Abstract
Protein phosphorylation regulates a wide range of cellular processes. Here, we report the proteome-wide mapping of in vivo phosphorylation sites in Arabidopsis by using complementary phosphopeptide enrichment techniques coupled with high-accuracy mass spectrometry. Using unfractionated whole cell lysates of Arabidopsis, we identified 2597 phosphopeptides with 2172 high-confidence, unique phosphorylation sites from 1346 proteins. The distribution of phosphoserine, phosphothreonine, and phosphotyrosine sites was 85.0, 10.7, and 4.3%. Although typical tyrosine-specific protein kinases are absent in Arabidopsis, the proportion of phosphotyrosines among the phospho-residues in Arabidopsis is similar to that in humans, where over 90 tyrosine-specific protein kinases have been identified. In addition, the tyrosine phosphoproteome shows features distinct from those of the serine and threonine phosphoproteomes. Taken together, we highlight the extent and contribution of tyrosine phosphorylation in plants.
Keywords: Arabidopsis, phosphoproteome, tyrosine kinase, tyrosine phosphorylation
Introduction
Protein phosphorylation is a critical regulatory step in signaling networks and is arguably the most widespread protein modification affecting almost all basic cellular processes in various organisms (Hunter, 2000; Manning et al, 2002).
Advances in mass spectrometry (MS)-based technologies accompanied with phosphopeptide enrichment methods paved the way for high-throughput, large-scale in vivo phosphorylation site mapping, and indeed, several pioneering plant phosphoproteome studies have been reported in the past 5 years (Nuhse et al, 2003, 2004, 2007; de la Fuente van Bentem et al, 2006; Benschop et al, 2007). Although these studies provided new insights into phosphorylation events in plants, the analyses were restricted to subfractionated samples, such as plasma membrane proteins, containing a few hundred phosphoproteins. No plant study has yet been reported using unfractionated whole cells to provide a wide-ranging view of cellular phosphorylation events.
More than 1000 phosphorylation sites have recently been identified in animal and yeast cells, using a combination of two or more methods for phosphopeptide enrichment coupled with mass spectrometric phosphopeptide-oriented techniques, such as neutral loss-triggered MS3 to generate fragment ions after elimination of labile phosphate groups, multistage activation, and electron transfer dissociation (Olsen et al, 2006; Bodenmiller et al, 2007a; Chi et al, 2007; Molina et al, 2007; Villen et al, 2007). We also reported the identification of more than 2000 in vivo phosphorylated sites in unstimulated HeLa cells employing an aliphatic hydroxy acid-modified metal oxide chromatography (HAMMOC) as a phosphopeptide enrichment method (Sugiyama et al, 2007). Since different phosphopeptide enrichment methods are likely to have distinct preferences for particular properties of phosphopeptides (Bodenmiller et al, 2007b), it is reasonable to use two or more phosphopeptide enrichment methods for evaluation of proteome-wide phosphorylation.
Comparative genome analyses revealed substantial differences in the ensembles of kinases (kinomes) in eukaryotes (Diks et al, 2007). The Arabidopsis genome encodes at least two times more protein kinases than the human genome (Manning et al, 2002; Champion et al, 2004). Importantly, the Arabidopsis genome (Initiative, 2000) does not contain any predicted human-type TKs (Rudrabhatla et al, 2006). However, plants are likely to utilize tyrosine phosphorylation signaling, as bona fide tyrosine-specific protein phosphatases do exist in Arabidopsis (Xu et al, 1998; Luan, 2003), and a few early studies detected tyrosine phosphorylation by using pY antibodies (Barizza et al, 1999; Kameyama et al, 2000; Luan, 2002). In addition, a previous Arabidopsis phosphoproteome study identified a small number of phosphorylated tyrosine residues, although the actual data sets were missing in the report (Benschop et al, 2007). Thus, evidence for tyrosine phosphorylation in plants is limited so far.
Here, we present a large-scale phosphoproteome analysis of Arabidopsis, providing an overview of in vivo phosphorylation events in Arabidopsis at the cellular level. Importantly, we show the extent of tyrosine phosphorylation in plants, which has been largely underestimated to date.
Results and discussion
Large-scale in vivo phosphorylation site mapping in Arabidopsis
To collect a comprehensive data set of Arabidopsis phosphorylation sites, we employed six distinct methods for phosphopeptide enrichment (Supplementary information). Our approach identified 2172 unique phosphorylation sites with very high confidence on 1346 proteins from unfractionated Arabidopsis cell lysates; this is one of the largest data sets available for a plant to date (Supplementary Table I and Supplementary information). A large majority (1155; 85.8%) of the identified phosphoproteins are novel, while 191 (14.2%) were reported in the previous phosphoproteome studies that focused on plasma membrane proteins (Nuhse et al, 2004; Benschop et al, 2007) (Supplementary Figure 2).
Arabidopsis phosphoproteome
To obtain an overview of phosphorylation events in Arabidopsis, protein abundance distribution, cellular localization, molecular function, and biological processes of identified phosphoproteins were analyzed and compared with those of all proteins encoded by the Arabidopsis genome (Supplementary Figures 3 and 4). Phosphoproteins were generally less abundant, as expected, even when we did not take account of the degree of phosphorylation (Supplementary information). Proteins from all subcellular compartments were found to be targets for phosphorylation. However, approximately 40% of phosphorylation occurred on predicted nuclear proteins. Since nuclear proteins account for only approximately 20% of all genome-encoded proteins and 15% of the experimentally identified proteins in this study, phosphorylation is likely to target nuclear proteins preferentially (Supplementary Figures 4A and 5). The distributions of the molecular function and biological processes of phosphoproteins and that of all genome-encoded proteins were relatively similar (Supplementary Figures 4B and C). This indicates that most cellular processes in Arabidopsis are likely to be regulated at least in part by various phosphorylation events.
To our surprise, of the 2172 identified phosphorylation sites, we found 94 sites to be tyrosine residues (Table I). The kinome of Arabidopsis does not contain any of the typical TKs found in humans, suggesting that plants and humans do not share mechanistic features of tyrosine phosphorylation. Nevertheless, the relative abundances of pS, pT, and pY in Arabidopsis were estimated to be 85.0, 10.7, and 4.3%, which are strikingly close to the human phosphoproteome profile recently reported. The proportion of pY among phospho-residues in human cells is estimated to be between 1.8 and 6.0%, depending on the analyzed samples (Olsen et al, 2006; Molina et al, 2007; Sugiyama et al, 2007). These data clearly indicate that the importance of tyrosine phosphorylation in plants has been greatly underestimated.
Table 1.
Numbers of identified phosphopeptides, phosphoproteins, phosphorylation sites, and the content of phosphorylated residues
| Items | Number |
|---|---|
| Number of phosphopeptidesa | 2597 |
| Number of phosphoproteinsb | 1346 |
| Number of unique phosphorylation sites | 2172 |
| Phosphorylated residues (Ser:Thr:Tyr) | 1847:231: 94 (85.0%) (10.6%) (4.3%) |
aThe number of phosphopeptides is based on unique sequences containing missed cleavage products, oxidization of methionine, and phosphorylation of different sites.
bMultiple distinct proteins except splicing variants matched against a single peptide are also counted.
Arabidopsis tyrosine phosphoproteome
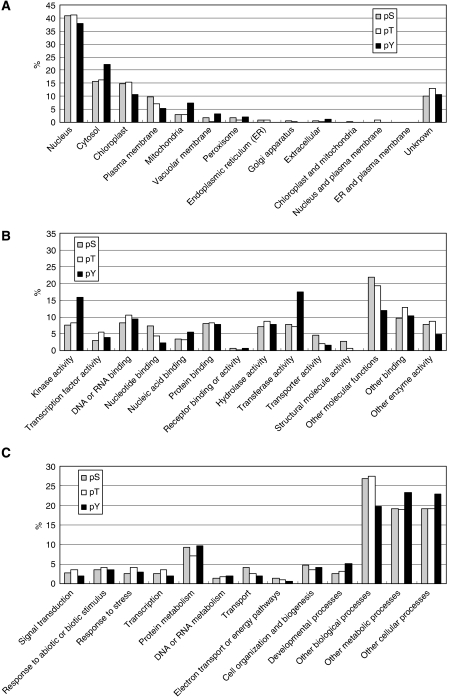
The 94 identified pY residues were mapped on 95 proteins (Supplementary Table II). The difference in the number of pY residues and corresponding proteins is due to matching of single phosphopeptides to several different proteins. Since the sequences surrounding tyrosine phosphorylation sites on the listed protein kinases are often well conserved, the number of protein kinases is over-represented. To investigate whether tyrosine phosphorylation is targeted to a specific subset of proteins, gene ontology analyses of serine-, threonine-, or tyrosine-phosphorylated proteins were performed as described (Figure 1). Tyrosine phosphorylation preferentially occurs on proteins that possess kinase activity or transferase activity (Figure 1B). Otherwise, no outstanding differences were found in the distributions.
Figure 1.
Gene ontology analysis of the serine-, threonine-, or tyrosine-phosphorylated proteins. (A) Cellular localization, (B) molecular function, and (C) biological process.
Location of phosphorylation sites on characterized protein domains
To assess whether trends or patterns exist in the position of tyrosine phosphorylation sites, we investigated whether these sites are located in conserved domains. Pfam search (Bateman et al, 2004) was used to extract domain information of the identified phosphoproteins. Of the 1346 proteins, we obtained domain information for the 1118 proteins. In these proteins, 77.95% of phosphorylation sites (1548 sites) were located outside of conserved domains (Table II). The tendency that the majority of phosphorylation occurs outside of conserved domains is consistent with the observations from the phosphoproteome study of plasma membrane proteins (Nuhse et al, 2004). Interestingly, however, nearly half (48.5%) of pYs were found to be located on conserved domains (Table II). These data indicate that tyrosine phosphorylation may have more impact on domain-associated function compared to serine and threonine phosphorylation.
Table 2.
Location of phosphorylation sites on characterized protein domains
| Number of proteins possessing Pfam domain | Number of phosphorylation sites |
|||
|---|---|---|---|---|
| Pfam domaina |
Total (%) | |||
| ON (%) | OUTb (%) | |||
| pS | 1014 | 317 (19.1) | 1340 (80.9) | 1657 (100) |
| pT | 195 | 74 (32.2) | 156 (67.8) | 230 (100) |
| pY | 87 | 49 (48.5) | 52 (51.5) | 101 (100) |
| All | 1118 | 440 (22.1) | 1548 (77.9) | 1988 (100) |
aWhether the phosphorylation sites are located on the conserved domains annotated in the Pfam database was analyzed.
bA single phosphorylation site was counted only once, regardless of how many domains are found in a single phosphoprotein.
Conservation of tyrosine phosphorylation sites in plant homologs
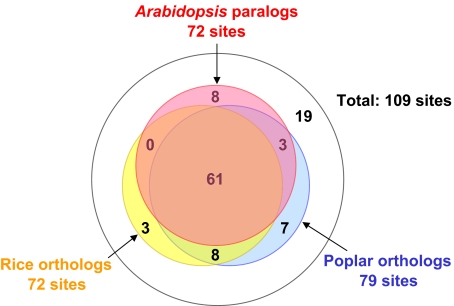
Conservation of the tyrosine phosphorylation sites between homologous proteins in Arabidopsis, rice (Oryza sativa), and poplar (Populus trichocarpa) was investigated to get an overview of tyrosine phosphorylation events in other plant species. Of the 95 tyrosine-phosphorylated proteins (109 tyrosine phosphorylation sites), 84 proteins (97 sites) were validated to possess homologs in Arabidopsis (paralogs), while 89 (103 sites) and 92 (106 sites) proteins had corresponding homologs (orthologs) in rice and poplar, respectively (Supplementary Table II). Multiple sequence alignments of the homologous proteins were created using ClustalW (Thompson et al, 1994), and the conservation of the phosphorylatable tyrosine residues was verified manually (Figure 2). In total, 72 sites are conserved within Arabidopsis paralogs, while 72 and 79 sites are conserved in rice and poplar orthologs, respectively. Most of these sites (61 sites) are conserved in all three plant species, indicating that the tyrosine phosphorylation sites are nearly equally conserved in paralogs and orthologs. This observation is in clear contrast to the case of serine phosphorylation sites, which are less conserved in paralogs compared to orthologs (Nuhse et al, 2004).
Figure 2.
Venn diagram showing the number of conserved tyrosine phosphorylation sites in homologs. Blue, yellow, and red circles indicate the conserved sites in Arabidopsis paralogs, rice orthologs, and poplar orthologs, respectively.
Distribution of the phosphorylation sites
We found that most (76.3%) of the pY-containing phosphopeptides are multiply phosphorylated, while the majority (80.9%) of phosphopeptides are singly phosphorylated (Table III). In other words, tyrosine phosphorylation seems to occur near other phospho-residues (Supplementary Table III). Since the amino acids surrounding the phosphorylation sites often contribute substantially to recognition by protein kinases, the phosphorylation status of neighboring residues is an essential factor in determining whether the phosphorylation site is targeted by a particular protein kinase. It would be very interesting to investigate whether and how the phosphorylation state of the neighboring residues affects tyrosine phosphorylation events in Arabidopsis.
Table 3.
Comparison of singly and multiply phosphorylated peptides
| All |
pS |
pT |
pY |
||||
|---|---|---|---|---|---|---|---|
| Single | Multi | Single | Multi | Single | Multi | Single | Multi |
| 1888 (80.9%) | 445 (19.1%) | 1712 (80.3%) | 419 (19.7%) | 153 (61.0%) | 98 (39.0%) | 23 (23.7%) | 74 (76.3%) |
Whether pS-, pT-, or pY-containing phosphopeptides are singly or multiply phosphorylated was analyzed.
Tyrosine phosphorylation motifs
An obvious question arising from our finding is, which kinases carry out tyrosine phosphorylation in plants? To address this question, we attempted to extract significant patterns surrounding the pY residues from our data set, assuming that conserved phosphorylation sites within functionally related proteins tend to be well targeted by structurally similar protein kinases. We have extracted 20 pY motifs through the substrate-driven approach (Schwartz and Gygi, 2005) (Supplementary information). Most of the identified pY motifs are novel and distinct from the human pS, and pT and pY motifs in the human protein reference database (Amanchy et al, 2007). These results indicate that tyrosine phosphorylation in plants is carried out by a novel class(es) of plant kinases. One candidate might be dual-specific serine/threonine/tyrosine protein kinases (Rudrabhatla et al, 2006). Other possible candidates would be tyrosine-specific protein kinase-like kinases (TKLs), which are especially abundant in plants: 776 in Arabidopsis and nearly 1000 in rice, compared to 55 in humans (Miranda-Saavedra and Barton, 2007). Tyrosine phosphorylation by human TKLs has not been reported. Functions of plant TKLs remain also unknown, but the large number of TKLs in plants may suggest that they carry out important and diverse plant-specific functions. In this sense, it is of particular interest to investigate if any of TKL possesses tyrosine phosphorylation activity.
Materials and methods
Plant material
Arabidopsis cell suspension line (ecotype Landsberg erecta) (Maor et al, 2007) was grown in Murashige and Skoog medium (pH 5.7) containing 3% sucrose, 0.59 g/l MES, 100 mg/l myo-inositol, 10 mg/l thiamine-HCl, 1 mg/l pyridoxine-HCl, 1 mg/l nicotinic acid, 0.5 mg/l 1-naphthaleneacetic acid, and 0.05 mg/l 6-benzylaminopurine under a 16-h light/8-h dark cycle at 22°C. Seven-day-old Arabidopsis suspension cultures were harvested by vacuum filtration, frozen immediately in liquid nitrogen, and kept at −80°C until the analysis.
Digestion of Arabidopsis cell cytoplasmic fraction
Arabidopsis cells (0.2 g, wet) were frozen in liquid nitrogen and then disrupted with a Multi-beads shocker (MB400U; Yasui Kikai). The disrupted cells were suspended in 0.1 M Tris–HCl (pH 8.0), containing protein phosphatase inhibitor cocktails 1 and 2 (Sigma) and protease inhibitors (Sigma). The homogenate was centrifuged at 1500 g for 10 min and the supernatant was reduced with dithiothreitol, alkylated with iodoacetamide, and digested with Lys-C, followed by dilution and trypsin digestion as described (Saito et al, 2006). These digested samples were desalted using StageTips with C18 Empore disk membranes (3 M) (Rappsilber et al, 2003). The peptide concentration of the eluates was adjusted to 1.0 mg/ml with 0.1% TFA and 80% acetonitrile.
Enrichment of phosphopeptides
HAMMOC using titania and zirconia was performed as described previously (Sugiyama et al, 2007) with some modifications. Custom-made MOC tips were prepared using C8-StageTips and metal oxide bulk beads (0.5 mg beads per 10 μl pipette tip), as described for SCX(beads)-C18 tips (Ishihama et al, 2006). Prior to loading samples, the MOC tips were equilibrated with 0.1% TFA, 80% acetonitrile, containing a hydroxy acid as a selectivity enhancer (solution A). As an enhancer, lactic acid was used at a concentration of 300 mg/ml for titania MOC tips and β-hydroxypropanoic acid at 100 mg/ml for zirconia MOC tips. The digested sample from 100 μg of Arabidopsis total proteins was diluted with 100 μl of solution and loaded onto the MOC tips. After successive washing with solution A and solution B (0.1% TFA and 80% acetonitrile), 0.5% ammonium hydroxide or 1.0% disodium hydrogen phosphate was used for elution. The eluted fraction was acidified with TFA, desalted using C18-StageTips as described above, and concentrated in a Tony CC-105 vacuum evaporator (Tokyo, Japan), followed by the addition of solution A for subsequent nanoLC-MS/MS analysis.
Fe-IMAC was conducted using Phos-Select (Sigma) as described previously (Kokubu et al, 2005; Ishihama et al, 2007), except for the use of C8-StageTips instead of C18-StageTips for packing Phos-Select beads. Briefly, after loading the sample solutions, the tips were rinsed with 0.5 ml of 50% ACN in 0.3% TFA. Then, 0.5% ammonium hydroxide or 1.0% disodium hydrogen phosphate was used for elution. The eluted fraction was acidified with TFA and desalted using C18-StageTips using HAMMOC methods. The eluted phosphopeptide fraction was concentrated in the vacuum evaporator and resuspended in solution A for nanoLC-MS/MS analysis.
NanoLC-MS system
A Finnigan LTQ-Orbitrap (Thermo Fisher Scientific, Bremen, Germany) coupled with a Dionex Ultimate3000 pump (Germering, Germany) and an HTC-PAL autosampler (CTC Analytics AG, Zwingen, Switzerland) was used for nanoLC-MS/MS analyses throughout this study. ReproSil C18 materials (3 μm; Dr Maisch, Ammerbuch, Germany) were packed into a self-pulled needle (150 mm length × 100 μm i.d., 6 μm opening) to prepare an analytical column needle with ‘stone-arch' frit (Ishihama et al, 2002). An x–y–z nanospray interface (Nikkyo Technos, Tokyo, Japan) was used to hold the column needle and to set the appropriate spray position. A spray voltage of 2400 V was applied. The injection volume was 5 μl and the flow rate was 500 nl/min. The mobile phases consisted of (A) 0.5% acetic acid and (B) 0.5% acetic acid and 80% acetonitrile. A three-step linear gradient of 5–10% B in 5 min, 10–40% B in 60 min, 40–100% B in 5 min and 100% B for 10 min was employed throughout this study. The MS scan range was m/z 300–1400, and the top 10 precursor ions were selected for subsequent MS/MS scans. A lock mass function was used for the LTQ-Orbitrap to obtain constant mass accuracy during gradient analysis (Olsen et al, 2005).
Database searching
Mass Navigator v1.2 (Mitsui Knowledge Industry, Tokyo, Japan) was used to create peak lists on the basis of the recorded fragmentation spectra. Peptides and proteins were identified by means of automated database search using Mascot v2.1 (Matrix Science, London) against TAIR7_pep_20070425 (ftp://ftp.arabidopsis.org/home/tair/Sequences/blast_datasets/TAIR7_blastsets/) with a precursor mass tolerance of 3 p.p.m., a fragment ion mass tolerance of 0.8 Da and strict trypsin specificity (Olsen et al, 2004), allowing for up to two missed cleavages. Carbamidomethylation of cysteine was set as a fixed modification, and oxidation of methionines and phosphorylation of serine, threonine, and tyrosine were allowed as variable modifications. Peptides were considered identified if the Mascot score was over the 95% confidence limit based on the ‘identity' score of each peptide and at least three successive y- or b-ions with a further two and more y-, b-, and/or precursor-origin neutral loss ions were observed, based on the error-tolerant peptide sequence tag concept (Mann and Wilm, 1994). A randomized decoy database created by a Mascot Perl program estimated a 2.1% false-positive rate for identified peptides within these criteria. Note that most sulfated peptides can be discriminated from phosphopeptides because of the ultrahigh accuracy of the Orbitrap instrument that we used.
Phosphorylated sites were unambiguously determined when y- or b-ions between which the phosphorylated residue exists were observed in the peak lists of the fragment ions.
Bioinformatics
We used the KAGIANA tool (http://pmnedo.kazusa.or.jp/kagiana/index.html) to extract cellular localization information of Arabidopsis proteins predicted by the WoLF PSORT program (Horton et al, 2007). For molecular function and biological process annotations extraction, the TAIR gene ontology annotation search tool (Berardini et al, 2004) was used.
For the homologs search, BlastP searches (Altschul et al, 1997) were performed against the protein databases, TAIR7_pep_2007425, rap1_all_orf_amino, and proteins.Poptr1_1.JamboreeModels for Arabidopsis, rice, and poplar, respectively (Ohyanagi et al, 2006; Tuskan et al, 2006). The E-value cutoff of 10−3 was used for the initial search and if there were no protein hits, the cutoff value was lowered stepwise to 10−2 and 10−1. In some cases, E-value cutoff of 10−6 was used for AT1G70520.1, E-value cutoff of 10−5 was used for AT3G05140.1, and E-value cutoff of 10−4 was used for AT2G30940.1. For multiple sequence alignment, ClustalW (Thompson et al, 1994) was performed with default parameter settings. The aligned sequences were further manually analyzed using the MEGA4 program (Tamura et al, 2007).
Pfam domain information was extracted from the database, TAIR7_all.domains (ftp://ftp.arabidopsis.org/home/tair/Proteins/Domains/).
Supplementary Material
Supplementary Information
Supplementary Figures
Supplementary Table 1
Supplementary Table 2
Supplementary data 1
Supplementary data 2
Supplementary data 3
Acknowledgments
We thank Sumiko Ohnuma (Keio University) for technical assistance, Alex Jones (The Sainsbury Laboratory, UK) for initial introduction of mass spectrometry at RIKEN, and Tetsuro Toyoda and Yoshiki Mochizuki (RIKEN) for kindly uploading the phosphorylation site data to a RIKEN OmicBrowse database. This study was supported by research funds from Yamagata Prefecture and Tsuruoka City to Keio University, MEXT Grants-in-Aid for Scientific Research (nos. 19678001 and 19039034 to KS and no. 19710169 to HN) and a grant from the Japan Society for the Promotion of Science to AD.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanchy R, Periaswamy B, Mathivanan S, Reddy R, Tattikota SG, Pandey A (2007) A curated compendium of phosphorylation motifs. Nat Biotechnol 25: 285–286 [DOI] [PubMed] [Google Scholar]
- Barizza E, Lo Schiavo F, Terzi M, Filippini F (1999) Evidence suggesting protein tyrosine phosphorylation in plants depends on the developmental conditions. FEBS Lett 447: 191–194 [DOI] [PubMed] [Google Scholar]
- Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, Studholme DJ, Yeats C, Eddy SR (2004) The Pfam protein families database. Nucleic Acids Res 32: D138–D141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop JJ, Mohammed S, O'Flaherty M, Heck AJ, Slijper M, Menke FL (2007) Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteomics 6: 1198–1214 [DOI] [PubMed] [Google Scholar]
- Berardini TZ, Mundodi S, Reiser L, Huala E, Garcia-Hernandez M, Zhang P, Mueller LA, Yoon J, Doyle A, Lander G, Moseyko N, Yoo D, Xu I, Zoeckler B, Montoya M, Miller N, Weems D, Rhee SY (2004) Functional annotation of the Arabidopsis genome using controlled vocabularies. Plant Physiol 135: 745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmiller B, Malmstrom J, Gerrits B, Campbell D, Lam H, Schmidt A, Rinner O, Mueller LN, Shannon PT, Pedrioli PG, Panse C, Lee HK, Schlapbach R, Aebersold R (2007a) PhosphoPep—a phosphoproteome resource for systems biology research in Drosophila Kc167 cells. Mol Syst Biol 3: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmiller B, Mueller LN, Mueller M, Domon B, Aebersold R (2007b) Reproducible isolation of distinct, overlapping segments of the phosphoproteome. Nat Methods 4: 231–237 [DOI] [PubMed] [Google Scholar]
- Champion A, Kreis M, Mockaitis K, Picaud A, Henry Y (2004) Arabidopsis kinome: after the casting. Funct Integr Genomics 4: 163–187 [DOI] [PubMed] [Google Scholar]
- Chi A, Huttenhower C, Geer LY, Coon JJ, Syka JE, Bai DL, Shabanowitz J, Burke DJ, Troyanskaya OG, Hunt DF (2007) Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc Natl Acad Sci USA 104: 2193–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente van Bentem S, Anrather D, Roitinger E, Djamei A, Hufnagl T, Barta A, Csaszar E, Dohnal I, Lecourieux D, Hirt H (2006) Phosphoproteomics reveals extensive in vivo phosphorylation of Arabidopsis proteins involved in RNA metabolism. Nucleic Acids Res 34: 3267–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diks SH, Parikh K, van der Sijde M, Joore J, Ritsema T, Peppelenbosch MP (2007) Evidence for a minimal eukaryotic phosphoproteome? PLoS ONE 2: e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35: W585–W587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T (2000) Signaling—2000 and beyond. Cell 100: 113–127 [DOI] [PubMed] [Google Scholar]
- Initiative TAG (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Ishihama Y, Rappsilber J, Andersen JS, Mann M (2002) Microcolumns with self-assembled particle frits for proteomics. J Chromatogr A 979: 233–239 [DOI] [PubMed] [Google Scholar]
- Ishihama Y, Rappsilber J, Mann M (2006) Modular stop and go extraction tips with stacked disks for parallel and multidimensional peptide fractionation in proteomics. J Proteome Res 5: 988–994 [DOI] [PubMed] [Google Scholar]
- Ishihama Y, Wei FY, Aoshima K, Sato T, Kuromitsu J, Oda Y (2007) Enhancement of the efficiency of phosphoproteomic identification by removing phosphates after phosphopeptide enrichment. J Proteome Res 6: 1139–1144 [DOI] [PubMed] [Google Scholar]
- Kameyama K, Kishi Y, Yoshimura M, Kanzawa N, Sameshima M, Tsuchiya T (2000) Tyrosine phosphorylation in plant bending. Nature 407: 37. [DOI] [PubMed] [Google Scholar]
- Kokubu M, Ishihama Y, Sato T, Nagasu T, Oda Y (2005) Specificity of immobilized metal affinity-based IMAC/C18 tip enrichment of phosphopeptides for protein phosphorylation analysis. Anal Chem 77: 5144–5154 [DOI] [PubMed] [Google Scholar]
- Luan S (2002) Tyrosine phosphorylation in plant cell signaling. Proc Natl Acad Sci USA 99: 11567–11569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S (2003) Protein phosphatases in plants. Annu Rev Plant Biol 54: 63–92 [DOI] [PubMed] [Google Scholar]
- Mann M, Wilm M (1994) Error-tolerant identification of peptides in sequence databases by peptide sequence tags. Anal Chem 66: 4390–4399 [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298: 1912–1934 [DOI] [PubMed] [Google Scholar]
- Maor R, Jones A, Nuhse TS, Studholme DJ, Peck SC, Shirasu K (2007) Multidimensional protein identification technology (MudPIT) analysis of ubiquitinated proteins in plants. Mol Cell Proteomics 6: 601–610 [DOI] [PubMed] [Google Scholar]
- Miranda-Saavedra D, Barton GJ (2007) Classification and functional annotation of eukaryotic protein kinases. Proteins 68: 893–914 [DOI] [PubMed] [Google Scholar]
- Molina H, Horn DM, Tang N, Mathivanan S, Pandey A (2007) Global proteomic profiling of phosphopeptides using electron transfer dissociation tandem mass spectrometry. Proc Natl Acad Sci USA 104: 2199–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuhse TS, Bottrill AR, Jones AM, Peck SC (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51: 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuhse TS, Stensballe A, Jensen ON, Peck SC (2003) Large-scale analysis of in vivo phosphorylated membrane proteins by immobilized metal ion affinity chromatography and mass spectrometry. Mol Cell Proteomics 2: 1234–1243 [DOI] [PubMed] [Google Scholar]
- Nuhse TS, Stensballe A, Jensen ON, Peck SC (2004) Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. Plant Cell 16: 2394–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyanagi H, Tanaka T, Sakai H, Shigemoto Y, Yamaguchi K, Habara T, Fujii Y, Antonio BA, Nagamura Y, Imanishi T, Ikeo K, Itoh T, Gojobori T, Sasaki T (2006) The Rice Annotation Project Database (RAP-DB): hub for Oryza sativa ssp. japonica genome information. Nucleic Acids Res 34: D741–D744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127: 635–648 [DOI] [PubMed] [Google Scholar]
- Olsen JV, de Godoy LM, Li G, Macek B, Mortensen P, Pesch R, Makarov A, Lange O, Horning S, Mann M (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol Cell Proteomics 4: 2010–2021 [DOI] [PubMed] [Google Scholar]
- Olsen JV, Ong SE, Mann M (2004) Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol Cell Proteomics 3: 608–614 [DOI] [PubMed] [Google Scholar]
- Rappsilber J, Ishihama Y, Mann M (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem 75: 663–670 [DOI] [PubMed] [Google Scholar]
- Rudrabhatla P, Reddy MM, Rajasekharan R (2006) Genome-wide analysis and experimentation of plant serine/threonine/tyrosine-specific protein kinases. Plant Mol Biol 60: 293–319 [DOI] [PubMed] [Google Scholar]
- Saito H, Oda Y, Sato T, Kuromitsu J, Ishihama Y (2006) Multiplexed two-dimensional liquid chromatography for MALDI and nanoelectrospray ionization mass spectrometry in proteomics. J Proteome Res 5: 1803–1807 [DOI] [PubMed] [Google Scholar]
- Schwartz D, Gygi SP (2005) An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat Biotechnol 23: 1391–1398 [DOI] [PubMed] [Google Scholar]
- Sugiyama N, Masuda T, Shinoda K, Nakamura A, Tomita M, Ishihama Y (2007) Phosphopeptide enrichment by aliphatic hydroxy acid-modified metal oxide chromatography for nano-LC-MS/MS in proteomics applications. Mol Cell Proteomics 6: 1103–1109 [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda T, Mochizuki Y, Player K, Heida N, Kobayashi N, Sakaki Y (2007) OmicBrowse: a browser of multidimensional omics annotations. Bioinformatics 23: 524–526 [DOI] [PubMed] [Google Scholar]
- Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, Schein J, Sterck L, Aerts A, Bhalerao RR, Bhalerao RP, Blaudez D, Boerjan W, Brun A, Brunner A, Busov V et al. (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313: 1596–1604 [DOI] [PubMed] [Google Scholar]
- Villen J, Beausoleil SA, Gerber SA, Gygi SP (2007) Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci USA 104: 1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Fu HH, Gupta R, Luan S (1998) Molecular characterization of a tyrosine-specific protein phosphatase encoded by a stress-responsive gene in Arabidopsis. Plant Cell 10: 849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figures
Supplementary Table 1
Supplementary Table 2
Supplementary data 1
Supplementary data 2
Supplementary data 3