Abstract
Background
The serotonin transporter 5-HTT mediates responses to serotonin reuptake inhibitors (SSRIs), a mainstay treatment in mood disorders. The amygdala, a key emotional processing center, has functional abnormalities in mood disorders, which resolve following successful SSRI treatment. To better understand the effects of SSRIs in mood disorders, we examined the distribution of 5-HTT labeled fibers relative to specific nuclear groups in the amygdala.
Methods
Immunocytochemical techniques were used to chart 5-HTT labeled fibers in the amygdala in coronal sections through the brain of six adult Macaques. Nissl staining was used to define nuclear groups in the amygdala.
Results
The serotonin transporter 5-HTT is distributed heterogeneously in the primate amygdala, with the lateral subdivision of the central nucleus, intercalated cell islands, amygdalohippocampal area, and the paralaminar nucleus showing the heaviest concentrations.
Conclusions
5HTT-labeled fibers are very densely concentrated in output regions of the amygdala. High concentrations of 5-HTT-positive fibers in the central nucleus indicate that tight regulation of serotonin is critical in modulating fear responses mediated by this nucleus. High concentrations of 5-HTT-labeled fibers in the intercalated islands and parvicellular basal nucleus/paralaminar nucleus, which contain immature -appearing neurons, suggest a potential trophic role for serotonin in these subregions.
Keywords: Central nucleus, intercalated islands, serotonin reuptake inhibitors, basolateral complex, amygdalohippocampal areas, anterior cortical nucleus
The link between serotonin (5-hydroxytryptamine, 5-HT) and mood disorders, particularly depression, has driven pharmaceutical development and modeled clinical thinking for many years. The serotonin hypothesis of depression was based on studies showing low levels of 5HT metabolites in response to probenecid in depressed individuals (Van Praag 1977), decreased central 5-HT in the brains of suicide victims (Pare et al 1969), and reports of antidepressant effects of tryptophan, a serotonin precursor (Agurell 1983; Asberg et al 1976; Berger 1975). Such ideas led to the development of selective serotonin reuptake inhibitors (SSRIs), drugs that competitively bind the serotonin transporter (5-HTT) on the presynaptic terminal and serve to acutely elevate synaptic levels of serotonin (Hyttel 1984; Tatsumi et al 1997). However, while SSRIs act to increase serotonin levels immediately (Guan and McBride 1988), clinical effects are observed 2–3 weeks later, suggesting additional mechanisms of response (for review see Asberg et al 1986). Recently, the ‘neurotrophic’ hypothesis suggests that enhanced serotonin stimulates neuronal growth and proliferation, which may in turn enhance function of emotional circuitry (Duman 1998; Kempermann and Kronenberg 2003).
The amygdala is a prominent limbic structure which plays a role in emotional processing. During major depressive episodes, the amygdala shows metabolic abnormalities, including both elevated resting cerebral blood flow and glucose metabolism in specific subgroups of depressed patients (Drevets et al 2002a, 2002b; Drevets and Raichle 1992; Ketter et al 2001; Nofzinger et al 1999). However, chronic effective antidepressant therapy with SSRIs normalizes these parameters (Drevets et al 2002a, 2002b; Sheline et al 2001). The correction of amygdaloid functional abnormalities correlates with clinical improvement and with known onset of action of the SSRIs (Drevets et al 2002a; Sheline et al 2001). This suggests that augmented serotonergic transmission correlates temporally with reversal of both symptoms and functional pathology of the amygdala.
While the amygdala has traditionally been thought of as a homogenous structure, it is composed of multiple nuclei, which are highly interconnected (Aggleton 1985; Pitkanen et al 1997). The basolateral nuclei (basal, lateral, and accessory basal nuclei) of the amygdala are generally regarded as the nuclei which link emotional meaning to complex sensory cues, for example in fear conditioning paradigms (Campeau and Davis 1995; Killcross et al 1997; Parkinson et al 2000; Burns et al 1996; LeDoux et al 1990). The basolateral complex receives converging inputs from the sensory association cortex, the orbital and medial prefrontal cortex, and the hippocampus (Aggleton et al 1980; Carmichael and Price 1996; Ghashghaei and Barbas 2002; Saunders et al 1988; Stefanacci and Amaral 2000, 2002; Turner et al 1980). The corticomedial nuclei include poorly differentiated cortical regions on the medial amygdaloid surface, which are thought to mediate emotional processing involving olfaction (Price 1973). The central nucleus is a key output area that receives inputs from virtually all other amygdaloid nuclei, and sends efferents to the hypothalamus and brainstem (Aggleton et al 1987; Amaral et al 1982; Fudge and Haber 2000; Price and Amaral 1981). Through its subcortical outputs, the central nucleus mediates fear responses, including freezing, startle, and autonomic changes (Applegate et al 1983; Campeau et al 1997; Gray 1993; Hitchcock and Davis 1991; Kalin et al 2004; Kapp et al 1979) (however, see Koo et al 2004). The intercalated cells are clusters of small neurons interspersed in fibers that course around the major amygdala nuclei. While relatively neglected compared to other amygdala regions, recent studies show that the intercalated cell islands are important in tightly regulating trafficking of information from the basolateral nuclei to the ‘output station’ of the central nucleus (Quirk et al 2003; Royer et al 1999). The morphology and chemical profile of the intercalated cells suggests that they are GABAergic (inhibitory) neurons, and are composed, in part, of immature neurons in the primate (Fudge 2004; Millhouse 1986; Pitkanen and Amaral 1994).
The serotonergic innervation of the primate amygdala has been established for years (Azmitia and Gannon 1986; Felten and Sladek 1983; Freedman and Shi 2001; Sadikot and Parent 1990). However, despite the fact that the amygdala contains some of the highest 5HT levels in the brain (Azmitia and Gannon 1986), there is little information on the specific innervation of discrete nuclei by fibers containing 5-HTT. Since different amygdaloid nuclei mediate distinct components of emotional processing, we examined the relative distribution of the 5-HTT positive fibers in specific amygdala subregions. We used immunocytochemistry for 5-HTT as a way to look at the precise anatomic innervation by serotonergic fibers, since 5HT is also found in extra-neuronal structures (for review see Azmitia 1999), and because 5-HTT is the site of SSRI action. The specific distribution of 5-HTT positive fibers provides one index of the relative effects of SSRIs on various amygdala subregions.
Methods and Materials
Tissue Preparation
Five adult male macaques (cases J1, J2, J3, J5, J6) and one adult female (case J7) were used in these studies. Two animals were approximately 2 years old (juvenile), two animals were 3 years old, one animal was 4 years old, and one animal was 8 years old (young adults). All experiments were carried out in accordance with National Institutes of Health guidelines. Experimental design and techniques were aimed at minimizing animal use and suffering, and were reviewed and approved by the University of Rochester Committee on Animal Research. Following initial anesthesia by an intramuscular injection of ketamine (10 mg/kg), animals were deeply anesthesized with intravenous pentobarbitol. They were perfused through the heart with saline, followed by a 4% paraformaldehyde solution in .1M phosphate buffer (pH 7.4). The brains were cryoprotected in 4% paraformaldehyde overnight and then in increasing gradients of sucrose (10%, 20%, and finally 30%). Serial sections of 50 μm were cut on a freezing microtome into .1M phosphate buffer or cryoprotectant solution. Adjacent sections were processed individually for 5-HTT and Nissl staining. Additional compartments were double-stained for 5-HTT and Nissl. Comparisons were also made with adjacent compartments immunostained for 5HT.
Immunocytochemistry
Sections to be immunoreacted for 5-HTT were rinsed thoroughly in .1 M phosphate buffer (pH 7.4) containing .3% Triton-X (PB-T), and then incubated for 4 nights in primary antibody (1:150,000 dilution [human anti-mouse, MAB Technologies, Inc., Stone Mountain, Georgia]) diluted in a solution containing 10% normal goat serum and .5% bovine serum albumin (Sigma, St. Louis, Missouri) in PB-T. The 5-HTT molecule was then visualized using the avidin-biotin reaction (Vectastain ABC kit, Vector Labs, Burlingame, California). Control sections in which the primary antibody was omitted were processed simultaneously and failed to show labeling. These sections were processed in parallel with 5-HT labeled sections according to the procedure above, using anti-5HT (rabbit polyclonal, 1:100,000 Immunostar, Hudson, Wisconsin). Staining for all reactions was enhanced by incubating the sections for 1–3 minutes in 3,3′ diaminobenzidine tetra-hydrochloride (DAB) and .01% H2O2 intensified with 1% cobalt chloride and 1% nickel ammonium sulfate. Sections were then mounted on gelatin-coated slides, dehydrated, and cover-slipped.
Analysis
Sections through the amygdala labeled with 5-HTT antibody were examined under bright field and dark field illumination. Fibers were charted by hand using the 10X objective and a drawing tube attached to the microscope. Charts were then scanned into the computer in the application Adobe Photoshop 6.0 using an Epson 3200 scanner. Scanned charts were converted to bitmaps and imported into the drawing program Adobe Illustrator 10.0. Nuclear boundaries were determined using Nissl and acetylcholinesterase (AChE)-stained adjacent sections. Additional compartments were counter-stained with cresyl violet. For analytical purposes, the relative concentrations of labeled fibers were classified along a gradient: + (light), ++ (moderate), +++ (moderately heavy), ++++ (heavy).
Results
Nomenclature of the Monkey Amygdaloidal Complex
Definitions of Subdivisions of the Amygdala (Figure 1A–D)
Figure 1.
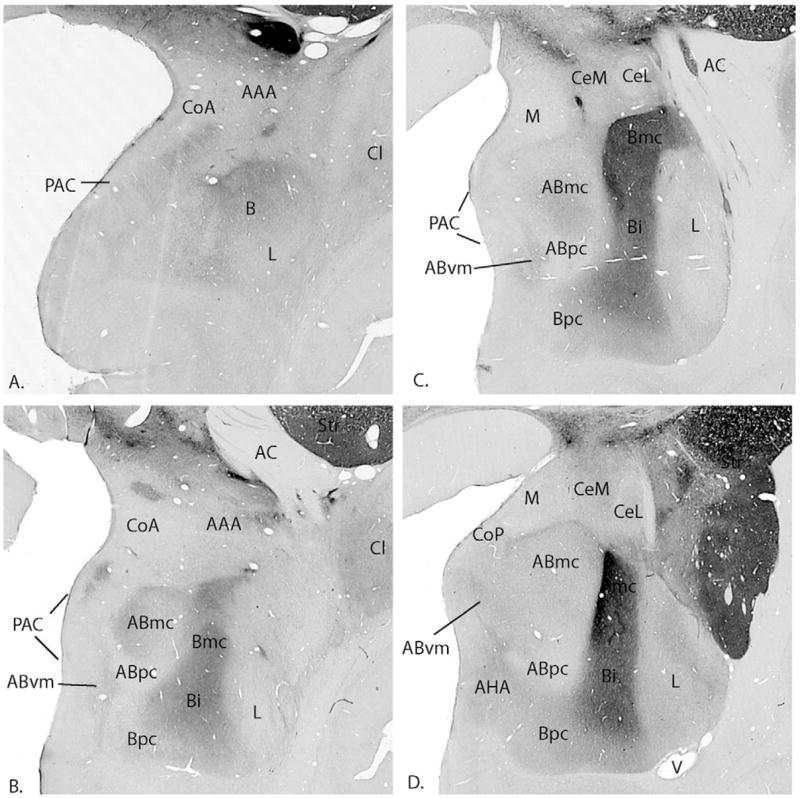
AChE stained sections show the various amygdaloid nuclei at four rostrocaudal levels. The section in (A) is the most rostral. AAA, anterior amygdaloid area; AC, anterior commissure; Abmc, accessory basal nucleus, magnocellular subdivision; Abpc, accessory basal nucleus, parvicellular subdivision; Abvm, accessory basal nucleus, ventromedial; ACA, amygdaloclaustral area; AHA, amygdalohippocampal area; Astr, amygdalostriatal area; Bi, basal nucleus, intermediate subdivision; Bmc, basal nucleus, magnocellular subdivision; Bpc, basal nucleus, parvicellular subdivision; CeL, Central nucleus, lateral core subdivision; CeM, central nucleus, medial subdivision; Cl, claustrum; CoA, cortical nucleus, anterior subdivision; CoP, cortical nucleus, posterior subdivision; L, lateral nucleus; M, medial nucleus; PAC, periamygdaloid cortex; Str, striatum; V, ventricle.
The amygdaloidal complex is detailed using the nomenclature of Price, Amaral, and colleagues, according to cytoarchitectural and histochemical characteristics described previously (Amaral and Bassett 1989; Price et al 1987). The lateral (L), basal (B), and accessory basal (AB) nuclei collectively make up the basolateral complex (Figure 1A–D). The lateral nucleus is differentiated from the basal nucleus by its smaller cells and lower concentrations of AChE. Caudally, the lateral nucleus can be subdivided into dorsomedial and ventrolateral divisions. The basal nucleus is composed of the parvicellular (Bpc), intermediate (Bi), and magnocellular (Bmc) subdivisions, which are differentiated both cytoarchitecturally and by an increasing gradient of AChE staining. The basal nucleus is bordered at its rostral and caudal poles by the paralaminar nucleus, a highly cellular nucleus that is best appreciated in Nissl-stained sections. The paralaminar nucleus has been considered part of the basal nucleus in the primate (Crosby and Humphrey 1941; Amaral and Price 1984). The accessory basal nucleus is subdivided into parvicellular (ABpc), magnocellular (ABmc), ventromedial (ABvm) subdivisions. The ABmc and ABvm have intermediate AChE staning while the parvicellular subdivision has little AChE activity. The anterior cortical nucleus (CoA), the medial nucleus (M), posterior cortical nucleus (CoP), and the periamygdaloid cortex (PAC) comprise the “corticomedial” structures. The PAC is a heterogeneous two to three-layered cortex, that merges caudally with the amygdalohippocampal area (AHA) and the rostral entorhinal cortex. The AHA, an undifferentiated cortical area, is dense with small cells and high AChE staining, and serves as a bridge between the caudal PAC and the subiculum of the hippocampus. The intercalated islands are groups of small, dark staining cells often surrounded by a fibrous capsule, which are lodged in fibers surrounding the major amygdaloid nuclei. Like the paralaminar nucleus, they are best appreciated in Nissl stained sections. The central nucleus is located in the caudal half of the primate amygdala and is contiguous with the anterior amygdaloid area rostrally. It is composed of two main subdivisions, medial and lateral. The medial subdivision of the central nucleus has small to medium sized cells and shows moderate staining with AChE. The lateral subdivision is an ovoid structure surrounded by a fibrous capsule and is characterized by low, homogeneous AChE activity.
5-HTT in the Amygdaloid Subregions
The serotonin transporter (5-HTT) is distributed throughout the primate amygdala with virtually no anatomic areas devoid of labeling by 5-HTT-positive fibers. However, there is a differential distribution of labeled fibers within specific subregions, as detailed below (Figure 2A–F). The majority of 5-HTT positive fibers are thin and very finely beaded, although thick nonvaricose fibers are seen coursing through the amygdala. The latter were interpreted as nonterminating fibers of passage and were not charted. The distribution of labeled fibers was similar in all animals studied, and there were no apparent gender differences based on comparisons with one female animal (J7). We also did not appreciate significant interhemispheric differences in the distribution of 5-HTT positive fibers. However, subtle but potentially meaningful differences in the quantities of 5-HTT protein cannot be detected by immunocytochemical techniques, which is most useful in detecting the fine anatomy and relative concentrations of labeled fibers.
Figure 2.
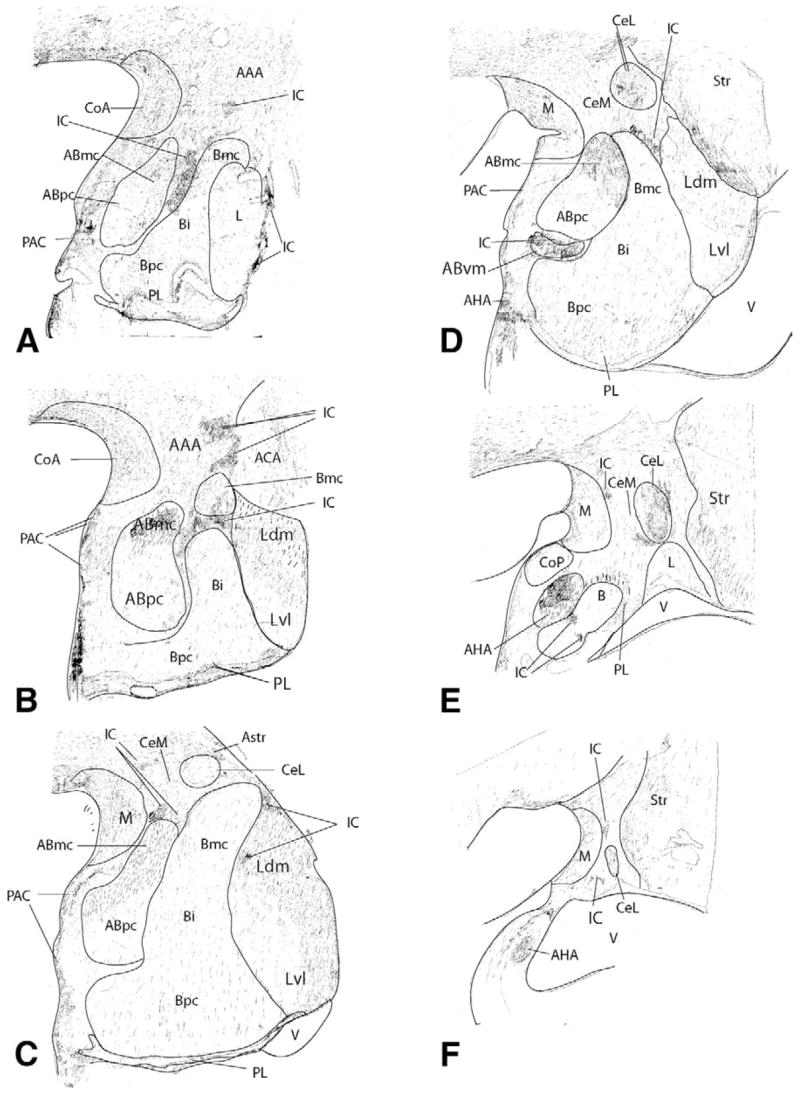
Hand-drawn charts of 5-HTT labeled sections through the rostrocaudal extent of the amygdala (case J1). AAA, anterior amygdaloid area; AC, anterior commissure; Abmc, accessory basal nucleus, magnocellular subdivision; Abpc, accessory basal nucleus, parvicellular subdivision; Abvm, accessory basal nucleus, ventromedial; ACA, amygdaloclaustral area; AHA, amygdalohippocampal area; Astr, amygdalostriatal area; Bi, basal nucleus, intermediate subdivision; Bmc, basal nucleus, magnocellular subdivision; Bpc, basal nucleus, parvicellular subdivision; CeL, Central nucleus, lateral core subdivision; CeM, central nucleus, medial subdivision; CI, claustrum; CoA, cortical nucleus, anterior subdivision; CoP, cortical nucleus, posterior subdivision; IC, intercalated cell islands; Ldm, lateral nucleus, dorsomedial subdivision; Lvl, lateral nucleus, ventrolateral subdivision; M, medial nucleus; PL, parlaminar nucleus; Str, striatum; V, ventricle.
Basal Nucleus/Paralaminar Nucleus
The basal nucleus showed variable concentrations of 5-HTT positive fibers throughout the rostrocaudal extent of the amygdala. At rostral levels, the magnocellular subdivision contained moderate to moderately heavy concentrations of labeled fibers (Figure 2A–B). The intermediate subdivision and parvicellular subdivisions contained light to moderate concentrations of labeled fibers, with the exception of the paralaminar nucleus, which was outlined with high concentrations of 5-HTT immunoreactive fibers. At central levels of the amygdala, 5-HTT immunoreactive fibers were seen in a light to moderate, diffuse meshwork across all subdivisions (Figure 2C–D). At caudal levels, 5-HTT labeled fibers were densely concentrated in the caudal paralaminar nucleus at its junction with the lateral ventricle (Figure 2E and 3B). The basal nucleus proper contained a moderate innervation of labeled fibers.
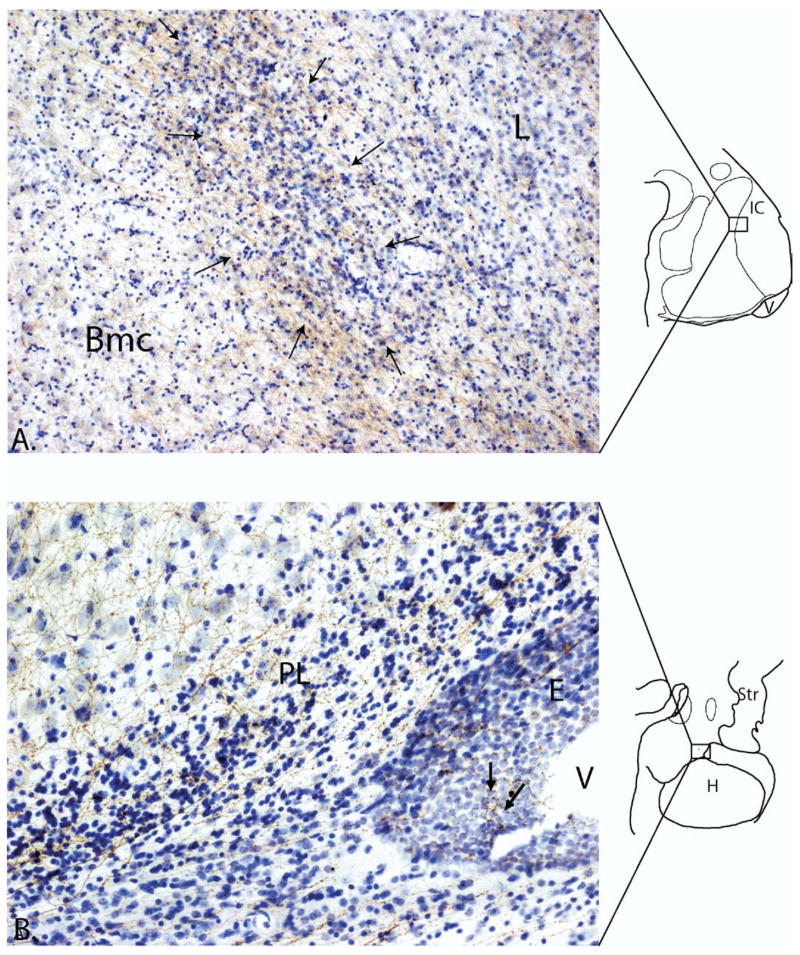
Figure 3.
(A) Photomicrograph of 5-HTT-positive fibers (brown) densely innervating an intercalated island (arrows). The basal nucleus to the left, and lateral nucleus to the right, both contain a lighter innervation by 5-HTT labeled fibers. The island is interposed between the basal and lateral nucleus which have larger, less densely packed cells (boxed area of the schematic shows position of the intercalated island). (B) Photomicrograph of 5-HTT labeled fibers (brown) in a Nissl stained caudal section through the amygdala. Labeled fibers in the paralaminar nucleus near the lateral ventricle extend to the ependymal lining (arrows). Bmc, basal nucleus, magnocellular subdivision; PL, paralaminar nucleus; V, ventricle; E, ependymal lining of ventricle; L, lateral nucleus; H, hippocampus; IC, intercalated cell islands; Str, striatum.
Lateral Nucleus
The lateral nucleus showed differential labeling in a rostro-caudal and dorsal-ventral gradient. In rostral sections, the lateral nucleus had light concentrations of 5-HTT labeled fibers (Figure 2A). However, at central levels where the two main subdivisions of the lateral nucleus are first seen, distinct staining patterns emerged. The ventrolateral subdivision of the lateral nucleus contained light concentrations of labeled fibers while the dorsomedial subdivision had moderate concentrations of 5-HTT positive fibers (Figure 2B–D).
Accessory Basal Nucleus
The magnocellular subdivision of the basal nucleus had moderately heavy concentrations of 5-HTT positive fibers (Figure 2A–D). In contrast, the parvicellular subdivision maintained light concentrations of labeled fibers throughout its rostrocaudal axis. The ventromedial subdivision, which is caudal and medial to the magnocellular and parvicellular subdivisions, had moderately heavy concentrations of labeled fibers similar to the magnocellular subdivision (Figure 2D).
Corticomedial Amygdala
The piriform cortex is just anterior to the anterior cortical nucleus and merges with it. Both structures are densely innervated by 5-HTT positive fibers (Figure 2A–B). Caudal to the anterior cortical nucleus, the medial nucleus had reduced densities of 5-HTT positive fibers, although the dorsal rostral medial nucleus had a high concentration of labeled fibers similar to that in the anterior cortical nucleus (Figures 2C–F, 4A–B). The posterior cortical nucleus also had relatively few 5-HTT labeled fibers (Figure 2E). The thin molecular layer (layer 1) of both the medial nucleus and posterior cortical nucleus contained moderate densities of labeled fibers, similar to the rest of the medial cortical structures (Figure 2D–E). The periamygdaloid cortex had moderately heavy concentrations of 5-HTT positive fibers in Layer 1, with occasional dense patches of labeled fibers in Layer II. The amygdaloid and hippocampal portions of the amygdalohippocampal (AHA) area, visible in the caudal sections (Figure 2E–F, respectively), showed a heavy concentration of 5-HTT labeled fibers (Figure 5A–B).
Figure 4.
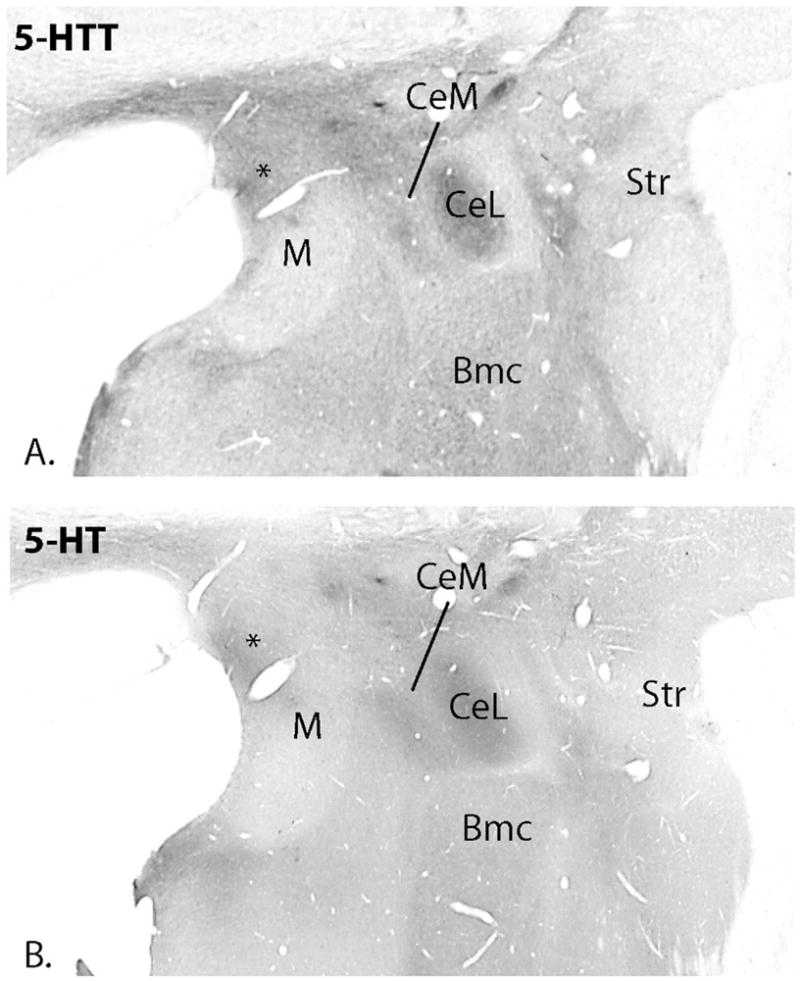
Low power photomicrographs of 5-HTT labeled (A) and 5-HT labeled (B) adjacent sections. Labeling patterns are similar but 5-HT immunoreactivity is less distinct, presumably because serotonin is also located in the extracellular space and glia. Note the relatively dense immunoreactivity in the central nucleus. The dorsal medial nucleus contains more immunoreactivity for both molecules than the ventral medial nucleus (*).
Figure 5.
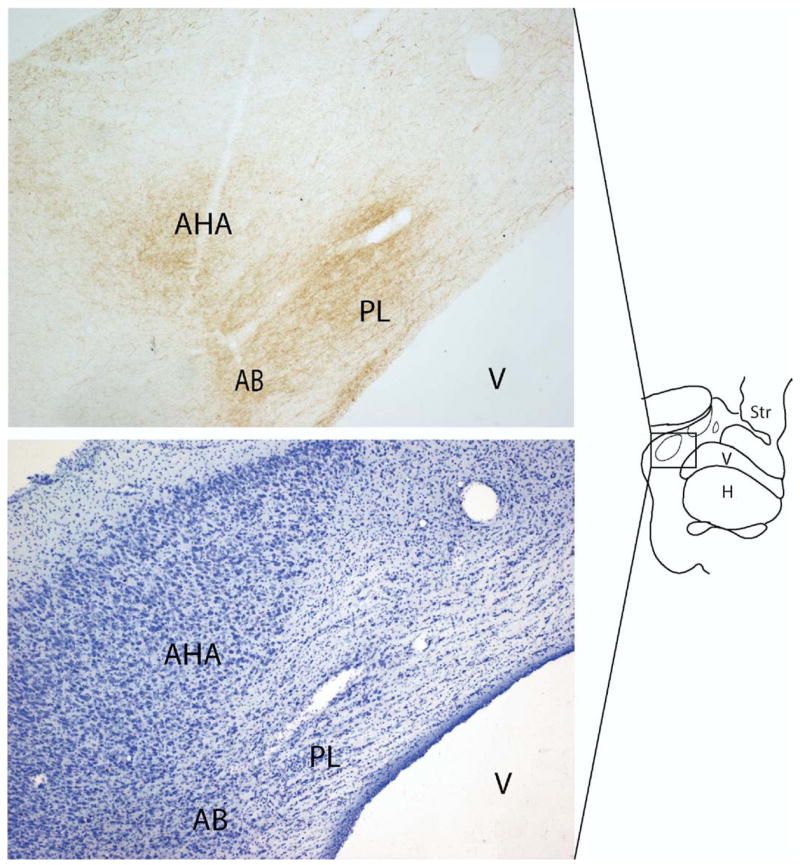
(A) The amygdalohippocampal area and paralaminar nucleus in the caudal amygdala contain a high density of 5-HTT labeled fibers. (B) is an adjacent Nissl stained section. AHA, amygdalohippocampal area; AB, accessory basal nucleus; PL, paralaminar nucleus; V, ventricle; H, hippocampus; Str, striatum.
Central Nucleus
The lateral subdivision of the central nucleus stood out as containing among the highest density of 5-HTT labeled fibers in the amygdala, particularly at its caudal pole (Figure 2D–F, Figure 4A–B). Only the rostral-most aspect of the lateral subdivision of the central nucleus contained a light concentration of labeled fibers (Figure 2C). In contrast, the medial subdivision of the central nucleus had more moderate concentrations of 5-HTT labeled fibers throughout.
Intercalated Islands
The intercalated islands showed heavy concentrations of 5-HTT immunoreactive fibers. At rostral levels, dense concentrations of 5-HTT labeled fibers overlapped a small intercalated island embedded in the anterior amygdaloid area and a much larger island interposed between the basal nucleus and accessory basal nucleus (Figure 2A) At levels slightly caudal to this, a relatively large intercalated island was seen in the anterior amygdaloid area, which again contained a high concentration of labeled fibers. Densely concentrated 5-HTT labeled fibers overlaid intercalated cells which were interspersed between the magnocellular and intermediate subdivisions of the rostral basal nucleus (Figure 2B). At central levels of the amygdala, small intercalated islands dotted the area between the central nucleus and the basal and magnocellular accessory basal nuclei. These contained many 5-HTT immunoreactive fibers. There were also intercalated islands containing 5-HTT labeled fibers between the lateral nucleus and magnocellular basal nucleus, and interposed between the parvicellular and ventromedial subdivisions of the accessory basal nucleus (Figure 2C–D, Figure 3A). In caudal sections, a small intercalated island containing labeled fibers was seen between the medial and central nucleus, and several small densely innervated intercalated islands were lodged in fiber tracts surrounding the basal nucleus (Figure 2E, F).
Discussion
Summary of Findings
While the entire primate amygdala is innervated by 5-HTT immunoreactive fibers, there are significant differences in the relative concentrations of 5-HTT labeled fibers within specific subregions (Table 1). Overall patterns of 5-HTT immunoreactivity were similar to those achieved with 5-HT labeling. However, the fine details of fiber plexi, particularly within small structures such as the intercalated islands, were much harder to appreciate with the latter. Based on our 5-HTT results, heavy concentrations of labeled fibers exist in the intercalated cell islands (Figure 3A), the paralaminar nucleus near the lateral ventricle, the amygdalohippocampal area (Figures 3B, Figure 5), and the lateral subdivision of the central nucleus (Figure 4). Moderately heavy concentrations of labeled fibers were found in the anterior cortical nucleus, the periamygdaloid cortex (layer I), and the magnocellular and ventromedial subdivisions of the accessory basal nucleus. The medial subdivision of the central nucleus, dorsal subdivision of the lateral nucleus, and rostral magnocellular and caudal parvicellular subdivisions of the basal nucleus contained moderate concentrations of labeled fibers. Several amygdaloid subregions had a relatively light, diffuse meshwork of 5-HTT labeled fibers: the posterior cortical nucleus, the ventrolateral subdivision of the lateral nucleus, the medial nucleus (except for its rostral dorsal sector), and the parvicellular subdivision of the accessory basal nucleus. 5-HTT labeled fibers in the paralaminar nucleus followed its sheet-like course under the basal nucleus to envelope the rostral dorsal pole of the basal nucleus. The density of labeled fibers in the paralaminar nucleus was variable, with relatively high concentrations of labeled fibers near the lateral ventricle (Figures 2E, 3B, 5A–B) and surrounding the dorsal basal nucleus (Figure 2A). Relatively lighter densities of labeled fibers occupied the paralaminar nucleus at central levels of the basal nucleus.
Table 1.
Relative Densities of 5-HTT Labeled Fibers in Amygdaloid Nuclei
| Nuclear Regions | 5-HTT Labeled Fibers |
|---|---|
| Basal Nucleus | |
| Magnocellular subdivision | +-+++ |
| Parvicellular subdivision | +-+++ |
| Intermediate subdivision | +-++ |
| Paralaminar nucleus | +-++++ |
| Lateral Nucleus | |
| Ventrolateral subdivision | + |
| Dorsomedial subdivision | ++ |
| Accessory Basal Nucleus | |
| Magnocellular subdivision | +++ |
| Parvicellular subdivision | + |
| Ventromedial subdivision | +++ |
| Corticomedial Amygdala | |
| Anterior cortical nucleus | +++ |
| Medial nucleusa | + |
| Posterior cortical nucleus | + |
| Periamygdaloid cortex | +++ |
| Central Nucleus | |
| Lateral subdivision | ++++ |
| Medial subdivision | ++ |
| Amygdalohippocampal Area | ++++ |
| Intercalated Cell Islands | ++++ |
Relative density of labeled fibers: + light, ++ moderate, +++ moderately heavy, ++++ heavy.
Except dorsal, rostral sector (see Figure 4).
Comparison with Previous Studies
While previous primate studies have examined the distribution of serotonin via immunofluorescence and immunostaining for 5HT (Azmitia and Gannon 1986; Sadikot and Parent 1990), this is the first study to examine the differential innervation of the amygdaloid nuclei by 5-HTT immunoreactive fibers. Our findings are generally consistent with one previous study of 5-HT immunoreactivity in New World monkeys, with several important exceptions (Sadikot and Parent 1990). Similarities include relatively high concentrations of 5HT- or 5HTT-labeled fibers in the amygdalohippocampal area, periamygdaloid cortex, and in the anterior cortical nucleus. Low concentrations of 5-HT and 5-HTT labeled fibers are found in the medial nucleus of each species, respectively, a result also consistent with one previous study of the Macaque ‘extended amygdala’ (Freedman and Shi 2001). However, in the New World monkey, the lateral subdivision of the central nucleus contains relatively low 5HT immunoreactivity, in contrast to very heavy concentrations of 5-HTT (and 5HT) labeled fibers in Macaques (Figure 5A and B) (see also Freedman and Shi 2001). Another discrepancy is the relative lack of 5HT immunoreactivity in the intercalated islands of the squirrel monkey, which stands in contrast to the present results in the Macaque (Figure 3A) (see also Freedman and Shi 2001). In rodents, 5HT levels in the central nucleus and intercalated islands are closer to the results in the squirrel monkey, rather than the Macaque, despite a similar distribution of 5HT labeled fibers in other amygdaloid nuclei (present results; Freedman and Shi 2001; Steinbusch 1984). One study shows relatively densely concentrated 5-HTT immunoreactive fibers in the basal nucleus of the rodent with moderate concentrations in the dorsal lateral nucleus; however, other nuclei are not described (Sur et al 1996). Differences in the relative density of 5-HTT/5HT in the central nucleus may be a true species difference, or reflect difficulties in identifying the components of the central nucleus across species. For example, in Macaques and humans, the lateral subdivision of the central nucleus has low, homogeneous levels of AchE (Amaral and Bassett 1989; DeOlmos 1990; present results), in contrast to the ‘moderately dense’ AChE staining described in New World monkeys (Sadikot and Parent 1990). The central nucleus in humans and Macaques shares a similar, though not identical, distribution of neuropeptides, which vary somewhat from the pattern found in the rodent (Martin et al 1991). While incompletely studied, there appears to be a significant degree of homology between the human and Macaque (Old World monkeys) in terms of the organization and histochemical characteristics of the amygdala as a whole (DeOlmos 1990; Heimer et al 1999; Martin et al 1991). In general, the basolateral complex, which accounts for most of the amygdala’s size, is disproportionately expanded in primates compared to other species, and parallels the massive expansion of the cortex, its main afferent source (Barton et al 2003; Stephan et al 1987).
The relative densities of 5-HTT labeled fibers in specific amygdaloid nuclei of the Macaque have been previously assessed using radiolabeled serotonin selective reuptake inhibitors ([3H]citalopram) (Smith et al 1999). [3H]Citalopram specifically binds 5-HTT, and can therefore be used to quantify the relative amount of this molecule in each nuclear region. While less sensitive for detailing the precise anatomic distribution of 5-HTT, [3H]citalopram binding provides an important quantifiable measure of 5-HTT activity in specific brain regions. Using this technique, Smith et al (1999) found the densest SSRI binding in the central nucleus, and relatively lower levels of binding in the lateral, basal, medial, and accessory basal nuclei, consistent with our immunocytochemical results. In addition there was differential activity in the accessory basal nucleus with activity in the magnocellular subdivision higher than that in the parvocellular division, further supporting the differential pattern of 5-HTT innervation found in the present study.
Functional Implications
Neurotransmission
The localization of 5-HTT labeled fibers within specific amygdaloid subdivisions has implications for the modulation of specific circuits. The basal, accessory basal, and lateral nuclei of the ‘basolateral complex’ are the main receiving nuclei. These nuclei are composed of pyramidal projection neurons containing glutamate, and nonpyramidal cells (interneurons) which contain GABA (Fuller et al 1987; McDonald and Augustine 1993). Sensory association afferents from the temporal cortex project to the lateral nucleus, and this information is in turn channeled to the basal and accessory basal nuclei (Aggleton et al 1980; Pitkanen and Amaral 1998; Turner et al 1980). Sensory association information is then further modulated by excitatory inputs from the orbital and medial prefrontal cortex and hippocampus (Aggleton 1986; Carmichael and Price 1996; Ghashghaei and Barbas 2002; Rosenkranz and Grace 2001; Saunders et al 1988). 5HT, arising from projections in the dorsal raphe, modulates these excitatory inputs, an effect that is blocked by GABA antagonists (Imai et al 1986; Jacobs et al 1978; Koyama et al 1999; Rainnie 1999; Stutzmann and LeDoux 1999; Wang and Aghajanian 1977). This suggests that 5HT stimulates GABA interneurons, which in turn damp excitatory responses in the basolateral amygdala projection neurons. The fine meshwork of 5-HTT fibers found throughout the basolateral complex indicates that serotonergic modulation of inhibitory tone is important to the normal processing of information in these receiving nuclei.
Although the receptor profile mediating 5HT transmission in the amygdala is far from delineated, the distribution of the 5HT1A receptor, which is a somatodendritic postsynaptic receptor in brain regions outside the dorsal raphe (Pompeiano et al 1992), shows some striking parallels to the distribution of 5-HTT labeled fibers, based on human ligand binding studies (Ohuoha et al 1993; Pazos et al 1987). Like the distribution of 5HTT in the present results, [3H]8-OH-DPAT, a 5HT1A ligand, is highly concentrated in the ‘granular’ subregion (corresponding to the parvicellular basal nucleus and paralaminar nucleus), and the ‘transitory zone’ (in the region of the amygdalohippocampal area), with more intermediate levels in the basal, accessory basal and lateral nucleus. Chronic SSRI treatment results in increased synaptic 5HT mediated by desensitization of 5HT1A autoreceptors in the raphe. Subsequently, enhanced 5HT levels result in altered neurotransmission, at least in the rat hippocampus, since 5HT1A post-synaptic receptors in this region do not desensitize (Chaput et al 1986; de Montigny and Blier 1984; Haddjeri et al 1998). The availability of 5HT1A receptors in amygdaloid subregions (Ohuoha et al 1993; Pazos et al 1987; Pompeiano et al 1992) which also express high concentrations of 5HTT labeled fibers (present results) suggests an anatomic substrate for how increased 5HT levels in specific amygdaloid nuclei might similarly affect 5HT1A-mediated neurotransmission. Whether the 5HT1A hypothesis of antidepressant efficacy can be broadened to include specific amygdaloid subregions, however, remains to be tested.
The basolateral complex sends excitatory projections to the central nucleus, a key ‘effector site’ that mediates fear responses via inputs to the brainstem and hypothalamus (Aggleton 1985; Fudge and Haber 2000; Hopkins 1975; Price and Amaral 1981). The intercalated islands, which are interposed between the basolateral complex and central nucleus (Millhouse 1986), play an important role in regulating he flow of information from the basolateral complex to central nucleus outputs. The intercalated islands are inhibitory (GABAergic), modulating the flow of excitatory information from the basolateral complex to the central nucleus, in an arrangement known as ‘feed forward inhibition’ (Collins and Pare 1999; Royer et al 1999). In this way, the intercalated islands serve as a ‘fine tuning’ inhibitory mechanism to gate emotionally relevant information reaching both the medial and lateral subdivisions of central nucleus. The strong 5-HTT-positive innervation of the intercalated islands suggests that tight control of serotonin transmission is important in this gating function. Finally, the central nucleus is itself internally organized such that the lateral subdivision exerts an inhibitory input to the medial subdivision (Pitkanen and Amaral 1994; Pitkanen et al 1997), which is the main output to the brainstem and hypothalamus (Applegate et al 1983; Kapp et al 1982). The presence of very high 5-HTT-positive fibers in the lateral subdivision of the central nucleus suggests that tight regulation of serotonin transmission is an important modulator of inhibitory interface from the lateral central nucleus to the medial subdivision (Figure 6). Thus 5HTT appears to be important in modulating several important sites that inhibit medial central nucleus outputs, namely, the intercalated islands and the lateral core of the central nucleus.
Figure 6.
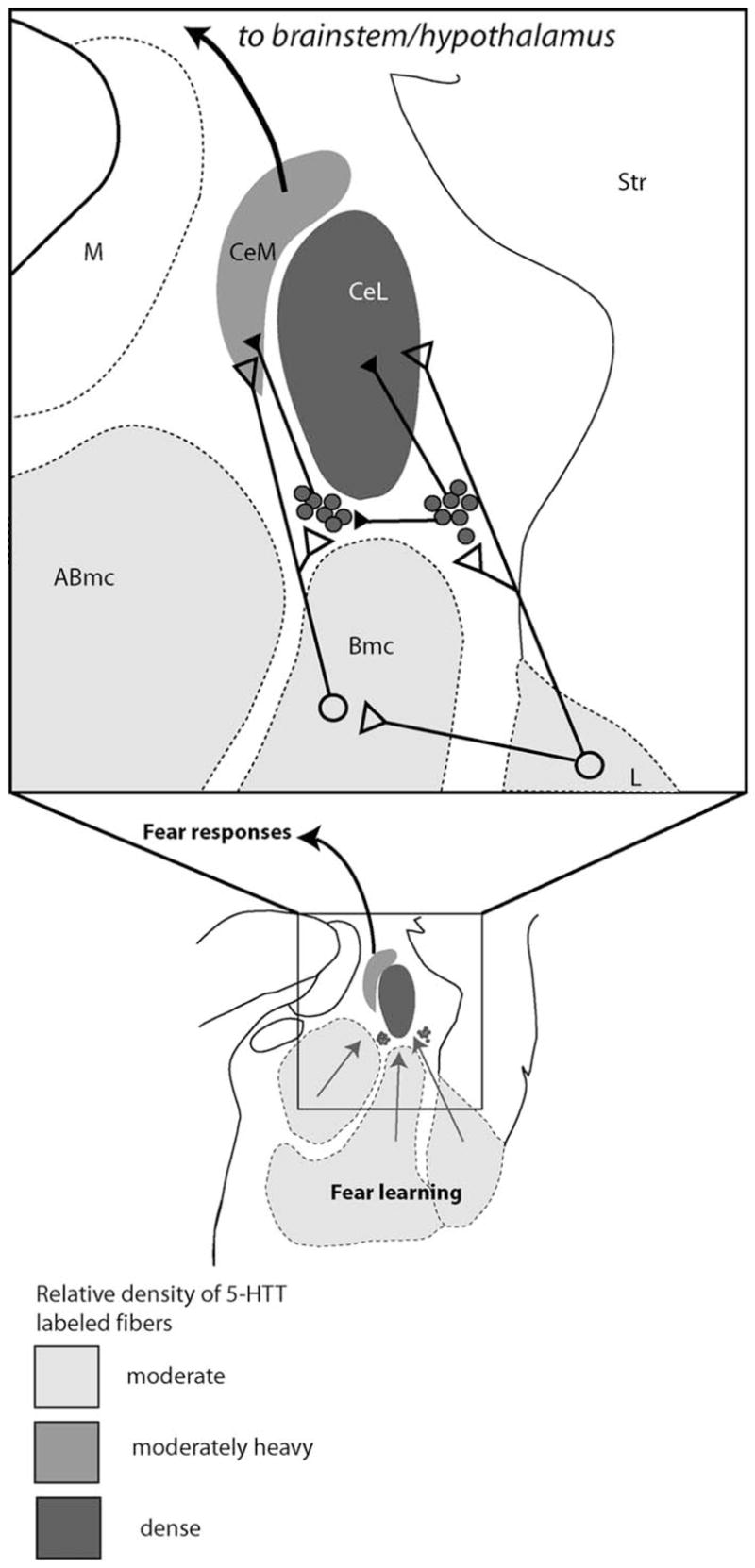
Intrinsic inputs from the basolateral complex to the central nucleus (modified with permission from Pare et al (2003). The intercalated islands (gray dots) serve as feed forward inhibitors of excitatory inputs from the basolateral complex to the central nucleus. The central nucleus, and the intercalated islands which modify its inputs, are both densely innervated by 5-HTT. Open circles/triangles are excitatory circuits; closed circles/triangles are inhibitory. CeM, central nucleus, medial subdivision; M, medial nucleus; Abmc, accessory basal nucleus, magnocellular subdivision; CeL, Central nucleus, lateral core subdivision; Str, striatum; Bmc, basal nucleus, magnocellular subdivision.
The central nucleus is the final relay out of the amygdala to the brainstem, mediating defensive responses to conditioned fear stimuli in animal models (Davis et al 1994; Hitchcock and Davis 1986; Kalin et al 2004; Winslow et al 2002). In humans, the ‘dorsomedial amygdala’ (corresponding to the central nucleus) is activated by switches from neutral to emotionally charged facial expressions (Breiter et al 1996; Etkin et al 2004; Liberzon et al 2003; Whalen et al 1998), and is also activated by graded increases in the intensity of emotional stimuli (Breiter et al 2001; Taylor et al 2000; Thomas et al 2001). Abnormalities in serotonin levels significantly affect the recognition of fearful facial expressions and startle responses, suggesting a key role of serotonin in this amygdaloid subregion (Bhagwagar et al 2004; Harmer et al 2004). Consistent with this, recent studies show that genetic variability in the promotor region of the serotonin transporter gene (serotonin transporter linked polymorphic region, HTTLPR) is associated with anxiety-related traits and may be a contributor to vulnerability to depressive illness (for review see Lotrich and Pollock 2004). Carriers of the ‘S’ (short) allele are thought to have lower 5-HTT protein levels relative to ‘L’ (long) allele homozygotes, indicating an impaired presynaptic regulation of serotonin (Heinz et al 2000; Lesch et al 1996). ‘S’ allele homozygotes and carriers also have increased amygdaloid activation in dorsal amygdala (in an area coinciding with the central nucleus), suggesting that alterations in serotonin reuptake in this region mediate this response (Hariri et al 2002). The central nucleus contains among the highest concentration of 5-HTT immunoreactive fibers in the amygdala based on the present results and previous binding studies (Smith et al l999). Given the dense concentration of 5-HTT containing fibers in the normal central nucleus, functional abnormalities resulting from changes in 5-HTT levels (due to polymorphisms or other causes) would be expected to be most sensitively detected in this amygdaloid area.
Neuroplasticity
The concept of mood disorders as resulting from aberrant neurotransmission has been recently expanded, given new knowledge about changes in intracellular signal transduction and gene expression patterns resulting from antidepressant therapy (for review see Duman 1998). Since mood and anxiety disorders are linked to chronic stress, reductions in brain volume associated with these disorders are hypothesized to be manifestations of accumulated stress events, mediated in part by glucocorticoids (Kaufman et al 2000). Since 5HT mediates a number of growth-related processes including neuronal proliferation, differentiation, migration, and synaptogenesis, it is hypothesized that serotonergic enhancement may help restore neuronal architecture, and even function, in affected brain regions (see reviews by Gaspar et al 2003; Azmitzia 2001). For example, brain-derived neurotrophic factor (BDNF) is one of the growth factors known to be induced by SSRIs (Nibuya et al 1995; Vaidya et al 1997), but this effect has been mainly established in rodent hippocampus. Similarly, neurogenesis induced by SSRIs and other antidepressant treatments has mainly been examined in hippocampal sites as well (Malberg et al 2000; Santarelli et al 2003). However, alterations in both hippocampal and amygdaloid volumes occur in depressed patient populations (Bremner et al 2000; Frodl et al 2003; Mervaala et al 2000; Sheline et al 1996, 1998). These morphologic changes implicate the amygdala, as well as the hippocampus, as a substrate for the deleterious effects of stress, and the potential trophic effects of serotonin, in higher primates (Drevets et al 2002a, 2002b; Sheline et al 2001).
Consistent with the hypothesis that the amygdala may also be a site for trophic effects of SSRIs and other mood stabilizers, our group and others have recently found that immature-appearing neurons exist in the amygdala of normal adult human and nonhuman primates. Bcl-2, a protein that has dual neuroprotective and neurotrophic properties, is abundantly expressed in these relatively undifferentiated cells (Bernier and Parent 1998; Fudge 2004; Yachnis et al 2000). Moreover, immature-appearing bcl-2 positive cells are specifically distributed in the parvicellular basal nucleus/paralaminar nucleus, intercalated cells islands, periamygdaloid cortex and amygdalohippocampal area–regions that have relatively high concentrations of 5-HTT labeled fibers based on the present results (Fudge 2004). The fact that amygdaloid subregions that contain relatively undifferentiated cellular components receive a significant innervation by the serotonin system suggests that serotonin may be one upstream regulator of intracellular cascades involved in cellular growth and differentiation in these cells. Serotonin can potentially mediate transcription of bcl-2 via stimulation of adenylyl cyclase-linked pathways and their associated 5HT receptors, or via serotonin receptors linked to calcium-dependent kinase cascades (for review see Duman et al 1999). Another possible mechanism for bcl-2 expression is its secondary induction by BDNF (Riccio et al 1999), after BDNF upregulation by 5HT. Experiments aimed at detecting co-localization of serotonin receptor subtypes with their intracellular targets will lay the groundwork for determining how specific amygdaloid circuits utilize serotonin for trophic support, and how SSRIs can influence these circuits.
Conclusion
The serotonin system and the amygdala are associated with functional abnormalities in depressive illnesses. However, normal distribution of 5-HTT containing fibers in the amygdala of higher primates has not previously been clarified. The present study shows that 5-HTT containing fibers are found throughout the primate amygdala, with differential densities in specific subregions. The central nucleus and intercalated islands show relatively high levels of 5-HTT/5HT in Macaques compared to lower species. In addition, 5-HTT positive fibers overlap in amygdaloid subregions previously shown to contain immature neurons in higher primates, suggesting a trophic role.
Acknowledgments
This work was supported in part by the Babigian Research Fellowship from the Department of Psychiatry at the University of Rochester Medical Center (HOR), and by CARES (Rochester, New York), and by MH63291 (JLF).
References
- Aggleton JP. A description of intra-amygdaloid connections in old world monkeys. Exper Brain Res. 1985;57:390–399. doi: 10.1007/BF00236545. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. A description of the amygdalo-hippocampal interconnections in the macaque monkey. Exper Brain Res. 1986;64:515–526. doi: 10.1007/BF00340489. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Friedman DP, Mishkin M. A comparison between the connections of the amygdala and hippocampus with the basal forebrain in the macaque. Exper Brain Res. 1987;67:556–568. doi: 10.1007/BF00247288. [DOI] [PubMed] [Google Scholar]
- Agurell S. The research and development of a 5-HT selective reuptake blocker. Preclinical aspects. Acta Psychiatrica Scand Suppl. 1983;308:19–24. [PubMed] [Google Scholar]
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: An immunohistochemical analysis with antisera to choline acetyl-transferase. J Comp Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Veazey RB, Cowan WM. Some observations on hypo-thalamo-amygdaloid connections in the monkey. Brain Res. 1982;252:13–27. doi: 10.1016/0006-8993(82)90974-x. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Beh. 1983;31:353–360. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- Asberg M, Eriksson B, Martensson B, Traskman-Bendz L, Wagner A. Therapeutic effects of serotonin uptake inhibitors in depression. J Clin Psychiatry. 1986;47 Suppl:23–35. [PubMed] [Google Scholar]
- Asberg M, Thoren P, Traskman L, Bertilsson L, Ringberger V. “Serotonin depression”–a biochemical subgroup within the affective disorders? Science. 1976;191:478 – 480. doi: 10.1126/science.1246632. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology. 1999;21:33S–45S. doi: 10.1016/S0893-133X(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv in Neurol. 1986;43:407–468. [PubMed] [Google Scholar]
- Barton RA, Aggleton JP, Grenyer R. Evolutionary coherence of the mammalian amygdala. Proceedings of the Royal Society of London -Series B. Biol Sci. 2003;270:539–543. doi: 10.1098/rspb.2002.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger FM. Commentary. Depression and antidepressant drugs. Clin Pharmacol Ther. 1975;18:241–248. doi: 10.1002/cpt1975183241. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Parent A. Bcl-2 protein as a marker of neuronal immaturity in postnatal primate brain. J Neurosci. 1998;18:2486–2497. doi: 10.1523/JNEUROSCI.18-07-02486.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. Amer J Psychiatry. 2004;161:166–168. doi: 10.1176/appi.ajp.161.1.166. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619 – 639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Amer J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: implication for limbic-striatal interactions. Behav Neurosci. 1996;110:60–73. doi: 10.1037//0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1996;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electro-physiological studies in the rat brain. Naunyn-Schmiedebergs Arch Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pare D. Reciprocal changes in the firing probability of lateral and central medial amygdala neurons [published erratum appears in J Neurosci 1999 19:2841] J Neurosci. 1999;19:836 – 844. doi: 10.1523/JNEUROSCI.19-02-00836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby EC, Humphrey T. Studies of the vertebrate telencephalon: the nuclear pattern of the anterior olfactory nucleus, tuberculum olfactorium and the amygdaloid complex in adult man. J Comp Neurol. 1941;74:309–347. [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–200. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- de Montigny C, Blier P. Effects of antidepressant treatments on 5-HT neurotransmission: electrophysiological and clinical studies. Adv Biochem Psychopharmacol. 1984;39:223–239. [PubMed] [Google Scholar]
- DeOlmos JS. Amygdala. In: Paxinos G, editor. The Human Nervous System. San Diego: Academic Press; 1990. pp. 583–710. [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Euro Neuropsychopharmacol. 2002a;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Beh. 2002b;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull. 1992;28:261–274. [PubMed] [Google Scholar]
- Duman RS. Novel therapeutic approaches beyond the serotonin receptor. Biol Psychiatry. 1998;44:324–335. doi: 10.1016/s0006-3223(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Regan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Felten DL, Sladek JR., Jr Monoamine distribution in primate brain. V. Monoaminergic nuclei: anatomy, pathways and local organization. Brain Res Bull. 1983;10:171–284. doi: 10.1016/0361-9230(83)90045-x. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Shi C. Monoaminergic innervation of the macaque extended amygdala. Neuroscience. 2001;104:1067–1084. doi: 10.1016/s0306-4522(01)00157-9. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Fudge JL. Bcl-2 immunoreactive neurons are differentially distributed in subregions of the amygdala and hippocampus of the adult macaque. Neuroscience. 2004;127:539–556. doi: 10.1016/j.neuroscience.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience. 2000;97:479–494. doi: 10.1016/s0306-4522(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamatergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258:317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann NY Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Guan XM, McBride WJ. Fluoxetine increases the extracellular levels of serotonin in the nucleus accumbens. Brain Res Bull. 1988;21:43–46. doi: 10.1016/0361-9230(88)90118-9. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala.[comment] Science. 2002;297:400 – 403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Amer J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Heimer L, De Olmos JS, Alheid GF, Person J, Sakamoto N, Shinoda K, et al. The human basal forebrain. Part II. Handbook of Chemical Neuroanatomy. In: Bloom FE, Bjorkland A, Hokfelt T, editors. The Primate Nervous System, Part III. Vol. 15. Amsterdam: Elsevier; 1999. pp. 57–226. [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity.[see comment] Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Beh Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Beh Neurosci. 1991;105:826 – 842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Hopkins DA. Amygdalotegmental projections in the rat, cat, and rhesus monkey. Neuroscience Lttr. 1975;1:263–270. doi: 10.1016/0304-3940(75)90041-5. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Experimental pharmacology of selective 5-HT reuptake inhibitors: differences and similarities. Clin Neuropharmacol. 1984;7:866 – 867. [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Foote SL, Bloom FE. Differential projections of neurons within the dorsal raphe nucleus of the rat: a horseradish peroxidase (HRP) study. Brain Res. 1978;147:149–153. doi: 10.1016/0006-8993(78)90779-5. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Beh. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Gallagher M, Underwood MD, McNall CL, Whitehorn D. Cardiovascular responses elicited by electrical stimulation of the amygdala central nucleus in the rabbit. Brain Res. 1982;234:251–262. doi: 10.1016/0006-8993(82)90866-6. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons–adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Koo J, Han J-S, Kim J. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Kubo C, Rhee JS, Akaike N. Presynaptic serotonergic inhibition of GABAergic synaptic transmission in mechanically dissociated rat basolateral amygdala neurons. J Physiol. 1999;518:525–538. doi: 10.1111/j.1469-7793.1999.0525p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region.[see comment] Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28:726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG. Meta-analysis of serotonin transporter polymorphisms and affective disorders. Psych Genetics. 2004;14:121–129. doi: 10.1097/00041444-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Powers RE, Dellovade TL, Price DL. The bed nucleus-amygdala continuum in human and monkey. J Comp Neurol. 1991;309:445–485. doi: 10.1002/cne.903090404. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;2:281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vaini P, Partanen K, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA, Nichols TE, Meltzer CC, Price J, Steppe DA, Miewald JM, et al. Changes in forebrain function from waking to REM sleep in depression: preliminary analyses of [18F]FDG PET studies. Psychiatry Res. 1999;91:59–78. doi: 10.1016/s0925-4927(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Ohuoha DC, Hyde TM, Kleinman JE. The role of serotonin in schizophrenia: an overview of the nomenclature, distribution and alterations of serotonin receptors in the central nervous system. Psychopharmacology. 1993;112:S5–15. doi: 10.1007/BF02245003. [DOI] [PubMed] [Google Scholar]
- Pare CM, Yeung DP, Price K, Stacey RS. 5-hydroxytryptamine, nor-adrenaline, and dopamine in brainstem, hypothalamus, and caudate nucleus of controls and of patients committing suicide by coal-gas poisoning. Lancet. 1969;2:133–135. doi: 10.1016/s0140-6736(69)92442-8. [DOI] [PubMed] [Google Scholar]
- Pare D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann NY Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain–III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience. 1987;21:97–122. doi: 10.1016/0306-4522(87)90326-5. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohistochemical and in situ hybridization study. J Neurosci. 1994;14:2200–2224. doi: 10.1523/JNEUROSCI.14-04-02200.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala [published erratum appears in Trends Neurosci (1998) 21:52] [see comments] Trends in Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol. 1973;150:87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Hokfelt BT, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Amsterdam: Elsevier; 1987. pp. 279–381. [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800 – 8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69 – 85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090 – 4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot AF, Parent A. The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience. 1990;36:431–447. doi: 10.1016/0306-4522(90)90439-b. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants.[see comment] Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL, Van Hoesen GW. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and nonreciprocal connections. J Comp Neurol. 1988;271:185–207. doi: 10.1002/cne.902710203. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression.[erratum appears in Neuroreport (1998) 9:2436] Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Nat Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Daunais JB, Nader MA, Porrino LJ. Distribution of [3H]citalopram binding sites in the nonhuman primate brain. Ann NY Acad Sci. 1999;877:700–702. doi: 10.1111/j.1749-6632.1999.tb09305.x. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. J Comp Neurol. 2000;421:52–79. doi: 10.1002/(sici)1096-9861(20000522)421:1<52::aid-cne4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Steinbusch HMW. Serotonin-immunoreactive neurons and their projectns in the CNS. In: Bjorklund A, Hokfelt T, Kuhar MJ, editors. Handbook of Chemical Neuroanatomy. Vol. 3. Amsterdam: Elsevier Science Publishers; 1984. pp. 68–125. [Google Scholar]
- Stephan H, Frahm HD, Baron G. Comparison of brain structure volumes in insectivora and primates VII. Amygdaloid components. J Hirn-forsch. 1987;5:571–584. [PubMed] [Google Scholar]
- Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19:RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Betz H, Schloss P. Immunocytochemical detection of the serotonin transporter in rat brain. Neuroscience. 1996;73:217–231. doi: 10.1016/0306-4522(96)00030-9. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human mono-amine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38:1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Turner BH, Mishkin M, Knapp M. Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. J Comp Neurol. 1980;191:515–543. doi: 10.1002/cne.901910402. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2a receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag HM. New evidence of serotonin-deficient depressions. Neuropsychobiology. 1977;3:56 – 63. doi: 10.1159/000117590. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Inhibition of neurons in the amygdala by dorsal raphe stimulation: mediation through a direct serotonergic pathway. Brain Res. 1977;120:85–102. doi: 10.1016/0006-8993(77)90499-1. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Parr LA, Davis M. Acoustic startle, prepulse inhibition, and fear-potentiated startle measured in rhesus monkeys. Biol Psychiatry. 2002;51:859 – 866. doi: 10.1016/s0006-3223(02)01345-8. [DOI] [PubMed] [Google Scholar]
- Yachnis AT, Roper SN, Love A, Fancey JT, Muir D. Bcl-2 immunoreactive cells with immature neuronal phenotype exist in the nonepileptic adult human brain. J Neuropath Exper Neurol. 2000;59:113–119. doi: 10.1093/jnen/59.2.113. [DOI] [PubMed] [Google Scholar]