Abstract
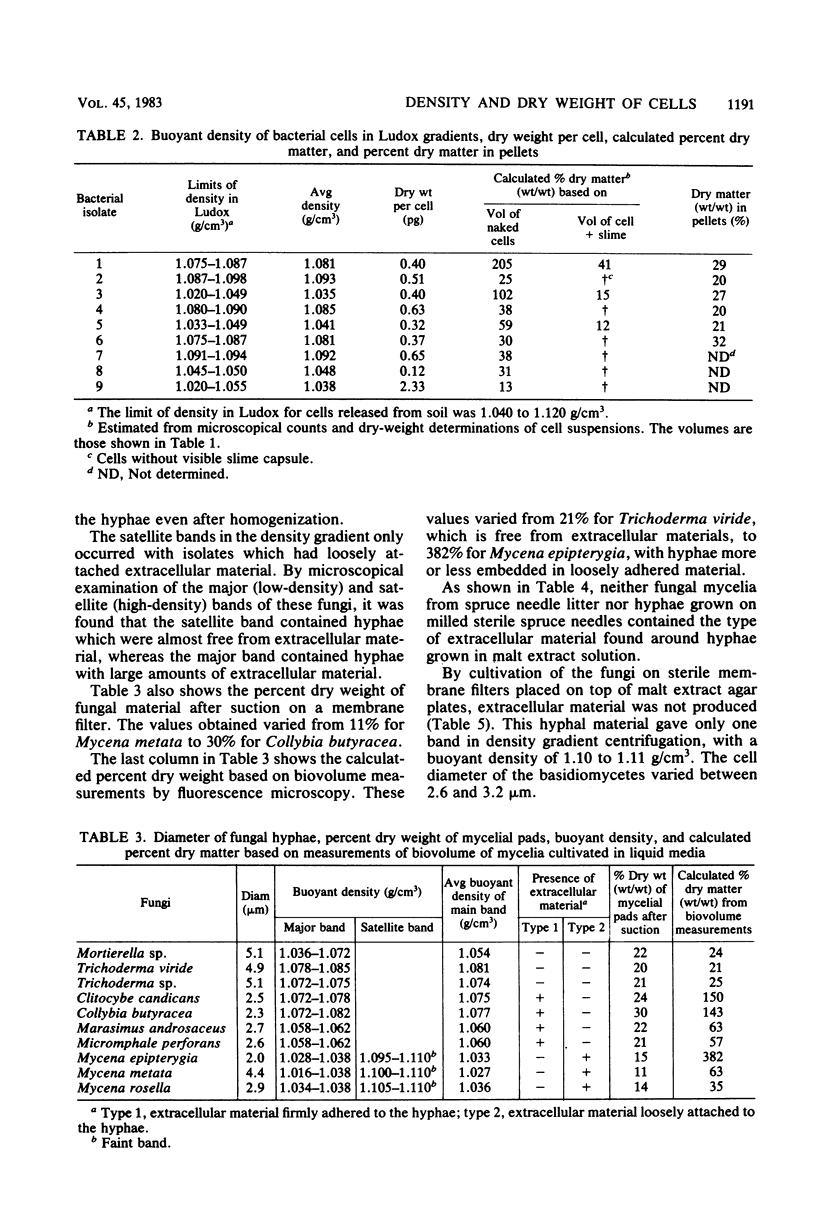
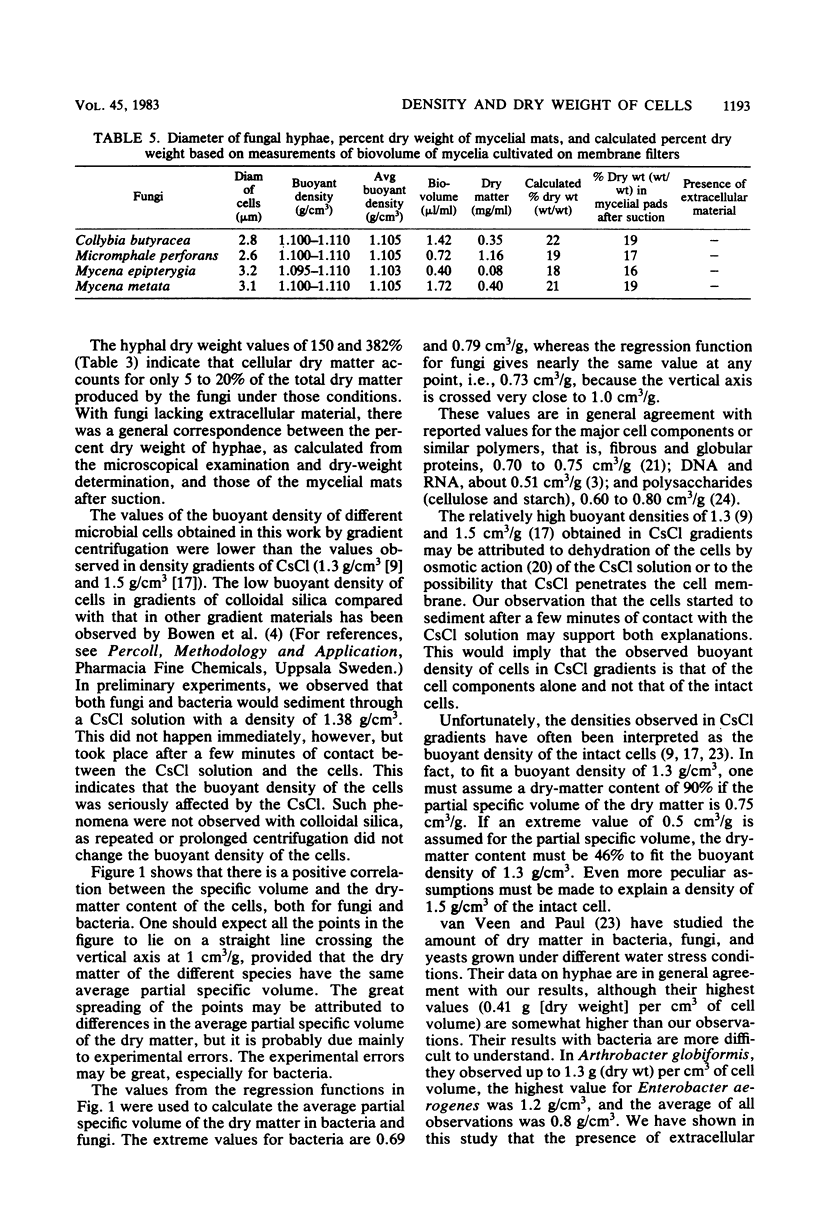
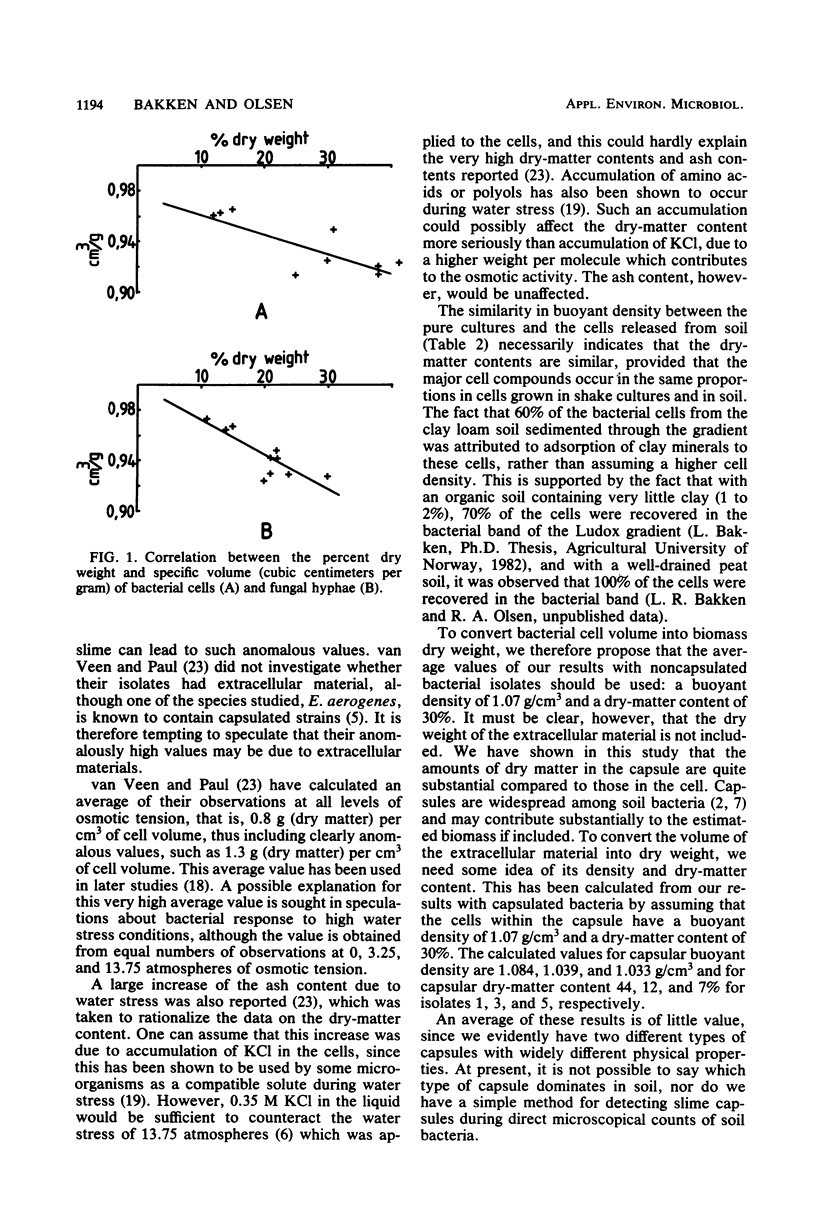
Several isolates of bacteria and fungi from soil, together with cells released directly from soil, were studied with respect to buoyant density and dry weight. The specific volume (cubic centimeters per gram) of wet cells as measured in density gradients of colloidal silica was correlated with the percent dry weight of the cells and found to be in general agreement with calculations based on the partial specific volume of major cell components. The buoyant density of pure bacterial cultures ranged from 1.035 to 1.093 g/cm3, and their dry-matter content ranged from 12 to 33% (wt/wt). Average values proposed for the conversion of bacterial biovolume into biomass dry weight are 1.09 g/cm3 and 30% dry matter. Fungal hyphae had buoyant densities ranging from 1.08 to 1.11 g/cm3, and their dry-matter content ranged from 18 to 25% (wt/wt). Average values proposed for the conversion of hyphal biovolume into biomass dry weight are 1.09 g/cm3 and 21% dry matter. Three of the bacterial isolates were found to have cell capsules. The calculated buoyant density and percent dry weight of these capsules varied from 1.029 g/cm3 and 7% dry weight to 1.084 g/cm3 and 44% dry weight. The majority of the fungi were found to produce large amounts of extracellular material when grown in liquid cultures. This material was not produced when the fungi were grown on either sterile spruce needles or membrane filters on an agar surface. Fungal hyphae in litter were shown to be free from extracellular materials.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Paul E. A. The use of fluorescein isothiocyanate in the determination of the bacterial biomass of grassland soil. Can J Microbiol. 1970 Feb;16(2):57–62. doi: 10.1139/m70-011. [DOI] [PubMed] [Google Scholar]
- Bae H. C., Casida L. E., Jr Responses of indigenous microorganisms to soil incubation as viewed by transmission electron microscopy of cell thin sections. J Bacteriol. 1973 Mar;113(3):1462–1473. doi: 10.1128/jb.113.3.1462-1473.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie G. D., Rickwood D., Hell A. Buoyant densities and hydration of nucleic acids, proteins and nucleoprotein complexes in metrizamide. Biochim Biophys Acta. 1973 Dec 7;331(2):283–294. doi: 10.1016/0005-2787(73)90441-3. [DOI] [PubMed] [Google Scholar]
- Casida L. E., Jr Microorganisms in unamended soil as observed by various forms of microscopy and staining. Appl Microbiol. 1971 Jun;21(6):1040–1045. doi: 10.1128/am.21.6.1040-1045.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff D. A. The separation of cells and subcellular particles by colloidal silica density gradient centrifugation. Methods Cell Biol. 1975;10:85–104. doi: 10.1016/s0091-679x(08)60731-1. [DOI] [PubMed] [Google Scholar]
- van Veen J. A., Paul E. A. Conversion of biovolume measurements of soil organisms, grown under various moisture tensions, to biomass and their nutrient content. Appl Environ Microbiol. 1979 Apr;37(4):686–692. doi: 10.1128/aem.37.4.686-692.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



