Abstract
Background
Our previous studies have shown that clinically relevant concentrations of inhalational anesthetics dose-dependently and specifically inhibit the PSD-95, Dlg, and ZO-1 (PDZ) domain-mediated protein interactions between postsynaptic density protein-95 (PSD-95) and N-methyl-d-aspartate receptors, and that the knockdown of spinal PSD-95 by intrathecal injection of PSD-95 antisense oligodeoxynucleotide significantly reduces the minimum alveolar anesthetic concentration for isoflurane in rats.
Methods
We constructed a fusion peptide Tat-PSD-95 PDZ2 comprising the second PDZ domain of PSD-95, which can specifically disrupt PSD-95 PDZ2-mediated protein interactions by binding to interaction partner. By intraperitoneal injection of this fusion peptide into mice, we investigated the effect of disrupting the PSD-95 PDZ2-mediated protein interactions on the threshold for halothane anesthesia.
Results
Systemically injected fusion peptide Tat-PSD-95 PDZ2 was delivered into the central nervous system, disrupted the protein-protein interactions between N-methyl-d-aspartate receptor NR2 subunits and PSD-95, and significantly reduced the minimum alveolar anesthetic concentration and righting reflex EC50 for halothane.
Conclusions
By disrupting PSD-95 PDZ2 domain-mediated protein interactions, intraperitoneal injection of cell-permeant fusion peptide Tat-PSD-95 PDZ2 dose-dependently reduces the threshold for halothane anesthesia. These results suggest that PDZ domain-mediated protein interactions at synapses in the central nervous system might play an important role in the molecular mechanisms of halothane anesthesia.
1. Introduction
N-methyl-d-aspartate receptor (NMDAR) activation has been demonstrated to play an important role in the processing of spinal nociceptive information1-4 and in the determination of the minimum alveolar anesthetic concentration (MAC) of inhalational anesthetics5-11. Postsynaptic density protein-95 (PSD-95), a scaffolding protein, has been identified to attach NMDARs to internal signaling molecules at neuronal synapses of the central nervous system (CNS)12;13. This function suggests that PSD-95 might be involved in physiological and pathophysiological actions triggered via the activation of NMDARs in the CNS. NMDAR/PSD-95 protein interactions are mediated by a PDZ domain (a term derived from the names of the first three proteins identified to contain the domain: PSD-95, Dlg, and ZO-1). PSD-95 possesses three PDZ domains. The second PDZ domain of PSD-95 (PSD-95 PDZ2) interacts with the seven-amino acid, COOH-terminal domain containing a terminal tSXV motif (where S is serine, X is any amino acid, and V is valine) common to NR2 subunits of NMDARs13. PSD-95 PDZ2 also interacts with the Shaker-type Kv1.4 potassium channel and this interaction regulates the clustering of PSD-95 with the Kv1.4 channel14.
Our previous studies have shown that clinically relevant concentrations of inhalational anesthetics dose-dependently and specifically inhibit the PDZ domain-mediated protein interaction between PSD-95 and NMDARs15. These inhibitory effects are immediate, potent, and reversible and occur at a hydrophobic peptide-binding groove on the surface of the PSD-95 PDZ2 in a manner relevant to anesthetic action15. These findings reveal the PDZ domain as a new molecular target for inhalational anesthetics. We have also found that PSD-95 knockdown significantly reduced MAC for isoflurane and attenuated the NMDA-induced increase in isoflurane MAC16.
To further define the role of PSD-95 PDZ2 domain-mediated protein interactions in the molecular mechanisms of inhalational anesthetics, we constructed a peptide comprising the PSD-95 PDZ2 and rendered it cell-permeable by fusing it to the protein transduction domain (PTD) of the human immunodeficiency virus-type 1 Tat protein to obtain the fusion peptide Tat-PSD-95 PDZ2. To investigate the effect of disrupting the PDZ domain-mediated protein interactions on the threshold for halothane anesthesia, we injected mice intraperitoneally with this fusion peptide and then measured their MAC and righting reflex EC50 (RREC50) for halothane.
2. Materials And Methods
2.1. Animal preparation
Male C57Bl/6J mice (8–10 weeks) were obtained from Jackson Laboratories (Bar Harbor, MA) and acclimated in our animal facility for a minimum of 1 week prior to use in experiments. The mice were housed under standard conditions with a 12-h light/dark cycle and allowed food and water ad libitum. All animal experiments were carried out with the approval of the Animal Care and Use Committee at Johns Hopkins University and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering. The animal assignment was blinded to the observer for all of in vivo testing including MAC measurement, RREC50 determination, and locomotor function test.
2.2. Construction and purification of Tat fusion peptides
The cDNA encoding the PSD-95 PDZ2 was prepared in our laboratory as described previously15. Here, we used sub-cloning to construct a Tat-PSD-95 PDZ2 plasmid by inserting PSD-95 PDZ2 cDNA into the pTAT-HA expression vector, which contains an amino-terminal, in-frame, 11-amino-acid, minimal transduction domain (residues 47-57 of human immunodeficiency virus Tat protein) termed Tat17. Two control plasmids were also constructed: mutated Tat-PSD-95 PDZ2, in which three sites critical for interactions between NMDARs and PSD-95 were mutated (K165T, L170R and H182L), and PSD-95 PDZ2, which contained the same sequences as Tat-PSD-95 PDZ2 but without Tat PTD. To produce the fusion peptides, these plasmids were transformed into Escherichia coli BL21 cells, and protein expression was induced by 0.5 mM isopropylthiogalactoside at 37°C for 4 h. The fusion peptides were purified using Ni-NTA agarose (Qiagen, Valencia, CA) according to a standard 6 × histidine-tagged protein purification protocol. The resulting fusion peptides were dialyzed twice against phosphate-buffered saline. The purified peptides were verified by Coomassie blue staining and Western blot analysis and then stored in 10% glycerol/phosphate-buffered saline at -80°C until use.
2.3. In vivo administration of Tat fusion peptides
The purified fusion peptides at the indicated amounts were injected intraperitoneally into mice in 300 μl of phosphate-buffered saline and 10% glycerol. The mice were given Tat-PSD-95 PDZ2 or control peptide (mutated Tat-PSD-95 PDZ2 or PSD-95 PDZ2 without Tat) 4 h before MAC measurement and righting reflex testing. All the animals were assigned randomly to experimental groups consisting of 6-8 animals each. Western blot analysis was then used to verify the CNS delivery of these fusion peptides after intraperitoneal injection.
2.4. Western blot analysis
Cerebral cortex, hippocampus, and lumbar spinal cord were harvested 4 h after intraperitoneal injection of the fusion peptides. Total proteins from these tissues were extracted. In brief, the tissues were removed and homogenized in homogenization buffer18 (10 mM Tris-HCl, 5 mM MgCl2, 2 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, 2 μM pepstatin A, and 320 mM sucrose, pH 7.4). The crude homogenates were centrifuged at 700 × g for 15 min at 4°C. The pellets were rehomogenized and spun again at 700 × g, and the supernatants were combined and diluted in resuspension buffer18 (10 mM Tris-HCl, 5 mM MgCl2, 2 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, 2 μM pepstatin A, and 250 mM sucrose, pH 7.4). Next, the protein extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrotransferred to nitrocellulose membranes, and then immunoblotted with monoclonal anti-His antibody (Sigma, St. Louis, MO) diluted (1:1,000) in blocking solution containing 3% nonfat dry milk and 0.1% Tween-20 in Tris-HCl-buffered saline for 1 h at room temperature. After extensive washing, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulin (Bio-Rad Laboratories, Hercules, CA) at a dilution of 1:3,000 for another 1 h. Specific proteins were detected by enhanced chemiluminescence (Amersham, Piscataway, NJ). Tubulin served as a loading control and cerebral cortex was used for its detection.
2.5. In vivo binding assay: Co-immunoprecipitation
5 μg of the affinity-purified rabbit NR2A/2B antibody (Chemicon, Temecula, CA) was incubated with 100 μl of protein A-Sepharose slurry for 1 h, and the complex was spun down at 2000 rpm for 4 min. The solubilized membrane fraction (500 μg) from the different groups of treated mice as mentioned-above then was added to the Sepharose beads, and the mixture was incubated for 2∼3 h at 4°C. The mixture was washed once with 1% Triton X-100 in immunoprecipitation buffer19 [containing (in mM): 137 NaCl, 2.7 KCl, 4.3 Na2HPO4, 1.4 KH2PO4, 5 EGTA, 1 sodium vanadate, 10 sodium pyrophosphate, 50 NaF, and 0.1 phenylmethylsulfonyl fluoride, and 20 U/ml Trasylol], twice with 1% Triton X-100 in immunoprecipitation buffer plus 300 mM NaCl, and three times with immunoprecipitation buffer. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and detected by NR2A/2B or PSD-95 antibody (Upstate, Lake Placid, NY). As a positive control (input), 50 μg of the solubilized membrane fraction was loaded onto the gel. The NR2A/2B antibody was preincubated with excess NR2 peptide (100 μg/ml) to verify its specificity.
2.6. Measurement of halothane MAC
Measurement of halothane MAC value was carried out as previously described with minor modification20-22. Mice were placed in individual Plexiglas chambers 3 h after the injection of the fusion peptides. Each chamber was fitted with a rubber stopper at one end through which the mouse's tail and a rectal temperature probe protruded. Groups of four mice were given halothane in oxygen (4 l/min total gas flow). A gas sample was continuously drawn, and the anesthetic concentration was measured with an agent analyzer (Ohmeda 5250 RGM, Louisville, CO). A rectal temperature probe was inserted under light general anesthesia, and temperature was kept at 36∼38°C with heat lamps throughout the experiment. Mice initially breathed approximately 1.5% halothane for 60 min. Next, a 15 cm hemostatic forceps was applied to the tail for 1 min, and the mice were observed for movement in response to the stimulation. In each case, the tail was stimulated proximal to the previous test site. Only the middle third of the tail was used for tail-clamping. The concentration of the anesthetic agent at which the mouse exhibited motor activity (gross movements of the head, extremities, and/or body) was considered one that permitted a positive motor response. The anesthetic concentration was increased (or decreased) in steps of 0.1% until the positive response disappeared (or vice versa), with 10 min for equilibration allowed after each change of anesthetic concentration. MAC is defined as the concentration midway between the highest concentration that permitted movement in response to the stimulus and the lowest concentration that prevented movement.
2.7. Determination of halothane RREC50
Following the measurement of MAC, the halothane concentration was halved for 10 min and the animal turned on its back to test the righting reflex defined as a return onto all four paws within 1 min20-22. The halothane concentration was reduced by 0.1% for 10 min if the animal failed to right itself and the righting reflex subsequently re-tested. RREC50 was calculated for each mouse as the mean value of the anesthetic concentrations that just permitted and just prevented the righting reflex.
2.8. Tests for locomotor function
The effects of Tat fusion peptides on locomotor function were examined 4 h after intraperitoneal injection. The following tests were performed as described previously23. 1) Placing reflex: The mouse was held with hind limbs slightly lower than forelimbs, and the dorsal surface of the hind paws was brought into contact with the edge of a table. The experimenter recorded whether the mouse placed its hind paws on the table surface reflexively; 2) Grasping reflex: The mouse was placed on a wire grid, and the experimenter recorded whether the hind paws grasped the wire on contact. Scores for these reflexes were based on counts of each normal reflex exhibited in six trials.
2.9. Statistical analysis
Data are expressed as mean ± S.E.M. and statistically analyzed with one-way analysis of variance followed by Student-Newman-Keuls method. Statistical significance was set at p < 0.05. Statistical analysis was conducted using SigmaStat 2.0 software.
3. Results
3.1. CNS delivery of Tat peptides after intraperitoneal injection
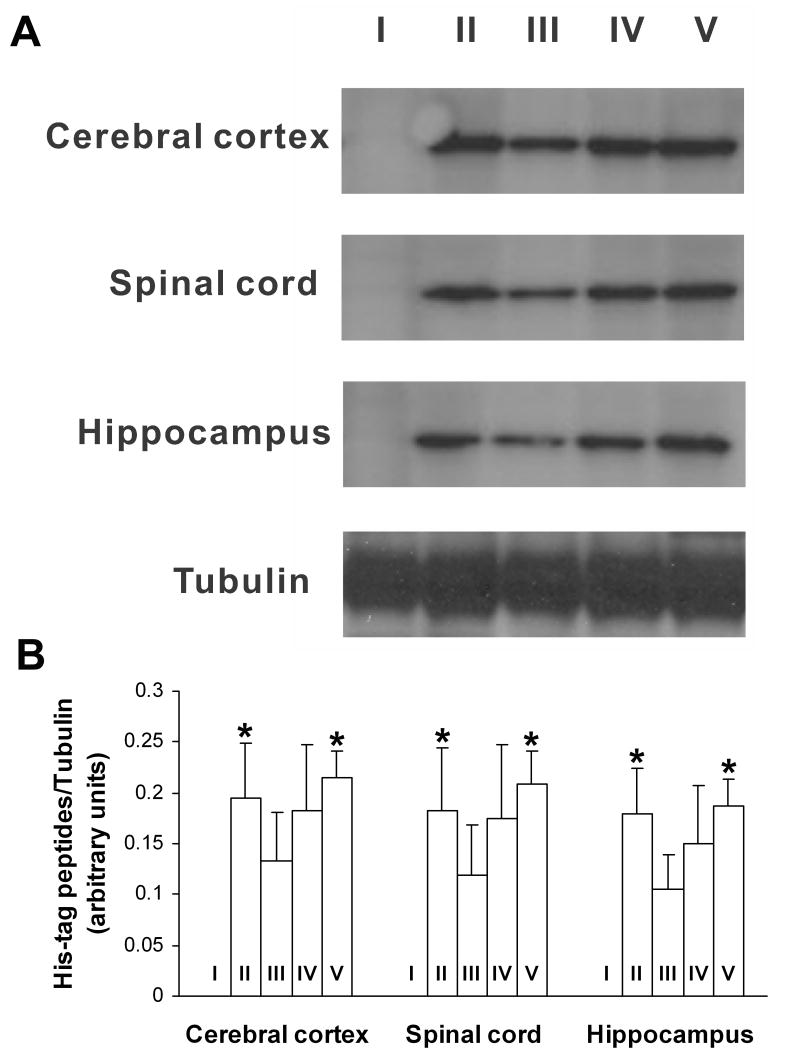
Western blotting showed that after intraperitoneal injection, Tat-linked fusion peptides (Tat-PSD-95 PDZ2 and mutated Tat-PSD-95 PDZ2), but not PSD-95 PDZ2 without Tat, were delivered into cerebral cortex, hippocampus and lumbar spinal cord of the mice (Fig. 1). Moreover, Tat-PSD-95 PDZ2 was delivered into the spinal cord in a dose-dependent manner (Fig. 1). No significant difference was observed in the PTD-mediated spinal delivery of Tat-PSD-95 PDZ2 and mutated Tat-PSD-95 PDZ2 (Fig. 1).
Fig. 1.
Tat peptides were delivered into CNS after intraperitoneal injection. I: PSD-95 PDZ2 (8 mg/kg); II: mutated Tat-PSD-95 PDZ2 (8 mg/kg); III: Tat-PSD-95 PDZ2 (2 mg/kg); IV: Tat-PSD-95 PDZ2 (4 mg/kg); V: Tat-PSD-95 PDZ2 (8 mg/kg). Tubulin served as a loading control. The data shown are representative of three independent experiments (A) and the results after statistical analysis (B). *p < 0.05 vs. group III.
3.2. Tat-PSD-95 PDZ2 markedly disrupted the interactions between NMDAR NR2 subunits and PSD-95
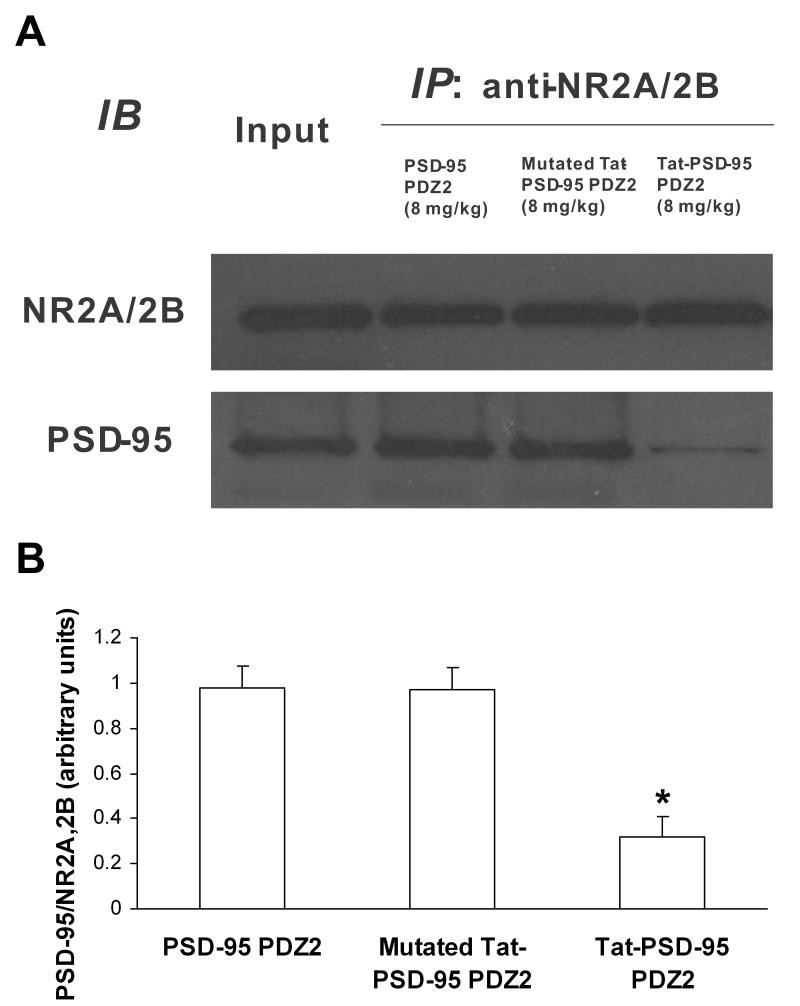
Co-immunoprecipitation assay was used to discover whether NMDAR/PSD-95 protein interactions were interrupted by Tat fusion peptides. We found that Tat-PSD-95 PDZ2 markedly disrupted the interactions between NMDAR NR2 subunits and PSD-95 (Fig. 2). However, mutated Tat-PSD-95 PDZ2 had no effect (Fig. 2).
Fig. 2.
Interaction of NMDA receptor NR2A/2B with PSD-95 in the spinal cord was disrupted by systemic administration with Tat-PSD-95 PDZ2. NR2A/2B antibody was used to immunoprecipitate NR2A/2B and its interacting proteins after intraperitoneal injection of Tat-PSD-95 PDZ2, mutated Tat-PSD-95 PDZ2 and PSD-95 PDZ2 without Tat. Note that Tat-PSD-95 PDZ2 (8 mg/kg) markedly blocked the interaction between NR2A/2B and PSD-95 and that mutated Tat-PSD-95 PDZ2 (8 mg/kg) had no effect on this interaction compared to the effect of PSD-95 PDZ2 (8 mg/kg). The amount of sample loaded for the input was 10% of that for the immunoprecipitation. The data shown are representative of three independent experiments (A) and the results after statistical analysis (B). *p < 0.05 vs. PSD-95 PDZ2 group. IP, immunoprecipitation; IB, immunoblotting.
After mice were given intraperitoneal injection of Tat-PSD-95 PDZ2, mutated Tat-PSD-95 PDZ2, or PSD-95 PDZ2 without Tat, NR2A/2B antibody was used to immunoprecipitate NR2A/2B and its interacting proteins from spinal cord homogenates (Fig. 2). We found that Tat-PSD-95 PDZ2 (8 mg/kg) markedly blocked the interaction between NR2A/2B and PSD-95 but that neither mutated Tat-PSD-95 PDZ2 (8 mg/kg) nor PSD-95 PDZ2 (8 mg/kg) had an effect on this interaction. The specificity of the NR2A/2B antibody was verified by preincubation with NR2 peptide, and no bands were detected in this condition (data not shown).
3.3. Effect of Tat fusion peptides on the threshold for halothane anesthesia
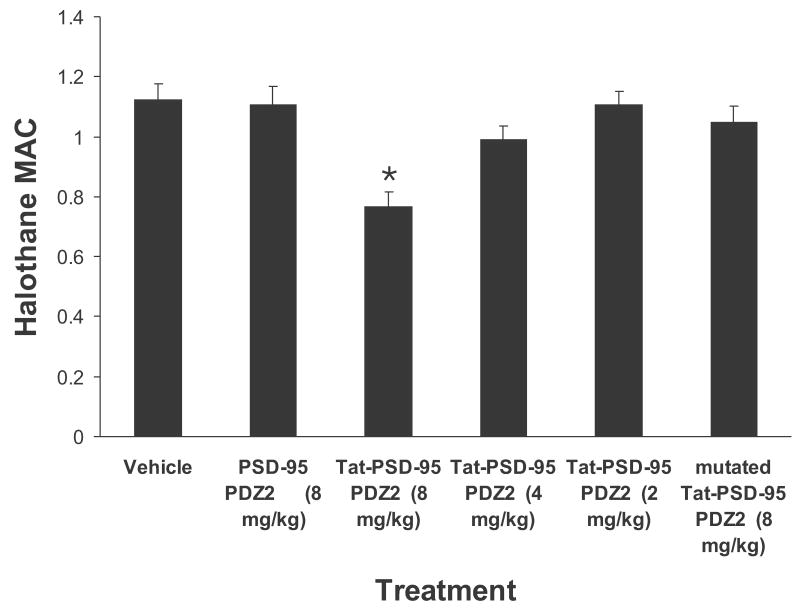
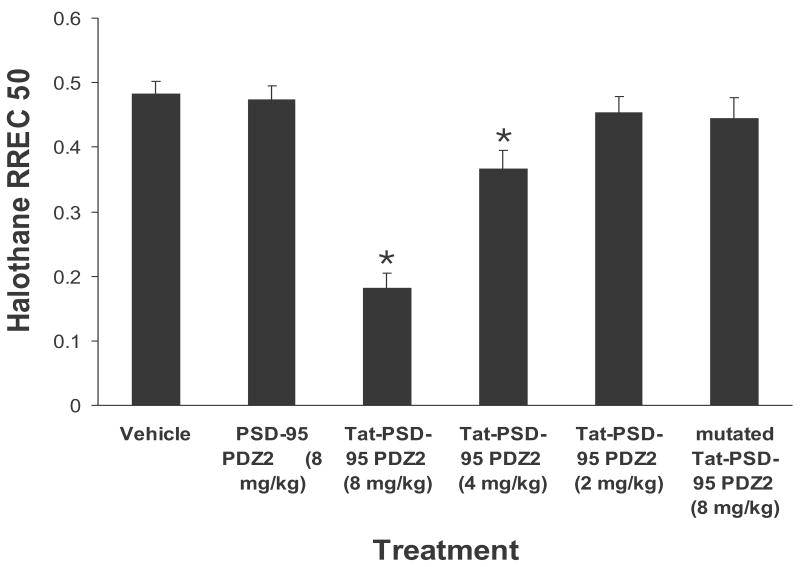
After mice were given intraperitoneal injection of the fusion peptides, halothane MAC and RREC50 were measured respectively. We found that Tat-PSD-95 PDZ2 dose-dependently reduced halothane MAC and RREC50 (Figs. 3, 4). However, mutated Tat-PSD-95 PDZ2 and PSD-95 PDZ2 without Tat had no effect (Figs. 3, 4). As a control, we observed that these peptides had no effect on locomotor function of unanesthetized mice (Table 1). The mice showed normal grooming behavior, normal levels of activity, and no significant change in either blood pressure or heart rate after intraperitoneal injection of these peptides.
Fig. 3.
Systemic pretreatment with Tat-PSD-95 PDZ2 significantly reduced halothane MAC. Halothane MAC was measured after intraperitoneal injection of Tat-PSD-95 PDZ2 (n = 8), mutated Tat-PSD-95 PDZ2 (n = 8) and PSD-95 PDZ2 without Tat (n = 6). Note that Tat-PSD-95 PDZ2 dose-dependently reduced halothane MAC. However, mutated Tat-PSD-95 PDZ2 and PSD-95 PDZ2 had no effect. *p < 0.05 vs. vehicle group.
Fig. 4.
Systemic pretreatment with Tat-PSD-95 PDZ2 significantly reduced halothane RREC50. Halothane RREC50 was determined following MAC measurement after intraperitoneal injection of Tat-PSD-95 PDZ2 (n = 8), mutated Tat-PSD-95 PDZ2 (n = 8) and PSD-95 PDZ2 without Tat (n = 6). Note that Tat-PSD-95 PDZ2 dose-dependently reduced halothane RREC50. However, mutated Tat-PSD-95 PDZ2 and PSD-95 PDZ2 had no effect. *p < 0.05 vs. vehicle group.
Table 1.
Intraperitoneal injection of Tat peptides had no effect on locomotor function of unanesthetized mice
| Agents | Placing | Grasping |
|---|---|---|
| Vehicle | 6 (0) | 6 (0) |
| PSD-95 PDZ2, 8 mg/kg | 6 (0) | 6 (0) |
| Tat- PSD-95 PDZ2, 8 mg/kg | 6 (0) | 6 (0) |
| Mutated Tat- PSD-95 PDZ2, 8 mg/kg | 6 (0) | 6 (0) |
Note: Data were expressed as mean (S.E.M). Six trials are taken for each testing. Score 1 for each normal response and score 0 when the animals fail. PDZ: PSD-95, Dlg, and ZO-1; PSD-95: postsynaptic density protein-95.
In the MAC study, the value for halothane MAC in vehicle-treated group was 1.12 ± 0.05. In the groups treated with Tat-PSD-95 PDZ2 at the doses of 2, 4, or 8 mg/kg, the halothane MAC values were 1.11 ± 0.05, 0.99 ± 0.05, or 0.77 ± 0.05, respectively (Fig. 3). One-way analysis of variance showed that halothane MAC was significantly altered after pretreatment with this peptide (p < 0.05, Fig. 3). The highest dose (8 mg/kg) of Tat-PSD-95 PDZ2 significantly reduced the halothane MAC compared to the vehicle-treated group (p < 0.05). In contrast, intraperitoneal injection with the same dose of mutated Tat-PSD-95 PDZ2 (8 mg/kg) or PSD-95 PDZ2 without Tat (8 mg/kg) had no effect on the halothane MAC (p > 0.05, Fig. 3).
In the RREC50 study, the value for halothane RREC50 in vehicle-treated group was 0.48 ± 0.02. In the groups treated with Tat-PSD-95 PDZ2 at the doses of 2, 4, or 8 mg/kg, the halothane RREC50 values were 0.45 ± 0.03, 0.37 ± 0.03, or 0.18 ± 0.02, respectively (Fig. 4). One-way analysis of variance showed that halothane RREC50 was significantly altered after pretreatment with this peptide (p < 0.05, Fig. 4). The two higher doses (4 and 8 mg/kg) of Tat-PSD-95 PDZ2 significantly reduced the halothane RREC50 compared to vehicle-treated group (p < 0.05, Fig. 4). In contrast, intraperitoneal injection of mutated Tat-PSD-95 PDZ2 (8 mg/kg) or PSD-95 PDZ2 without Tat (8 mg/kg) had no effect on the halothane RREC50 (p > 0.05, Fig. 4).
4. Discussion
Results from our present studies indicate that intraperitoneally injected fusion peptide Tat-PSD-95 PDZ2 (1) can be delivered into the CNS; (2) dose-dependently disrupts the protein-protein interactions between NMDAR NR2 subunits and PSD-95; and (3) significantly reduces halothane MAC and RREC50. These results suggest that PDZ domain-mediated protein interactions at synapses in the CNS might play an important role in the molecular mechanisms of halothane anesthesia.
PTD-mediated in vivo delivery of biologically active peptides represents a novel and promising strategy to treat CNS diseases. Although the exact mechanism of transduction across the cellular membrane is currently unknown, the first step of the transduction appears to involve a charge-charge interaction of the basic PTD with acidic motifs on the cellular membrane. It has been demonstrated that fusion peptides containing the PTD sequence derived from human immunodeficiency virus Tat protein can be transduced into the CNS after systemic administration24. In our current study, we found that after intraperitoneal injection, Tat-PSD-95 PDZ2 and mutated Tat-PSD-95 PDZ2 were detected in cerebral cortex, hippocampus, and lumbar spinal cord of mice, but PSD-95 PDZ2 lacking Tat was not seen in these tissues. These results support the conclusion that a wide variety of cargo, including peptides and full-length proteins, can be delivered into cells when linked to the PTD sequence25.
The interactions between NMDAR NR2 subunits and PSD-95 are mediated by the second PDZ domain of PSD-95 protein13. The Shaker-type potassium channel, Kv1.4, also binds to the PSD-95 PDZ214. Thus, we hypothesized that competition with a peptide consisting of PSD-95 PDZ2 could disrupt this PDZ domain-mediated protein interaction. Our current results support this hypothesis. By in vivo binding assay, we show here that fusion peptide Tat-PSD-95 PDZ2 dose-dependently suppresses the NMDAR/PSD-95 protein interaction. However, mutation of three critical aminal acids (K165T, L170R and H182L) of the PDZ2 domain in the fusion peptide eliminated its ability to affect the interaction. The mutated Tat-PSD-95 PDZ2 and PSD-95 PDZ2 without Tat served as controls for Tat-PSD-95 PDZ2 in our studies.
Inhalational anesthetics have been in widespread use in modern surgical procedures, but their molecular mechanisms remain poorly understood. PDZ domain-mediated protein interactions play a central role in organizing signaling complexes around synaptic receptors for efficient signal transduction. Our previous studies have demonstrated that halothane dose-dependently and reversibly inhibits PSD-95 PDZ domain-mediated protein interactions, and that the halothane binding site on PSD-95 PDZ2 completely overlaps with the binding pocket of PSD-95 for NMDAR NR2 subunits15, suggesting a new concept that affecting PDZ domain-mediated protein interactions at synapses in the CNS might be one of molecular mechanisms by which the general anesthetic state is achieved. By knocking down PSD-95 expression in the spinal cord, we have shown that the deficiency of spinal PSD-95 reduced isoflurane MAC in rats16. In the present study, we found that fusion peptide Tat-PSD-95 PDZ2, but not mutated Tat-PSD-95 PDZ2 or PSD-95 PDZ2, dose-dependently reduced halothane MAC and RREC50 in mice by disrupting the PDZ domain-mediated protein interactions. These results provide in vivo evidence to support this concept. On the other hand, a key concern with inhalational anesthetics is the narrow relationship between the therapeutic and toxic doses. This concern has negative impact on clinical administration of the inhalational anesthetics. Tat-PSD-95 PDZ2, a novel agent, markedly reduces the amount of inhalational anesthetics needed to induce anesthesia, thereby reducing the dose-dependent toxic side effects of the inhalational anesthetics.
In conclusion, this study demonstrates that by disrupting PDZ domain-mediated protein interactions, intraperitoneal injection of cell-permeable fusion peptide Tat-PSD-95 PDZ2 dose-dependently reduces the threshold for halothane anesthesia. These results provide a novel insight into the molecular mechanisms that underlie the inhalational anesthetic state and a new target for development of anesthetics.
Acknowledgments
The authors thank Dr. Steven Dowdy (Ph.D.) at University of California, San Diego for providing the pTAT-HA expression vector. The authors also thank Drs. Yuanxiang Tao (M.D., Ph.D.), Qingning Su (Ph.D.), and Yun Xu (M.D.) at Johns Hopkins University for their assistance on Tat plasmid construction.
Financial support: This work was supported by National Institutes of Health (Bethesda, Maryland) R01 grants GM049111 and NS44219.
Reference List
- 1.Ren K, Hylden JL, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–44. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- 2.Mao J, Price DD, Hayes RL, Lu J, Mayer DJ. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain Res. 1992;598:271–8. doi: 10.1016/0006-8993(92)90193-d. [DOI] [PubMed] [Google Scholar]
- 3.Garry MG, Malik S, Yu J, Davis MA, Yang J. Knock down of spinal NMDA receptors reduces NMDA and formalin evoked behaviors in rat. Neuroreport. 2000;11:49–55. doi: 10.1097/00001756-200001170-00010. [DOI] [PubMed] [Google Scholar]
- 4.Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci. 2001;4:164–9. doi: 10.1038/83993. [DOI] [PubMed] [Google Scholar]
- 5.Lukatch HS, Kiddoo CE, Maciver MB. Anesthetic-induced burst suppression EEG activity requires glutamate-mediated excitatory synaptic transmission. Cereb Cortex. 2005;15:1322–31. doi: 10.1093/cercor/bhi015. [DOI] [PubMed] [Google Scholar]
- 6.Nagele P, Metz LB, Crowder CM. Nitrous oxide (N(2)O) requires the N-methyl-D-aspartate receptor for its action in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2004;101:8791–6. doi: 10.1073/pnas.0402825101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittson S, Himmel AM, MacIver MB. Multiple synaptic and membrane sites of anesthetic action in the CA1 region of rat hippocampal slices. BMC Neurosci. 2004;5:52. doi: 10.1186/1471-2202-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranft A, Kurz J, Deuringer M, Haseneder R, Dodt HU, Zieglgansberger W, Kochs E, Eder M, Hapfelmeier G. Isoflurane modulates glutamatergic and GABAergic neurotransmission in the amygdala. Eur J Neurosci. 2004;20:1276–80. doi: 10.1111/j.1460-9568.2004.03603.x. [DOI] [PubMed] [Google Scholar]
- 9.Irifune M, Takarada T, Shimizu Y, Endo C, Katayama S, Dohi T, Kawahara M. Propofol-induced anesthesia in mice is mediated by gamma-aminobutyric acid-A and excitatory amino acid receptors. Anesth Analg. 2003;97:424–9. doi: 10.1213/01.ANE.0000059742.62646.40. [DOI] [PubMed] [Google Scholar]
- 10.Stover JF, Sakowitz OW, Kroppenstedt SN, Thomale UW, Kempski OS, Flugge G, Unterberg AW. Differential effects of prolonged isoflurane anesthesia on plasma, extracellular, and CSF glutamate, neuronal activity, 125I-Mk801 NMDA receptor binding, and brain edema in traumatic brain-injured rats. Acta Neurochir (Wien) 2004;146:819–30. doi: 10.1007/s00701-004-0281-9. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead KJ, Rose S, Jenner P. Halothane anesthesia affects NMDA-stimulated cholinergic and GABAergic modulation of striatal dopamine efflux and metabolism in the rat in vivo. Neurochem Res. 2004;29:835–42. doi: 10.1023/b:nere.0000018858.64265.e9. [DOI] [PubMed] [Google Scholar]
- 12.Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–73. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 13.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–40. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 14.Imamura F, Maeda S, Doi T, Fujiyoshi Y. Ligand binding of the second PDZ domain regulates clustering of PSD-95 with the Kv1.4 potassium channel. J Biol Chem. 2002;277:3640–6. doi: 10.1074/jbc.M106940200. [DOI] [PubMed] [Google Scholar]
- 15.Fang M, Tao YX, He F, Zhang M, Levine CF, Mao P, Tao F, Chou CL, Sadegh-Nasseri S, Johns RA. Synaptic PDZ domain-mediated protein interactions are disrupted by inhalational anesthetics. J Biol Chem. 2003;278:36669–75. doi: 10.1074/jbc.M303520200. [DOI] [PubMed] [Google Scholar]
- 16.Tao YX, Johns RA. Effect of the deficiency of spinal PSD-95/SAP90 on the minimum alveolar anesthetic concentration of isoflurane in rats. Anesthesiology. 2001;94:1010–5. doi: 10.1097/00000542-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24:247–56. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- 18.Tao F, Skinner J, Su Q, Johns RA. New role for spinal Stargazin in alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated pain sensitization after inflammation. J Neurosci Res. 2006;84:867–73. doi: 10.1002/jnr.20973. [DOI] [PubMed] [Google Scholar]
- 19.Tao YX, Huang YZ, Mei L, Johns RA. Expression of PSD-95/SAP90 is critical for N-methyl-D-aspartate receptor-mediated thermal hyperalgesia in the spinal cord. Neuroscience. 2000;98:201–6. doi: 10.1016/s0306-4522(00)00193-7. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda T, Saito S, Sato S, Harukuni I, Toyooka H. Halothane minimum alveolar anesthetic concentration and neuronal nitric oxide synthase activity of the dorsal horn and the locus ceruleus in rats. Anesth Analg. 1999;89:1035–9. doi: 10.1097/00000539-199910000-00040. [DOI] [PubMed] [Google Scholar]
- 21.Engelhardt T, Lowe PR, Galley HF, Webster NR. Inhibition of neuronal nitric oxide synthase reduces isoflurane MAC and motor activity even in nNOS knockout mice. Br J Anaesth. 2006;96:361–6. doi: 10.1093/bja/ael010. [DOI] [PubMed] [Google Scholar]
- 22.Ichinose F, Huang PL, Zapol WM. Effects of targeted neuronal nitric oxide synthase gene disruption and nitroG-L-arginine methylester on the threshold for isoflurane anesthesia. Anesthesiology. 1995;83:101–8. doi: 10.1097/00000542-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Tao F, Tao YX, Mao P, Johns RA. Role of postsynaptic density protein-95 in the maintenance of peripheral nerve injury-induced neuropathic pain in rats. Neuroscience. 2003;117:731–9. doi: 10.1016/s0306-4522(02)00801-1. [DOI] [PubMed] [Google Scholar]
- 24.Denicourt C, Dowdy SF. Protein transduction technology offers novel therapeutic approach for brain ischemia. Trends Pharmacol Sci. 2003;24:216–8. doi: 10.1016/S0165-6147(03)00074-9. [DOI] [PubMed] [Google Scholar]
- 25.Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. 2002;13:52–6. doi: 10.1016/s0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]