Abstract
Corticotropin Releasing Hormone (CRH) or Corticotropin Releasing Factor (CRF) and its family of related naturally occurring endogenous peptides and receptors are becoming recognized for their actions within central (CNS) and peripheral (PNS) nervous systems. It should be recognized that the term ‘CRH’ has been displaced by ‘CRF’ (Guillemin 2005). However, to maintain uniformity among contributions to this special issue we have used the original term, CRH. The term ‘CRF’ has been associated recently with CRH receptors and designated with subscripts by the IUPHAR nomenclature committee (Hauger R.L. et al. 2003) to denote the type and subtype of receptors activated or antagonized by CRH ligands. CRH, as a hormone, has long been identified as the regulator of basal and stress-induced ACTH release within the hypothalamo-pituitary-adrenal axis (HPA axis). But the concept, that CRH and its related endogenous peptides and receptor ligands have non-HPA axis actions to regulate CNS synaptic transmission outside the HPA axis, is just beginning to be recognized and identified (Orozco-Cabal et al. 2006). It is especially noteworthy that since the synapse has become a prime focus for a variety of mental diseases, e.g. schizophrenia (Fischbach 2007), and neurological disorders, e.g., Alzheimer’s disease (Bell and Cuello 2006), we suggest that “THE STRESSED SYNAPSE” has been overlooked (c.f., Kim and Diamond 2002; Radley and Morrison 2005) as a major contributor to many CNS disorders. We present data demonstrating CRH neuroregulatory and neuromodulatory actions at three limbic synapses, the basolateral amygdala to central amygdala synapse; the basolateral amygdala to medial prefrontal cortex synapse, and the lateral septum mediolateral nucleus synapse. A novel stress circuit is presented involving these three synapses. We suggest that CRH ligands and their receptors are significant etiological factors that need to be considered in the pharmacotherapy of mental diseases associated with CNS synaptic transmission.
Index Words: Corticotropin Releasing Hormone (CRH), Corticotropin Releasing Factor (CRF), CNS glutamatergic synaptic transmission, CRH1 receptor, CRH2 receptor, Neuroregulator, Central amygdala nucleus, Lateral septal mediolateral nucleus, Medial prefrontal cortex
1. INTRODUCTION
CRH, as an endogenous signaling molecule, has existed and functioned phylogenetically even prior to the evolution of tetrapods and teleosts (Chang and Hsu 2004). Such a genetic history suggests that CRH and its family of structurally related peptides are essential ingredients for the maintenance of an organism’s well-being or homeostasis (Valdez et al. 2005; Lovejoy and Balment 1999). A primary hypophysiotropic ‘releasing function’ for CRH was described initially in vitro (Guillemin and Rosenberg 1955), and its endogenous functions reviewed (Guillemin 1967; Saffran and Schally 1977). A major contribution to further understanding the roles of CRH was its characterization and synthesis by Vale and colleagues (Vale et al. 1981). Over the past 30+ years, CRH, originally implicated within the HPA axis as the “stress hormone”, has also been considered for its role within the CNS (Ito and Miyata, 1999; Bale and Vale 2004) outside the HPA axis (Guillemin 2005), within GIT (Tache and Bonaz 2007), heart (Kimura et al. 2002), and lung (Wu et al. 2006).
1.1 CRH as a mediator of organismic homeostasis
Why consider that CRH ligands have functional roles within CNS synapses outside of the HPA? Immunolabeling, radioimmunoassay, and mRNA expression studies have demonstrated that CRH and its receptors are widely distributed in brain, e.g., human (Charlton et al. 1987), rat (Fischman and Moldow 1982), and mouse (Nakane et al. 2007). Within the CNS, CRH is synthesized and stored at specific synapses, and, under appropriate conditions may be released or co-released along with classical neurotransmitters. Immunohistochemical studies demonstrated a nerve terminal localization for CRH (Cain et al. 1991). Within the hippocampus, CRH has been demonstrated within GABAergic neurons (Yan et al. 1998). In addition, CRH has also been identified at the electron microscopic level within both glutamate and GABA terminals of the rat locus coeruleus (Valentino et al. 2001). Significantly, synaptic peptide release, previously considered to be minimal and requiring extraordinary stimuli, in terms of both intensity and frequency of stimuli, has recently been demonstrated to exhibit comparable release properties and kinetics as that of biogenic amines (Whim 2006). Thus, CRH is available for release at CNS synapses, but its functions within specific synapses are only beginning to be elucidated. Importantly, CRH actions and associated functions differ depending on the particular synapse.
1.2 Extra-HPA axis roles for CRH
In the brain, two main sources of CRH can be distinguished, one within the HPA-axis, and others in non-HPA axis sites. The production of anxiety-like behavioral and autonomic effects after centrally administered (intracerebroventricularly, icv) CRH (Dunn and File 1987; Britton et al. 1982; Koob and Bloom 1985) have been associated with non-HPA axis-CRH, CNS sources of CRH, since these anxiety-related outcomes persist in hypophysectomized rats (Eaves et al. 1985).
Abnormalities in extra-HPA axis CRH homeostasis have been associated with prevalent neuropsychiatric conditions including anxiety disorders, depression, and Alzheimer disease. Earlier studies (Reul and Holsboer 2002; McCarthy et al. 1999; Arborelius et al. 1999) suggest that CRH receptor antagonists may be useful as therapeutic agents to treat these stress-related disorders.
Bremmer et al. (Bremner et al. 1997) reported higher levels of CRH (29.0 pg/ml; ~37% increase) in CSF of patients diagnosed with chronic combat-related posttraumatic stress disorder versus CSF-CRH in comparison subjects (21.9 pg/ml). Subsequently, Vythilingam (Vythilingam et al. 2000) concluded that in healthy humans, CSF-CRH represented CRH derived primarily from non-HPA axis (hypothalamic-pituitary-adrenal axis) CRH neurons rather than HPA-axis-CRH neurons projecting from the paraventricular nucleus of the hypothalamus. Plasma CRH (1.8 pmole\L) is elevated by 100 to 255% above normal values in patients diagnosed with mild to severe depressive disorder (Catalan et al. 1998); these elevated levels of plasma CRH occur without any accompanying changes in plasma ACTH. Plasma and CSF levels of CRH are diminished in the elderly, especially as noted in patients diagnosed with Alzheimer’s disease. Earliest studies demonstrated that in individuals with Alzheimer disease, CRH immunoreactivity is reduced in neocortex (Bissette et al. 1985). CRH-immunoreactivity in spinal fluid was also reduced in Alzheimer disease (Mouradian et al. 1986). A later study in which cerebrospinal fluid (CSF) CRH-immunoreactivity was measured correlated a lower CSF-CRH-immunoreactivity with a greater cognitive impairment in Alzheimer disease patients. Powers (Powers et al. 1987) demonstrated abnormal CRH-immunoreactive axons, as well as neurites associated with deposits of amyloid in brain regions showing senile plaques. Behan (Behan et al. 1996) demonstrated that in Alzheimer disease there are dramatic reductions in human CRH concentrations and reciprocal increases in CRH receptor density in the cortex.
These results also point to different variables that should be considered when comparing the actions of CRH receptor ligands. First, is the state/condition of the subject (Heilig and Koob 2007; Griebel et al. 2002; Contarino et al. 1999; Henry et al. 2006), i.e., animals exposed to acute, chronic, or no stress will establish different baseline CRH levels from which one could expect a tonic or phasic action by CRH at its receptors. Second, is brain area, e.g., central amygdala nucleus vs lateral septum mediolateral nucleus (Liu et al. 2004). It is important to note that one of the first anatomical sites exposed to the most common means of central administration, namely, icv injection of CRH or CRH-ligands, is the lateral septum mediolateral nucleus. Excitatory transmission within this nucleus is affected by CRH ligands (Liu, et al. 2004; Liu et al. 2005).
1.3 CRH receptors
As a means of determining synaptic function(s) for CRH, the presence and characterization of CRH receptors was essential. The ability to characterize CRH receptors followed upon the purification of CRH from ovine hypothalamic extracts and concomitant determination of its 41-residue peptide structure (Vale et al. 1981). Subsequent isolation of additional endogenous CRH receptor ligands, namely, Urocortin 1, Urocortin 2, and Urocortin 3, and, synthesis of peptide and non-peptide ligands for CRH receptors have provided essential tools necessary to investigate the roles of CRH within CNS synapses.
Briefly, mammalian CRH receptors have been differentiated broadly into two major types, CRH1 and CRH2; each type exhibiting a 70% sequence homology (Perrin and Vale 1999) and also possessing molecular splice variants. Consistent with structural differences, CRH1 and CRH2 receptors display distinct pharmacological profiles (Dautzenberg and Hauger 2002; De Souza 1995; Gulyas et al. 1995; Fekete and Zorrilla 2007; Chatzaki et al. 2006). CRH1 receptor immunoreactivity has been detected in cholinergic, dopaminergic, and noradrenergic neurons of the murine basal forebrain and brainstem nuclei (Sauvage and Steckler 2001). CRH receptors have also been associated with serotonin pathways and 5-HT release (Valentino and Commons 2005). We demonstrated CRH1 and CRH2 receptor functions both at pre- and post-synaptic CNS sites within three different CNS synapses, Fig. 2–4 (Liu et al. 2004;Orozco-Cabal et al. In press).
Fig. 2.
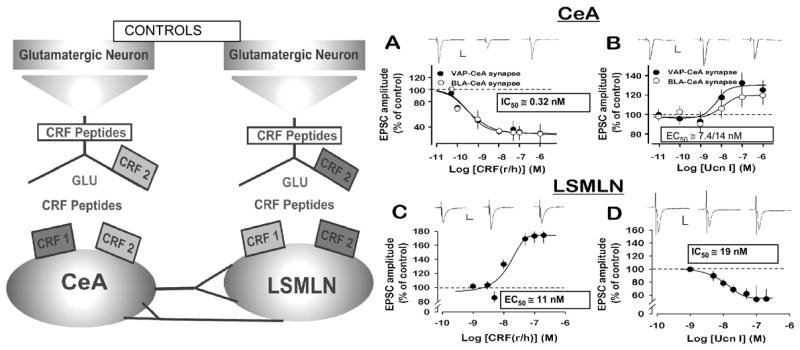
Distribution and regulatory functions depicted for CRH1 and CRH2 synaptic receptors upon excitatory synaptic transmission, [facilitatory-light gray and depressant-dark grey]. CRH1 and CRH2 receptors regulate glutamatergic transmission within synapses in the central amygdala nucleus, Left, and lateral septum medial lateral nucleus, Right. R\hCRF and Ucn I (Urocortin I), CRH1 and CRH2 receptor agonists, respectively –each produce opposite effects to inhibit or facilitate excitatory transmission-monitored as excitatory postsynaptic currents (EPSCs)-in the two different limbic nuclei, central amygdala nucleus and lateral septum mediolateral nucleus. Note low nanomolar effective concentrations. Adapted From: J. Neurosci.,24, 4020–4029.
Fig. 4.
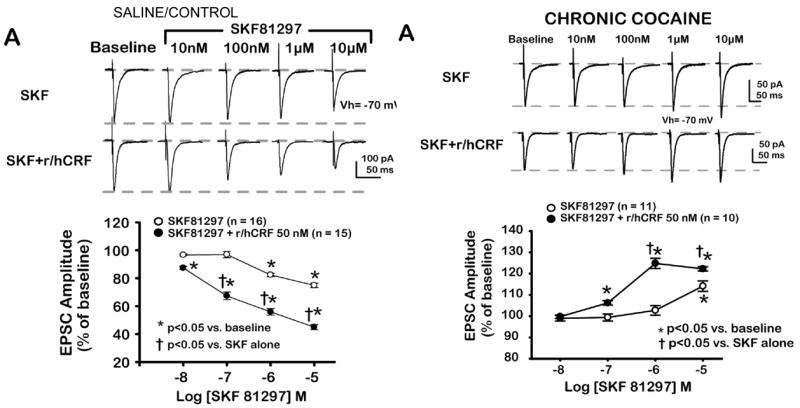
Switch in dopamine and CRH actions after acute withdrawal from chronic cocaine. A. In saline control slices D1-like activation (SKF 81297) inhibited excitatory postsynaptic current (EPSC) amplitude; SKF 81297 effects were enhanced by addition of CRH at a concentration of CRH that did not itself affect basolateral amygdala-medial prefrontal cortex EPSCs. B. After acute withdrawal from chronic cocaine, SKF 81297 and CRH synergistically enhanced EPSC amplitude at medial prefrontal cortex synapses. Comparable results to SKF 81297 obtained with dopamine.
CRH receptors belong to the family of class ‘B’ G-protein coupled receptors. Although CRH receptors primarily couple to Gs protein and adenylate cyclases to increase cAMP production, CRH receptors have the ability to interact with other G-protein systems including Gq, Gi, Go, Gil/2, and Gz (Grammatopoulos et al. 2001). Thus, CRH receptors can modulate various signaling pathways and kinases including phosphokinase A (PKA), phosphokinase B (PKB), phosphokinase C (PKC), mitogen activated protein (MAP) kinases (e.g., ERK1/2 – p42,44), and intracellular Ca2+ concentrations in a tissue-specific manner, and activate these various G-protein systems in a concentration-dependent manner (Grammatopoulos and Chrousos 2002). These latter results also suggest that there are various degrees of coupling potency between CRH receptors and their respective G-protein systems. We propose that at the single cell level, the net effect of a CRH receptor ligand upon synaptic transmission is determined by its ability to modify the existing balance between the possible signaling cascades it may activate, rather than the effect of CRH upon one pathway exclusively (Orozco-Cabal et al. 2006a; Arzt and Holsboer 2006).
Another possibility we considered was that CRH receptors may also share the property of constitutive activity, a property of many G-protein coupled receptors. Constitutive activity of G-protein coupled receptors would modulate the baseline activity/sensitivity of the cell on which that receptor is localized in the absence of ligand, i.e., an intrinsic property of many G-protein coupled receptors. However, to identify this receptor property on a given neuron an inverse agonist for a CRH receptor is required. Currently, no inverse agonist of a CRH1 or CRH2 receptor has been identified. Moreover, CRH receptor ligands - at given concentrations - may have different effects at different loci, not only at different sites within the CNS but also at peripheral sites. Finally, different actions of endogenous and exogenously applied CRH receptor ligands can be expected based upon their different receptors.
1.4 Semantics associated with CRH synaptic functions
As electrophysiologists we propose that semantics in terminology have contributed to the delay in considering the synaptic role by which CRH activation of its receptors serves a neuroregulatory function (Orozco-Cabal et al. 2006a). Non-HPA- axis CRH was initially identified as a putative neurotransmitter (Valentino 1988; Valentino 1989; Valentino and Foote 1988; Thomas et al. 2003; Valentino et al. 2001; Dunn and Berridge 1990). These earlier reports suggesting CRH as a neurotransmitter were based primarily on behavioral assessments and extracellular in vivo electrophysiological recordings. Instead, we propose that CRH was acting either as a possible neuromodulator or neuroregulator in each of these earlier referenced instances, and may indeed have been acting within specific synapses to affect synaptic transmission. Subsequently, and based on intracellular electrophysiological recordings, we have limited the definition of a neurotransmitter to be an endogenous neuroactive substance which when released from a nerve terminal or transported in a volume, paracrine fashion activates a synaptic membrane receptor that causes a change in that neuron’s membrane potential. Examples of classical ionotropic-receptor-coupled neurotransmitters would be glutamate, GABA, acetylcholine, etc. A property associated with a neurotransmitter is the speed by which it induces membrane potential changes in the neuron where its receptor is located. The kinetics of neurotransmitters’ actions is typically in the tens of milliseconds range or less. CRH, at physiological concentrations, does not induce a membrane potential change irrespective of its exposure time. Applying these considerations to CRH, neither endogenous CRH nor CRH receptor ligands-applied exogenously and at concentrations comparable to those measured in plasma or cerebrospinal fluid (≤ 10nM ~ 20pg/ml in CSF, i.e., physiological concentrations)-should be considered a neurotransmitter.
Historically, another function assigned to CRH and its family of related peptides is that of a neuromodulator (Radulovic et al. 1999; Merchenthaler 1984). Neuromodulators can balance more delicately the net drive at a given synapse, and in so doing, regulate the function (excitation or inhibition) of a given neuronal circuit and its associated behaviors. From an electrophysiologist’s perspective, a neuromodulator would be an endogenous substance that modifies the action of a neurotransmitter by either enhancing or depressing the primary membrane change induced by a neurotransmitter. The modulatory action is brought about by the neuromodulator changing membrane potential, and/or, electrical excitability of the neuronal membrane where its receptor and that of the neurotransmitter is located. Other modulatory actions, typically not measured by an electrophysiologist could include effects mediated by receptor modification, such as phosphorylation of a neurotransmitter receptor, or changes in the number of receptors expressed in the neuronal membrane, etc.
These modulatory actions are typically mediated by activation of a G-protein coupled receptor (GPCR) associated with a particular neuromodulator. Neuromodulator receptors are considered to be metabotropic as opposed to ionotropic. The speed at which a neuromodulator affects a response is in the hundred to thousands of milliseconds range. Examples of neuromodulators include the biogenic amines, opiates, tachykinins, neuropeptides, etc. The unique synaptic attribute of neuroregulation we have assigned to CRH (and possibly other endogenous molecules), is the neuroregulator’s ability, by activating one of its two G-protein coupled receptors (CRH1 and CRH2), to affect (‘prime’) the subsequent actions of a neurotransmitter or neuromodulator without itself, the neuroregulator, inducing any apparent membrane potential change or change in electrical excitability of the neuronal membrane on which its receptors are located (Orozco-Cabal et al. 2006a). We consider this neuroregulator role of CRH, its primary, most basic function in a hierarchy of actions. This primary, neuroregulator function results in the facilitation or depression of a neurotransmitter’s action. In its primary role, the neuroregulator does not exhibit any affect upon membrane potential or membrane excitability, rather it acts in a ‘silent’ process that affects a transmitter’s action. As neuroregulators, CRH receptor ligands may affect the actions of: 1) neurotransmitters, e.g., glutamate (Koenig and Luthi 2002; Liu et al. 2004), GABA (Nie et al. 2004); 2) neuromodulators, e.g., serotonin (Tan et al. 2004), dopamine (Orozco-Cabal et al. 2005), endocannabinoids (Bayatti et al. 2005; Hermann and Lutz 2005); and/or, 3) possibly other neuroregulators, e g., Brain Derived Neurotropic Factor (BDNF, Traver et al. 2006). CRH has already been reported to modulate actions of norepinephrine (Valentino et al. 1983) and opiates measured during a stress reaction. A secondary role for CRH receptor ligands (#2 above) would be to affect the action of a neuromodulator which subsequently affects the action of a neurotransmitter. As an example, Price et al. (2000) demonstrated CRH regulation of serotonin release at the lateral septal nucleus of swim-stressed rats. Thus, in this case CRH modulates the effects of serotonin.
A recent review (Leach et al. 2007) characterizes a group of molecules which they identify as allosteric GPCR modulators. An allosteric GPCR modulator is defined as a ligand that increases or decreases the action of an (primary or orthosteric) agonist or antagonist by combining with a distinct (allosteric) site on the receptor macromolecule, while having no effect of its own (Schwartz and Holst 2007). We suggest that CRH receptor ligands acting as neuroregulators may also be identified as GPCR allosteric ligands, i.e., as allosteric GPCR agonists or modulators. In this role, CRH may also contribute to the process of synaptic plasticity which has been associated with cellular models of learning and memory. CRH has been suggested to be responsible for “Priming” (Blank et al. 2002; Rainnie et al. 2004), and as such may also contribute to a functional synonym for priming, namely, “Metaplasticity” (Abraham and Bear 1996). CRH has been implicated directly in long-term potentiation (LTP, Rebaudo et al. 2001; Fu et al. 2006; Pollandt et al. 2006; Wang et al. 2000; Wang et al. 1998) and long-term depression (LTD, Miyata et al. 1999; Schmolesky et al. 2007).
2. CNS SYNAPTIC ACTIONS OF CRH LIGANDS RECORDED INTRACELLULARLY
Outside of the cerebellum, there are nine in vitro reports of electrophysiological intracellular investigations of specific CRH ligand effects upon synaptic transmission, namely, synapses of the central amygdala nucleus and lateral septum mediolateral nucleus (Liu et al. 2004); lateral septum mediolateral nucleus (Liu et al. 2005); basolateral amygdala (Rainnie et al. 2004); central amygdala nucleus (Nie et al. 2004); ventral tegmental area (Ungless et al. 2003); dorsal vagal complex (Lewis et al. 2002); hippocampus (Blank et al. 2002; Schierloh et al. 2007; and medial prefrontal cortex (Orozco-Cabal et al. In press). These reports represent all information utilizing intracellular electrophysiological techniques with an in vitro brain preparation that demonstrate a synaptic location and function for CRH and its related family of peptides. There are several reports indicating a synaptic role for CRH ligands in the cerebellum, v.i.
2.1 Synapses of the central amygdala nucleus and lateral septum mediolateral nucleus
Our report (Liu et al. 2004) was the first to demonstrate functional data - at the cellular level - that CRH and its related family of peptides act differentially at CRH1 vs. CRH2 synaptic receptors to facilitate or depress excitatory transmission. Notably, the effects of CRH and its ligands occurred without any apparent direct action on membrane potential or membrane excitability. As a result, we have suggested that the role of CRH at these limbic synapses is that of ‘neuroregulator’. Furthermore, at the two limbic nuclei we investigated - the central nucleus of the amygdala and the lateral septal medial lateral nucleus - we concluded that CRH1 and CRH2 receptors were present on the same post-synaptic neuron, while only CRH2 receptors were located pre-synaptically (Fig. 2). Moreover, the functions of these receptors were different depending on the synapse and synaptic locus. Our data utilized new pharmacological tools (Rivier et al. 2002; Rivier et al. 2007) to characterize the CRH receptor types responsible for these functional synaptic effects. Selective and potent CRH1 and CRH2 receptor agonists and antagonists had been a limiting factor in identifying CRH synaptic actions. Lewis (Lewis et al. 2002) reported facilitation of excitatory postsynaptic currents (EPSCs) at the rat dorsal vagal complex by a pre-synaptic action of CRH; a CRH2 receptor was suggested as being responsible. Similarly, if a CRH receptor type was inferred from other reports (Rainnie et al. 2004; Ungless et al. 2003; Lawrence et al. 2002; Smagin et al. 2001), a CRH2, not CRH1 receptor, was suggested at the pre-synaptic site.
We suggested (Fig. 2, Left) pre- and post-synaptic loci for CRH1 and CRH2 receptors within two limbic synapses, the central amygdala nucleus, and lateral septum mediolateral nucleus. Note, although both synapses exhibit a comparable pre- and post-synaptic location of CRH1 and CRH2 receptors, their functions (facilitation vs. depression of glutamatergic transmission) is opposite within each synapse. Importantly, the results (Fig. 2 Right) from which we derived the synaptic locations and functions of CRH1 and CRH2 receptors at these synapses yielded apparent receptor association values for r\hCRH and urocortin I at low nanomolar concentrations, concentrations equivalent to those measured endogenously.
Our (Liu et al. 2004) findings with CRH and Ucn I and those of Rainnie (Rainnie et al. 2004) with Ucn demonstrated that CRH-peptides, at nanomolar concentrations do NOT affect membrane potential or neuronal input resistance and point to a regulatory role rather than a transmitter role for the CRH ligands. Our data also demonstrated that endogenous CRH ligands could induce a tonic effect on excitatory glutamatergic transmission at synapses within both these nuclei since application of competitive, selective CRH1 or CRH2 receptor antagonists resulted in an enhancement or depression of glutamatergic EPCS (Liu et al 2004). A similar tonic endogenous action of CRH ligands was not observed under control conditions in the medial prefrontal cortex (Orozco-Cabal et al. In press). This latter result further emphasizes that CRH effects are different depending upon the CNS synapse being investigated. We also observed different effects of CRH during in vitro brain slice investigations which were conducted with brains obtained from rats administered cocaine in vivo chronically when compared with brain slices from drug naïve subjects (Fig. 4, 5). A role of CRH in cocaine addiction has been reviewed (Sarnyai et al. 2001; Koob 1999).
Fig. 5.
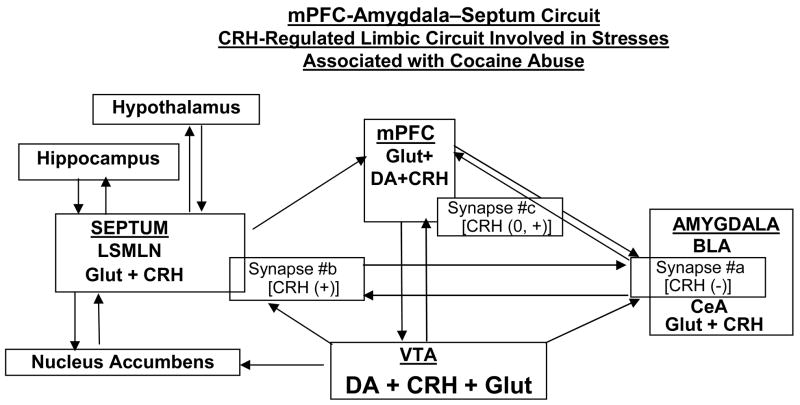
Diagram depicting a novel stress network
[medial prefrontal cortex ↔ amygdala ↔ septum]
implicating CRH AND DOPAMINE (DA) as regulator and modulator of excitatory synaptic transmission (glutamate -GLUT) between these three nuclei, and other major limbic nuclei. Facilitatory or depressant regulatory roles of CRH upon excitatory glutamatergic transmission under control conditions at two of the three synapses (#a=basolateral nucleus (BLA) to central amygdala nucleus (CeA); #b=central amygdala nucleus (CeA) to lateral septum mediolateral nucleus (LSMLN) are depicted as (+) or (−), respectively. At basolateral amygdala nucleus (BLA) to medial prefrontal cortex (mPFC), synapse #c, and under control conditions CRH does not regulate glutamatergic transmission (0), but rather modulates positively (+) the depressant action of dopamine upon glutamatergic transmission.
We had demonstrated that following the stresses associated with chronic cocaine administration and its acute withdrawal, the distributions and functions of both CRH1 and CRH2 receptors within the lateral septum mediolateral nucleus changed (Liu et al. 2005). ‘Normal’ regulation of glutamatergic transmission by CRH ligands was altered after chronic cocaine and its withdrawal (Fig. 3) and led to a functional loss of pre- and postsynaptic CRH2 receptors – both CRH2 receptors being responsible normally for depression of excitatory glutamatergic transmission within the lateral septum mediolateral nucleus (Fig. 3 and 6). Following chronic cocaine and its acute withdrawal there was a ‘switch’ within the lateral septum mediolateral nucleus synapse from the normal balance of facilitation and depression by CRH-ligands acting at their respective receptors to facilitation only. We concluded that the signaling pathways associated with both CRH receptors switched from a dominant PKA process to become PKC dominant.
Fig. 3.
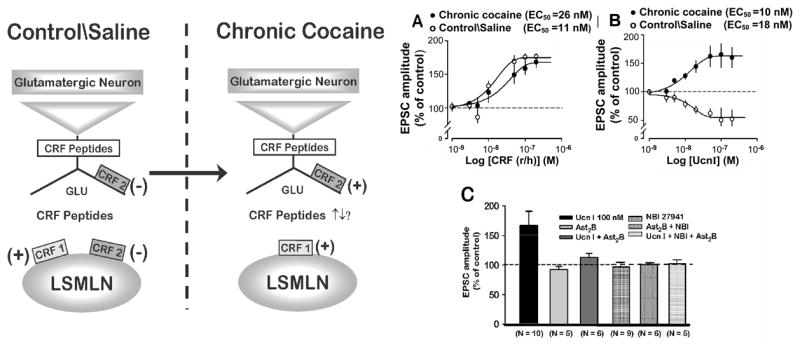
Following chronic cocaine administrations and its withdrawal there are changes in sensitivities and functions of CRH and Ucn 1 within the lateral septal mediolateral nucleus. CRH1 receptor mediated facilitation of glutamatergic transmission persists, albeit CRH is less potent compared to control, whereas the former CRH2-mediated depression by Ucn 1 is switched to facilitation, and at comparable potency. Diagram depicts receptor distributions and functions before and after chronic cocaine at lateral septum mediolateral nucleus synapse. Adapted From: J. Neurosci.,25, 577–583.
Fig. 6.
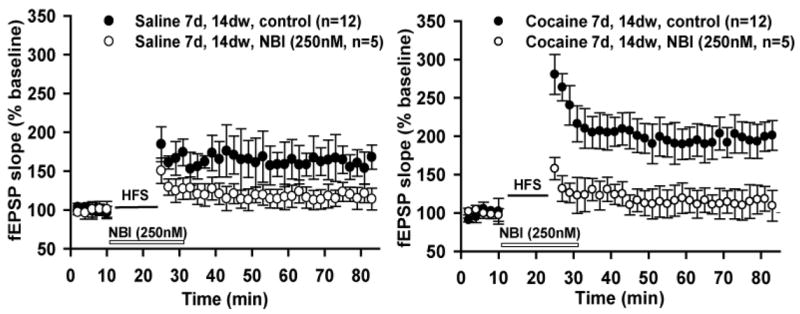
Long-Term Potentiation (LTP) in basolateral amygdala-central amygdala nucleus pathway (Fig. 5,#a) is dependent on CRF1 receptors in saline and cocaine-treated preparations. A.,B. Orthodromic stimulation of basolateral amygdala-central amygdala nucleus pathway with high frequency stimulation (HFS) induced LTP in saline-treated animals (Left) or chronic cocaine treated animals (Right). HFS-LTP is blocked by selective CRF1 antagonist (NBI27914, 250 nM) in both preparations. NOTE: Higher magnitude LTP in brains from cocaine-treated animals (Right).
2.2 Excitatory transmission at the basolateral amygdala to medial prefrontal cortex synapse is affected by dopamine, CRH, and their combination
Since both CRH and dopamine systems have been implicated as stress-sensitive modulators of synaptic transmission within limbic reward circuits (Sarnyai 1998; Koob and Heinrichs 1999), we initiated studies with a novel brain slice preparation (Orozco-Cabal et al. 2006b) containing a putative glutamatergic (Bacon et al. 1996) amygdala to medial prefrontal cortex synapse. We confirmed the glutamatergic nature of this excitatory synapse (Orozco-Cabal et al. 2006b). Additional investigations compared the actions of dopamine, CRH, and their combination on excitatory transmission at this putative basolateral amygdala to Layer V pyramidal neuron synapse (basolateral amygdala-medial prefrontal cortex synapse). We chose this pathway, since output from the amygdala to the medial prefrontal cortex plays a significant role in human executive functions (Fuster 2000). Our goal was to determine the normal actions of dopamine, CRH, and their combination upon excitatory transmission at this synapse, and furthermore, examine if chronic administration of a stressor (cocaine) and its acute removal - as we had demonstrated for CRH at the lateral septum mediolateral nucleus synapse (Liu et al. 2005) - altered excitatory transmission (and as a possible inferred corollary - decision-making processing) at this putative basolateral amygdala-medial prefrontal cortex synapse.
Dopamine via D1-like receptor activation depressed glutamatergic transmission at a putative basolateral amygdala to Layer V medial prefrontal cortex pyramidal neuron synapse within the rat medial prefrontal cortex (Orozco-Cabal et al. In press). This depressant action of dopamine was potentiated with co-administration of CRH - although CRH, itself, was without any apparent effect on basolateral amygdala-medial prefrontal cortex glutamatergic transmission. However, following administration of cocaine chronically, dopamine no longer depressed EPSCs, rather dopamine facilitated EPSCs and this facilitation was potentiated by co-administration of CRH. Additional changes in dopamine and CRH receptor distribution and function also occurred subsequent to cocaine.
2.3 Summary of effects of CRH1 and CRH2 activation upon excitatory CNS synaptic transmission at three different limbic synapses
CRH ligands, interacting with CRH synaptic receptors, produced specific effects upon excitatory glutamatergic transmission at three different but anatomically connected limbic synapses, namely, basolateral amygdala-central amygdala nucleus (Fig. 5, #a), lateral septum mediolateral nucleus (Fig. 5, #b), and basolateral amygdala-medial prefrontal cortex (Fig. 5, #c). At the basolateral amygdala-central amygdala synapse (Fig. 5, #a) and under control conditions, activation of postsynaptic CRH1 receptors resulted in a net depression (−)of evoked glutamatergic transmission despite weak pre- and postsynaptic facilitatory actions mediated by CRH2 activation. On the other hand and under control conditions, evoked excitatory synaptic transmission at the lateral septum mediolateral nucleus (Fig. 5, #b) was facilitated (+) primarily by activation of a postsynaptic CRH1 receptor, despite potential concomitant depressant actions by CRH ligands acting at pre- and post-synaptic CRH2 receptors. Finally, and different from its effects at the two previously described synapses, CRH does not affect (0) evoked glutamatergic transmission at the putative basolateral amygdala-medial prefrontal cortex (Fig. 5, #c) synapse. Although CRH had no effect on glutamatergic transmission at the basolateral amygdala-medial prefrontal cortex synapse under control conditions, a postsynaptic CRH1-mediated potentiation (+) of a pre- and postsynaptic dopamine 1-like receptor (D1-like) mediated depression occurred following exogenous application of CRH plus dopamine. Thus, at this synapse CRH was acting to modulate the depressant action of dopamine resulting from the combined activation of both receptors (D1-like plus CRH1).
In addition, the “state” of a particular synapse, i.e. under control conditions vs. under a “stressed” state, e.g., due to exposure to cocaine administered chronically and its acute withdrawal, altered a typical CRH effect (Fig. 3, 4). We suggest a novel CRH-regulated limbic circuit (Fig. 5) which would result in positive or negative net signal depending on the presence of a stressor, i.e., the state of the system, as may occur following exposure to chronic cocaine.
3. CRH and LTP
One of the net outcomes that could be affected from this circuit would be a learned or remembered behavior. Long-term potentiation (LTP) is used as a cellular model of learning and memory. CRH, itself, can induce LTP (Wang et al. 1998; Wang et al. 2000; Pollandt et al. 2006) or potentiate the magnitude of LTP induced by other means (Pollandt et al. 2006).
We (Fu and Shinnick-Gallagher 2007) demonstrated at the basolateral amygdala to central amygdala nucleus (Fig. 5, #a) synapse that endogenous CRH co-released during high frequency stimulation was blocked by the selective CRH1 antagonist NBI30775. We had previously demonstrated (Pollandt et al. 2006) at the lateral amygdala (LA) to central amygdala nucleus synapse that exogenously applied CRH (12.5 nM) was sufficient to induce LTP in both untreated rats and rats that had been administered cocaine chronically. Interestingly, the exogenously applied CRH induced an LTP of greater magnitude when rats were withdrawn from cocaine for a period of two weeks. This CRH-induced LTP was dependent on CRH1 receptors and involved PKA. These results may have direct implications regarding learning and memory processing under stress (Joëls et al. 2006).
4. CEREBELLUM
A series of electrophysiological and immunocytochemical studies examining rat (Swinny, et al. 2003; Swinny et al. 2004) and mouse (King and Bishop 2002; Bishop, 2002; Bishop, et al. 2000) cerebellum have concluded that CRH1 and CRH2 are expressed differentially in pre- and post-synaptic elements. Furthermore, Swinny et al. (Swinny et al. 2003) concluded that CRH2 is membrane bound at synapses, while CRH1 is not. As a result, they suggest that CRH-peptide ligands couple to CRH2 receptors via synaptic transmission, whereas these same ligands couple to CRH1 receptors via volume transmission. The most recent electrophysiological study (Schmolesky et al. 2007) concluded that by regulating climbing fiber input to Purkinje cells, CRH facilitated LTD-induction at this synapse. This latter result is the most recent demonstration of CRH affecting synaptic transmission at a specific CNS synapse.
5. CONCLUSIONS and FUTURE DIRECTIONS
This special issue contains reviews (see: McEwen, Korosi & Baram, and Holsboer & Ising) supporting the concept that CRH receptor antagonists may be useful as therapeutic agents to treat a variety of mental illnesses often associated with stress, namely, anxiety, fear, depression.
Our results showing enhanced CRH actions in rats receiving chronic cocaine, and those reporting elevation of CRH with cocaine via activation of the HPA axis (Rivier and Vale 1987; Goeders et al. 1990; Richter et al. 1995; Sarnyai et al. 1995) support the possible use of CRH receptor antagonists in the pharmacotherapy for substance abuse. Multiple reports support a close association between stress, CRH, and drug addiction (Smagin and Dunn 2000; Smagin et al. 2002). Thus, CRH receptor ligands hold a bright future as pharmacotherapy in the treatment of substance abuse and other comorbid mental disorders.
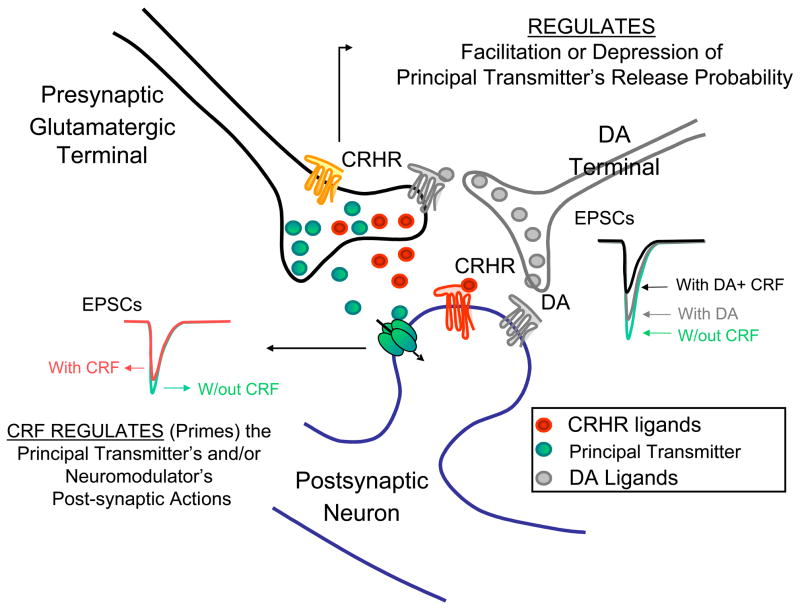
Fig. 1. Roles for CRH, CRH Receptor (CRHR) Ligands as Neuroregulators (‘Primers’) within CNS Synapses.
Tonic role of endogenous CRH as demonstrated following application of a CRH receptor antagonist results in an enhancement or depression of the primary transmitter’s action, e.g., upon glutamate transmission at central amygdala nucleus or lateral septum mediolateral nucleus synapses;
OR,
Phasic role of CRH acting either by evoked or volume transmission to modulate the action of a principal transmitter, e.g., glutamate; or, a modulator, e.g., enhance dopamine’s affects on basolateral amygdala to medial prefrontal cortex glutamatergic synaptic transmission.
Acknowledgments
The authors gratefully acknowledge research support from the National Institutes of Health, Grants: DA011991, DA017727, MH066996, and MH058327. We also appreciate our discussions with and reagents provided by W.W. Vale, J. Rivier, and D. Grigoriadis.
The authors have no potential conflicts of interest relevant to the contents of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinology. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Arzt E, Holsboer F. CRF signaling: molecular specificity for drug targeting in the CNS. Trends Pharmacol Sci. 2006;27:531–538. doi: 10.1016/j.tips.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex in the rat: a light and electron microscope study. Brain Res. 1996;720:211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Hermann H, Lutz B, Behl C. Corticotropin-Releasing Hormone-Mediated Induction of Intracellular Signaling Pathways and Brain-Derived Neurotrophic Factor Expression Is Inhibited by the Activation of the Endocannabinoid System. Endocrinology. 2005;146:1205–1213. doi: 10.1210/en.2004-1154. [DOI] [PubMed] [Google Scholar]
- Behan DP, Grigoriadis DE, Lovenberg T, Chalmers D, Heinrichs S, Liaw C, De Souza EB. Neurobiology of corticotropin releasing factor (CRF) receptors and CRF-binding protein: implications for the treatment of CNS disorders. Mol Psychiatry. 1996;1:265–277. [PubMed] [Google Scholar]
- Bishop GA. Development of a corticotropin-releasing factor-mediated effect on the firing rate of Purkinje cells in the postnatal mouse cerebellum. Exp Neurol. 2002;178:165–174. doi: 10.1006/exnr.2002.8046. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Seelandt CM, King JS. Cellular localization of corticotropin releasing factor receptors in the adult mouse cerebellum. Neuroscience. 2000;101:1083–1092. doi: 10.1016/s0306-4522(00)00413-9. [DOI] [PubMed] [Google Scholar]
- Bissette G, Reynolds GP, Kilts CD, Widerlov E, Nemeroff CB. Corticotropin-releasing factor-like immunoreactivity in senile dementia of the Alzheimer type. Reduced cortical and striatal concentrations. JAMA. 1985;254:3067–3069. [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sciences. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Cain ST, Owens MJ, Nemeroff CB. Subcellular distribution of corticotropin-releasing-factor-like immunoreactivity in rat central nervous system. Neuroendocrinology. 1991;54:36–41. doi: 10.1159/000125848. [DOI] [PubMed] [Google Scholar]
- Catalan R, Gallart JM, Castellanos JM, Galard R. Plasma corticotropin-releasing factor in depressive disorders. Biol Psychiatry. 1998;44:15–20. doi: 10.1016/s0006-3223(97)00539-8. [DOI] [PubMed] [Google Scholar]
- Charlton BG, Ferrier IN, Perry RH. Distribution of corticotropin-releasing factor-like immunoreactivity in human brain. Neuropeptides. 1987;10:329–334. doi: 10.1016/s0143-4179(87)90083-7. [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Minas V, Zoumakis E, Makrigiannakis A. CRF receptor antagonists: utility in research and clinical practice. Curr Med Chem. 2006;13:2751–2760. doi: 10.2174/092986706778521977. [DOI] [PubMed] [Google Scholar]
- Contarino A, Heinrichs SC, Gold LH. Understanding corticotropin releasing factor neurobiology: contributions from mutant mice. Neuropeptides. 1999;33:1–12. doi: 10.1054/npep.1999.0001. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Is corticotropin-releasing factor a mediator of stress responses? Annals New York Academy of Sciences. 1990;579:183–191. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Hormones and Behavior. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Eaves M, Thatcher-Britton K, Rivier J, Vale W, Koob G. Effects of corticotropin-releasing factor on locomotor activity in hypophysectomized rats. Peptides. 1985;6:923–926. doi: 10.1016/0196-9781(85)90323-7. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Frontiers in Neuroendocrinology. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman AJ, Moldow RL. Extrahypothalamic distribution of CRF-like immunoreactivity in the rat brain. Peptides. 1982;3:149–153. doi: 10.1016/0196-9781(82)90044-4. [DOI] [PubMed] [Google Scholar]
- Fu Y, Pollandt S, Liu J, Krishnan B, Genzer K, Orozco-Cabal L, Gallagher JP, Shinnick-Gallagher P. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors. 2007. J Neurophysiol. 2006;97(1):937–41. doi: 10.1152/jn.00349.2006. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Prefrontal neurons in networks of executive memory. Brain Res Bull. 2000;52:331–336. doi: 10.1016/s0361-9230(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Bienvenu OJ, De Souza EB. Chronic cocaine administration alters corticotropin-releasing factor receptors in the rat brain. Brain Res. 1990;531:322–328. doi: 10.1016/0006-8993(90)90794-c. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva H, Levine M, Kanellopoulou K, Hillhouse E. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J Neurochem. 2001;76:509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine Hydrochloride (SSR125543A), a Potent and Selective Corticotrophin-Releasing Factor1 Receptor Antagonist. II. Characterization in Rodent Models of Stress-Related Disorders. Journal of Pharmacology and Experimental Therapeutics. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Gulyas J, Rivier C, Perrin M, Koerber SC, Sutton S, Corrigan A, Lahrichi SL, Craig AG, Vale W, Rivier J. Potent, Structurally Constrained Agonists and Competitive Antagonists of Corticotropin-Releasing Factor. Proc Natl Acad Sci USA. 1995;92:10575–10579. doi: 10.1073/pnas.92.23.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Corticotrophin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30(8):399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann H, Lutz B. Coexpression of the cannabinoid receptor type 1 with the corticotropin-releasing hormone receptor type 1 in distinct regions of the adult mouse forebrain. Neurosci Lett. 2005;375:13–18. doi: 10.1016/j.neulet.2004.10.080. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The stress hormone system is back on the map. Curr Psychiatry Rep. 2000;2:454–456. doi: 10.1007/s11920-000-0001-y. [DOI] [PubMed] [Google Scholar]
- Ito M, Miyata M. Corticotropin Releasing Factor (CRF) and Its Role in the Central Nervous system. Results Probl Cell Differ. 1995;26:43–66. doi: 10.1007/978-3-540-49421-8_3. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends in Cognitive Sciences. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, Suzuki T, Ebina M, Nukiwa T, Sasano H. Expression of Urocortin and Corticotropin-Releasing Factor Receptor Subtypes in the Human Heart. J Clin Endocrinol Metab. 2002;87:340–346. doi: 10.1210/jcem.87.1.8160. [DOI] [PubMed] [Google Scholar]
- King JS, Bishop GA. The distribution and cellular localization of CRF-R1 in the vermis of the postnatal mouse cerebellum. Exp Neurol. 2002;178:175–185. doi: 10.1006/exnr.2002.8052. [DOI] [PubMed] [Google Scholar]
- Koenig R, Luthi A. Chronic treatment with CRF enhances excitatory synaptic transmission in medial prefrontal cortex slice cultures. Society for Neurosci Abst. 2002;864.2 [Google Scholar]
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Annals New York Academy of Sciences. 1999;897:445–460. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Corticotropin-releasing factor and behavior. Fed Proc. 1985;44:259–263. [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Krstew EV, Dautzenberg FM, Ruhmann A. The highly selective CRF(2) receptor antagonist K41498 binds to presynaptic CRF(2) receptors in rat brain. Br J Pharmacol. 2002;136:896–904. doi: 10.1038/sj.bjp.0704783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K, Sexton P, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci. 2007;28(8):382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol. 2002;543:135–146. doi: 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-Releasing Factor and Urocortin I Modulate Excitatory Glutamatergic Synaptic Transmission. J Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher J. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577–583. doi: 10.1523/JNEUROSCI.4196-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JR, Heinrichs SC, Grigoriadis DE. Recent advances with the CRF1 receptor: design of small molecule inhibitors, receptor subtypes and clinical indications. Curr Pharm Des. 1999;5:289–315. [PubMed] [Google Scholar]
- McEwen BS. The neurobiology and neuroendocrinology of stress implications for post-traumatic stress disorder from a basic science perspective. Psychiatr Clin North Am. 2002;25:469–494. doi: 10.1016/s0193-953x(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5 Suppl 1:53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Miyata M, Okada D, Hashimoto K, Kano M, Ito M. Corticotropin-releasing factor plays a permissive role in cerebellar long-term depression. Neuron. 1999;22:763–775. doi: 10.1016/s0896-6273(00)80735-7. [DOI] [PubMed] [Google Scholar]
- Mouradian MM, Farah JM, Jr, Mohr E, Fabbrini G, O’Donohue TL, Chase TN. Spinal fluid CRF reduction in Alzheimer’s disease. Neuropeptides. 1986;8:393–400. doi: 10.1016/0143-4179(86)90010-7. [DOI] [PubMed] [Google Scholar]
- Nakane T, Audhya T, Hollander CS, Schlesinger DH, Kardos P, Brown C, Passarelli J. Corticotrophin-releasing factor in extra-hypothalamic brain of the mouse: demonstration by immunoassay and immunoneutralization of bioassayable activity. J Endocrinol. 2007;111:143–149. doi: 10.1677/joe.0.1110143. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Orozco-Cabal L, Liu J, Pollandt S, Schmidt K, Shinnick-Gallagher P, Gallagher JP. Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala to medial prefrontal cortex synaptic transmission: Functional switch after chronic cocaine administration. J Neuroscience. doi: 10.1523/JNEUROSCI.2666-07.2008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. Regulation of Synaptic Transmission by CRF Receptors. Reviews in the Neurosciences. 2006a;17:279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- Orozco-Cabal L, Shinnick-Gallagher P, Gallagher JP. Combined Effects of a stress-related synaptic regulator, CRH, and synaptic modulator, dopamine, change excitatory synaptic responses of neocortical pyramidal cells in the rat: an in vitro study. American Endocrinology Society Annual Meeting; San Diego, CA. 2005. [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Vergara L, Shinnick-Gallagher P, Gallagher JP. A novel rat medial prefrontal cortical slice preparation to investigate synaptic transmission from amygdala to layer V prelimbic pyramidal neurons. Journal of Neuroscience Methods. 2006b;151:148–158. doi: 10.1016/j.jneumeth.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Powers RE, Walker LC, DeSouza EB, Vale WW, Struble RG, Whitehouse PJ, Price DL. Immunohistochemical study of neurons containing corticotropin-releasing factor in Alzheimer’s disease. Synapse. 1987;1:405–410. doi: 10.1002/syn.890010504. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebaudo R, Melani R, Balestrino M, Izvarina N. Electrophysiological effects of sustained delivery of CRF and its receptor agonists in hippocampal slices. Brain Res. 2001;922:112–117. doi: 10.1016/s0006-8993(01)03160-2. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Richter RM, Pich EM, Koob GF, Weiss F. Sensitization of cocaine-stimulated increase in extracellular levels of corticotropin-releasing factor from the rat amygdala after repeated administration as determined by intracranial microdialysis. Neurosci Lett. 1995;187:169–172. doi: 10.1016/0304-3940(95)11365-4. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Cocaine stimulated adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res. 1987;422:403–406. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi J-C, Waser B, Koerber SC, Martinez V, Wang L, Tache Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) Receptor 2 peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Kunitake K, DiGruccio M, Cantle JP, Perrin MH, Donaldson C, Vaughan J, Million M, Gourcerol G, Adelson DW, Rivier C, Tache Y, Vale W. Stressin1-A, a potent corticotropin releasing factor receptor 1 (CRF1)-selective peptide agonist. J Med Chem. 2007;50:1668–1674. doi: 10.1021/jm0613875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of Corticotropin-Releasing Factor in drug addiction. Pharmacol Rev. 2001;53:209–244. [PubMed] [Google Scholar]
- Sarnyai Z. Neurobiology of stress and cocaine addiction. Studies on corticotropin-releasing factor in rats, monkeys, and humans. Ann NY Acad Sci. 1998;851:371–387. doi: 10.1111/j.1749-6632.1998.tb09011.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, De Ruiter MM, De Zeeuw CI, Hansel C. The neuropeptide corticotropin-releasing factor regulates excitatory transmission and plasticity at the climbing fibre-Purkinje cell synapse. Eur J Neurosci. 2007;25:1460–1466. doi: 10.1111/j.1460-9568.2007.05409.x. [DOI] [PubMed] [Google Scholar]
- Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol Sci. 2007 Aug;28(8):366–73. doi: 10.1016/j.tips.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Selye H. Syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Dunn AJ. The role of CRF receptor subtypes in stress-induced behavioural responses. Eur J Pharmacol. 2000;405:199–206. doi: 10.1016/s0014-2999(00)00553-7. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Heinrichs SC, Dunn AJ. The role of CRH in behavioral responses to stress. Peptides. 2001;22:713–724. doi: 10.1016/s0196-9781(01)00384-9. [DOI] [PubMed] [Google Scholar]
- Smagin G, Heinrichs SC, Dunn AJ. The role of CRH in behavioral responses to stress. Peptides. 2002;22:713–724. doi: 10.1016/s0196-9781(01)00384-9. [DOI] [PubMed] [Google Scholar]
- Swinny JD, Kalicharan D, Blaauw EH, Ijkema-Paassen J, Shi F, Gramsbergen A, van der Want JJL. Corticotropin-releasing factor receptor types 1 and 2 are differentially expressed in pre- and post-synaptic elements in the post-natal developing rat cerebellum. Eur J Neurosci. 2003;18:549–562. doi: 10.1046/j.1460-9568.2003.02776.x. [DOI] [PubMed] [Google Scholar]
- Swinny JD, Metzger F, Ijkema-Paassen J, Gounko NV, Gramsbergen A, van der Want JJL. Corticotropin-releasing factor and urocortin differentially modulate rat Purkinje cell dendritic outgrowth and differentiation in vitro. Eur J Neurosci. 2004;19:1749–1758. doi: 10.1111/j.1460-9568.2004.03279.x. [DOI] [PubMed] [Google Scholar]
- Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. Journal of Clinical Investigation. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Zhong P, Yan Z. Corticotropin-Releasing Factor and Acute Stress Prolongs Serotonergic Regulation of GABA Transmission in Prefrontal Cortical Pyramidal Neurons. J Neurosci. 2004;24:5000–5008. doi: 10.1523/JNEUROSCI.0143-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E, Pernar L, Lucki I, Valentino RJ. Corticotropin-releasing factor in the dorsal raphe nucleus regulates activity of lateral septal neurons. Brain Res. 2003;960:201–208. doi: 10.1016/s0006-8993(02)03882-9. [DOI] [PubMed] [Google Scholar]
- Traver S, Marien M, Martin E, Hirsch EC, Michel PP. The Phenotypic Differentiation of Locus Coeruleus Noradrenergic Neurons Mediated by BDNF is Enhanced by Corticotropin Releasing Factor through the Activation of a cAMP-dependent Signaling Pathway. Molecular Pharmacology. 2006;70:30–40. doi: 10.1124/mol.106.022715. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-Releasing Factor Requires CRF Binding Protein to Potentiate NMDA Receptors via CRF Receptor 2 in Dopamine Neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphione. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ. CRH effects on central noradrenergic neurons: relationship to stress. Adv Exp Med Biol. 1988;245:47–64. doi: 10.1007/978-1-4899-2064-5_5. [DOI] [PubMed] [Google Scholar]
- Valentino RJ. Corticotropin-releasing factor: putative neurotransmitter in the noradrenergic nucleus locus ceruleus. Psychopharmacol Bull. 1989;25:306–311. [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci. 1988;8:1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Rudoy C, Saunders A, Liu X-B, Van Bockstaele EJ. Corticotropin-releasing factor is preferentially colocalized with excitatory rather than inhibitory amino acids in axon terminals in the peri-locus coeruleus region. Neuroscience. 2001;106:375–384. doi: 10.1016/s0306-4522(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Anderson GM, Owens MJ, Halaszynski TM, Bremner JD, Carpenter LL, Heninger GR, Nemeroff CB, Charney DS. Cerebrospinal fluid corticotropin-releasing hormone in healthy humans: Effects of yohimbine and naloxone. J Clin Endocrinol Metab. 2000;85:4138–4145. doi: 10.1210/jcem.85.11.6968. [DOI] [PubMed] [Google Scholar]
- Wang HL, Tsai LY, Lee EHY. Corticotropin-releasing factor produces a protein synthesis-dependent long-lasting potentiation in dentate gyrus neurons. J Neurophysiol. 2000;83:343–349. doi: 10.1152/jn.2000.83.1.343. [DOI] [PubMed] [Google Scholar]
- Wang HL, Wayner MJ, Chai CY, Lee EH. Corticotrophin-releasing factor produces a long-lasting enhancement of synaptic efficacy in the hippocampus. Eur J Neurosci. 1998;10:3428–3437. doi: 10.1046/j.1460-9568.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- Whim MD. Near Simultaneous Release of Classical and Peptide Cotransmitters from Chromaffin Cells. J Neurosci. 2006;26:6637–6642. doi: 10.1523/JNEUROSCI.5100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xu Y, Zhou H, Tao J, Li S. Expression of urocortin in rat lung and its effect on pulmonary vascular permeability. J Endocrinol. 2006;189:167–178. doi: 10.1677/joe.1.06607. [DOI] [PubMed] [Google Scholar]
- Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]