Abstract
The ventral striatum mediates goal-directed behavior through limbic afferents. One well-established afferent to the ventral striatum is the amygdaloid complex, which projects throughout the shell and core of the nucleus accumbens, the rostral ventromedial caudate nucleus, and rostral ventromedial putamen. However, striatal regions caudal to the anterior commissure also receive inputs from the amygdala. These caudal areas contain histochemical and cytoarchitectural features that resemble the shell and core, based on our recent studies. Specifically, there is a calcium binding protein (CaBP)-poor region in the lateral amygdalostriatal area that resembles the “shell.” To examine the idea that the caudal ventral striatum is part of the “classic” ventral striatum, we placed small injections of retrograde tracers throughout the caudal ventral striatum/amygdalostriatal area and charted the distribution of specific amygdaloid inputs. Amygdaloid inputs to the CaBP-poor zone in the lateral amygdalostriatal area arise from the basal nucleus, the magnocellular subdivision of the accessory basal nucleus, the periamygdaloid cortex, and the medial subdivision of the central nucleus, resembling that of the shell of the ventral striatum found in our previous studies. There are also amygdaloid inputs to CaBP-positive areas outside the shell, which originate mainly in the basal nucleus. Taken together, the “limbic-related” striatum forms a continuum from the rostral ventral striatum through the caudal ventral striatum/lateral amygdalostriatal area based on histochemical and cellular similarities, as well as inputs from the amygdala.
Keywords: shell, nucleus accumbens, central nucleus, amygdalostriatal area, extended amygdala, limbic
The concept of the “ventral striatum” originated with Heimer (Heimer and Wilson, 1975; Heimer, 1978) and has been further elaborated over the last decade. In addition to the “nucleus accumbens,” the ventromedial caudate nucleus and ventromedial putamen are considered part of the ventral striatum in primates, based on afferent inputs from brain regions mediating motivation and reward (Haber et al., 1995; Chikama et al., 1997; Fudge et al., 2002; Kunishio and Haber, 1994). Consistent with its anatomic connections, the ventral striatum mediates operant responses to natural and drug rewards (Dickinson, 1994; Kelley and Holahan, 1997; Corbit et al., 2001). Functional neuroimaging studies confirm that the ventral striatum is involved in goal-directed behaviors in humans, for example, in tasks involving financial or other rewards (Koepp et al., 1998; Elliott et al., 2000; Breiter et al., 2001). Across species, the ventral striatum contains a unique subregion known as the “shell,” which is tightly connected to the limbic system (Kunishio and Haber, 1994; Haber et al., 1995; McFarland and Haber, 1995; Chikama et al., 1997; Haber et al., 2000). The shell is best characterized by its relatively low levels of calbindin-D28k immunoreactivity but is also distinguished by other cytoarchitectural and histochemical features (Meredith et al., 1996; Heimer et al., 1997; Holt et al., 1997; Fudge and Haber, 2002).
The amygdala is a defining input of the classic ventral striatum (Russchen et al., 1985b; Price et al., 1987; Fudge et al., 2002) and is considered a key afferent influencing goal-directed behaviors (Cador et al., 1989; Burns et al., 1996; Parkinson et al., 1999). However, the amygdala is a heterogeneous structure with several nuclear subdivisions (Price et al., 1987). We recently found that the various amygdaloid subdivisions differentially project to the ventral striatal subterritories in the primate (Fudge et al., 2002). Whereas the ventral striatum outside the shell mainly receives inputs from the basal and accessory basal amygdaloid nuclei, the shell is innervated by all major subregions of the amygdala. Thus, the shell receives the strongest and most diverse influence from the entire amygdaloid complex.
Although the classic ventral striatum has been extensively studied, comparatively little is known about amygdaloid influences on striatal domains caudal to this region. Several anatomic studies in primates suggest that brain regions involved in motivation and reward also project to striatal regions caudal to the anterior commissure (Russchen et al., 1985b; Selemon and Goldman-Rakic, 1985; Price et al., 1987; Saint-Cyr et al., 1990; Fudge et al., 2002). Based on these collective studies, this caudal striatal region includes the amygdalostriatal area and caudoventral putamen and medial tail and genu of the caudate nucleus. This region has histochemical and cytoarchitectural features characteristic of the “classic ventral striatum” (Heimer et al., 1999; Fudge et al., 2002). Specifically there is a calcium binding protein (CaBP)-poor area (the lateral amygdalostriatal region) similar to the “shell” of the ventral striatum (Fudge and Haber, 2002).
In the present study we sought to determine whether the caudoventral striatum, including the “shell-like” zone of the lateral amygdalostriatal area, can be considered a part of the classic ventral striatum based on afferents from the amygdala. We placed multiple small injections of retrograde tracers throughout the caudal striatum and amygdalostriatal area in Old World monkeys and analyzed the distribution of retrogradely labeled cells in specific amygdaloid nuclei. Cases were analyzed with respect to injection site placement within the “shell-like” lateral amygdalostriatal area or conventional striatum, based on CaBP immunoreactivity. We subsequently placed anterograde tracers into the amygdala and examined the distribution of labeled fibers throughout the rostrocaudal extent of the striatum.
MATERIALS AND METHODS
Injection sites
Injections of the bidirectional tracers horseradish peroxidase conjugated to wheat germ agglutinin (HRP-WGA; 10%, Sigma, St. Louis, MO), Lucifer Yellow conjugated to dextran amine (LY; 10%, Molecular Probes, Eugene, OR), fluorescein conjugated to dextran amine (FS; 10%, Molecular Probes), or Fluoro Ruby conjugated to dextran amine (FR; 4%, Molecular Probes) were placed throughout the amygdalostriatal area and caudal ventral putamen. Several injections were also placed in the central amygdaloid nucleus for comparison of afferent inputs. The location of injection sites within the “shell-like” caudal ventral striatum, or striatum outside the “shell-like” zone, was determined using double labeling for tracer and calbindin-D28k. Anterograde injections were then placed in the amygdala. The location of injection sites within specific nuclei was determined by counterstaining tracer-labeled sections for Nissl or acetylcholinesterase (AChE).
Surgery
Five Old World monkeys (Macaca nemestrina and Macaca fascicularis) were used in these studies. All experiments were carried out in accordance with National Institutes of Health guidelines. Experimental design and techniques were aimed at minimizing animal use and suffering and were reviewed and approved by the University of Rochester Committee on Animal Research. Initial anesthesia was administered by an intramuscular injection of ketamine (10 mg/kg), and a deep anesthesia was induced by intravenous injection of pentobarbital (initial dose 20 mg/kg IV) maintained as needed. Electrophysiological mapping was performed to locate appropriate injection sites (Fudge and Haber, 2000). Small deposits of tracers (40–50 nL) were pressure-injected into discrete regions of the central nucleus, amygdalostriatal area, and caudal striatum using a 0.5-mm Hamilton syringe under stereotaxic guidance. Following each injection, the syringe remained in situ for 20 minutes to prevent leakage up the needle track. Following analysis of retrograde studies, injections of bidirectional tracers were placed in the amygdala in separate cases using electrophysiologic mapping and stereotaxic placement of tracers (50 nL) by pressure injection.
Ten to 13 days after surgery, animals were deeply anesthetized and killed by perfusion through the heart with saline containing 0.5 mL of heparin sulfate, followed by 4% paraformaldehyde solution in 0.1 M phosphate buffer (pH 7.4). The brains were then removed and cryoprotected in increasing gradients of sucrose (10, 20, and 30%). Serial sections of 50 µm were cut on a freezing microtome into 0.1 M phosphate buffer or cryoprotectant solution.
Tracers
Sections were first thoroughly rinsed in 0.1 M phosphate buffer (pH 7.2) with 0.3% Triton X-100 (PB-T). Following treatment with endogenous peroxidase inhibitor, and more PB-T rinses, tissue was preincubated in 10% normal goat serum (NGS) diluted with PB-T (NGS-PB-T) for 30 minutes. Tissue was then placed in primary antisera to HRP-WGA (1:10,000, Sigma, anti-rabbit), LY (1:2,000 Molecular Probes, anti-rabbit), FS (1:2,000, Molecular Probes, anti-rabbit) or FR (1:1,000, Molecular Probes, anti-rabbit) for four nights at 4°C. Following rinses with PB-T, the tracers were visualized using the avidin-biotin complex reaction (Vectastain ABC kit, Vector, Burlingame, CA). Staining was enhanced by incubating sections for 1–3 minutes in 3,3′ diaminobenzidine tetrahydrochloride and 0.03% hydrogen peroxide intensified with 1% cobalt chloride and 1% nickel ammonium sulfate.
Double labeling
Sections double-labeled for tracer and CaBP immunoreactivity were first labeled for tracer by using the nickel intensification technique described above. Sections were then rinsed overnight and placed in antisera to CaBP (1:10,000, Sigma, anti-mouse) in NGS-PB-T for four nights at 4°C. The sections were then developed without intensification to yield a light brown reaction product. Tracer-labeled sections counterstained for AChE were first processed for tracer using the nickel intensification technique and then processed for AChE using a modified Geneser technique (Geneser-Jensen and Blackstad, 1971).
Analysis
Cases with retrograde tracer injections into the central nucleus, amygdalostriatal area, and caudal ventral striatum were examined for labeled cells in the amygdala, which were charted under brightfield illumination using a 10× objective. The boundaries of the amygdala on sections counterstained for AChE were traced under bright- and darkfield illumination. Charts were entered into the computer using a drawing tablet in conjunction with the program Canvas 5.0. For anterograde cases, the distribution of anterogradely labeled fibers in the striatum and amygdalostriatal area was charted by hand under bright- and darkfield illumination using a 25× objective and a drawing tube. Charts of labeled fibers were then scanned into the computer by using an Epson scanner at 300 dpi and visualized with Adobe Photoshop 6.0. Photomicrographs were digitally captured with an Olympus CCD camera attached to the microscope, by using the program Image Pro 5.0. Color images were converted to grayscale in Adobe Photoshop 6.0.
RESULTS
Defining the caudal ventral striatum
We define the caudal ventral striatum based on cytoarchitectural and histochemical features found in the “classic ventral striatum,” as previously reported (Fudge and Haber, 2002). The lateral amygdalostriatal area and contiguous caudal ventral putamen and tail of the caudate nucleus are considered part of the striatum, based on the presence of the striatal markers acetylcholinesterase (AChE) and tyrosine hydroxylase (TH) (Sarnat and Netsky, 1981). Within this region, the lateral amygdalostriatal area resembles the ventral striatal “shell” based on its low CaBP immunoreactivity compared with the rest of the caudoventral striatum (Fig. 1A,B). The irregularly shaped, CaBP-poor (“shell-like”) region is bordered medially by the medial amygdalostriatal area, ventrally by the lateral ventricle, and dorsolaterally by the CaBP-positive ventromedial putamen and tail of the caudate nucleus (Fig. 1B). The medial amygdalostriatal area is not considered part of the striatum due to its lack of AChE and TH staining, as well as the presence of cellular and histochemical features more related to those in the central nucleus (Heimer et al., 1999; Fudge and Haber, 2002).
Fig. 1.
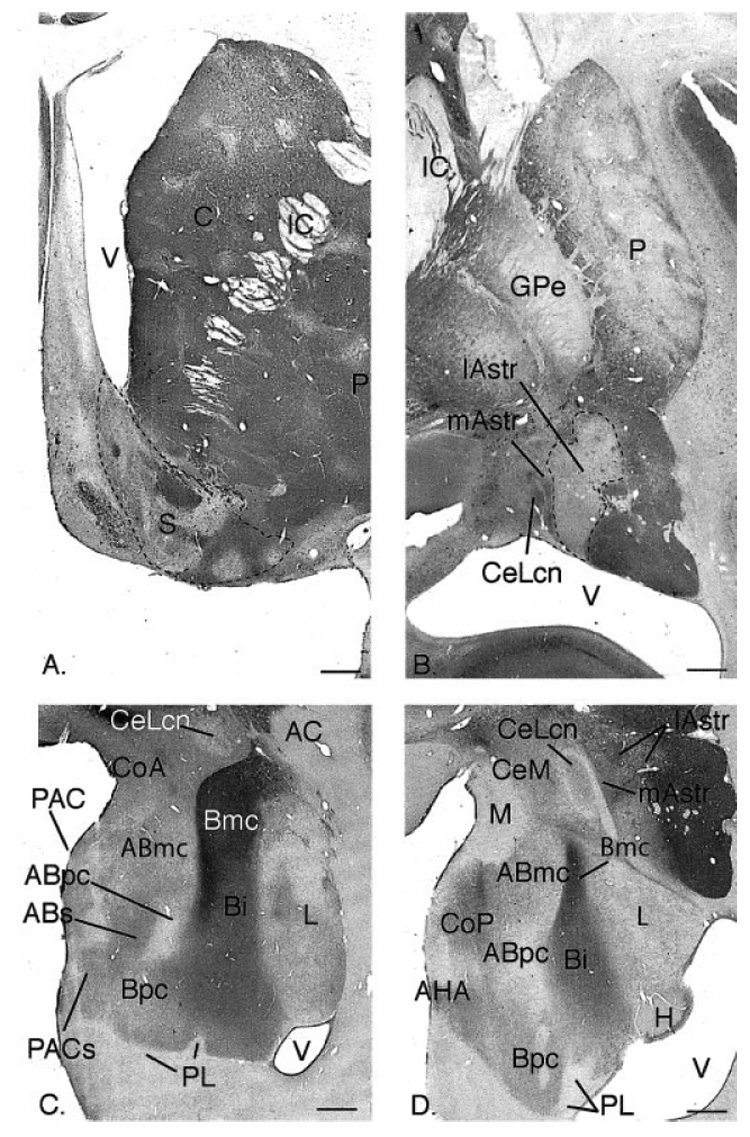
A,B: Coronal sections through the rostral ventral striatum (A) and caudal ventral striatum (B) of the macaque, immunoreacted for CaBP. The CaBP-poor shell in (A) and the CaBP-poor “shell-like” lateral amygdalostriatal area in (B) are outlined. Note that the medial amygdalostriatal area is CaBP-positive, similar to the lateral core of the central nucleus (CeLcn). C,D: Coronal sections through the central (C) and caudal (D) amygdala stained for acetylcholinesterase (AChE), and labeled for various nuclei. For other abbreviations, see list. Scale bars = 1 mm in A–D.
Amygdaloid subregions
The amygdaloid nuclei are best identified by cytoarchitectural criteria on Nissl stained sections and by AChE activity (Fig. 1C,D). With the exception of the central nucleus, we use the nomenclature of Amaral and Price to describe the primate amygdala (Price et al., 1987; Amaral and Bassett, 1989). The basolateral nuclear group includes the basal nucleus, the lateral nucleus, and the accessory basal nucleus. The basal nucleus is divided into magnocellular (Bmc), intermediate (Bi), and parvicellular (Bpc) subdivisions, identified by high, intermediate, and low levels of AChE activity, respectively. The lateral nucleus forms the lateral aspect of the amygdala. The accessory basal nucleus includes a ventral, parvicellular subdivision (very low AChE) and magnocellular (ABmc) and sulcal (ABs) subdivisions (which contain moderate AChE levels). The corticomedial amygdala includes the anterior cortical nucleus, the medial nucleus, the posterior cortical nucleus, and the periamygdaloid cortex. The sulcal region of the periamygdaloid cortex (PACs) merges with the ventromedial tip of the Bpc. Further caudal, the amygdalohippocampal area (AHA) bridges the ventral, caudomedial amygdala and the hippocampus (Fig. 1D). The paralaminar nucleus is a group of tightly packed cells that surround the basal nucleus. The intercalated cell islands are islands of small cells, embedded in the fiber tracts surrounding the major nuclei. Both the paralaminar nucleus and intercalated islands are best appreciated with Nissl preparations (not shown).
The central nucleus is located in the caudal half of the dorsomedial amygdala and is composed of medial and lateral subdivisions. The medial subdivision of the central nucleus (CeM) contains a heterogeneous cell population and intermediate levels of AChE. The lateral central nucleus contains a central core subdivision (CeLcn), which is surrounded by a fibrous capsule (DeOlmos, 1990; Martin et al., 1991). The region immediately lateral to the lateral core has been referred to as the “lateral capsular” subdivision and in primates contains “paracapsular” islands of medium-sized cells resembling those in the CeLcn (DeOlmos, 1990). The paracapsular islands contain moderate CaBP staining, similar to the CeLcn. We refer to this heterogeneous area as the “medial amygdalostriatal area” (mAstr) and consider it more closely related to the central nucleus than the striatum, based on histochemical and cellular criteria (Fudge and Haber, 2002). The lateral amygdalostriatal area (lAstr) contains intermediate levels of AChE and TH staining and low CaBP immunoreactivity and is considered more striatal, as described above (Fig. 1D).
Location of injection sites
Eight retrograde injections were placed in the central amygdaloid nucleus, the medial and lateral amygdalostriatal area, and the caudal ventral striatum (Fig. 2A–C). Injection sites were classified as being mainly in the central nucleus if they were centered on the central nucleus or medial amygdalostriatal area. Injection sites were classified as being in the “shell-like” lateral amygdalostriatal area if they were centered in the CaBP-poor lateral amygdalostriatal area. Injections in the CaBP-positive ventromedial putamen and tail of the caudate nucleus were considered to be in the conventional striatum.
Fig. 2.
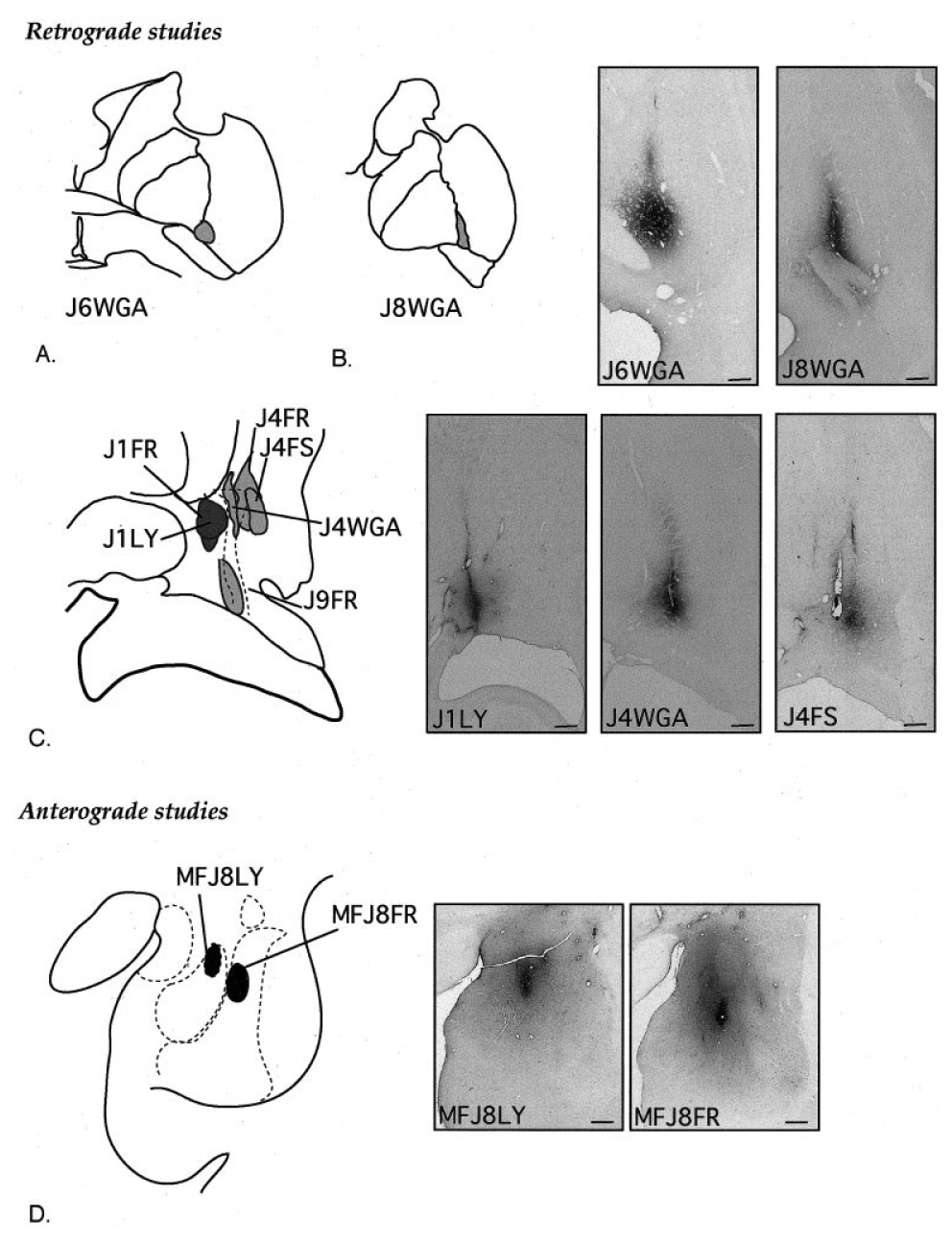
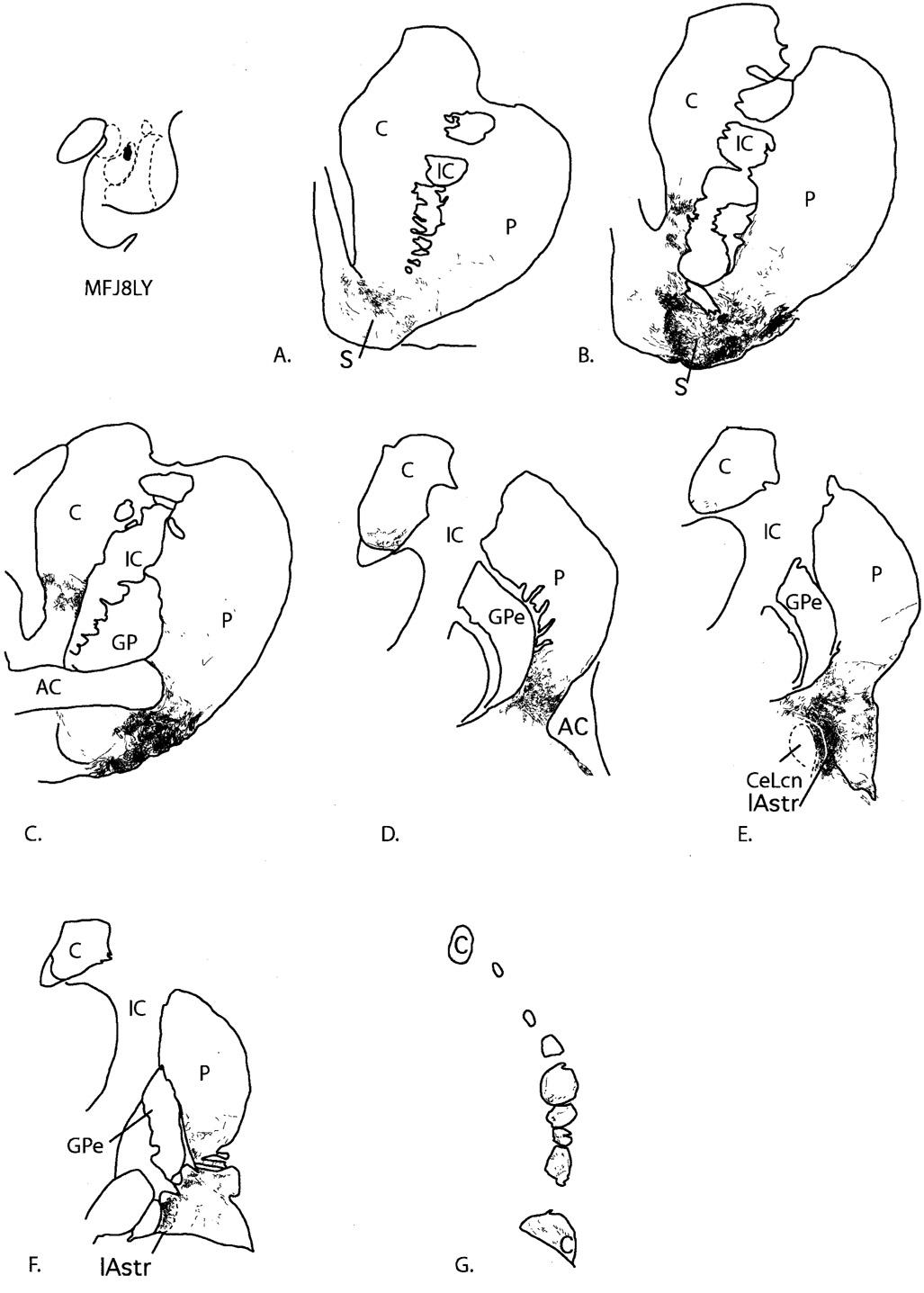
A–C: Schematic of retrograde injection sites in the central (A,B) and caudal (C) ventral striatum and central nucleus, with photomicrographs of representative injection sites. DAB staining of electrode tracks is a nonspecific response to gliosis and is not due to leakage of tracer. The dotted line in C represents the CaBP-poor zone. D: Schematic of anterograde tracer injection site placement in the amygdala, with photomicrographs of each. Scale bars = 1 mm in B–D.
Two injection sites were centered in the central amygdaloid nucleus (J1LY and J1FR). Case J1FR encroached slightly on the adjacent medial amygdalostriatal area (Fig. 2C). Two injection sites were centered mainly in the CaBP-poor (“shell-like”) lateral amygdalostriatal area (J4WGA and J9FR). The injection site in J9FR was caudal to the injection site in case J4WGA and encroached slightly on the medial amygdalostriatal area. Four injections were placed in the CaBP-positive ventromedial putamen: two injections were in the ventromedial putamen at the level of the crossing of the anterior commissure (J6WGA and J8WGA) (Fig. 2A,B), and two injection sites were centered mainly in the CaBP-rich area of the ventral, caudal putamen (J4FR and J4FS; Fig. 2C). The injection site in J4FR encroached slightly on the CaBP-poor zone, whereas the injection site in J4FS was confined to the CaBP-positive striatum. There were two anterograde injection sites in the amygdala (Fig. 2D). The injection site in J8FR was centered in the magnocellular subdivision of the basal nucleus (J8FR). The injection site in J8LY was medial and ventral to this and centered in the magnocellular subdivision of the accessory basal nucleus (J8LY).
Retrograde injections into the caudal ventral striatum
CaBP-poor lateral amygdalostriatal area
Case J9FR (Fig. 3A–C)
Fig. 3.
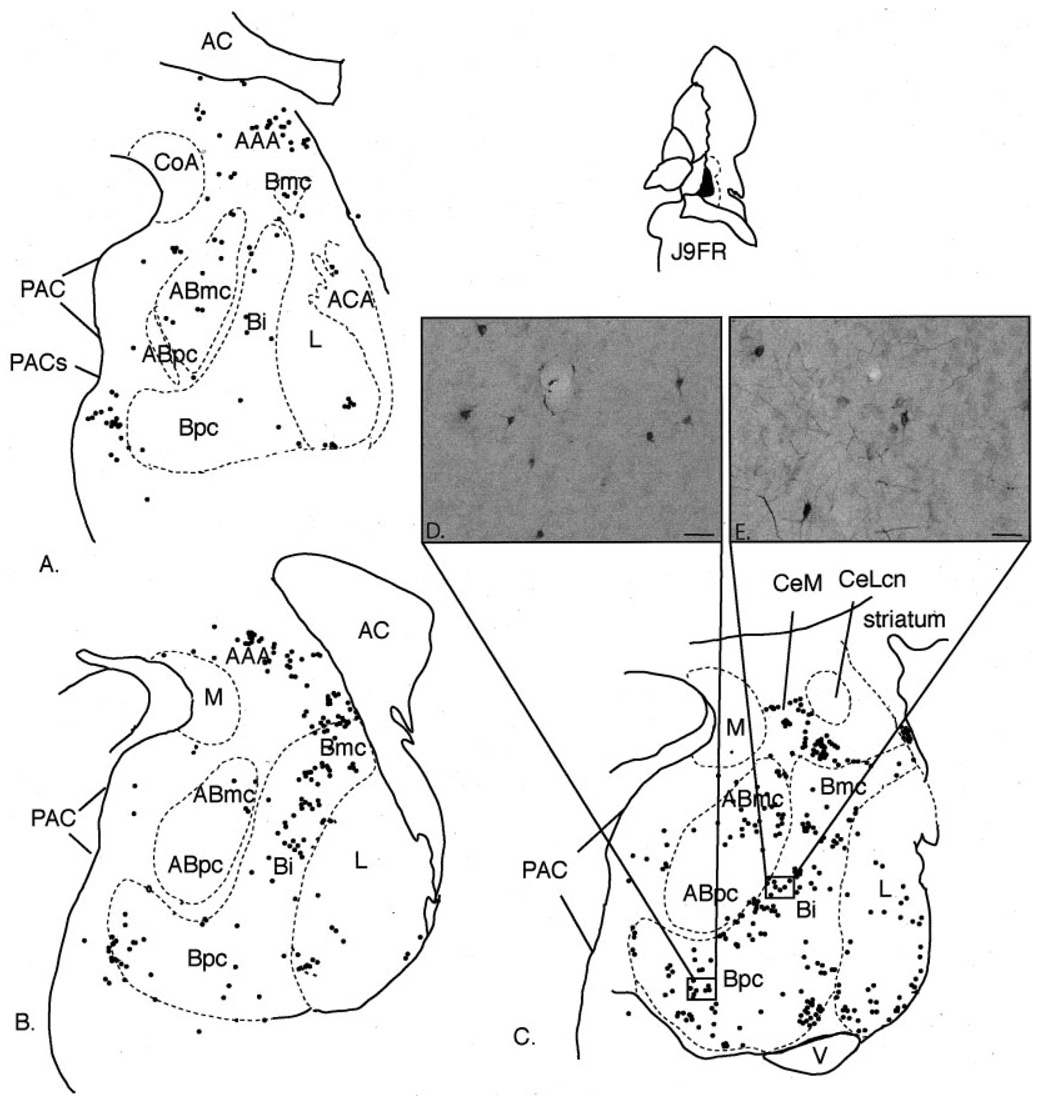
Case J9FR. A–C: Schematic of the distribution of retrogradely labeled cells at three rostrocaudal levels of the amygdala following an injection in the ventral CaBP-poor zone. Each dot = 1 cell. D–E: Photomicrograph of retrogradely labeled cells in the Bpc (D) and Bi (E). For abbreviations, see list. Scale bar = 25 µm in D,E.
This injection site was located in the CaBP-poor lateral amygdalostriatal area at its ventral boundary with the horn of the lateral ventricle. The injection site encroached slightly into the medial amygdalostriatal area. Labeled cells were mainly concentrated in the caudal half of the amygdala. The basal nucleus contained the majority of labeled cells, which were found in all subdivisions (Fig. 3A–E). Clusters of labeled cells were also distributed in fiber tracts surrounding the basal nucleus, and in the basal nucleus at its boundary with the paralaminar nucleus, particularly at caudal levels (Fig. 3C). The lateral nucleus contained a moderate number of labeled cells. As in the basal nucleus, clusters of FR-immunoreactive cells were concentrated caudally near the lateral nucleus’ boundary with the paralaminar nucleus. There was a moderate concentration of labeled cells in the PAC, mainly in the PACs. The accessory basal nucleus contained a high density of labeled cells in the magnocellular subdivision. There were few to no labeled cells in the parvicellular subdivision of the accessory basal nucleus. FR-positive cells also were relatively highly concentrated in the anterior amygdaloid area and medial subdivision of the central nucleus. There were few labeled cells in the medial nucleus, posterior cortical nucleus, or anterior cortical nucleus.
Case J4WGA (Fig. 4A–C)
Fig. 4.
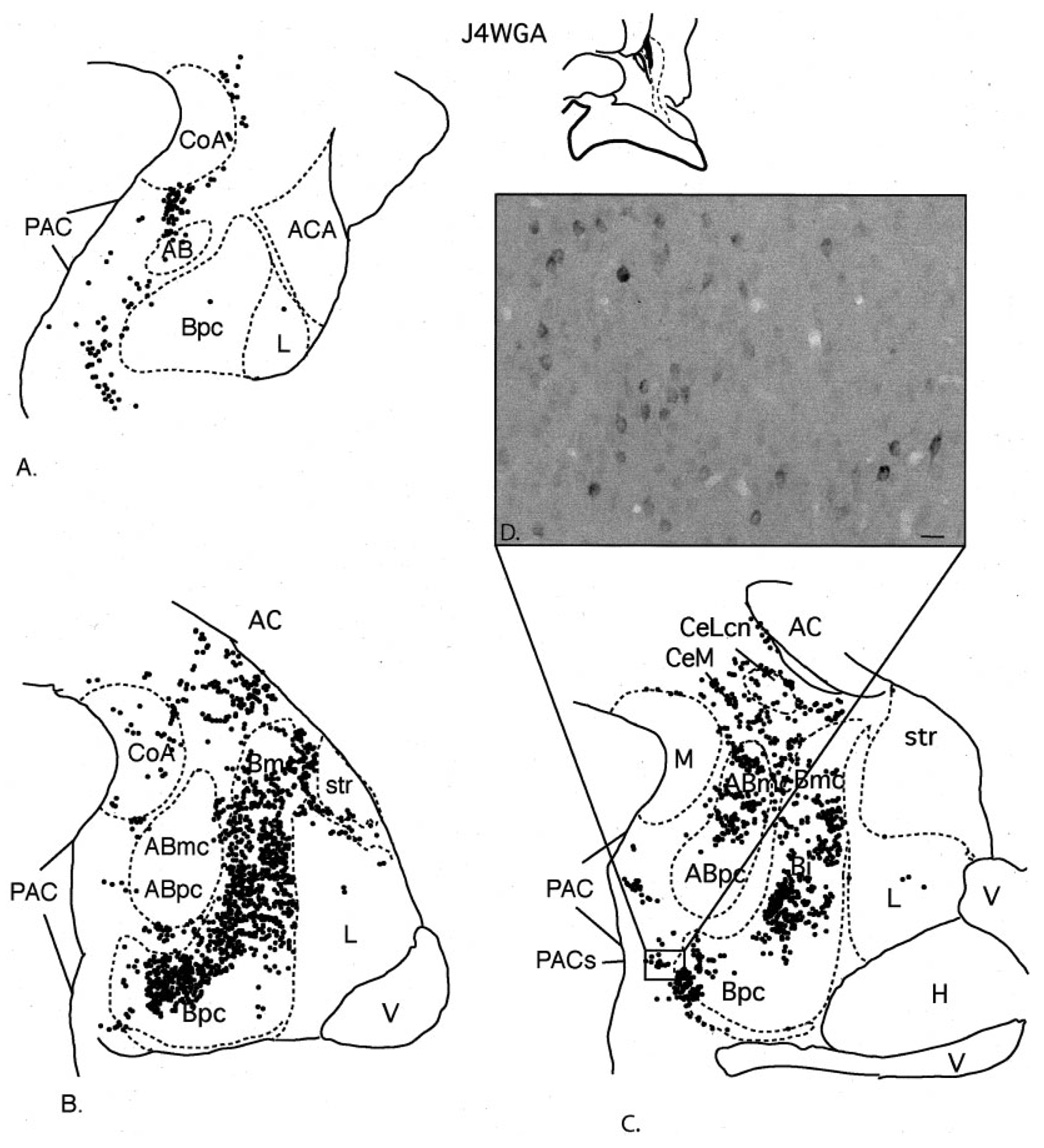
Case J4WGA. A–C: Schematic of the distribution of retrogradely labeled cells at three rostrocaudal levels of the amygdala following an injection in the CaBP-poor zone. Each dot = 1 cell. D: Photomicrograph of labeled cells in the PACs transition with the Bpc. For abbreviations, see list. Scale bar = 25 µm in D.
The injection site in this case was centered in the CaBP-poor zone, rostral and dorsal to the injection site in J9FR (above). The highest density of labeled cells was in the basal nucleus at central to caudal levels. All basal nucleus subdivisions contained many labeled cells. The accessory basal nucleus contained a moderate to dense distribution of HRP-labeled cells in the magnocellular subdivision, mainly at caudal levels. In contrast, the parvicellular subdivision of the accessory basal nucleus was relatively devoid of labeled cells. The PAC also contained a moderate concentration of labeled cells along its rostrocaudal extent. The PACs in the caudal amygdala contained prominent clusters of labeled cells at the transition with the Bpc (Fig. 4C,D). The anterior amygdaloid area and the central nucleus contained a moderate distribution of labeled cells. Within the central nucleus, the medial subdivision contained the majority of labeled cells, with the central core (CeLcn) containing a relatively light distribution. There were scattered labeled cells in the lateral nucleus and anterior cortical nucleus, and few to no labeled cells in the medial nucleus and the posterior cortical nucleus.
CaBP-positive ventral striatum
Cases J6WGA (Fig. 5) and J8WGA (not shown)
Fig. 5.
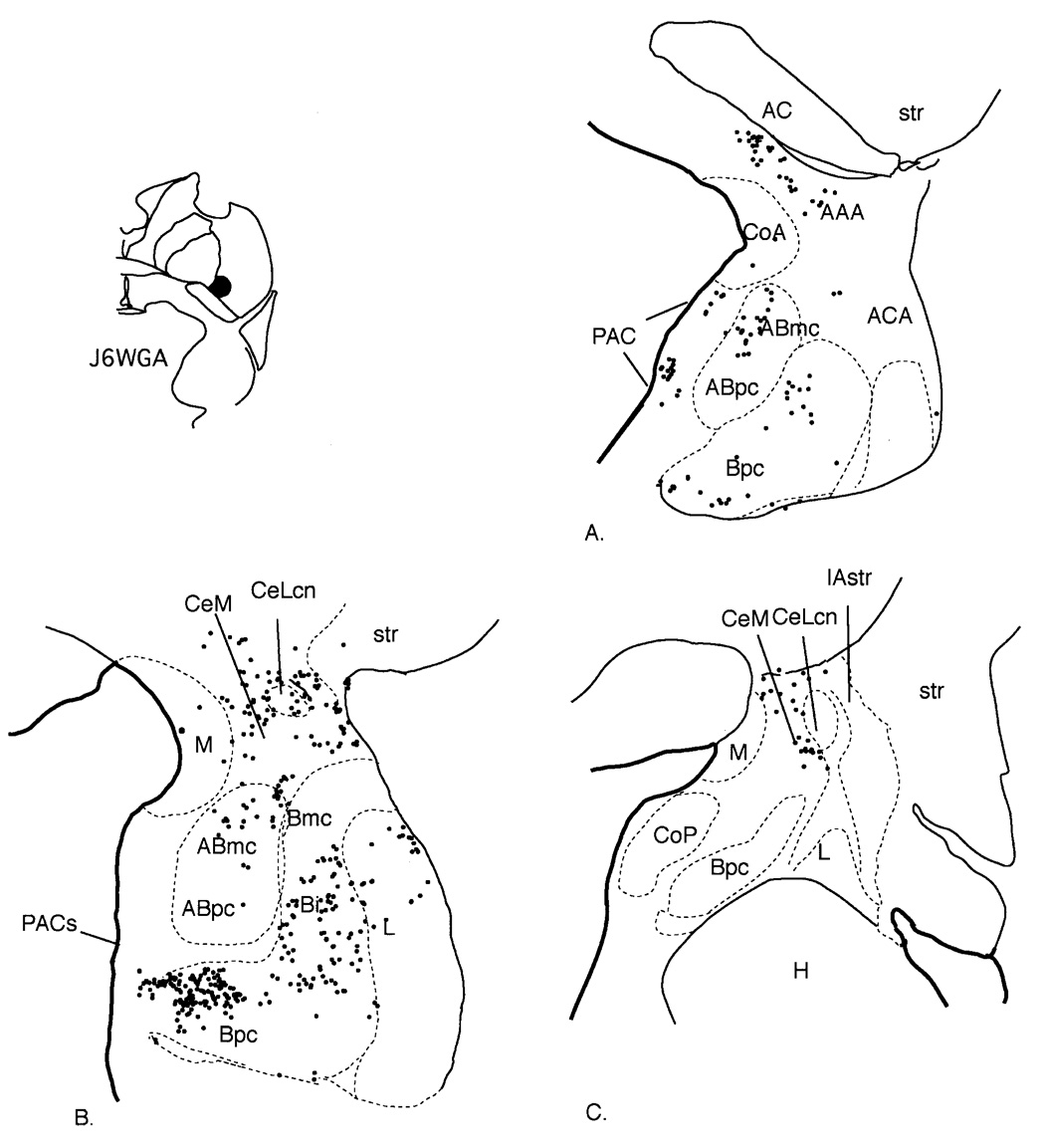
Case J6WGA. A–C: Schematic of the distribution of retrogradely labeled cells at three rostrocaudal levels of the amygdala. Each dot = 1 cell. For abbreviations, see list.
The injection sites in these cases were located in the ventromedial putamen at the level of the crossing of the anterior commissure, with the injection site in J6 slightly rostral to that in J8. The distribution of labeled cells was similar in each case, and they are described together. Labeled cells were concentrated throughout the rostrocaudal extent of the amygdala; however, they were most concentrated at central levels. The basal nucleus contained the majority of labeled cells, mainly in the parvicellular and intermediate subdivisions. The accessory basal nucleus contained moderate to heavy concentrations of HRP-labeled cells in its magnocellular subdivision. There were few to no labeled cells in the parvicellular subdivision of the accessory basal nucleus. Labeled cells were found in the PAC, and, as with the cases involving injections in the CaBP-poor zone (cases J4WGA and J9FR), they were especially concentrated in the PACs, traversing the boundary into the Bpc. The lateral nucleus contained a light distribution of labeled cells. The anterior amygdaloid area and medial subdivision of the central nucleus contained a moderate number of labeled cells, which extended dorsally into the basal forebrain. In contrast, there were relatively few labeled cells in the CeLcn. There were scattered labeled cells in the medial nucleus, anterior cortical nucleus, and posterior cortical nucleus.
Case J4FR (Fig. 6A,B)
Fig. 6.
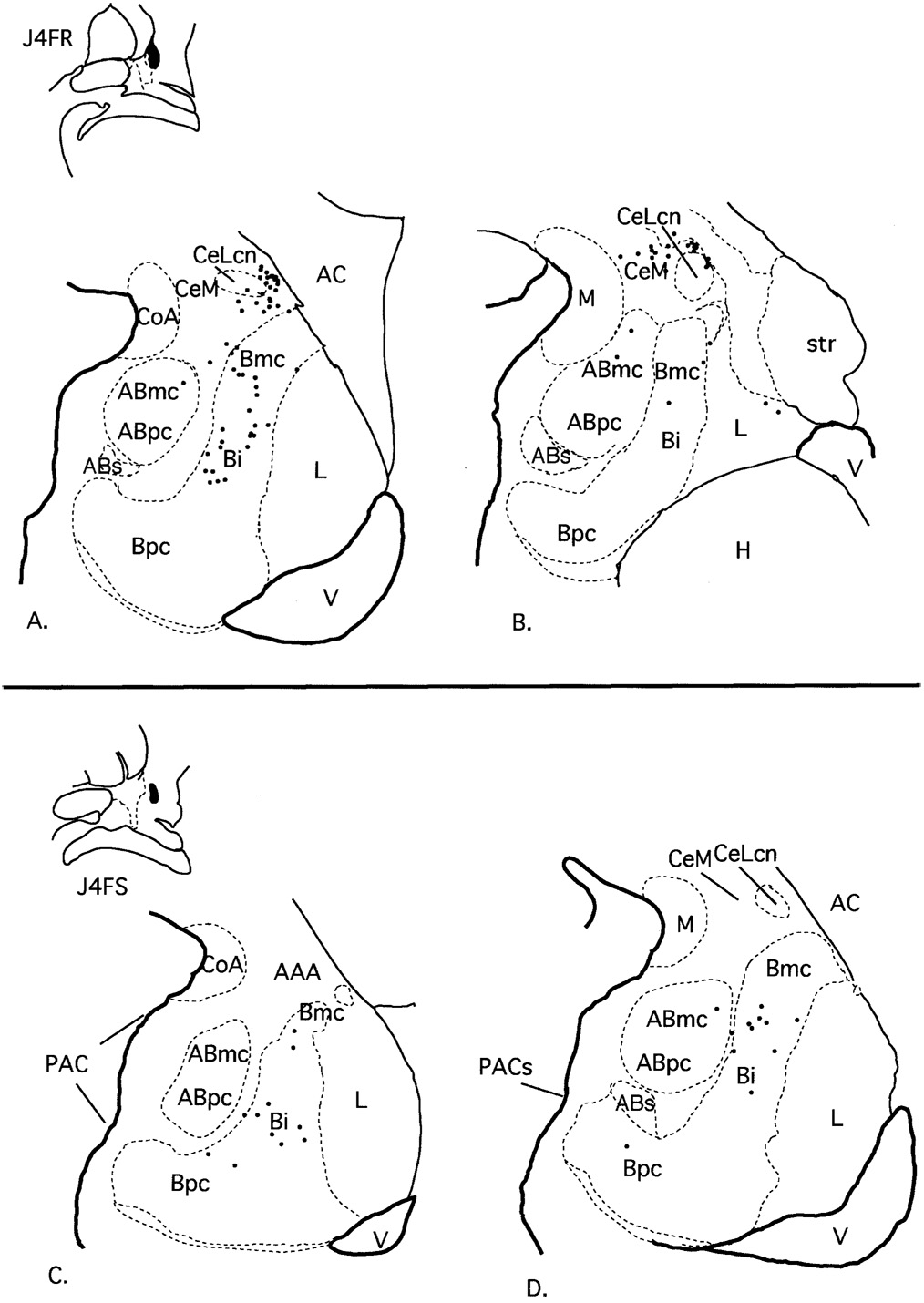
Case J4FR. A,B: Schematic of the distribution of retrogradely labeled cells at two rostrocaudal levels of the amygdala following an injection in the CaBP-positive ventral striatum. Case J4FS. C,D: Schematic of the distribution of retrogradely labeled cells at two rostrocaudal levels of the amygdala following an injection in the CaBP-positive ventral striatum, slightly lateral to the injection in case J4FR above. Each dot = 1 cell. For abbreviations, see list.
The injection site in case J4FR was lateral to the injection site in J4WGA, at the same rostrocaudal level. It was centered in the CaBP-positive striatal zone with slight encroachment on the CaBP-poor lateral amygdalostriatal area. In general there was a narrower distribution of labeled cells compared with the more medially placed injection sites (J9FR, J4WGA, J6WGA, and J8WGA). Labeled cells were concentrated in the caudal amygdala. The magnocellular and intermediate subdivisions of the basal nucleus contained the majority of cells, and there were few to no labeled cells in the parvicellular subdivision of the basal nucleus. The accessory basal nucleus also had relatively fewer labeled cells that were confined to the magnocellular subdivision. The PAC contained no labeled cells. Within the central nucleus, there were scattered labeled cells in the medial subdivision, with no labeled cells in the lateral core subdivision. There were also few to no labeled cells in the medial nucleus, anterior cortical nucleus, or posterior cortical nucleus.
Case J4FS (Fig. 6C,D)
The injection site in this case was situated lateral to the injection site in J4FR (above) at the same anterior-posterior level, with no encroachment on the CaBP-poor lateral amygdalostriatal area. Labeled cells were confined mainly to the intermediate subdivision of the basal nucleus as in case J4FR. The accessory basal nucleus contained only scattered labeled cells in the magnocellular subdivision and none in the parvicellular subdivision. There were few to no labeled cells in the PAC, medial nucleus, central nucleus, anterior or posterior cortical nuclei, or lateral nucleus.
Retrograde injections into the central nucleus/medial amygdalostriatal area
Cases J1LY (Fig. 7A,B) and J1FR (Fig. 7E,F)
Fig. 7.
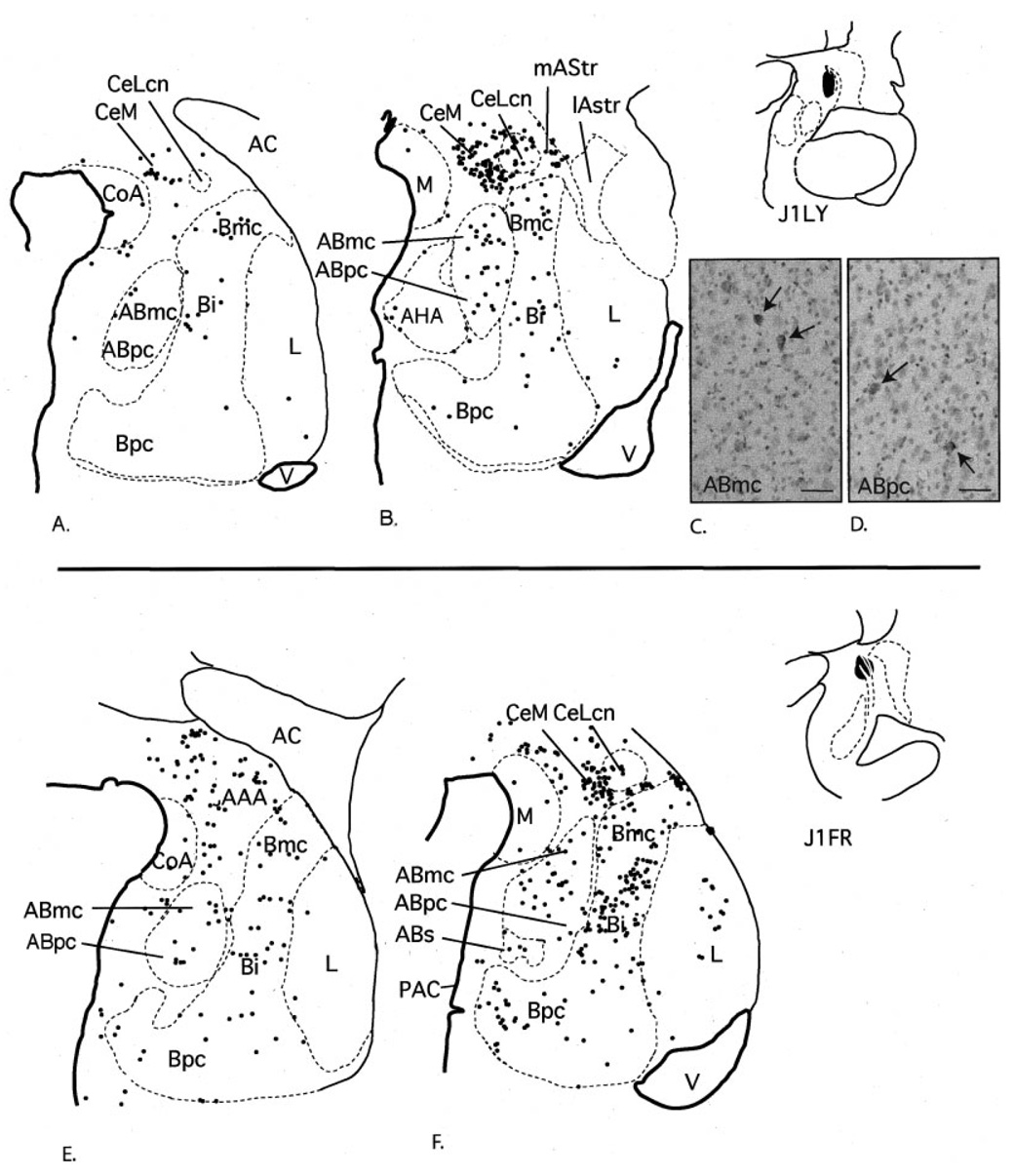
Case J1LY. A,B: Schematic of the distribution of retrogradely labeled cells at two rostrocaudal levels of the amygdala following an injection into the central nucleus (A,B). Each dot = 1 cell. C,D: Photomicrographs of labeled cells in the ABmc (C) and ABpc (D). Case J1FR. E,F: Schematic of the distribution of retrogradely labeled cells at two rostrocaudal levels of the amygdala after a slightly larger injection into the central nucleus. Each dot = 1 cell. For abbreviations, see list. Scale bar = 25 µm in C,D.
For comparison with injection sites in the lateral amygdalostriatal area and caudal ventral striatum, we analyzed the distribution of labeled cells following two injection sites in the central nucleus, which were centered in the lateral core subdivision at caudal levels. In case J1LY, the injection site was confined to the lateral core of the central nucleus. In case J1FR, the injection was placed slightly rostral to that in J1LY and included small areas of the medial central nucleus and medial amygdalostriatal area.
There was little to no encroachment on the lateral amygdalostriatal area in either case. The resulting pattern of labeled cells was similar in each case, although there was a relatively higher number of labeled cells in case J1 FR compared with J1LY. Compared with cases with injections into lateral amygdalostriatal area and caudal ventral striatum, labeled cells were more diffusely distributed throughout the amygdala. The basal nucleus contained many labeled cells, which were relative evenly dispersed through all basal nucleus subdivisions. The accessory basal nucleus also contained a moderate number of labeled cells, which were also relatively evenly distributed throughout all subdivisions (Fig. 7D,E). There was a light distribution of labeled cells in the lateral nucleus and the periamygdaloid cortex. There were scattered labeled cells in the medial nucleus, the anterior and posterior cortical nuclei, and the amygdalohippocampal area.
Anterograde injections into the amygdala
Case J8FR (Fig. 8A–H, Fig. 9A–D)
Fig. 8.
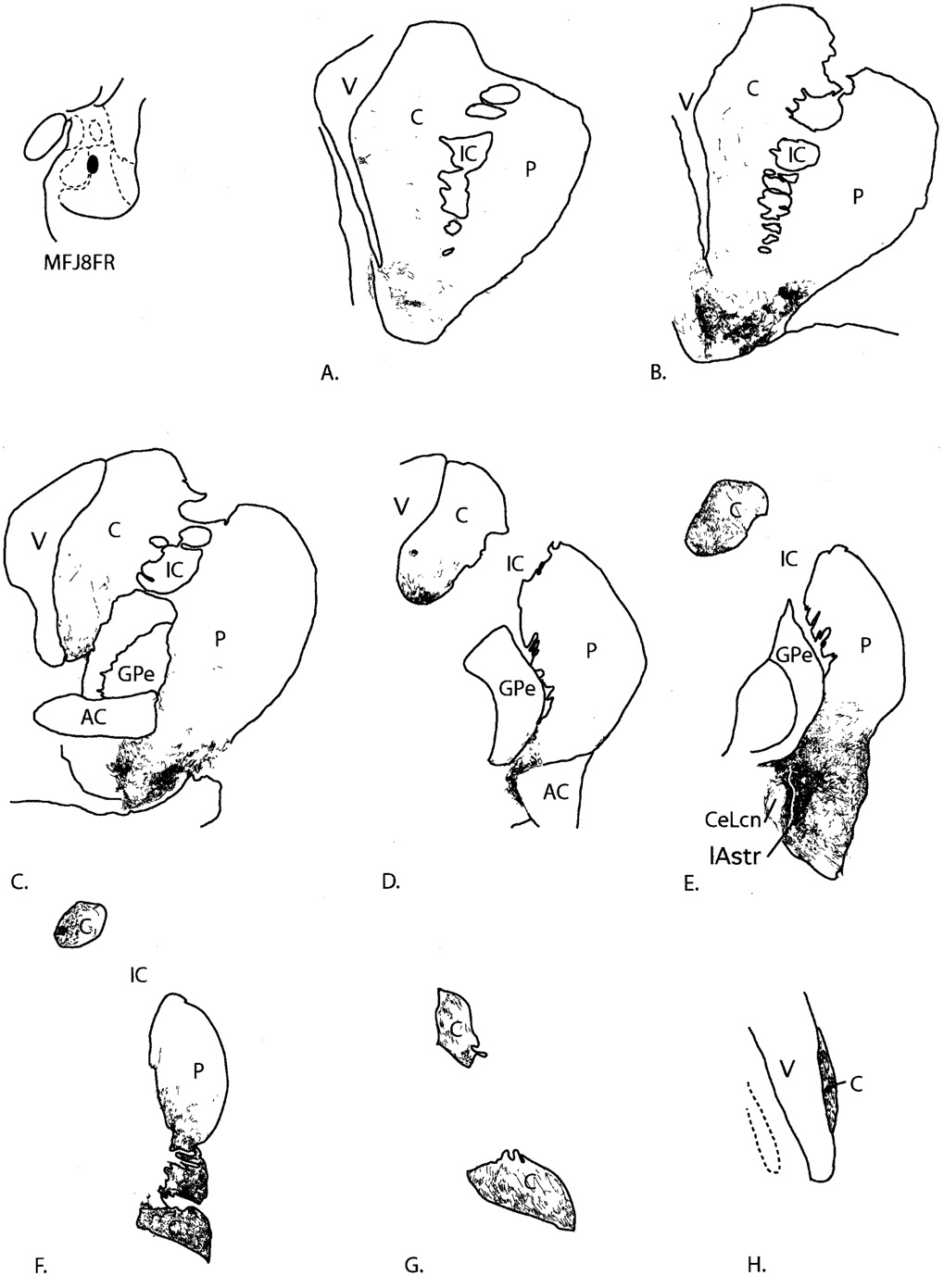
Case J8FR. A–H: Distribution of anterogradely labeled fibers in the striatum following an injection into the basal nucleus, magnocellular subdivision. In D, labeled fibers in the ventromedial putamen are in the region of the putative IPAC. For abbreviations, see list.
Fig. 9.
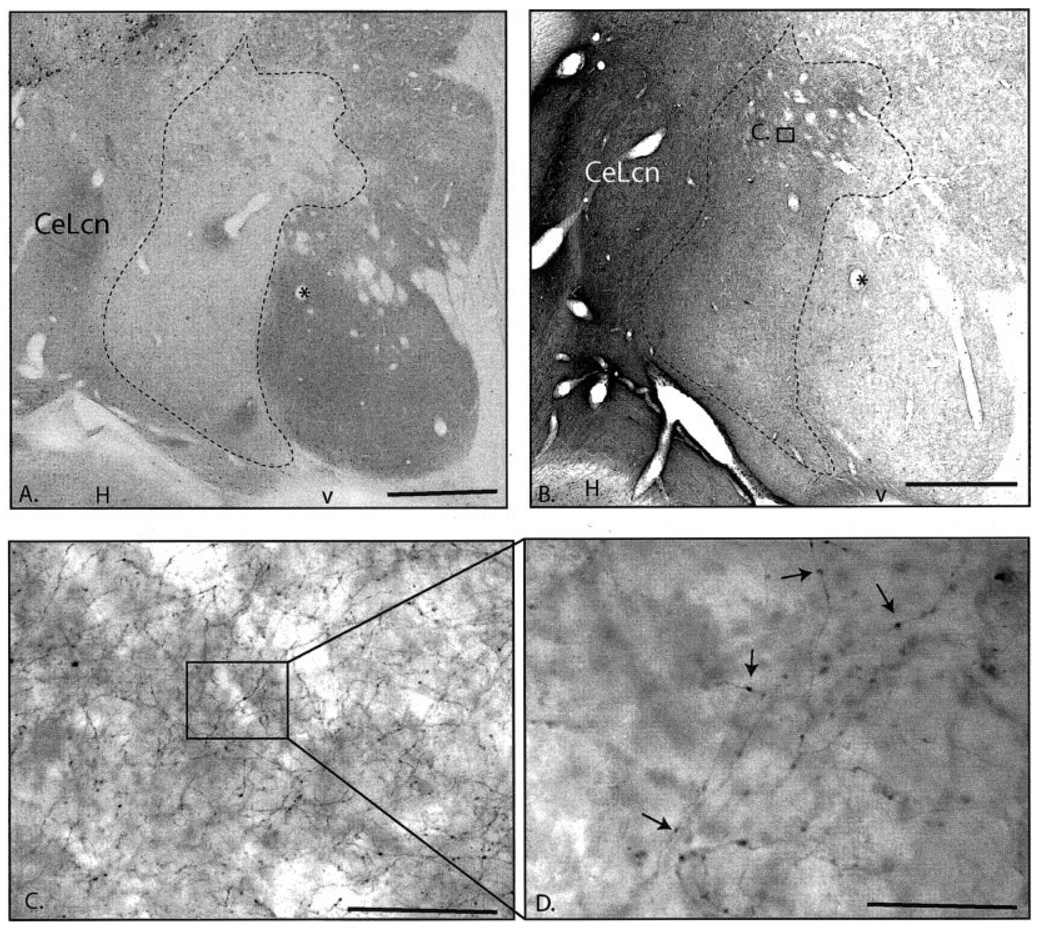
Case J8FR. A,B: Macroscopic photomicrographs of adjacent sections through the caudal ventral striatum in Case J8FR immunoreacted for CaBP (A) and anterograde tracer (B). Asterisk indicates same blood vessel. Dense patches of anterogradely labeled in the CaBP-poor zone compared with the CaBP-positive striatum is apparent even at low magnification. C: Higher power image of labeled fibers in the CaBP-poor zone (boxed area in B). D: Photomicroscopic image at 100× of labeled fibers (boxed area in C) showing numerous boutons. For abbreviations, see list. Scale bar = 1 mm in A,B; 25 µm in C; 10 µm in D.
The injection site was located at the medial edge of the magnocellular subdivision of the basal nucleus, with no encroachment on adjacent structures. Anterogradely labeled fibers were distributed along a large rostrocaudal extent of the striatum, from the ventral striatum through the genu of the caudate nucleus. Rostrally, fine varicose fibers were mainly distributed in patches in the ventral and lateral shell region, with few labeled fibers in the dorsomedial shell. Patches of labeled fibers also extended into the ventral putamen outside the shell (Fig. 8A,B). There were few labeled fibers in the core of the nucleus accumbens or caudate nucleus. At the level of the crossing of the anterior commissure, a light distribution of labeled fibers terminated along the ventromedial edge of the caudate nucleus, after passing through the bed nucleus of the stria terminalis (Fig. 8C). At this level, patches of labeled fibers were most densely concentrated in the ventrolateral putamen beneath the commissure. There were few FR-labeled fibers in the central or dorsolateral caudate nucleus and putamen at this level.
At the level of the posterior limb of the anterior commissure, the majority of fine, labeled fibers were concentrated along the ventromedial body of the caudate nucleus and along the ventromedial edge of the caudal putamen (Fig. 8D). Caudally, the highest concentrations of labeled fibers extended laterally from the central nucleus into the lateral (CaBP-negative) amygdalostriatal area (Fig. 8E, Fig 9A–D). From the CaBP-poor lateral amygdalostriatal area, fine FR-labeled fibers extended dorsolaterally into the ventromedial and ventral, central (CaBP-positive) putamen, where they were more moderately concentrated. Small patches of relatively dense FR-immunoreactive fibers were concentrated at the lateral edge of the caudal ventral striatum; however, the dorsolateral putamen was devoid of labeling. Moderate concentrations of labeled fibers were also seen in the ventromedial third of the body of the caudate nucleus. Further caudal, labeled fibers terminated in the ventromedial half of the body, and throughout the tail, of the caudate nucleus and throughout the ventral half of the putamen (Fig. 8F,G). The dorsolateral putamen and dorsolateral body of the caudate nucleus contained few labeled fibers. At the genu of the caudate nucleus, there was a moderate concentration of labeled fibers, oriented in dorsal-ventral strips.
Case J8 LY (Fig. 10)
Fig. 10.
Case J8LY. A–G: Distribution of anterogradely labeled fibers in the striatum following an injection into the accessory basal nucleus, magnocellular subdivision. Dense patches of labeled fibers near the posterior limb of the anterior commissure (D) are in the vicinity corresponding to the IPAC in the rodent. For abbreviations, see list.
The injection site in this case was rostral and medial to that in J8FR and was confined to the accessory basal nucleus (magnocellular subdivision). Overall, the projection was more confined rostrocaudally compared with the projection resulting from the injection into the magnocellular subdivision of the basal nucleus (case J8FR, above). Fine, varicose labeled fibers were mainly distributed along the border between the shell and core of the rostral ventral striatum (Fig. 10A). At slightly more caudal levels, labeled fibers were densely concentrated in the ventral and lateral shell. Labeled fibers extended into the rostral ventromedial putamen and ventromedial caudate nucleus, similar to the projection from the magnocellular subdivision of the basal nucleus (case J8FR, above; Fig. 10B). There were few to no labeled fibers in the central or dorsolateral striatum. At the level of the crossing of the anterior commissure, beaded labeled fibers were very densely concentrated in the ventromedial putamen, just beneath the commissure. There was also a light distribution of labeled fibers in the ventromedial caudate nucleus (Fig. 10C). Few to no labeled fibers were seen in the central or dorsolateral striatum.
At the level of the posterior limb of the anterior commissure, labeled fibers were concentrated in the ventral putamen. A small patch of densely concentrated labeled fibers was found just beneath the commissure (Fig. 10D). There was a relatively lighter concentration of labeled fibers at the ventromedial edge of the caudate nucleus. Further caudal, labeled fibers overlaid the medial amygdalostriatal area and lateral (CaBP-poor) amygdalostriatal area, where they were very densely concentrated (Fig. 10E). Small dense patches of labeled fibers were scattered through the CaBP-positive caudal ventral striatum, particularly along lateral edge of the ventral putamen. Fine, labeled fibers were mainly confined to the ventromedial edge of the putamen and tail of the caudate nucleus at the caudal end of the projection field (Fig. 10F,G).
DISCUSSION
Amygdaloid afferents define a caudal “limbic-related” striatum
The amygdala has a robust projection to the caudal ventral striatum, including the CaBP-poor lateral amygdalostriatal area, in the primate. The basal nucleus and magnocellular subdivision of the accessory basal nucleus form the main projection to this entire caudal ventral striatal region, consistent with previous primate studies. In contrast, there are few inputs from the parvicellular subdivision of the accessory basal nucleus. This overall projection pattern is similar to the organization of amygdaloid afferents to the “classic” rostral ventral striatum, in which the amygdala also projects across both the CaBP-poor and CaBP-positive zones (Fudge et al., 2002). Taken together, the basal nucleus and magnocellular subdivision of the accessory basal nucleus project in a continuum from the “classic” ventral striatum, to the ventromedial putamen at central levels, and to the lateral amygdalostriatal area, ventromedial putamen, and caudate nucleus at caudal levels. Although the function of these caudal striatal regions is undetermined, they can be considered continuations of the “limbic-related” striatum based on modulation by emotionally relevant stimuli channeled from the amygdala.
Previous studies of the amygdalostriatal projection have focused mainly on inputs to the nucleus accumbens. However, inputs to the central, ventral, and caudoventral striatum are reported in several previous studies (Russchen et al., 1985b; Saint-Cyr et al., 1990; McDonald, 1991; Alheid et al., 1999; McDonald et al., 1999). In rodents, several studies report retrograde labeling in the basal nucleus after injections into the caudal, ventral striatum (McDonald, 1991; McDonald et al., 1999). Furthermore, the striatal region known as the “interstitial nucleus of the posterior limb of the anterior commissure (IPAC)” in rodents (deOlmos and Ingram, 1972) receives robust amygdaloid inputs (Shammah-Lagnado et al., 1999). This striatal area is closely associated with the posterior limb of the commissure, and has been referred to as the “ventral striatal pocket” in older anatomic literature (Crosby and Humphrey, 1941; Nauta et al., 1978). Although a homologous region has not been defined in the primate, the central, ventromedial striatal area targeted by the amygdala lies in this vicinity (Fig. 5, Fig. 8D, Fig. 10D). In the monkey, Russchen et al. (1985b) concluded that the entire basal nucleus projects to the caudoventral putamen and tail of the caudate in the primate, based on multiple injections of anterograde tracers into the amygdala. Saint-Cyr et al. (1990) also found inputs from the basal nucleus after a series of retrograde tracer injections into the caudal ventral striatum in the monkey.
Comparison of amygdaloid afferents to the central nucleus (extended amygdala) versus the ventral striatum
The central nucleus has traditionally been considered part of the amygdala but has more recently been conceptualized as part of a separate macrostructure known as the “central extended amygdala.” In this concept, the extended amygdala is a unified continuum that includes the central nucleus, the bed nucleus of the stria terminalis, and the cell columns that bridge them in the basal forebrain (deOlmos and Ingram, 1972; Alheid and Heimer, 1988; Heimer et al., 1997). Distinguishing the extended amygdala from the adjacent ventral striatum based on connectional criteria has been problematic because, in general, their inputs are similar (McDonald, 1991a; Zahm et al., 1999). Our studies are generally consistent with previous work showing a similar afferent input to the two structures (Krettek and Price, 1978; Aggleton, 1985; Russchen et al., 1985a, b; Bonda, 2000). Amygdaloid inputs to the central nucleus originate in the basal nucleus, the magnocellular accessory basal nucleus, the PAC, and, to a lesser extent, the lateral nucleus, similar to afferents to the ventral striatum. However, the parvicellular subdivision of the accessory basal nucleus projects to the central nucleus but has few inputs to the striatum. These findings are consistent with previous studies detailing intrinsic amygdaloid connections in the primate (Aggleton, 1985; Price et al., 1987; Bonda, 2000), and our previous study showing that the parvicellular accessory basal nucleus has few inputs to the “classic” ventral striatum (Fudge et al., 2002).
Functional implications
The striatum has been divided into functional domains, based on classic anatomic and physiologic studies (for review, see Haber and Fudge, 1997). The ventromedial striatum is considered “limbic-related,” the central striatum is considered “association-related,” and the dorsolateral striatum is considered “sensorimotor-related” (Fig. 11A). Selemon and Goldman-Rakic (1985) first proposed that the ventromedial (limbic) to dorsolateral (sensorimotor) functional gradient of the striatum is maintained along the rostrocaudal axis based on the topography of corticostriatal afferents. This organizational schema is supported and extended by the present results. The amygdala, a key structure for determining the emotional valence of environmental stimuli, projects robustly throughout the rostrocaudal extent of the ventromedial striatum (Fig. 11B), indicating that the “limbic” striatum is not confined to rostral striatal levels. Furthermore, the lateral amygdalostriatal area is a caudal ventral striatal subterritory with histochemical and cellular features of the “shell,” which also has strong amygdaloid inputs.
Fig. 11.
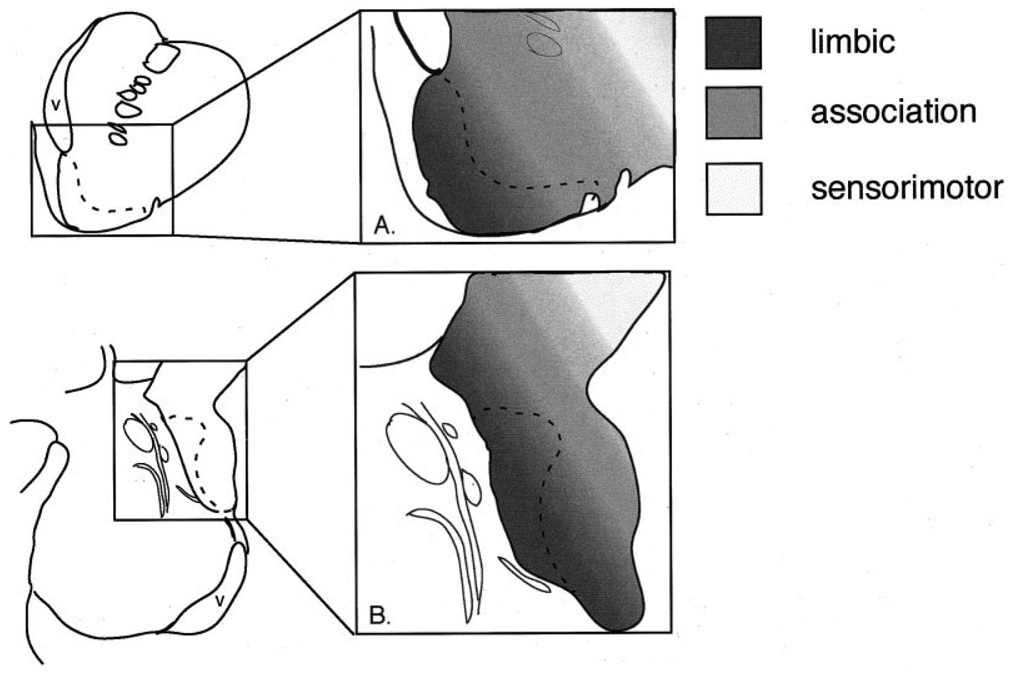
The functional gradient formed in the rostral ventral striatum (A) is reflected in the caudal ventral striatum (B), based on amygdaloid inputs. The “shell” (dotted line) is in close proximity to the ventricle both rostrally and caudally, suggesting another rostrocaudal symmetry. Schematic of the ventral striatum adapted from Haber and Fudge (1997).
The classic ventral striatum mediates appropriate response selection based on inputs from the amygdala and other “limbic” related structures (Cador et al., 1989; Burns et al., 1996). The classic shell, which has specific connections with the limbic system (Zahm and Brog, 1992; Haber et al., 1995, 2000; Fudge et al., 2002), mediates distinct aspects of goal-directed behavior (Berridge and Robinson, 1998; Sokolowski et al., 1998; Ahn and Phillips, 1999; Corbit et al., 2001; Bassareo et al., 2002). For example, exposure to novel stimuli preferentially evokes dopamine release in the shell, compared with the rest of the ventral striatum (Rebec et al., 1997; Sokolowski et al., 1998; Bassareo and Di Chiara, 1999; Parkinson et al., 1999). Although the function of the caudal ventral striatum is not known, recent studies show that subjective emotional responses to an amphetamine challenge in humans are correlated with changes in DA binding in the caudal ventral putamen, as well as in the rostral ventral striatum (Drevets et al., 2001; Martinez et al., 2003). These data may be best interpreted by taking into account the entire continuum of the “limbic-related” striatum. The present results provide a plausible interpretation for such functional data because they indicate that the caudal ventral striatal region is in a position—like the classic ventral striatum—to mediate responses to drug or natural rewards via afferents from the amygdala. Future behavioral, physiologic, and imaging studies should include the caudal ventral striatum as a region of interest to determine its functional similarities to, and differences from, the “classic” ventral striatum.
Acknowledgments
Grant sponsor: National Institute of Mental Health; Grant number: R01 MH 63291.
Abbreviations
- AAA
anterior amygdaloid area
- AB
accessory basal nucleus
- ABmc
accessory basal nucleus, magnocellular subdivision
- ABpc
accessory basal nucleus, parvicellular subdivision
- ABs
accessory basal nucleus, sulcal subdivision
- AC
anterior commissure
- ACA
amygdalo-claustral area
- AHA
amygdalohippocampal area
- Bi
basal nucleus, intermediate subdivision
- Bmc
basal nucleus, magnocellular subdivision
- Bpc
basal nucleus, parvicellular subdivision
- C
caudate nucleus
- CeLcn
central nucleus, lateral core subdivision
- CeM
central nucleus, medial subdivision
- CoA
anterior cortical nucleus
- CoP
posterior cortical nucleus
- GPe
globus pallidus, external segment
- H
hippocampus
- IC
internal capsule
- L
lateral nucleus
- lAstr
lateral amygdalostriatal area
- M
medial nucleus
- mAstr
medial amygdalostriatal area
- P
putamen
- PAC
periamygdaloid cortex
- PACs
periamygdaloid cortex, sulcal subdivision
- S
shell
- Str
striatum
- V
ventricle
LITERATURE CITED
- Aggleton JP. A description of intra-amygdaloid connections in old world monkeys. Exp Brain Res. 1985;57:390–399. doi: 10.1007/BF00236545. [DOI] [PubMed] [Google Scholar]
- Ahn S, Phillips AG. Dopaminergic correlates of sensory-specific satiety in the medial prefrontal cortex and nucleus accumbens of the rat. J Neurosci. 1999;19:RC29. doi: 10.1523/JNEUROSCI.19-19-j0003.1999. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Shammah-Lagnado SJ, Beltramino CA. The interstitial nucleus of the posterior limb of the anterior commissure: a novel layer of the central division of extended amygdala. Ann NY Acad Sci. 1999;877:645–654. doi: 10.1111/j.1749-6632.1999.tb09294.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–642. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bonda E. Organization of connections of the basal and accessory basal nuclei in the monkey amygdala. Eur J Neurosci. 2000;12:1971–1992. doi: 10.1046/j.1460-9568.2000.00082.x. [see comments] [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: implication for limbic-striatal interactions. Behav Neurosci. 1996;110:60–73. doi: 10.1037//0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Chikama M, McFarland N, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby EC, Humphrey T. Studies of the vertebrate telencephalon: the nuclear pattern of the anterior olfactory nucleus, tuberculum olfactorium and the amygdaloid complex in adult man. J Comp Neurol. 1941;74:309–347. [Google Scholar]
- deOlmos JS. Amygdala. In: Paxinos G, editor. The human nervous system. San Diego: Academic Press; 1990. pp. 583–710. [Google Scholar]
- deOlmos JS, Ingram WR. The projection field of the stria terminalis in the rat brain. J Comp Neurol. 1972;146:303–333. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience. 2000;97:479–494. doi: 10.1016/s0306-4522(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Fudge J, Haber S. Defining the caudoventral striatum in primates: cytoarchitectural and histochemical features. J Neurosci. 2002;22:10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen FA, Blackstad TW. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. Z Zellforsch. 1971;114:460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland N. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L. The olfactory cortex and the ventral striatum. In: Livingston KE, Hornykiewicz O, Livingston KE, editors. Limbic mechanisms. New York: Plenum Press; 1978. pp. 95–187. [Google Scholar]
- Heimer L, Wilson RD. The subcortical projections of the allocortex: similarities in the neural associations of the hippocampus, the piriform cortex, and the neocortex. In: Santini M, editor. Golgi Centennial Symposium: Perspectives in Neurobiology. New York: Raven Press; 1975. pp. 177–193. [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Heimer L, De Olmos JS, Alheid GF, Person J, Sakamoto N, Shinoda K, Marksteiner J, Switzer RC. The human basal forebrain. Part II. In: Bloom FE, Bjorkland A, Hokfelt T, editors. Handbook of chemical neuroanatomy. Amsterdam: Elsevier; 1999. pp. 57–226. [Google Scholar]
- Holt DJ, Graybiel AM, Saper CB. Neurochemical architecture of the human striatum. J Comp Neurol. 1997;384:1–25. doi: 10.1002/(sici)1096-9861(19970721)384:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Holahan MR. Enhanced reward-related responding following cholera toxin infusion into the nucleus accumbens. Synapse. 1997;26:46–54. doi: 10.1002/(SICI)1098-2396(199705)26:1<46::AID-SYN5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol. 1994;350:337–356. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Powers RE, Dellovade TL, Price DL. The bed nucleus-amygdala continuum in human and monkey. J Comp Neurol. 1991;309:445–485. doi: 10.1002/cne.903090404. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann NY Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Pattiselanno A, Groenewegen HJ, Haber SN. Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin-D28k. J Comp Neurol. 1996;365:628–639. doi: 10.1002/(SICI)1096-9861(19960219)365:4<628::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Smith GP, Faull RLM, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Hokfelt BT, Swanson LW, editors. Handbook of chemical neuroanatomy. Amsterdam: Elsevier; 1987. pp. 279–381. [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J Comp Neurol. 1985a;242:1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985b;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Ungerleider LG, Desimone R. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallidonigral complex in the monkey. J Comp Neurol. 1990;298:129–156. doi: 10.1002/cne.902980202. [DOI] [PubMed] [Google Scholar]
- Sarnat H, Netsky M. Evolution of the nervous system. New York: Oxford University Press; 1981. Telencephalon; pp. 321–378. [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience. 1999;94:1097–1123. doi: 10.1016/s0306-4522(99)90280-4. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Conlan AN, Salamone JD. A microdialysis study of nucleus accumbens core and shell dopamine during operant responding in the rat. Neuroscience. 1998;86:1001–1009. doi: 10.1016/s0306-4522(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [Review] [118 refs] [DOI] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, Martin JR., 3rd Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur J Neurosci. 1999;11:1119–1126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]


