Abstract
This parametric functional magnetic resonance imaging (fMRI) study investigates the balance of negative and positive fMRI signals in the brain. A set of visual attention (VA) and working memory (WM) tasks with graded levels of difficulty was used to deactivate separate but overlapping networks that include the frontal, temporal, occipital, and limbic lobes; regions commonly associated with auditory and emotional processing. Brain activation (% signal change and volume) was larger for VA tasks than for WM tasks, but deactivation was larger for WM tasks. Load‐related increases of blood oxygenation level‐dependent (BOLD) responses for different levels of task difficulty cross‐correlated strongly in the deactivated network during VA but less so during WM. The variability of the deactivated network across different cognitive tasks supports the hypothesis that global cerebral blood flow vary across different tasks, but not between different levels of task difficulty of the same task. The task‐dependent balance of activation and deactivation might allow maximization of resources for the activated network. Hum Brain Mapp, 2005. © 2006 Wiley‐Liss, Inc.
Keywords: visual attention, working memory, fMRI, activation, deactivation, PET, CBF
INTRODUCTION
Functional magnetic resonance imaging (fMRI) studies of visual stimulation in cats [Harel et al.,2002] and humans [Shmuel et al.,2002] demonstrated negative blood oxygenation level‐dependent (BOLD) responses (NBR) in nonstimulated areas of the visual cortex. Similarly, fMRI studies using cross‐modal stimuli showed deactivation of the auditory cortices during visual stimulation, whereas deactivation of the visual cortices occurred during auditory stimulation [Laurienti et al.,2002; Lewis et al.,2000]. These findings suggest that the concomitant deactivation could represent cross‐modal inhibition (an active suppression of neural activity), to minimize potentially distracting, task‐irrelevant neural processes. Studies in rodents, however, using optical imaging of hemoglobin oxygenation and physiological recordings of spiking activity and local field potentials suggest that the negative hemodynamic activity might not correspond to changes in neuronal activity [Devor et al.,2005].
Functional MRI deactivation may also represent a direct hemodynamic response (“blood stealing”) in the vascular system in response to changes in adjacent regional cerebral blood flow (rCBF), because negative BOLD signals are accompanied by decreases in rCBF [Hoge et al.,1999b; Raichle,1998; Shmuel et al.,2002; Stefanovic et al.,2004]. In this model, rCBF‐increases (Δ+) in activated regions would necessitate synchronous rCBF‐decreases (Δ−) in other brain regions, i.e. Δ+ = k Δ−, where the constant k depends on the vascular distribution. This hypothesis is supported by the finding that global brain metabolism, which is proportional to global CBF, is remarkably constant despite varying mental and motor activity [Raichle and Gusnard,2002]. The BOLD signal depends in a complex manner on CBF, cerebral blood volume (CBV), and cerebral metabolic rate of oxygen consumption CMRO2 [Buxton,2002]. Hoge et al. [1999a], however, have suggested that during visual stimulation (flickering checkerboard), Δ± and the BOLD± signals in the human occipital cortex are coupled; Δ± is proportional to CMRO2 increases, and the BOLD± signal ∝ Δ± 0.9.
Positron emission tomography (PET) also showed rCBF‐decreases in visual [Kawashima et al.,1995] and auditory [Shulman et al.,1997] cortices during spatial‐, visual‐ or selective‐attention tasks, and in auditory and somatosensory cortices during tasks that did not require attention [Born et al.,2002; Haxby et al.,1994; Shulman et al.,1997].
Brain deactivation also has been reported during attention‐requiring tasks [Deary et al.,2004; Hester et al.,2004; Lawrence et al.,2003]; however, whether it reflects neural inhibition of task‐irrelevant neural processing or hemodynamic compensatory mechanisms in the brain remains unclear. Deficits in attention and memory are common in many brain disorders, including Alzheimer's disease, attention deficit hyperactivity disorder, schizophrenia, Parkinson's disease, human immunodeficiency virus (HIV) infection, and drug addiction. A better understanding of the mechanism for deactivation may therefore be important if fMRI is used to evaluate brain pathophysiology or to monitor treatments.
In a series of fMRI studies on the effect of scanner noise on visual attention and working memory processing in healthy subjects and HIV patients [Tomasi et al.,2005a,b,c], we observed similar patterns of brain deactivation for both tasks. The goal of this study was therefore to investigate if the activation–deactivation balance during attention‐demanding tasks (working memory [WM] and visual attention [VA] tasks with graded levels of difficulty [Chang et al.,2001,2004]) is task dependent.
We hypothesized that if global CBF is constant across tasks, then larger fMRI activation (% BOLD signal change) should be counterbalanced by larger fMRI deactivation. This hypothesis assumes the same (constant) global CBF during the tasks (Δ+ = k Δ−), and that fMRI signals and hemodynamic responses are coupled (BOLD± ∝ Δ, where α is a constant). With these assumptions, BOLD activation should be accompanied by synchronous proportional BOLD deactivation, i.e., BOLD+ = q BOLD− where q is a constant. Two different tasks (WM and VA) with parametric changes of cognitive load therefore were used to modulate brain activation and deactivation to allow a comparative analysis of activation and deactivation. The study was conducted at high magnetic field strength (4 Tesla) in a cohort of 22 healthy volunteers.
MATERIALS AND METHODS
Subjects
Twenty‐two healthy, nonsmoking, right‐handed volunteers (10 men, 12 women; age 30 ± 8 years; education: 16 ± 2 years) with normal vision participated in the study. The subjects signed a written consent, approved by the Institutional Review Board at Brookhaven National Laboratory. Subjects were screened carefully with a detailed medical history, physical and neurological examination, and blood‐ and urine‐screening tests to ensure they fulfilled all inclusion (age 18 years or older, English as their first language, healthy, and on no medications) and exclusion criteria (history of head injury, current or past drug abuse or dependence including positive urine toxicology, any past or current medical or neuropsychiatric illnesses, significant abnormalities on screening blood tests, pregnancy tested by urine test or breast‐feeding if female subjects, any contraindications for MRI).
Neuropsychological Tests
All subjects were evaluated with a battery of neuropsychological tests to evaluate attention [Symbol Digit Modalities; Smith,1982], WM (forward and backward digit span and letter‐number sequencing; Wechsler Adult Intelligence Scale‐Third Edition [WAIS‐III]), and executive function [Stroop Color Interference Test; Stroop,1935].
Visual Attention Paradigm
Subjects carried out a set of three VA tasks that involved mental tracking of 2, 3, or 4 of 10 moving balls [Culham et al.,1998; Jovicich et al.,2001; Tomasi et al.,2004]. During the TRACK periods, the target balls (2, 3, or 4) were briefly highlighted and then all 10 balls started to move. Subjects were instructed to fixate on the cross while mentally tracking the target balls as they moved randomly across the display. At the end of TRACK periods, the balls stopped moving and a new set of balls was highlighted, and subjects were trained to press a button if these balls are the same as the target set. Reaction times and accuracy in performance thus were recorded. After 0.5‐sec delay, the original target balls were then rehighlighted to refocus the subjects' attention on these balls, and the sequence was repeated five times. During DO NOT TRACK periods, all 10 balls moved in the same manner; however, no balls were highlighted. The subjects were instructed to stop tracking the balls and view them passively. These tasks activate a neural network that includes primarily the dorsolateral prefrontal cortex (DLPFC), inferior and superior parietal lobes (IPL and SPL, respectively), cerebellum, and motion areas V5/MT+ [Culham et al.,1998; Jovicich et al.,2001]. The stimuli were created as movies in audio video interleave (AVI) format using MATLAB (The Mathworks, Inc., Natick, MA), and presented to the subjects on MRI‐compatible LCD goggles connected to a personal computer. The display software was synchronized precisely with the MR acquisition using an MRI trigger pulse.
Working Memory Paradigm
Three sequential letter tasks were used to test WM. The zero‐back task was a simple reaction task: During the 30‐sec‐long task block, a letter was flashed for 500 msec at random times (10 events). The task was to push a response button as soon as a letter appeared on the screen. During the control periods of 30 sec, only a fixation cross was displayed. For the one‐ and two‐back tasks, random alphabetical letters were presented sequentially at a rate of 1 per second. The subjects were instructed to push a button as quickly as possible when the current letter was the same as the one before (one‐back task) or two before (two‐back task). During each task period of 30 sec, five targets were presented at random time points. During the resting period (30 sec), nonsense characters were displayed randomly at the same size, rate, and luminance, and the subjects were instructed not to respond but to maintain fixation at the center cross.
Task order was counterbalanced to minimize habituation effects. Half of the studies started with WM tasks; the remaining studies started with VA tasks. The stimuli were presented to the subjects on MRI‐compatible LCD goggles connected to a personal computer. All response button events during stimulation were recorded to determine task performance.
The subjects were briefly trained outside the scanner (for approximately 10 min), using shortened versions of the paradigms, to ensure that they understood and were able to carry out the tasks.
DATA ACQUISITION
Subjects underwent MRI in a 4 Tesla whole‐body Varian/Siemens MRI scanner (VARIAN, Inc., Palo Alto, CA; Siemens, Munich, Germany), equipped with a self‐shielded whole‐body SONATA gradient set. A T2*‐weighted single‐shot gradient‐echo echo‐planar imaging (EPI) sequence with ramp‐sampling (echo time/repetition time [TE/TR] = 25/3,000 msec, 4‐mm slice thickness, 1‐mm gap, typically 33 coronal slices covering the whole brain, 48 × 64 matrix size, 4.1 × 3.1 mm in‐plane resolution, 90 degree flip angle, and time points of 84 for WM, 124 for VA) was used to measure BOLD responses. The entire battery was carried out twice to increase statistical power. Padding was used to minimize motion. Task performance and subject motion were determined immediately after each fMRI trial to assure performance accuracy better than 80%, and motion <1‐mm translations and <1‐degree rotations [Caparelli et al.,2003].
A T1‐weighted 3D‐MDEFT sequence [Lee et al.,1995] (TE/TR = 7/15 msec, 0.94 × 0.94 × 3 mm spatial resolution, axial orientation, 256 readout and 192 × 48 phase‐encoding steps, and 8‐min scan time) and a modified T2‐weighted Hyperecho sequence [Hennig and Scheffler,2001] (TE/TR = 42/10,000 msec, echo train length = 16, 256 × 256 matrix size, 30 coronal slices, 0.86 × 0.86 mm in‐plane resolution, 5‐mm thickness, 1‐mm gap, and 2‐min scan time) were used to obtain anatomical images.
Data Processing
The first four volumes in the time series were discarded to avoid non‐equilibrium effects of the MR signal. The statistical parametric mapping package SPM99 (Wellcome Department of Cognitive Neurology, London UK) was used for fMRI analyses. A six‐parameter rigid body transformation was used for image realignment to correct for head motion. Only scans with head motion less than 1‐mm translations and 1‐degree rotations were included in the analysis. The realigned datasets were normalized to a Talairach template using a 3 × 3 × 3 mm3 voxel size and an affine transformation [Ashburner et al.,1997]. The data were smoothed using an 8‐mm full‐width half‐maximum (FWHM) Gaussian kernel. A general linear model [Friston et al.,1995], and a boxcar design convolved with a canonical hemodynamic response function (HRF) were used to calculate the activation maps. Because global normalization in SPM can produce false deactivation signals [Aguirre et al.,1998; Gavrilescu et al.,2002], the BOLD signal strength was estimated without the removal of global effects. The time series were band‐pass filtered with the HRF as low‐pass filter and an additional high‐pass filter (cut‐off frequency of 1/126 Hz for WM and 1/256 for VA).
Statistical Analyses
BOLD responses (% signal change maps) for each trial and subject were included in a voxel‐by‐voxel repeated‐measures analysis of variance (ANOVA) model with six conditions (WM: zero‐, one‐, and two‐back; VA: 2, 3, and 4 balls) to identify significantly activated and deactivated brain areas during WM and VA tasks. Masks of networks that activated or deactivated during WM or VA tasks were created using the repeated‐measures ANOVA model and a threshold P = 0.05. Simple regression analyses of BOLD signals and behavioral measurements during fMRI (reaction times, performance accuracy, and task difficulty, as measured by the reaction times‐to‐performance accuracy ratio) were conducted across subjects and tasks to complement the statistical analyses of brain activation. Activation maps for group analyses were calculated using a voxel‐level threshold (uncorrected) of P < 0.05 and a minimum cluster size of 15 voxels (400 mm3). Clusters with at least 15 voxels (400 mm3) and P < 0.05, corrected for multiple comparisons, were considered significant in the group analysis.
Region‐of‐Interest Analysis
To validate the SPM results, functional regions of interest (ROIs) with volume of 729 mm3 (cubic, 27 voxels) were defined at the cluster centers of brain activation (see Tables I and II) to extract the average BOLD signal from these regions, using a customized program written in IDL (Research Systems, Boulder, CO). The position, shape, and size of the ROIs were invariant across subjects, tasks, and conditions. A repeated‐measures ANOVA was conducted for each ROI to validate the voxel‐by‐voxel statistical analyses described above. Additional cross‐correlation analyses between load effects (differential BOLD responses; WM load: two‐back − zero‐back; VA load: 4 balls − 2 balls) in different ROIs were carried out across subjects to study: (1) potential interconnections in the networks, and (2) if a larger dynamic range of hemodynamic responses in the activated network is balanced out by a larger dynamic range of hemodynamic responses in the deactivated network. Statistical significance for ROI analyses was defined as P = 0.05 (uncorrected).
Table I.
Location of major areas of brain activation in the Talairach frame of reference, and statistical significance of BOLD responses in these voxel locations
| Brain region | Side | Coordinates, mm (x, y, z) | T‐scores | |||||
|---|---|---|---|---|---|---|---|---|
| VA | WM | VA Load | WM Load | VA > WM | WM > VA | |||
| IFG (BA47) | L | −36, 15, −3 | 5.7a | 8.0a | 2.4a | 3.2b | NS | NS |
| R | 27, 18, 0 | 6.1a | 5.4a | NS | 2.1b | NS | NS | |
| MFG (BA6) | L | −24, 6, 60 | 8.8a | NS | NS | NS | 2.3a | NS |
| R | 33, −12, 57 | 4.0a | NS | NS | NS | 3.1a | NS | |
| MFG (BA9) | L | −36, 21, 15 | 2.9a | 7.7a | 2.7a | 2.8b | NS | 2.7b |
| R | 39, 15, 18 | 7.9a | 8.3a | NS | 2.3b | NS | NS | |
| medFG (BA8) | C | 3, 21, 39 | 5.1a | 7.0a | NS | 2.2b | NS | NS |
| FusG (BA19) | L | −39, −63, −6 | 8.7a | 10.0a | 4.9a | NS | NS | NS |
| R | 39, −66, −15 | 10.1a | 4.5a | 3.4a | NS | 2.0 | NS | |
| IPL (BA40) | L | −36, −36, 45 | 10.1a | 7.7a | 4.2a | 2.3b | 2.7a | NS |
| R | 39, −36, 45 | 9.1a | 4.8a | 5.3a | NS | 3.9a | NS | |
| SPL (BA7) | L | −30, −51, 45 | 10.0a | 13.1a | 3.8 | 4.6 | NS | NS |
| R | 30, −54, 51 | 12.0a | 12.2a | 2.3 | 2.8 | 3.7a | NS | |
| L | −21, −57, 66 | 19.4a | 5.7a | 3.6 | NS | 10.9a | NS | |
| R | 15, −66, 60 | 13.6a | 2.8a | NS | 2.3 | 8.4a | NS | |
| PostCG (BA7) | L | −3, −51, 69 | 8.8a | NS | NS | NS | 7.7a | NS |
| R | 3, −51, 69 | 10.4a | NS | NS | NS | 9.8a | NS | |
| PCL | C | 0, −42, 63 | 4.6a | NS | −2.5a | NS | 6.4a | NS |
| SOG (BA19) | L | −27, −81, 27 | 4.7a | NS | 5.5a | NS | NS | NS |
| R | 33, −81, 21 | 13.2a | NS | 5.9a | NS | 6.2a | NS | |
| IOG (BA19) | L | −36, −66, −6 | 6.4a | 7.1a | 5.4a | NS | NS | NS |
| R | 36, −66, −6 | 7.4a | 5.2a | 2.0 | NS | 2.2a | NS | |
| Thalamus | L | −9, −18, 9 | 8.3a | 8.2a | 3.4a | NS | NS | NS |
| R | 3, −21, 9 | 10.1a | 8.2a | 2.0 | NS | 2.3a | NS | |
| CER declive | L | −33, −66, −21 | 4.7a | 5.5a | 5.1a | NS | NS | NS |
| R | 33, −66, −21 | 8.1a | 12.0a | 4.2a | NS | NS | NS | |
| CER vermis | C | −3, −78, −15 | 10.9a | 11.0a | 4.5a | NS | NS | NS |
Sample size: 22 healthy subjects. Repeated‐measures ANOVA (random‐effects) analyses. IFG, inferior frontal gyrus; MFG, middle frontal gyrus; medFG, medial frontal gyrus; FusG, fusiform gyrus; IPL, inferior parietal lobe; SPL, superior parietal lobe; PostCG, postcentral gyrus; PCL, paracentral lobule; SOG, superior occipital gyrus; IOG, inferior occipital gyrus; CER, cerebellum; BA, Brodmann area; NS, not significant.
Bonferroni corrections:
P < 0.001
P < 0.05 cluster‐level corrected.
Table II.
Location of major areas of brain deactivation in the Talairach frame of reference, and statistical significance of BOLD responses in these voxel locations
| Brain region | Side | Coordinates, mm (x, y, z) | T‐scores | |||||
|---|---|---|---|---|---|---|---|---|
| VA | WM | VA Load | WM Load | VA > WM | WM > VA | |||
| SFG (BA9) | L | −12, 54, 36 | −2.0 | −2.8b | NS | NS | NS | NS |
| R | 12, 57, 33 | NS | −2.8b | NS | NS | NS | NS | |
| ACG (BA24) | C | −6, 36, 0 | −4.9b | −4.5b | NS | 3.6 | NS | NS |
| Insula (BA13) | L | −36, −18, 12 | −4.3a | −5.5a | NS | −3.5b | NS | NS |
| R | 42, −9, 3 | −2.8b | −4.0a | NS | NS | NS | NS | |
| PHG (BA35) | L | −21, −24, −18 | NS | −2.7b | 3.1b | NS | NS | NS |
| R | 24, −21, −18 | NS | −3.2b | NS | NS | NS | −2.0 | |
| (BA36) | L | −24, −42, −9 | NS | −2.6b | 3.0b | NS | NS | NS |
| (BA30) | L | −9, −48, 0 | NS | −2.9a | 4.0a | NS | NS | NS |
| R | 9, −48, 0 | NS | −3.7a | 2.7a | NS | NS | NS | |
| PreCG (BA4) | L | −24, −21, 57 | NS | −2.1b | NS | NS | NS | −2.3 |
| R | 24, −21, 57 | NS | NS | NS | NS | NS | −2.0 | |
| MTG (BA39) | L | −45, −63, 27 | NS | −2.3a | 3.8 | NS | NS | NS |
| R | 45, −63, 27 | NS | −4.8a | 2.0 | NS | NS | −2.0 | |
| CG (BA31) | C | 0, −48, 27 | −7.2a | −10.9a | NS | −4.8a | NS | NS |
| (BA 24) | C | 3, −6, 42 | NS | −4.8a | NS | NS | NS | −2.4b |
| PCL (BA5) | C | 0, −42, 63 | NS | −4.5a | 2.5a | 2.5 | NS | −6.4a |
| PCG (BA30) | L | −9, −48, 6 | NS | −3.1a | 3.9a | 4NS | NS | NS |
| R | 12, −54, 6 | −2.5a | −5.4a | 2.0a | NS | NS | NS | |
| Precuneus (BA7) | L | −15, −63, 27 | NS | −3.0a | 4.9a | NS | NS | NS |
| R | 15, −63, 27 | 2.4a | −5.0a | 4.0a | NS | NS | NS | |
Sample size: 22 healthy subjects. Repeated‐measures ANOVA (random‐effects) analyses. SFG, superior frontal gyrus; ACG, anterior cinculate gyrus; PHG, parahippocampal gyrus; PreCG, precentral gyrus; MTL, middle temporal gyrus; CG, cingulated gyrus; PCL, paracentral lobule; PCG, postcentral gyrus; BA, Brodmann area; NS, not significant.
Bonferroni corrections:
P < 0.001
P < 0.05 cluster‐level corrected.
RESULTS
Performance and Reaction Times
Figure 1 shows the average values of task performance and reaction time (RT) during fMRI. Subjects were able to carry out the tasks with high performance accuracy (>90%). Performance accuracy was significantly lower for two‐back compared to zero‐ and one‐back (P‐value < 0.0001), but not for one‐back versus zero‐back (P‐value = 0.08). RT increased from zero‐back to one‐back, and from one‐back to two‐back (P < 0.0001). Performance accuracy during 4‐ball tracking was significantly lower compared to 3‐ and 2‐ball tracking (P‐value < 0.0001); there was no difference in RTs between 2‐ball tracking (P‐value = 0.5). Although RT increased with increasing number of balls tracked, the increases were not statistically significant. Performance accuracy for WM and VA were similar; however, RT was slower during VA tasks compared to that during WM tasks (Fig. 1). These task‐dependent increases in RT and decreases in performance suggest increasing difficulty of the WM and VA tasks (WM load, and VA load, respectively). Task difficulty during fMRI in individual subjects was defined as the reaction time‐to‐performance accuracy ratio (reaction time/performance accuracy). The subjects' performance as measured by this task difficulty was associated with poorer performance (lower scores) on digit‐symbol [Smith,1982] (R = −0.67) and on WAIS tests (R < −0.42), as well as slower (longer time) performance on the Stroop tests (R > 0.56) during neuropsychological testing outside the scanner. These correlations support the use of this parameter as a measure of task difficulty, because subjects that performed worse on common neurocognitive tasks also experienced the fMRI tasks as more difficult.
Figure 1.
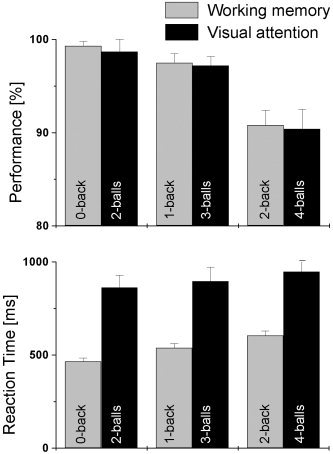
Performance accuracy and reaction times for working memory and visual attention tasks.
Activation
The WM tasks activated a network (Table I) that includes the prefrontal (PFC) cortex (inferior [IFG; Brodmann area (BA) 47], medial [medFG; BA 8], middle [MFG; BA 9] frontal gyri), inferior (IPL; BA 40) and superior (SPL; BA 7) parietal lobes, the inferior occipital (IOG; BA 19), and fusiform (FusG; BA 19) gyri, as well as the thalami and the cerebellum [declive and vermis], in agreement with our prior observations [Chang et al.,2001]. VA tasks activated a network that involves the same brain regions and additionally the MFG (BA 6), PostCG (BA 7), and SOG (BA 19), also in agreement with our previous studies [Chang et al.,2004; Jovicich et al.,2001]. Brain activation was highly significant in all these regions for both WM and VA tasks.
Figure 2 shows the activation patterns (top panel, red) and the extent of common activation (bilateral) produced by both paradigms. Brain activation in the MFG (BA 9) was larger overall for WM tasks compared to VA tasks (Fig. 2, middle panel, cyan), whereas brain activation in the SPL, IPL, MFG (BA 6), PostCG, IOG, SOG, and the thalamus was greater during VA than during WM tasks (Fig. 2, middle panel, yellow). The volume of the activated network was larger for VA tasks than for WM tasks (430 ± 10 vs. 310 ± 10 cm3).
Figure 2.
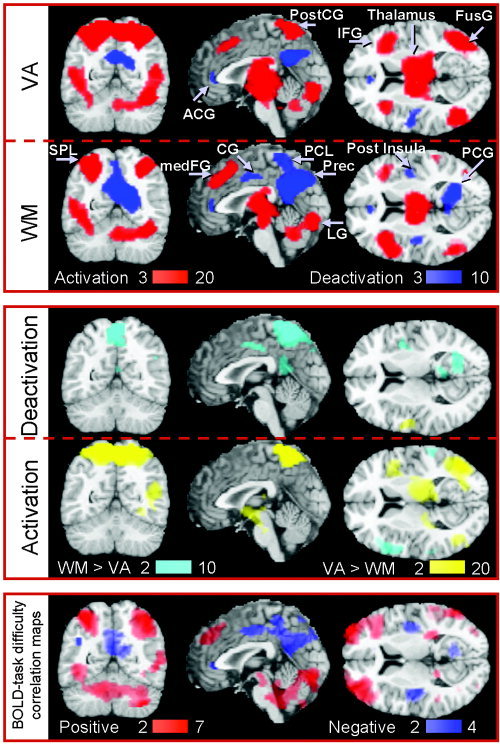
Statistical maps of positive (top; red) and negative (top; blue) BOLD signals during working memory (WM) and visual attention (VA) tasks, differential BOLD signals between tasks (middle), and positive and negative correlation of BOLD signals with task difficulty (Bottom). The color bars are t‐score windows; for the bottom panel they indicate the significance of the voxel‐wise fit of the linear regression with parametric variation of the task difficulty, i.e., reaction time/performance accuracy. Sample size: Twenty‐two healthy subjects, random‐effects analyses (repeated ANOVA). IFG, inferior frontal gyrus; medFG, medial frontal gyrus; FusG, fusiform gyrus; SPL, superior parietal lobe; PostCG, postcentral gyrus; PCL, paracentral lobule; Prec, precuneus; ACG, anterior cingulate gyrus; CG, cingulate gyrus; PCG, posterior cingulate gyrus; LG, lingual gyrus;
For WM tasks, increased RT and task difficulty, and decreased performance accuracy, were associated with increased activation of the activated network. For VA tasks, however, increased task difficulty was coupled to increased activation in the cerebellum, IPL, SOG, and IOG (P corrected < 0.001; Fig. 2, bottom panel, red).
Deactivation
Figure 2 also shows the pattern of deactivation for WM and VA tasks (top panel, blue). WM tasks (zero‐, one‐, and two‐back combined) deactivated a bilateral network (Table II) that comprises the frontal (superior frontal [SFG; BA 9], precentral [PreCG; BA 4], and anterior cingulate gyri [ACG; BA 24], paracentral lobule [PCL; BA 5], and the posterior insula), temporal (middle temporal gyrus [MTG; BA 39]), limbic (cingulate [CG; BA 24 and 31], parahippocampal [PHG; BA 30, 35, and 36], posterior cingulate [(PCG; BA 30] gyri), and occipital (precuneus [BA 7]) lobes. VA tasks (2, 3, and 4 balls combined) deactivated the insula, ACG, CG, PCG, and the precuneus (Table II).
The amplitude of the negative BOLD responses was larger during WM than during VA in the right PHG (BA 30), CG (BA 24 and 31), and the PCG (Fig. 2, middle panel, cyan). Furthermore, the PCL deactivated during WM tasks but activated during VA tasks (Fig. 2, middle panel). The volume of the deactivated network was larger for WM tasks than for VA tasks (100 ± 5 vs. 40 ± 4 cm3).
Furthermore, increased RT and task difficulty, and reduced performance accuracy during WM tasks were associated with increased deactivation of the deactivation network (P corrected < 0.008; Fig. 2, bottom panel, blue). Specifically, BOLD signals correlated stronger with task difficulty than with either RT or performance accuracy. For VA tasks, increased task difficulty produced larger deactivation in the CG (BA 31; P corrected = 0.008). This demonstrates that the RT‐to‐performance accuracy ratio predicts both activation and deactivation for WM tasks, but not for VA tasks.
Region‐of‐Interest Results
VA tasks produced larger positive BOLD responses than WM tasks did in an occipitoparietal network comprising the FusG, PostCG, SOG, IPL, and SPL (P < 0.001; gray box in Fig. 3), but no differences were observed in the PFC (IFG and MFG) and the subcortical brain regions (thalamus and cerebellum). In the PostCG, the average BOLD responses were positive during VA tasks but negative during WM tasks (see Fig. 3), and the responses did not modulate with either WM load or VA load. In the PFC (IFG, MFG, and medFG), BOLD responses modulated with WM load (P < 0.004) but did not modulate significantly with VA load. BOLD signals in other brain regions that activated during the tasks, however, modulated with WM load and VA load (P < 0.001). The load effects in the medFG and those in other regions of the PFC were correlated strongly across subjects for VA tasks (0.84 < R < 0.92; Table III), but less so for WM tasks (0.51 < R < 0.79). In general, VA load and WM load effects exhibited remarkably similar cross‐correlation coefficients in activated regions (Table III). In these regions, the average BOLD responses in the ROIs followed a normal distribution (Gaussian) across subjects and trials; the mean of the distribution was different but its FWHM was similar across all ROIs (FWHM = 3.1 ± 0.4 and 3.0 ± 0.4% for VA and WM tasks, respectively) for VA and WM tasks.
Figure 3.
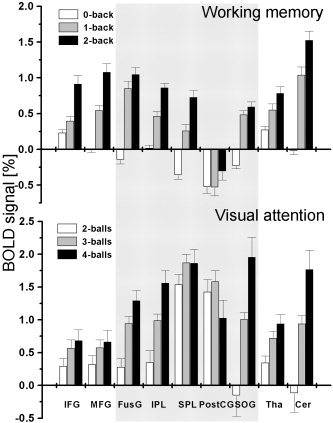
Average BOLD signals at specific regions of interest in the activated network (see Table I).
Table III.
Cross correlation factors of load responses in the activated networks during working memory (WM) and visual attention (VA) tasks
| WM | VA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFG | MFG6 | MFG9 | MedFG | FusG | IPL | SPL | PostCG | SOG | IOG | Thalamus | CER | |
| IFG | — | 0.89a | 0.94a | 0.84a | 0.59a | 0.76a | 0.29 | 0.21 | 0.56a | 0.66a | 0.84a | 0.57a |
| MFG6 | 0.73a | — | 0.85a | 0.84a | 0.53a | 0.75a | 0.27 | 0.35 | 0.43 | 0.62a | 0.76a | 0.54a |
| MFG9 | 0.71a | 0.58a | — | 0.92a | 0.52a | 0.74a | 0.11 | 0.35 | 0.39 | 0.62a | 0.80a | 0.47 |
| MedFG | 0.75a | 0.51a | 0.79a | — | 0.37 | 0.66a | 0.15 | 0.15 | 0.56a | 0.48 | 0.73a | 0.35 |
| FusG | 0.58a | 0.69a | 0.60a | 0.49 | — | 0.79a | 0.66a | 0.41 | 0.82a | 0.96a | 0.49 | 0.90a |
| IPL | 0.48 | 0.43 | 0.59a | 0.44 | 0.58a | — | 0.53a | 0.42 | 0.76a | 0.86a | 0.70a | 0.77a |
| SPL | 0.50a | 0.42 | 0.72a | 0.64a | 0.56a | 0.47 | — | 0.74a | 0.71a | 0.62a | 0.28 | 0.69a |
| PostCG | 0.52a | 0.52a | 0.24 | 0.40 | 0.51a | 0.16 | 0.42 | — | 0.53a | 0.40 | 0.29 | 0.58a |
| SOG | 0.53a | 0.48 | 0.42 | 0.37 | 0.73a | 0.68a | 0.41 | 0.35 | — | 0.81a | 0.46 | 0.78a |
| IOG | 0.45 | 0.50a | 0.49 | 0.40 | 0.90a | 0.48 | 0.41 | 0.29 | 0.65a | — | 0.55a | 0.90a |
| Thalamus | 0.68a | 0.56a | 0.56a | 0.55a | 0.35 | 0.36 | 0.52a | 0.46 | 0.28 | 0.15 | — | 0.56a |
| Cerebellum | 0.45 | 0.52a | 0.49 | 0.49 | 0.86a | 0.52a | 0.58a | 0.53a | 0.68a | 0.80a | 0.32 | — |
R > 0.6.
WM tasks produced larger average negative BOLD responses (across trials and subjects) than did VA tasks in the PHG, MTG, PreCG, PCL, and PCG (P < 0.05; paired t‐test; Fig. 4). Conversely, VA tasks did not produce larger deactivation than WM tasks did in any brain region. The PreCG, PCL, and the precuneus deactivated during WM tasks (P < 0.0001) but activated during VA tasks (P < 0.0006). Increased WM load produced increased deactivation in the SFG, ACG, insula, CG, PreCG, PCG, and precuneus (P < 0.05; t‐tests for differential [two‐back − zero‐back] BOLD amplitudes; Fig. 4). For VA tasks, however, increased VA load produced decreased deactivation in the PCG, MTG, and the precuneus (P < 0.05). Cross‐correlations of load responses (% signal change) in the deactivated network were larger for VA tasks compared to that for WM tasks (Table IV). Figure 5A and 5B exemplify the linear correlations (across subjects) between load effects in the PCG and the precuneus, for VA and WM tasks. Figure 5C and 5D plot the distribution of BOLD signal amplitudes across subjects and tasks, demonstrating that WM and VA tasks produce different Gaussian distributions of BOLD signals. The negative average of the distribution is larger during WM tasks, but its FWHM is larger during VA tasks (FWHM = 1.55 ± 0.1 and 0.95 ± 0.1% for VA and WM tasks, respectively). The position (see Tables I and II), shape, and size of the ROIs were invariant across subjects, tasks, and conditions.
Figure 4.
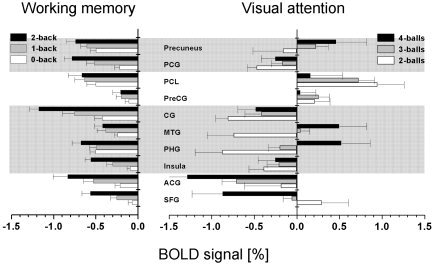
Average BOLD signals at specific regions of interest in the deactivated network (see Table II).
Table IV.
Cross correlation factors of load responses in the deactivated network for working memory (WM) and visual attention (VA) tasks
| WM | VA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SFG | ACG | Insula | PHG | PreCG | MTG | CG | PCL | PCG | Precuneus | |
| SFG | — | 0.37 | 0.40 | 0.65a | 0.52a | 0.59a | 0.71a | 0.22 | 0.69a | 0.49 |
| ACG | 0.22 | — | 0.68a | 0.57a | 0.49 | 0.72a | 0.70a | 0.16 | 0.62a | 0.25 |
| Insula | 0.24 | 0.05 | — | 0.84a | 0.70a | 0.76a | 0.79a | 0.49 | 0.85a | 0.49 |
| PHG | 0.14 | −0.07 | 0.17 | — | 0.73a | 0.86a | 0.82a | 0.63a | 0.96a | 0.89a |
| PreCG | 0.29 | 0.08 | 0.54a | 0.46 | — | 0.56a | 0.73a | 0.50a | 0.66a | 0.60a |
| MTG | 0.37 | −0.04 | 0.09 | 0.48 | 0.38 | — | 0.80a | 0.56a | 0.86a | 0.80a |
| CG | 0.39 | −0.08 | 0.32 | 0.77a | 0.41 | 0.62a | — | 0.54a | 0.89a | 0.83a |
| PCL | 0.15 | −0.12 | 0.09 | 0.66a | 0.41 | 0.56a | 0.58a | — | 0.63a | 0.69a |
| PCG | 0.23 | 0.01 | 0.20 | 0.96a | 0.45 | 0.50a | 0.80a | 0.68a | — | 0.94a |
| Precuneus | 0.27 | 0.00 | 0.25 | 0.85a | 0.48 | 0.55a | 0.81a | 0.71a | 0.86a | — |
R > 0.6.
Figure 5.
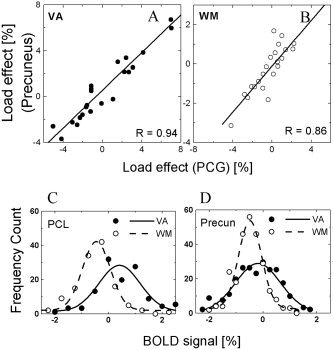
Correlation of load effects in regions of interest (ROIs) that deactivated for visual attention (VA; A) and working memory (WM; B) tasks, and BOLD signal distribution in the paracentral lobule (PCL; C) and the precuneus (D) for WM and VA tasks. Average signal amplitudes in bilateral cubic ROIs (27 3 × 3 × 3 mm3 voxels) for all subjects and conditions (22 subjects × 2 repetitions × 2 sides [left and right] × 3 tasks [zero‐, one‐, and two‐back] = 264 measurements [full and open circles]).
DISCUSSION
This study provides the first intra‐subject comparison of fMRI deactivation during two different cognitive tasks. The main findings are: (1) VA and WM tasks commonly deactivate a network that includes the frontal, temporal, occipital, and limbic lobes; (2) although WM tasks caused lower overall activation, they produced larger overall deactivation than VA tasks did; and (3) specific regions in the frontal lobes (PreCG and PCL) deactivated during WM but activated during VA tasks.
These findings suggest that global CBF is not constant across the tasks, for two reasons. First, VA tasks produced greater activation but lesser amplitude of deactivation than WM tasks do (Figs. 2, 3, 4). Consequently, the BOLD− in the deactivated network is not proportional to BOLD+ in the activated network; i.e., Δ+ ≠ k Δ− across tasks. Second, although cross‐correlation of load responses in the activated network were similar during VA and WM tasks (Table III), in the deactivated network the load responses cross‐correlated better for VA tasks than for WM tasks (Table IV and Fig. 5A,B). The correlation differences between tasks result from the lower dynamic range of negative BOLD signals during WM tasks compared to that during VA tasks. Similarly, in deactivated brain regions, the Gaussian distribution of BOLD responses has different FWHM (Fig. 5C,D) for WM and VA tasks. In activated regions, however, the FWHM is the same for both tasks. This is also inconsistent with proportional blood flow changes in the activated and the deactivated networks across tasks.
The parametric increases of VA load enhanced the positive BOLD signals in the IPL, SOG, IOG, thalamus, cerebellum, and the left DLPFC (IFG and MFG), and the negative BOLD signals in the PCG, PHG, and the precuneus (see Tables I, II, and Figs. 3, 4). Similarly, parametric increases of WM load enhanced brain activation bilaterally in the PFC and left IPL, and brain deactivation in the CG and the insula (see Tables I, II, and Figs. 3, 4). These corresponding increases of activation and deactivation support the notion that global CBF is constant across varying task difficulties within the same task. Our results are supported by one previous fMRI study that used an auditory target detection task with parametric changes of task difficulty [McKiernan et al.,2003], and found that increasing task difficulty resulted in greater degrees of brain deactivation in the ACG, SFG, MFG, PCG, SPL, and precuneus. This study suggested that the ongoing internal information processing during the conscious resting state is suspended during the task to allow for reallocation of processing resources. The present study therefore suggests that the hemodynamic responses in activated and deactivated networks are proportional across load conditions but not across different tasks (WM and VA).
There are two potential mechanisms underlying deactivation on BOLD fMRI. Model 1 assumes a local reduction of rCBF in less active brain regions to compensate for rCBF increases in activated brain regions, without central involvement (“blood stealing”). Conversely, Model 2 postulates stimulus‐correlated, centrally mediated inhibition of neural processes in task‐irrelevant brain regions.
Model 1 relates primarily to shunting of blood flow to activated brain regions. Because increased neural activity in the activated network requires increased rCBF and oxygen consumption (CMRO2) and the total metabolism of the brain is approximately constant over a wide range of mental and motor activities [Raichle and Gusnard,2002], increased rCBF in the activated network might require a synchronous decrease of rCBF in adjacent regions of the brain (i.e., a hemodynamic response). Consequently, these adjacent task‐irrelevant regions might present negative rather than positive BOLD responses. In this purely hemodynamic model, regions with fMRI deactivation would reflect a transition from decreased rCBF‐supply during “task” periods to normal rCBF‐supply during “resting” periods. Despite greater global activation during VA tasks in this study, the PCL, PreCG, and precuneus deactivated during WM but activated during VA. This seems to be inconsistent with Model 1, because the cerebrovasculature is constant in anatomy and location within each subject; therefore, these areas also should deactivate during VA because all brain areas activated by WM tasks also activated during VA tasks, within the same cerebrovasculature in each subject.
Model 2 explains deactivation as a consequence of cross‐modal inhibition mechanisms that reduce potentially distracting neural processes [Laurienti et al.,2002]. With respect to the present study, Model 2 advocates that deactivation in the posterior insula, PCL, ACG, MTG, CG, PHG, PCG, and precuneus during WM but less so during VA is a result of direct neural inhibition. This inhibition may serve the purpose of optimizing performance by minimizing interference. Deactivation of these brain areas during rapid visual information processing [Lawrence et al.,2003] and auditory target detection tasks [McKiernan et al.,2003] were associated with the need for focused attention toward more difficult tasks. For instance, competing neural processes such as those produced by the stimulation of the auditory cortices (from scanner noise) or by attention to introspective or emotional factors (i.e., anxiety during fMRI) could interfere with cognitive task performance. Consequently, neural processing in task‐irrelevant networks might be partially inhibited (during task periods but not during rest periods) to increase efficiency of the task‐activated network. In this model, deactivation reflects the transition from an inhibited neural state (during task periods) to a less inhibited state (during resting periods). Several regions in the deactivated networks in our study seem to be related to auditory and emotional tasks. For instance, passive music listening [Brown et al.,2004] and facial emotion processing [Gur et al.,2002; Kircher et al.,2000; Lennox et al.,2004; Pessoa et al.,2002; Pierce et al.,2004] engage the same regions in the limbic and paralimbic systems (insula, ACG, CG, PCG, PHG, MTG, and the retrosplenial cortex). In addition, event‐related fMRI studies on inhibition using go/no‐go tasks have shown that errors during the tasks are associated with activation of the CG, suggesting an important function of the CG in the dynamic control of behavior [Fassbender et al.,2004; Garavan et al.,2002,2003]. The spatial specificity of deactivated brain regions in our current study makes a simple redistribution of blood supply unlikely, because these regions support neural processing that can interfere with attention processing. Furthermore, brain deactivation during WM tasks correlated with the behavioral data (RT and performance accuracy during the fMRI tasks; Fig. 2); the linear increase of deactivation with task difficulty might reflect greater suppression of neural processes during more demanding tasks, which is consistent with the inhibition model. Recent optical imaging studies in the rodent somatosensory cortex, however, found no changes in neuronal activity in brain regions that showed a negative hemodynamic response. This suggests that local shunting of rCBF may occur within a few millimeters within neuronal activity due to a complex spatially distributed neuronal activity pattern [Devor et al.,2005]. In our current study, however, deactivation occurred at much greater distance (e.g., insula cortices, cingulated gyrus, and occipital cortices), and across different vascular distributions, from the activated brain regions (e.g., lateral prefrontal regions, parietal cortices, cerebellum; see Fig. 2). Neural inhibition therefore may play a important role in the negative BOLD responses observed on fMRI, although local changes in rCBF adjacent to activated brain regions are also possible.
In addition to neural inhibition, brain deactivation might also be due to a simple reduction of neuronal activity in deactivated regions as other brain regions become more active [McKiernan et al.,2003]. Lastly, deactivation could also result from subjects' anxiety and discomfort in the MRI environment. Our MRI system is based on an older 4 Tesla magnet that has a very long (3 meters) bore, which increases the risk for claustrophobic reactions (two other subjects did not carry out the fMRI study for this reason), and produces loud sound pressure levels of acoustic noise (98 dB at the entrance of the tube). Functional MRI studies on emotional pain modulation have shown that anxiety about pain activates the CG, posterior insula, and the hippocampus [Ploghaus et al.,2001]. PET studies on anticipatory anxiety (painful shocks to subject's fingers) found that activation at the ACG correlates linearly with the anxiety ratings, suggesting that the rCBF in the medial PFC might reflect a combined effect of attentional demands causing reductions of rCBF, and accompanying performance anxiety that attenuate those reductions [Simpson et al.,2001]. During the resting periods, neural processing in the limbic regions therefore might have been enhanced due to greater awareness of the confined MR scanner environment. During the task periods, however, the subjects might have inhibited the interfering neural processing in the limbic system while focusing their attention on the tasks. Similarly, during the resting periods, neural processing in the auditory cortices (the posterior insula adjacent to the primary auditory cortex, BA 41) might have been enhanced by the loud scanner noise. During task periods, the interfering auditory processing might have been partially inhibited to maximize attention to the tasks.
Limitations of the Study
Our study could have been improved by using an additional task that activates the insula and the limbic lobe (for instance an emotional task) to test if the areas activated for this task are the same “interfering” areas deactivated by cognitive tasks (WM and VA tasks). In addition, matching the number and duration of tasks and resting blocks could have minimized the number of design feature differences between the tasks. We did not make these improvements because the study was based on a reanalysis of existing data. Furthermore, the order of task‐difficulty levels was not counterbalanced (zero‐, one‐, and two‐back for WM; 2, 3, and 4 balls for VA; although the WM/VA order was counterbalanced) in this work to minimize variance due to differential practice effects [Tomasi et al.,2004]. This approach, however, could have reduced the effect of cognitive load, i.e., WM load and VA load [Tomasi et al.,2004].
In summary, there are multiple findings of this study (the first intra‐subject comparison of fMRI deactivation during different cognitive tasks). First, distinct cognitive paradigms (WM and VA) commonly deactivated a network that comprises the frontal (SFG, PreCG, ACG, PCL, and posterior insula), temporal (MTG), occipital (precuneus), and limbic (CG, PHG, and PCG) lobes. Second, WM tasks produced larger deactivation than did VA tasks. Third, the PreCG and PCL deactivated during WM tasks, but activated during VA tasks. WM and VA tasks both activated a network that includes prefrontal, parietal, and occipital cortices, thalamus, and the cerebellum, as reported previously. In this network, positive BOLD signals probably reflect increased local oxygen consumption and increased rCBF. The larger deactivation during WM tasks compared to that during VA tasks suggests that global CBF is task dependent. Brain deactivation seems to occur predominantly in brain regions that potentially interfere with or are unimportant for carrying out the required tasks and is probably a compensatory (primarily neuronal inhibitory) response to optimize task performance due to limitations in processing bandwidth.
Acknowledgements
We thank Dr. R. Goldstein for helpful discussions on emotional processing, and Dr. K. Leckova and Ms. K. Warren for their help in subject recruitment, assessment and coordination.
REFERENCES
- Aguirre GK, Zarahn E, D'Esposito M (1998): The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage 8: 302–306. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Neelin P, Collins DL, Evans AC, Friston KJ (1997): Incorporating prior knowledge into image registration. Neuroimage 6: 344–352. [DOI] [PubMed] [Google Scholar]
- Born AP, Law I, Lund TE, Rostrup E, Hanson LG, Wildschiodtz G, Lou HC, Paulson OB (2002): Cortical deactivation induced by visual stimulation in human slow‐wave sleep. Neuroimage 17: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez M, Parsons L (2004): Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport 15: 2033–2037. [DOI] [PubMed] [Google Scholar]
- Buxton RB. 2002. Introduction to functional magnetic resonance imaging: principles and techniques. Cambridge: Cambridge University Press. [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T (2003): k‐Space based summary motion detection for functional magnetic resonance imaging. Neuroimage 20: 1411–1418. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller E, Braun A, Jovicich J, Koch C, Itti L, Ernst T (2001): Neural correlates of attention and working memory deficits in HIV patients. Neurology 57: 1001–1007. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T (2004): Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 56: 259–272. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB (1998): Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol 80: 2657–2670. [DOI] [PubMed] [Google Scholar]
- Deary I, Simonotto E, Meyer M, Marshall A, Marshall I, Goddard N, Wardlaw J (2004): The functional anatomy of inspection time: an event‐related fMRI study. Neuroimage 22: 1466–1479. [DOI] [PubMed] [Google Scholar]
- Devor A, Ulbert I, Dunn A, Narayanan S, Jones S, Andermann M, Boas D, Dale A (2005): Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci USA 102: 3822–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe J, Wylie G, Javitt D, Robertson I, Garavan H (2004): A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res 20: 132–143. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Franckowiak RS (1995): Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Garavan H, Ross T, Kaufman J, Stein E (2003): A midline dissociation between error‐processing and response‐conflict monitoring. Neuroimage 20: 1132–1139. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross T, Murphy K, Roche R, Stein E (2002): Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage 17: 1820–1829. [DOI] [PubMed] [Google Scholar]
- Gavrilescu M, Shaw ME, Stuart GW, Eckersley P, Svalbe ID, Egan GF (2002): Simulation of the effects of global normalization procedures in functional MRI. Neuroimage 17: 532–542. [PubMed] [Google Scholar]
- Gur R, Schroeder L, Turner T, McGrath C, Chan R, Turetsky B, Alsop D, Maldjian J, Gur R (2002): Brain activation during facial emotion processing. Neuroimage 16: 651–662. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee S, Nagaoka T, Kim D, Kim S (2002): Origin of negative blood oxygenation level‐dependent fMRI signals. J Cereb Blood Flow Metab 22: 908–917. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL (1994): The functional organization of human extrastriate cortex: a PET‐rCBF study of selective attention to faces and locations. J Neurosci 14: 6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig J, Scheffler K (2001): Hyperechoes. Magn Reson Med 46: 6–12. [DOI] [PubMed] [Google Scholar]
- Hester R, Murphy K, Foxe J, Foxe D, Javitt D, Garavan H (2004): Predicting success: patterns of cortical activation and deactivation prior to response inhibition. J Cogn Neurosci 16: 776–785. [DOI] [PubMed] [Google Scholar]
- Hoge R, Atkinson J, Gill B, Crelier G, Marrett S, Pike G (1999a): Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA 96: 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB (1999b): Investigation of BOLD signal flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med 42: 849–863. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Peters RJ, Koch C, Braun J, Chang L, Ernst T (2001): Brain areas specific for attentional load in a motion tracking task. J Cogn Neurosci 13: 1048–1058. [DOI] [PubMed] [Google Scholar]
- Kawashima R, O'Sullivan BT, Roland PE (1995): Positron‐emission tomography studies of cross‐modality inhibition in selective attentional tasks: closing the “mind's eye.” Proc Natl Acad Sci USA 92: 5969–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher T, Senior C, Phillips M, Benson P, Bullmore E, Brammer M, Simmons A, Williams S, Bartels M, David A (2000): Towards a functional neuroanatomy of self processing: effects of faces and words. Brain Res Cogn Brain Res 10: 133–144. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, Wallace MT, Yen YF, Field AS, Stein BE (2002): Deactivation of sensory‐specific cortex by cross‐modal stimuli. J Cogn Neurosci 14: 420–429. [DOI] [PubMed] [Google Scholar]
- Lawrence N, Ross T, Hoffmann R, Garavan H, Stein E (2003): Multiple neuronal networks mediate sustained attention. J Cogn Neurosci 15: 1028–1038. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K (1995): High contrast and fast three‐dimensional magnetic resonance imaging at high fields. Magn Reson Med 34: 308–312. [DOI] [PubMed] [Google Scholar]
- Lennox B, Jacob R, Calder A, Lupson V, Bullmore E (2004): Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol Med 34: 795–802. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Beauchamp MS, DeYoe EA (2000): A comparison of visual and auditory motion processing in human cerebral cortex. Cereb Cortex 10: 873–888. [DOI] [PubMed] [Google Scholar]
- McKiernan K, Kaufman J, Kucera‐Thompson J, Binder J (2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider L (2002): Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA 99: 11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E (2004): The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain 127: 2703–2716. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann C, Clare S, Bantick S, Wise R, Matthews P, Rawlins J, Tracey I (2001): Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 21: 9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME (1998): Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA 95: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA (2002): Appraising the brain's energy budget. Proc Natl Acad Sci USA 99: 10237–10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K (2002): Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36: 1195–1210. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE (1997): Top‐down modulation of early sensory cortex. Cereb Cortex 7: 193–206. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME (2001): Emotion‐induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci USA 98: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. 1982. Symbol digit modalities test: Western Psychological Services.
- Stefanovic B, Warnking J, Pike G (2004): Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 22: 771–778. [DOI] [PubMed] [Google Scholar]
- Stroop J (1935): Studies of interference in serial verbal reaction. J Exp Psychol 18: 643–662. [Google Scholar]
- Tomasi D, Caparelli E, Chang I, Ernst T, Telang F (2005a): Acoustic noise changes fMRI activation during visual attention tasks. Presented at the 11th Meeting of the Organization for Human Brain Mapping, June 12–16, 2005, Toronto, Canada.
- Tomasi D, Caparelli EC, Chang L, Ernst T (2005b): fMRI‐acoustic noise alters brain activation during working memory tasks. Neuroimage 27: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Chang I, Caparelli EC, Foerster B, Ernst T, Telang F (2005c): Acoustic interference on working memory in HIV patients. Presented at the SMRT 14th Annual Meeting, May 7–13, 2005, Miami Beach, Florida.
- Tomasi D, Ernst T, Caparelli EC, Chang L (2004): Practice‐induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. Neuroimage 23: 1414–1421. [DOI] [PubMed] [Google Scholar]


