, Gallegos et al., 1999, Stobbs et al., 2004, Margolis et al., 2006, Xiao et al., 2007). Second, these two agents significantly suppress GABAergic synaptic transmission onto VTA DA neurons. That is, decrease eIPSC amplitude and sIPSC frequency, yet, no effect on mIPSCs. Third, these two agents excite VTA DA neurons (Gessa et al., 1985, Johnson and North, 1992a, Margolis et al., 2003, Xiao et al., 2007) via a mechanism of disinhibition (Johnson and North, 1992a, Xiao et al., 2007). Fourth, opioid receptor antagonist blocks or dramatically attenuates ethanol-induced inhibition of VTA GABAergic neurons and excitation of VTA DA neurons (Xiao et al., 2007). Fifth, saturating concentration of a MOR agonist occludes ethanol-induced excitation of VTA DA neurons (Xiao et al., 2007), probably resulted from a maximal inhibition of GABAergic synaptic transmission. These results are in line with previous observations that ethanol increases the release of β-endorphin in the brain, which activates MORs (Stein, 1993, Herz, 1997, Marinelli et al., 2004).
Interestingly, in the presence of saturating concentration of DAMGO, ethanol potentiated GABAergic IPSCs in VTA DA neurons. The mechanisms underlying this observation warrant further investigation. Based on the results presented in this report and the relevant literature, we propose that VTA DA neurons receive GABAergic inputs from several sources, the effects of ethanol on GABAergic IPSCs may vary according to their sources. While ethanol inhibits VTA GABAergic neurons by acting on the µ–opioid receptors expressed on their soma and dendritic area (Xiao et al., 2007), and consequently inhibits the GABAergic IPSCs in VTA DA neurons, ethanol potentiates the GABAergic IPSCs from other sources, in keeping with the potentiation effect of ethanol on the GABAergic IPSCs observed in other brain regions (Siggins et al., 2005, Weiner and Valenzuela, 2006). As the GABAergic neurons in the VTA are the major source of GABAergic inputs to VTA DA neurons, ethanol inhibition of GABAergic IPSCs is dominated. Ethanol potentiation of IPSCs appears only when GABAergic IPSCs from VTA GABAergic neurons are suppressed by DAMGO. This ethanol inhibition of GABAergic synaptic transmission to VTA DA neurons may have a critical role in ethanol addiction.
It is important to study drug abuse in developing brain
In very young animals, neuronal circuits in the VTA are still being refined. Therefore, findings in preparations at P14 to P21 are very relevant from the point of view of brain development. Although most studies of drug addiction are on adults, better knowledge of how drugs of abuse affect brain development is of critical importance, given that the use of addictive substances during childhood and adolescence is disturbingly common (Johnston et al., 2005). According to Izumi et al (2005), a single day of ethanol exposure during development has persistent effects on bi-directional plasticity, NMDA receptor function and ethanol sensitivity. Young individuals exposed to drugs of abuse are also at increased risk of developing major psychiatric problem (Famy et al., 1998), and alcohol use among adolescents, particularly preteen alcohol use initiation, is an important risk factor for both suicide ideation and suicide attempts among boys and girls (Swahn and Bossarte, 2007).
In conclusion, our findings support growing evidence that VTA GABAergic transmission is a crucial target of ethanol.
GRANT
This work was supported by grant AA015925 from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Mole B, Hodge CW. GABAA receptor regulation of voluntary ethanol drinking requires PKCepsilon. Synapse. 2006;60:411–419. doi: 10.1002/syn.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491(Pt 1):163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, North RA. Neurobiology of opiate abuse. Trends Pharmacol Sci. 1992;13:185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- Dilts RP, Kalivas PW. Autoradiographic localization of mu-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res. 1989;488:311–327. doi: 10.1016/0006-8993(89)90723-3. [DOI] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry. 1998;155:552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology (Berl) 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC. Adaptive responses of gamma-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther. 1999;291:1045–1053. [PubMed] [Google Scholar]
- Garzon M, Pickel VM. Plasmalemmal mu-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse. 2001;41:311–328. doi: 10.1002/syn.1088. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Hernandez A, Floran B, Galarraga E, Tapia D, Valdiosera R, Erlij D, Aceves J, Bargas J. Control of the subthalamic innervation of substantia nigra pars reticulata by D1 and D2 dopamine receptors. J Neurophysiol. 2006;95:1800–1811. doi: 10.1152/jn.01074.2005. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Kohl RR, McBride WJ. GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997a;69:137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABA(A) antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997b;111:369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992a;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992b;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Terry-McEllrath YM, O'Malley PM, Wakefield M. Trends in recall and appraisal of anti-smoking advertising among American youth: national survey results, 1997–2001. Prev Sci. 2005;6:1–19. doi: 10.1007/s11121-005-1249-6. [DOI] [PubMed] [Google Scholar]
- Jones S, Kauer JA. Amphetamine depresses excitatory synaptic transmission via serotonin receptors in the ventral tegmental area. J Neurosci. 1999;19:9780–9787. doi: 10.1523/JNEUROSCI.19-22-09780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M, Fassio A, Gemignani A, Bonanno G, Raiteri M. CGP 52432: a novel potent and selective GABAB autoreceptor antagonist in rat cerebral cortex. Eur J Pharmacol. 1993;237:191–195. doi: 10.1016/0014-2999(93)90268-m. [DOI] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci. 2007;27:6531–6541. doi: 10.1523/JNEUROSCI.1678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcohol Clin Exp Res. 2006;30:119–126. doi: 10.1111/j.1530-0277.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS. Properties of isolated GABAB-mediated inhibitory postsynaptic currents in hippocampal pyramidal cells. Neuroscience. 1994;63:937–944. doi: 10.1016/0306-4522(94)90561-4. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res. 1994;643:245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–784. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ. Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int. 1999;35:115–123. doi: 10.1016/s0197-0186(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Morl F, Leemhuis J, Lindemeyer K, Grass N, Norenberg W, Meyer DK. Stimulation of GABAB receptors increases the expression of the proenkephalin gene in slice cultures of rat neocortex. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:640–647. doi: 10.1007/s00210-003-0746-z. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Blocking GABA(A) receptors in the anterior ventral tegmental area attenuates ethanol intake of the alcohol-preferring P rat. Psychopharmacology (Berl) 1998;139:108–116. doi: 10.1007/s002130050695. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA. Ventral tegmental self-stimulation selectively induces opioid peptide release in rat CNS. Synapse. 1993;13:63–73. doi: 10.1002/syn.890130109. [DOI] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Sugita S, Johnson SW, North RA. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci Lett. 1992;134:207–211. doi: 10.1016/0304-3940(92)90518-c. [DOI] [PubMed] [Google Scholar]
- Swahn MH, Bossarte RM. Gender, early alcohol use, and suicide ideation and attempts: findings from the 2005 youth risk behavior survey. J Adolesc Health. 2007;41:175–181. doi: 10.1016/j.jadohealth.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington JL, Cross AJ. Neurochemical changes following kainic acid lesions of the nucleus accumbens: implications for a GABAergic accumbal-ventral tegmental pathway. Life Sci. 1978;22:1011–1014. doi: 10.1016/0024-3205(78)90367-3. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABA A receptor subtypes. Pharmacol Ther. 2006;112:513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wu PH, Poelchen W, Proctor WR. Differential GABAB Receptor Modulation of Ethanol Effects on GABA(A) synaptic activity in hippocampal CA1 neurons. J Pharmacol Exp Ther. 2005;312:1082–1089. doi: 10.1124/jpet.104.075663. [DOI] [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjevic K, Ye JH. Effects of ethanol on midbrain neurons: role of opioid receptors. Alcohol Clin Exp Res. 2007;31:1106–1113. doi: 10.1111/j.1530-0277.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Ye JH, Wang F, Krnjevic K, Wang W, Xiong ZG, Zhang J. Presynaptic glycine receptors on GABAergic terminals facilitate discharge of dopaminergic neurons in ventral tegmental area. J Neurosci. 2004;24:8961–8974. doi: 10.1523/JNEUROSCI.2016-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Zhang J, Xiao C, Kong JQ. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods. 2006;158:251–259. doi: 10.1016/j.jneumeth.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]