Abstract
DO was used in an aged mouse model to determine if systemically and/or locally administered rhIGF-I improved osteoblastogenesis and new bone formation. Local and systemic rhIGF-I treatment increased new bone formation. However, only systemic delivery produced measurable concentrations of rhIGF-I in the circulation.
Introduction
Human and rodent research supports a primary role for IGF-I in bone formation. Significant roles for both endocrine and paracrine/autocrine IGF-I have been suggested for normal osteoblastogenesis and bone formation. We have assessed, using a mouse model of distraction osteogenesis (DO), the impact of continuous administration of recombinant human (rh)IGF-I, delivered either locally to the distraction site or absorbed systemically, on bone formation in an aged mouse model.
Materials and Methods
DO was performed in aged mice (18-month-old C57BL/6 male mice), which were distracted at 0.15 mm daily. At the time of osteotomy, miniosmotic pumps were inserted subcutaneously to (1) deliver vehicle or rhIGF-I subcutaneously for systemic delivery or (2) deliver vehicle or rhIGF-I directly to the newly forming bone through infusion tubing routed subcutaneously from the pump to the distraction site. Serum concentrations of mouse IGF-I, human IGF-I, and osteocalcin were determined at the end of the study.
Results
New bone formation observed in DO gaps showed a significant increase in new bone formation in rhIGF-I–treated mice, irrespective of delivery route. However, detectable levels of human IGF-I were found only in the serum of animals receiving rhIGF-I systemically. Osteocalcin levels did not differ between controls and rhIGF-I–treated groups.
Conclusions
Locally and systemically delivered rhIGF-I both produce significant increases in new bone formed in an aged mouse model in which new bone formation is normally markedly impaired, suggesting that rhIGF-I may improve senile osteoporosis. Because systemic administration of IGF-I can result in untoward side effects, including an increased risk for cancer, the findings that locally delivered IGF-I improves bone regeneration without increasing circulating IGF-I levels suggests that this delivery route may be preferable in an at-risk, aged population.
Keywords: osteoporosis, limb lengthening, distraction osteogenesis, anabolic agents, aging
INTRODUCTION
The mechanisms controlling bone formation are complex, involving a variety of growth factors, hormones and cytokines, extracellular matrix molecules, endopeptidases and proteases, and mineral availability.(1,2) For over two decades, research has supported a primary role for growth hormone (GH) and the IGFs (IGF-I and IGF-II) in anabolic bone formation.(3) Studies in both animals and humans have shown that both GH and IGF-I can stimulate longitudinal bone growth when given to GH-deficient individuals.(3) However, it has only been in recent years that an essential role of IGFs in normal bone development has been confirmed through the elimination of IGF-I and the type I IGF receptor in mice through homologous recombination.(4,5) Profound growth retardation, growth plate abnormalities, and decreased bone calcification were observed in these animals. Recent studies have further refined how elimination of IGF-I affects bone physiology not only through the dwarfing of bones, but also by significantly decreasing bone formation rate and cortical thickness, resulting in more compact bone.(6)
Whereas IGFs play an important role in normal bone development, the source of IGFs is complex. GH increases serum concentrations of IGF-I by stimulating IGF production by the liver; this mechanism likely accounts for many of the anabolic effects of GH on bone.(3) The great majority of serum IGFs are packaged in a large multimeric protein complex consisting of the major serum carrier protein for IGFs, IGF-binding protein-3 (IGFBP-3), and an 85-kDa acid-labile protein (ALS), providing a ready depot of IGFs for transport when needed at distant sites, such as bone. Indeed, recent studies suggest that scavenging of IGFs from this large complex by smaller IGFBPs, such as IGFBP-4, may shuttle IGFs from the vascular compartment into skeletal tissues.(7) However, systemic IGFs as a source for regulating bone homeostasis has come under scrutiny, in that several studies have shown that significant reductions in their circulating concentrations yield only marginal affects on bone.(8) Alternatively, numerous studies have shown that both IGF-I(9–13) and IGF-II(14–17) are produced by both murine and human osteoblasts, suggesting that IGFs may function in a paracrine/autocrine manner within bone. In bone, unlike liver, IGF production is not primarily under the control of GH, but is regulated by a variety of agents including estrogen, PTH, cortisol, and a host of local growth factors and cytokines.(3) Another source of IGFs is from the large reservoir of IGFs complexed with bone matrix molecules.(2,3,12,18,19) This abundant supply of IGFs is likely critical to ongoing bone formation, bone repair, and bone cell proliferation. Indeed, recent data showed that skeletal IGF-I content in human bone declines almost 60% between the ages of 20 and 60 years,(20) suggesting that this essential pool of IGFs may not be sufficiently available for new bone formation as aging ensues.
To better clarify the role of local versus systemic effects of IGF-I in direct bone formation and osteoblastogenesis in the aging skeleton, we studied in a model of mouse distraction osteogenesis (DO) how the continuous exogenous application of recombinant human IGF-I impacts regenerating bone. Whereas longitudinal bone growth results from endochondral ossification from growth plates in long bones, DO produces intramembranous ossification, providing for isolated zones of osteoblast proliferation and differentiation. For these studies, we used DO in an aged mouse model, because this animal shows very poor new bone formation.(21) We show that local application of recombinant human (rh)IGF-I promotes marked improvements in bone formation in this model without impacting circulating concentrations of IGF-I, yet systemically delivered rhIGF-I, whereas also promoting enhanced de novo bone formation, can expose extraskeletal tissues to circulating rhIGF-I.
MATERIALS AND METHODS
Animal model
Male, 18-month-old C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and acclimatized to 12-h light/dark cycles in climate-controlled rooms before surgery. All research protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Arkansas for Medical Sciences.
Each mouse was anesthetized with intraperitoneal sodium pentobarbital at a dose of 71 mg/kg. A titanium two-ring fixator was secured on the left tibia by four transosseous wires. A mid-diaphyseal osteotomy of the tibia and fibula was created, as previously described.(21) Figure 1A shows the distraction apparatus and how it is applied around the osteotomy before distraction. After the osteotomy, Alzet Model 1002 miniosmotic pumps (Durect, Cupertino, CA, USA) were filled with either (1) PBS (vehicle) or (2) (vehicle) + recombinant human IGF-I (rhIGF-I; delivery rate: 1 μg/7μl, delivering 0.25 μl/hr ± 0.05 μl/day) and were inserted subcutaneously on the back according to the manufacturer’s recommendations to (1) deliver vehicle or rhIGF-I subcutaneously where it would be absorbed into the systemic circulation or (2) to deliver vehicle or rhIGF-I directly at the distraction site (Fig. 1B). For local delivery to the distraction gap, a catheter was attached to the pump, the catheter’s opening was placed adjacent to the osteotomy (Fig. 1B,inset), and two sutures were used to secure the catheter, one near the site of the osteotomy and one midway between the osteotomy and the pump. Infusions of rhIGF-I or vehicle were begun at the beginning of the latency phase, and the pumps remained in place throughout the entire distraction phase. Buprenex (0.1 mg/kg) was given postoperatively by intramuscular injection for analgesia.
FIG. 1.
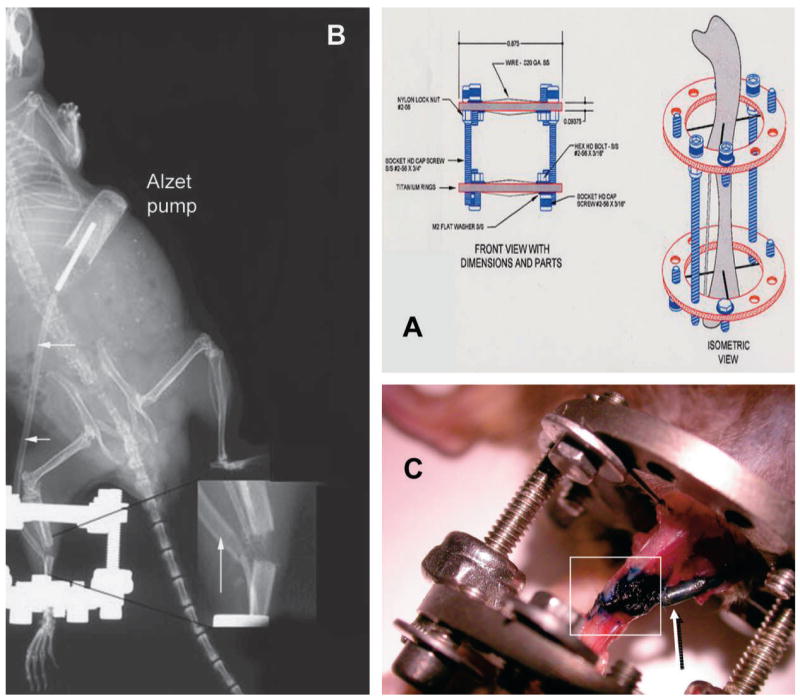
(A) Fixator used for DO in the mouse model and how it is applied to the tibia around the osteotomy site. (B) As shown in this whole animal radiograph, Alzet Model 1002 miniosmotic pumps were inserted subcutaneously, and infusion tubing (arrows) was secured and routed subcutaneously from the pump to the distraction site for local delivery. (Inset) The catheter’s opening was placed adjacent to the osteotomy. (C) Methylene blue was infused for 14 days by local infusion. At death, surrounding soft tissues were dissected away from the surgical site, exposing the distraction gap, adjacent bone, and the infusion catheter tip. Blue dye was seen only in the distraction gap (boxed area) and in the catheter tip (arrow).
Two study designs were carried out. In the first study (Figs. 2 and 3), locally delivered vehicle (i.e., PBS) was compared with locally delivered rhIGF-I. Distraction began 1 day after surgery at 0.075 mm b.i.d. (0.15 mm/day) for 13 days. In the second study (Fig. 4), locally delivered vehicle or systemically delivered vehicle were compared with locally delivered rhIGF-I or systemically delivered rhIGF-I, respectively. Distraction began 3 days after surgery at 0.075 mm b.i.d. (0.15 mm/day) for 14 days total. Mice were killed at the completion of distraction. At death, the distracted tibias were harvested and fixed in 10% neutral buffered formalin for radiographic and histological studies as detailed below. In a pilot study to assess the local delivery and spread of the contents delivered through miniosmotic pumps to the distraction gap, methylene blue (10 mg/ml) was infused locally for 14 days in two animals. Figure 1C shows the infusion site from an animal treated with methylene blue for 14 days, showing that the dye was only observed to be present within the distraction gap per se and not in adjacent tissues surrounding the gap, suggesting that this mode of exogenous application could deliver a local and sustained infusion that permeated into the DO gap of the mouse.
FIG. 2.
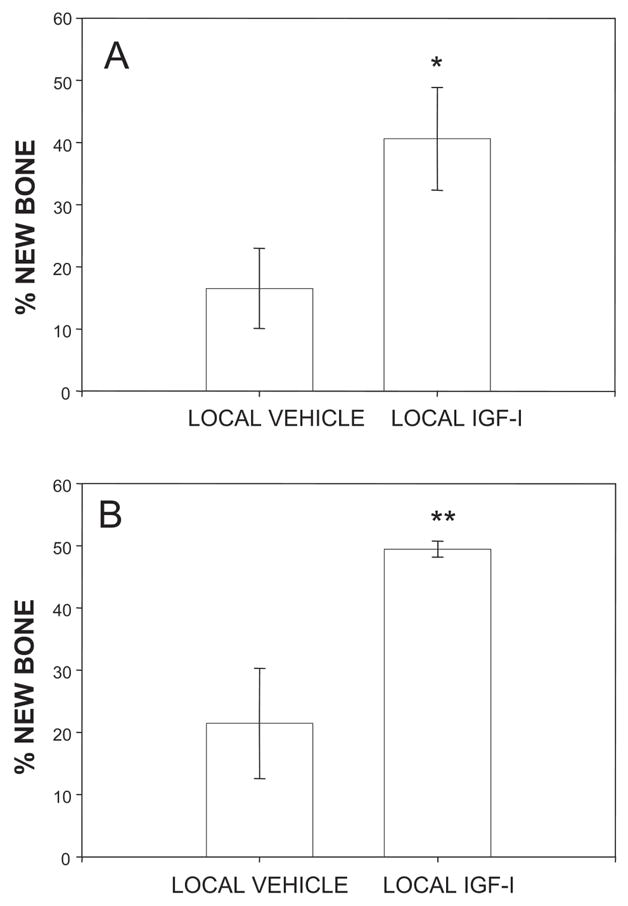
(A) Radiological and (B) histological quantification of new bone formed in aligned distraction gaps from mice receiving either locally delivered vehicle or rhIGF-I. *p < 0.05 and **p < 0.01 (local vehicle vs. local rhIGF-I).
FIG. 3.
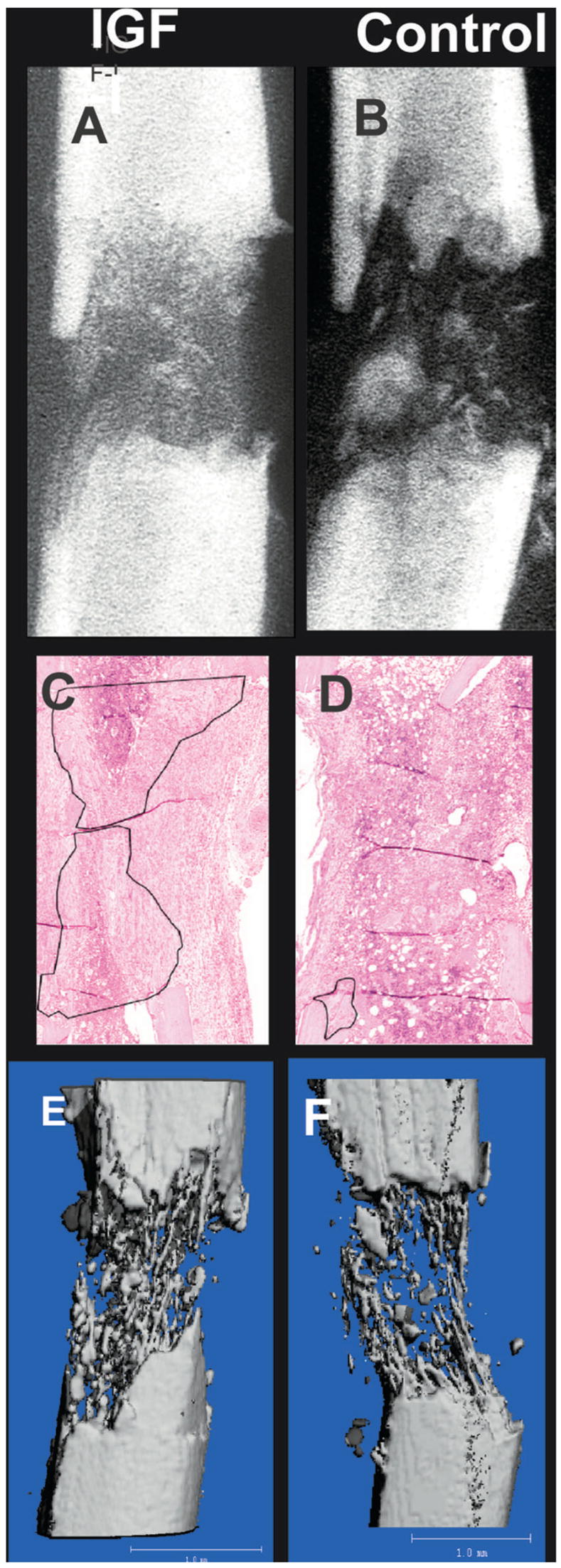
Representative radiographs of a distraction gap from (A) an rhIGF-I–treated mouse and (B) a vehicle-treated mouse (control). Histological sections from representative animals treated (C) with or (D) without rhIGF-I are presented, showing new bone formation outlined in black. (E and F) Effects of rhIGF-I infusion on bone formation was refined further using μCT. Representative μCT reconstructions of distraction gaps from (E) an rhIGF-I–treated and (F) a vehicle-treated mouse are shown.
FIG. 4.
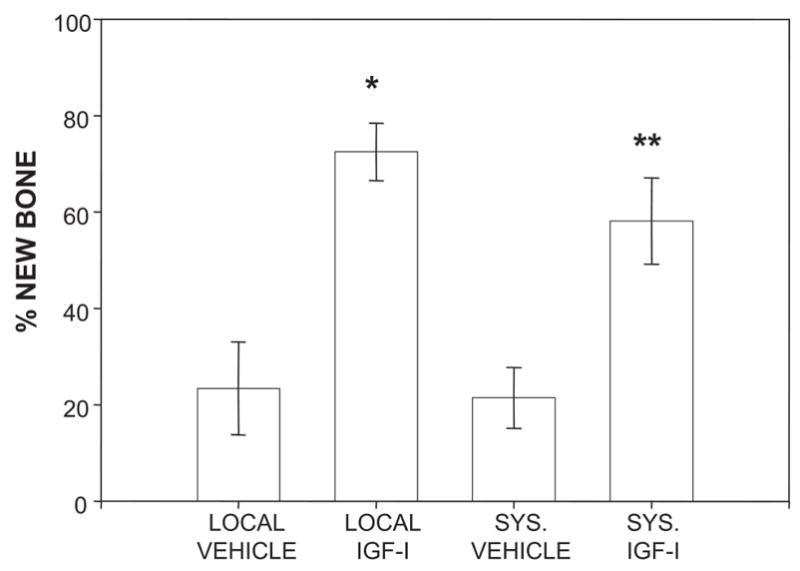
Quantification of new bone formed in aligned distraction gaps from mice receiving either locally delivered vehicle, locally delivered rhIGF-I, systemic vehicle, or systemic rhIGF-I. **p < 0.05 (systemic vehicle vs. systemic rhIGF-I); *p < 0.001 (local vehicle vs. local rhIGF-I).
Biochemical analyses
Measurements were performed on serum samples obtained at the time of death. Human IGF-I was measured using two different IGF-I ELISAs: (1) nonextraction IGF-I ELISA, Diagnostic Systems Laboratories, Webster, TX, USA (sensitivity 0.01 ng/ml) and/or hIGF-I IDS Octeia IGF-I, Immunodiagnostics Systems, Fountain Hills, AZ, USA (sensitivity 1.9 ng/ml). Mouse IGF-I was measured using a rodent-specific ELISA (Rat/Mouse Octeia IGF-I; Immunodiagnostic Systems; sensitivity, 82 ng/ml). Murine osteocalcin was measured by enzyme immunosorbent assay (Mouse EIA kit; Biomedical Technologies, Stoughton, MA, USA).
Radiographic and histological analysis
After 48 h of fixation in 10% neutral buffered formalin, the tibias were removed from the fixators for high-resolution single beam radiography: a Xerox Micro50 closed system radiography unit (Xerox, Pasadena, CA USA) was used at 40 kV (3 mA) for 20 s using Kodak X-OMAT film. For quantification, the radiographs were video recorded under low power (× 1.25 objective) magnification, and the area and density of mineralized new bone in the distraction gaps were evaluated using NIH Image J software. The distraction gap was outlined from the outside corners of the two proximal and the two distal cortices forming a quadrilateral region of interest (ROI). The mineralized new bone area in the gap was determined by outlining regions with radio density equivalent to or greater than the adjacent medullary bone. The percentage of new mineralized bone within the distraction gap (percent new bone) was calculated by dividing mineralized bone area by total gap area.
After radiography, select distracted tibias from the first study were decalcified in 5% formic acid, dehydrated, and embedded in paraffin, as previously described.(22,23) Longitudinal sections (5–7 μm) were stained with hematoxylin and eosin. Sections were selected for analysis to represent a central gap location. As detailed above, a ROI was outlined and recorded. New bone was defined as all organized osteoid/sinusoid columns, and any marrow elements within the gap were excluded. Both the proximal and distal endosteal new bone matrix was outlined, and the area was recorded. The percentage of new bone area within the DO gap (percent new bone) was calculated by dividing the new bone matrix area by the total distraction gap area.
μCT
Representative specimens of distracted tibias from the first study were determined from the 2D radiographs and imaged by μCT using a μCT-40 (Scanco, Bassersdorff, Switzerland) and the manufacturer’s supplied software, as previously described.(24)
Statistical analysis
For statistical analysis, the unpaired Student’s t-test was used to compare differences between groups for all serum analyses and skeletal parameter comparisons. All data are reported as mean ± SE, and differences were considered statistically significant at p < 0.05.
RESULTS
Biochemical parameters
Locally delivered rhIGF-I by miniosmopump could theoretically lead to both increases in rhIGF-I concentrations at the site of application and increases in serum concentrations through leaching of rhIGF-I into the systemic circulation. If the latter did occur, the relative contribution of locally delivered rhIGF-I compared with systemically delivered rhIGF-I could not be clearly elucidated in the model described herein. Therefore, to determine if locally delivered rhIGF-I did gain access to the systemic circulation, we measured human IGF-I in serum of animals from the first study using two human-specific IGF-I ELISAs. Using the IDS hIGF-I ELISA, no human IGF-I was detected in the serum of any animal treated with locally delivered vehicle (n = 7) or rhIGF-I–treated animals (n = 8); the DSL human IGF-I assay detected extremely low levels of IGF-I in several animals, yet these very low values were not statistically different between the vehicle-treated and rhIGF-I–treated groups (0.63 ± 0.13 [n = 7] versus 1.58 ± 0.52 ng/ml [n = 8], respectively). These data suggest that the DSL assay likely has very low cross-reactivity with mouse IGFs because it detected IGF-I even in animals that did not receive any exogenous rhIGF-I. Therefore, the IDS hIGF-I ELISA was used in all ensuing studies. In the first study, serum levels of mouse IGF-I in both groups were not significantly different (296.0 ± 74.8 versus 234.4 ± 74.0 ng/ml, respectively), suggesting that endogenous mouse IGF-I production was not altered in either locally delivered rhIGF-I–treated or locally delivered vehicle-treated mice. When serum was assayed for hIGF-I in the second study, no hIGF-I was detected in systemic vehicle-treated (n = 4), locally delivered vehicle-treated (n = 8), or locally delivered rhIGF-I–treated (n = 8) mice. In contrast, hIGF-I was detected in serum of seven of nine animals receiving systemic rhIGF-I (8.3 ± 2.5 ng/ml), showing that the assay was capable of detecting the ligand of interest and that systemic delivery of rhIGF-I resulted in measurable amounts of hIGF-I in the circulation.
Serum levels of osteocalcin were not significantly different when comparing locally delivered vehicle versus locally delivered rhIGF-I in serum from animals in the first study (37.44 ± 15.3 versus 20.8 ± 10.5 ng/ml, respectively). Similarly, serum assayed for osteocalcin in animals in the second study receiving vehicle systemically or locally, or hIGF-I systemically or locally, showed that osteocalcin levels were not significantly different among any of the groups (16.15 ± 16.15 or 23.0 ± 8.6 versus 37.3 ± 8.5 or 28.0 ± 10.8 ng/ml, respectively).
Assessment of new bone formation
The impact of locally delivered rhIGF-I on new bone formation was addressed in the first study using radiographic quantification of only aligned distraction gaps from mice receiving either locally delivered vehicle or locally delivered rhIGF-I. These data showed a significant improvement in new bone formation in distraction gaps from mice treated locally with rhIGF-I (40.6 ± 8.3%; n = 7) compared with 16.5 ± 6.4% (n = 6) in animals treated with locally applied vehicle (p < 0.05; Fig. 2A). A second measure of new bone formation in the first study was performed using histological analyses. Again, an increase in the formation of new bone columns was apparent by histological analysis in aged mice receiving locally delivered rhIGF-I (49.5 ± 1.28%; n = 5) compared with mice receiving only locally delivered vehicle (21.4 ± 8.9%; n = 5; p < 0.01; Fig. 2B). The modest discrepancy between radiographic and histological quantification is expected because single-beam radiographic analysis superimposes endosteal and periosteal new bone formation, whereas histological analysis can differentiate endosteal from periosteal new bone and includes unmineralized bone matrix.(21,25)
Representative radiographs, histological sections, and μCT images of distraction gaps from the first study from mice treated with locally delivered rhIGF-I (Figs. 3A, 3C, and 3E) or locally delivered vehicle (Figs. 3B, 3D, and 3F) are shown in Fig. 3. Figure 3A shows significantly more radiodense material present within the distraction gap compared with a control aged animal (Fig. 3B). These findings were corroborated histologically as evidenced in Fig. 3C, showing that locally delivered rhIGF-I treatment results in significantly more regenerate bone compared with vehicle (Fig. 3D); new endosteal bone is outlined in Figs. 3C and 3D. Finally, the significant improvement in new bone volume observed with locally delivered rhIGF-I treatment was confirmed when specimens were analyzed for microarchitecture by μCT. Figure 3E shows significantly more bone columns bridging the distraction gap compared with vehicle treatment (Fig. 3F). Taken together, these three modalities complement each other in providing evidence that locally delivered rhIGF-I treatment improves accrued bone formation throughout the distraction process.
To compare the effects of systemically delivered rhIGF-I with that of locally delivered rhIGF-I on de novo bone formation, 18-month-old mice were randomized to receive either locally delivered vehicle, locally delivered rhIGF-I, systemic vehicle, or systemic rhIGF-I. Miniosmotic pumps used to deliver rhIGF-I either systemically or locally were loaded with the same amount of rhIGF-I and were set to deliver at the same rate. Figure 4 shows that new bone formation, as assessed by radiography, was again increased in mice receiving locally administered rhIGF-I compared with mice receiving locally delivered vehicle (72.5 ± 9.6% [n = 8] versus 23.4 ± 9.6% [n = 8], respectively; p < 0.001). A significant effect on bone formation of systemically delivered rhIGF-I was also observed compared with animals systemically treated with vehicle (58.1 ± 8.9% [n = 9] versus 21.4 ± 6.3% [n = 4], respectively; p < 0.05). Comparing the route of rhIGF-I delivery revealed no statistically significant differences between locally delivered rhIGF-I versus systemically delivered rhIGF-I (p = 0.2).
DISCUSSION
Debate remains as to the relative roles of systemic versus paracrine/autocrine and locally stored IGF-I in bone homeostasis. Studies specifically designed to examine how relative degrees of peripheral (i.e., hepatic production) IGF-I affect bone formation have revealed effects on cortical periosteal bone growth along the cortex.(8) Further reductions in circulating IGF-I concentrations (to 10–15% of controls) achieved by crossing mice null for hepatic production of IGF-I with animals null for the acid-labile subunit of the IGFBP-3/IGF complex (all three components form the major 150-kDa complex responsible for carrying IGFs in the vascular compartment in mammals) results in a 10% decrease in BMD and a 35% decrease in periosteal circumference and cortical thickness.(26) Similar findings regarding a positive correlation in serum IGF-I, a quantitative trait locus on chromosome 6, and BMD has been established in congenic mouse models.(27) Together, these studies showed that circulating (i.e., endocrine) IGF-I can have a significant effect on several parameters of bone density and formation, as well as the actual size of the bone. Exactly how IGF-mediated affects may involve anabolic effects on osteoblast activity in vivo has been studied by Zhang et al.,(28) who, through tissue-specific gene targeting, ablated the type I IGF (IGFR) receptor in osteoblasts using the osteocalcin promoter. These studies showed that mice deficient in IGFR are of normal size, yet show a marked decrease in cancellous bone volume, connectivity, and trabecular number, as well as a striking decrease in the rate of mineralization of osteoid matrix. Thus, a significant amount of the bone-forming and mineralization actions of IGF-I seem to be mediated through effects of IGF-I specifically on the osteoblast. Indeed, autocrine overexpression of IGF-I in vivo under the control of the osteocalcin promoter results in the opposite phenotype, in which BMD, as measured by DXA and QCT, is significantly increased in IGF-I transgenic mice compared with controls, and histomorphometric measurements reveal a marked increase in femoral cancellous bone volume.(29) Complimentary to this model is one described recently showing that transgenic mice expressing osteoblast-targeted IGF-I under control of the Col1a1 3.6 kb 5= upstream regulatory sequence show increased femur length, cortical width, and cross-sectional area.(30)
Several studies support a role for local exogenous IGF-I delivered by various means on improving indices of bone homeostasis.(31–33) The studies described herein were designed to understand better how local application of rhIGF-I over a sustained period of time would affect bone formation and osteoblastogenesis and how the route of delivery of rhIGF-I might impact these measures. We examined these effects in aged mice who normally display severe deficits in forming bone.(21) DO is germane to the study of de novo intramembranous bone formation, because DO involves osteogenesis occurring exclusively as a consequence of osteoblast-mediated events, requiring no cartilaginous scaffolding or endochondral bone activity or remodeling. Thus, DO provides a pure model in which to study the actions of exogenous agents on osteoblast activity. The concept of combining both DO and sustained locally delivered or systemic administration of a growth factor by a miniosmopump in a mouse model has not been reported to our knowledge. Thus, these studies provide proof-of-concept that such an approach can be effectively used in a mouse model and that application of an agent (rhIGF-I in this instance) by miniosmopump can be used to assess the impact of the delivery route of the agent on bone development.
In our studies examining peripheral levels of human IGF-I in animals treated with local delivery of rhIGF-I, no IGF-I was detected in serum, suggesting that it was primarily retained within the gap and adjacent areas. This was also supported by observations of mice treated with methylene blue as a tracer. Why exogenous rhIGF-I did not penetrate beyond the regenerating bone tissue is not completely understood; however, both IGF-I and its homolog IGF-II are found in biological fluids and/or in tissue matrices bound to at least six distinct high affinity IGF binding proteins (IGFBPs).(34–38) Various studies have shown that bone cells produce most IGFBPs; however, two of the IGFBPs, IGFBP-4 and IGFBP-5, have clearly been shown to play important and possibly unique roles in controlling IGF action in bone. Specifically, IGFBP-4 is the principal IGFBP produced by human bone cells and inhibits IGF-mediated proliferation of osteoblasts.(2,39) In contrast to IGFBP-4, numerous studies have shown that IGFBP-5 can enhance IGF action, especially in human and rodent osteoblasts.(2,18,40–45) IGFBP-5 seems unique among the IGFBPs in that it is found in great quantity in human bone tissues where it functions to store IGFs.(42) IGFBP-5 has been shown to bind several extracellular matrix (ECM) molecules prominent in bone,(46,47) and it is likely that through this mechanism IGFBP-5 provides a “trapping” mechanism for extracellular matrix IGFs. Thus, in the model presented herein, it is possible that IGF-I administered to the gap or reaching the gap from the circulation is sequestered by matrix-bound IGFBPs, and then released in a regulated manner to interact with IGF receptors. We and others have shown that various proteases, including matrix metalloproteinases (MMPs), can degrade IGFBPs and can liberate IGFs, resulting in activation of the IGF receptor.(48–51) Interestingly, recent data also show that, throughout DO, MMPs are significantly upregulated, thereby providing a mechanism by which IGFBP-bound IGF-I could be released throughout the distraction period.(52)
Together, these studies support a role for both locally delivered and systemic IGF-I in osteoblastogenesis and bone formation in the aged mouse. We have tested these hypotheses using DO to determine if osteoblastogenesis and bone regeneration can be positively affected by rhIGF-I delivered either though a local or systemic route. In these studies, rhIGF-I delivered by either route and using the same dosing strategy, achieved a similar result. Whereas rhIGF-I was measurable in the serum of the majority of mice receiving systemic rhIGF-I, it is possible that IGF-I could have been shuttled preferentially to the regenerating bone, achieving a similar goal as that of local delivery. Whereas the studies as designed do not specifically address this possibility, the lack of suppression of endogenous IGF-I and no increase in osteocalcin levels in mice treated with systemic rhIGF-I suggest little impact of this dose and delivery route on peripheral tissues. Thus, the two routes of rhIGF-I delivery might provide for a similar result in regards to bone formation. Indeed, systemic administration of IGF-I to animals and humans has resulted in improved bone homeostasis(53–55); however, systemic administration of IGF-I can result in hypoglycemia,(56,57) and there is also a strong positive correlation between circulating IGF-I levels and the risk for breast, prostate, and colon cancer,(58) all tumorigenic conditions associated with the aging population. Therefore, our findings that locally delivered IGF-I improves bone formation and regeneration without increasing circulating IGF-I levels in an aged animal model may hold promise for the treatment of senile bone disease with IGF-I without the potential side effects of systemic IGF-I administration.
Acknowledgments
We acknowledge the excellent technical assistance of Robert A Skinner and the generous gifts of the rodent and human IGF-I kits from IDS and Susan Durham. The authors thank Genentech for supplying the rhIGF-I. This work was supported by a grant from the National Institutes of Health (R01 DK055653 to JLF) to the Arkansas Children’s Hospital Research Institute and a grant from the Arkansas Biosciences Institute (to JLF), the major research component of the Tobacco Settlement Proceeds Act of 2000.
Footnotes
The authors state that they have no conflicts of interest.
References
- 1.Margolis RN, Canalis E, Partridge NC. Invited review of a workshop: Anabolic hormones in bone: Basic research and therapeutic potential. J Clin Endocrinol Metab. 1996;81:872–877. doi: 10.1210/jcem.81.3.8772542. [DOI] [PubMed] [Google Scholar]
- 2.Mohan S, Baylink DJ. Insulin-like growth factor system components and the coupling of bone formation to resorption. Horm Res. 1996;45(Suppl 1):59–62. doi: 10.1159/000184833. [DOI] [PubMed] [Google Scholar]
- 3.Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 4.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 5.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 6.Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16:2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- 7.Miyakoshi N, Qin X, Kasukawa Y, Richman C, Srivastava AK, Baylink DJ, Mohan S. Systemic administration of insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) increases bone formation parameters in mice by increasing IGF bioavailability via an IGFBP-4 protease-dependent mechanism. Endocrinology. 2001;142:2641–2648. doi: 10.1210/endo.142.6.8192. [DOI] [PubMed] [Google Scholar]
- 8.Sjogren K, Sheng M, Moverare S, Liu JL, Wallenius K, Tornell J, Isaksson O, Jansson JO, Mohan S, Ohlsson C. Effects of liver-derived insulin-like growth factor I on bone metabolism in mice. J Bone Miner Res. 2002;17:1977–1987. doi: 10.1359/jbmr.2002.17.11.1977. [DOI] [PubMed] [Google Scholar]
- 9.Wong GL, Kotliar D, Schlaeger D, Brandes SJ. IGF-I production by mouse osteoblasts. J Bone Miner Res. 1990;5:133–140. doi: 10.1002/jbmr.5650050206. [DOI] [PubMed] [Google Scholar]
- 10.Thrailkill KM, Siddhanti SR, Fowlkes JL, Quarles LD. Differentiation of MC3T3-E1 osteoblasts is associated with temporal changes in the expression of IGF-I and IGFBPs. Bone. 1995;17:307–313. doi: 10.1016/8756-3282(95)00223-z. [DOI] [PubMed] [Google Scholar]
- 11.Slootweg MC, van Buul-Offers SC, Hoogerbrugge CM, Herrmann-Erlee MP, van den Eijndenvan Raaij AJ, Duursma SA, van Zoelen EJ. Characterization of growth factor activity produced by fetal mouse osteoblasts. J Endocrinol. 1990;124:301–309. doi: 10.1677/joe.0.1240301. [DOI] [PubMed] [Google Scholar]
- 12.Mohan S, Baylink DJ. Bone growth factors. Clin Orthop. 1991;263:30–48. [PubMed] [Google Scholar]
- 13.Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989;83:60–65. doi: 10.1172/JCI113885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swolin D, Brantsing C, Matejka G, Ohlsson C. Cortisol decreases IGF-I mRNA levels in human osteoblast-like cells. J Endocrinol. 1996;149:397–403. doi: 10.1677/joe.0.1490397. [DOI] [PubMed] [Google Scholar]
- 15.Slootweg MC, Hoogerbrugge CM, de Poorter TL, Duursma SA, van Buul-Offers SC. The presence of classical insulin-like growth factor (IGF) type-I and -II receptors on mouse osteoblasts: Autocrine/paracrine growth effect of IGFs? J Endocrinol. 1990;125:271–277. doi: 10.1677/joe.0.1250271. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki R, Conover CA, Harris SA, Spelsberg TC, Riggs BL. Normal human osteoblast-like cells consistently express genes for insulin-like growth factors I and II but transformed human osteoblast cell lines do not. J Bone Miner Res. 1995;10:788–795. doi: 10.1002/jbmr.5650100516. [DOI] [PubMed] [Google Scholar]
- 17.Kanzaki S, Baxter RC, Knutsen R, Baylink DJ, Mohan S. Evidence that human bone cells in culture secrete insulin-like growth factor (IGF)-II and IGF binding protein-3 but not acid-labile subunit both under basal and regulated conditions. J Bone Miner Res. 1995;10:854–858. doi: 10.1002/jbmr.5650100605. [DOI] [PubMed] [Google Scholar]
- 18.Schmid C, Schlapfer I, Gosteli-Peter MA, Froesch ER, Zapf J. Expression, effects, and fate of IGFBP-5 are different in normal and malignant osteoblastic cells. Prog Growth Factor Res. 1995;6:167–173. doi: 10.1016/0955-2235(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 19.Bautista CM, Baylink DJ, Mohan S. Isolation of a novel insulin-like growth factor (IGF) binding protein from human bone: A potential candidate for fixing IGF-II in human bone. Biochem Biophys Res Commun. 1991;176:756–763. doi: 10.1016/s0006-291x(05)80249-9. [DOI] [PubMed] [Google Scholar]
- 20.Mohan S, Baylink DJ. Serum insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5 levels in aging and age-associated diseases. Endocrine. 1997;7:87–91. doi: 10.1007/BF02778070. [DOI] [PubMed] [Google Scholar]
- 21.Aronson J, Liu L, Liu Z, Gao GG, Perrien DS, Brown EC, Skinner RA, Thomas J, Morris K, Suva L, Badger TM, Lumpkin CK., Jr Decreased endosteal intramembranous bone formation accompanies aging in a mouse model of distraction osteogenesis. J Regenerative Med. 2002;3:7–16. [Google Scholar]
- 22.Aronson J, Shen XC, Gao GG, Miller F, Quattlebaum T, Skinner RA, Badger TM, Lumpkin CK., Jr Sustained proliferation accompanies distraction osteogenesis in the rat. J Orthop Res. 1997;15:563–569. doi: 10.1002/jor.1100150412. [DOI] [PubMed] [Google Scholar]
- 23.Perrien DS, Brown EC, Aronson J, Skinner RA, Montague DC, Badger TM, Lumpkin CK., Jr Immunohistochemical study of osteopontin expression during distraction osteogenesis in the rat. J Histochem Cytochem. 2002;50:567–574. doi: 10.1177/002215540205000414. [DOI] [PubMed] [Google Scholar]
- 24.Thrailkill KM, Liu L, Wahl EC, Bunn RC, Perrien DS, Cockrell GE, Skinner RA, Hogue WR, Carver AA, Fowlkes JL, Aronson J, Lumpkin CK. Bone Formation Is Impaired in a Model of Type 1 Diabetes. Diabetes. 2005;54:2875–2881. doi: 10.2337/diabetes.54.10.2875. [DOI] [PubMed] [Google Scholar]
- 25.Aronson J. Modulation of distraction osteogenesis in the aged rat by fibroblast growth factor. Clin Orthop Relat Res. 2004;425:264–283. doi: 10.1097/01.blo.0000138186.53426.f9. [DOI] [PubMed] [Google Scholar]
- 26.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouxsein ML, Rosen CJ, Turner CH, Ackert CL, Shultz KL, Donahue LR, Churchill G, Adamo ML, Powell DR, Turner RT, Muller R, Beamer WG. Generation of a new congenic mouse strain to test the relationships among serum insulin-like growth factor I, bone mineral density, and skeletal morphology in vivo. J Bone Miner Res. 2002;17:570–579. doi: 10.1359/jbmr.2002.17.4.570. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 29.Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted over-expression of insulin-like growth factor I to osteoblasts of transgenic mice: Increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, Lichtler AC, Gronowicz GA, Adams DJ, Clark SH, Rosen CJ, Kream BE. Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone. 2006 doi: 10.1016/j.bone.2006.02.068. in press. [DOI] [PubMed] [Google Scholar]
- 31.Schmidmaier G, Wildemann B, Bail H, Lucke M, Fuchs T, Stemberger A, Flyvbjerg A, Haas NP, Raschke M. Local application of growth factors (insulin-like growth factor-1 and transforming growth factor-beta1) from a biodegradable poly(D,L-lactide) coating of osteosynthetic implants accelerates fracture healing in rats. Bone. 2001;28:341–350. doi: 10.1016/s8756-3282(00)00456-7. [DOI] [PubMed] [Google Scholar]
- 32.Wakisaka A, Tanaka H, Barnes J, Liang CT. Effect of locally infused IGF-I on femoral gene expression and bone turnover activity in old rats. J Bone Miner Res. 1998;13:13–19. doi: 10.1359/jbmr.1998.13.1.13. [DOI] [PubMed] [Google Scholar]
- 33.Meinel L, Zoidis E, Zapf J, Hassa P, Hottiger MO, Auer JA, Schneider R, Gander B, Luginbuehl V, Bettschart-Wolfisberger R, Illi OE, Merkle HP, von Rechenberg B. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33:660–672. doi: 10.1016/s8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 34.Bach LA, Rechler MM. Insulin-like growth factor binding proteins. Diabetes Rev. 1995;3:38–61. [Google Scholar]
- 35.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: Biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 36.Oh Y, Nagalla SR, Yamanaka Y, Kim HS, Wilson E, Rosenfeld RG. Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7. Recombinant human mac25 protein specifically binds IGF-I and -II. J Biol Chem. 1996;271:30322–30325. doi: 10.1074/jbc.271.48.30322. [DOI] [PubMed] [Google Scholar]
- 37.Rechler MM. Insulin-like growth factor binding proteins. Vitamin Horm. 1993;47:1–114. doi: 10.1016/s0083-6729(08)60444-6. [DOI] [PubMed] [Google Scholar]
- 38.Rechler MM. Non-receptor-binding proteins for insulin-like growth factors and other cytokines: Modulators of peptide action. In: Weintraub BD, editor. Molecular Endocrinology: Basic Concepts and Clinical Correlations. Raven Press; New York, NY, USA: 1995. pp. 155–180. [Google Scholar]
- 39.Mohan S, Bautista CM, Wergedal J, Baylink DJ. Isolation of an inhibitory insulin-like growth factor (IGF) binding protein from bone cell-conditioned medium: A potential local regulator of IGF action. Proc Natl Acad Sci USA. 1989;86:8338–8342. doi: 10.1073/pnas.86.21.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andress DL, Birnbaum RS. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J Biol Chem. 1992;267:22467–22472. [PubMed] [Google Scholar]
- 41.Andress DL, Loop SM, Zapf J, Kiefer MC. Carboxytruncated insulin-like growth factor binding protein-5 stimulates mitogenesis in osteoblast-like cells. Biochem Biophys Res Commun. 1993;195:25–30. doi: 10.1006/bbrc.1993.2004. [DOI] [PubMed] [Google Scholar]
- 42.Campbell PG, Novak JF, Yanosick TB, McMaster JH. Involvement of the plasmin system in dissociation of the insulin-like growth factor-binding protein complex. Endocrinology. 1992;130:1401–1412. doi: 10.1210/endo.130.3.1371448. [DOI] [PubMed] [Google Scholar]
- 43.Conover CA, Kiefer MC. Regulation and biological effect of endogenous insulin-like growth factor binding protein-5 in human osteoblastic cells. J Clin Endocrinol Metab. 1993;76:1153–1159. doi: 10.1210/jcem.76.5.7684391. [DOI] [PubMed] [Google Scholar]
- 44.Hakeda Y, Kawaguchi H, Hurley M, Pilbeam CC, Abreu C, Linkhart TA, Mohan S, Kumegawa M, Raisz LG. Intact insulin-like growth factor binding protein-5 (IGFBP-5) associates with bone matrix and the soluble fragments of IGFBP-5 accumulated in culture medium of neonatal mouse calvariae by parathyroid hormone and prostaglandin E2-treatment. J Cell Physiol. 1996;166:370–379. doi: 10.1002/(SICI)1097-4652(199602)166:2<370::AID-JCP15>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 45.Mohan S, Nakao Y, Honda Y, Landale E, Leser U, Dony C, Lang K, Baylink DJ. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem. 1995;270:20424–20431. doi: 10.1074/jbc.270.35.20424. [DOI] [PubMed] [Google Scholar]
- 46.Nam TJ, Busby WH, Jr, Rees C, Clemmons DR. Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology. 2000;141:1100–1106. doi: 10.1210/endo.141.3.7386. [DOI] [PubMed] [Google Scholar]
- 47.Jones JI, Gockerman A, Busby WH, Jr, Camacho-Hubner C, Clemmons DR. Extracellular matrix contains insulin-like growth factor binding protein-5: Potentiation of the effects of IGF-I. J Cell Biol. 1993;121:679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fowlkes JL, Enghild JJ, Suzuki K, Nagase H. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem. 1994;269:25742–25746. [PubMed] [Google Scholar]
- 49.Fowlkes JL, Serra DM, Bunn RC, Thrailkill KM, Enghild JJ, Nagase H. Regulation of insulin-like growth factor (IGF)-I action by matrix metalloproteinase-3 involves selective disruption of IGF-I/IGF-binding protein-3 complexes. Endocrinology. 2004;145:620–626. doi: 10.1210/en.2003-0636. [DOI] [PubMed] [Google Scholar]
- 50.Fowlkes JL, Suzuki K, Nagase H, Thrailkill KM. Proteolysis of insulin-like growth factor binding protein-3 during rat pregnancy: A role for matrix metalloproteinases. Endocrinology. 1994;135:2810–2813. doi: 10.1210/endo.135.6.7527335. [DOI] [PubMed] [Google Scholar]
- 51.Thrailkill KM, Quarles LD, Nagase H, Suzuki K, Serra DM, Fowlkes JL. Characterization of insulin-like growth factor-binding protein 5-degrading proteases produced throughout murine osteoblast differentiation. Endocrinology. 1995;136:3527–3533. doi: 10.1210/endo.136.8.7543045. [DOI] [PubMed] [Google Scholar]
- 52.Carvalho RS, Einhorn TA, Lehmann W, Edgar C, Al-Yamani A, Apazidis A, Pacicca D, Clemens TL, Gerstenfeld LC. The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone. 2004;34:849–861. doi: 10.1016/j.bone.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 53.Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87:2883–2891. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- 54.Kasukawa Y, Stabnov L, Miyakoshi N, Baylink DJ, Mohan S. Insulin-like growth factor I effect on the number of osteoblast progenitors is impaired in ovariectomized mice. J Bone Miner Res. 2002;17:1579–1587. doi: 10.1359/jbmr.2002.17.9.1579. [DOI] [PubMed] [Google Scholar]
- 55.Boonen S, Rosen C, Bouillon R, Sommer A, McKay M, Rosen D, Adams S, Broos P, Lenaerts J, Raus J, Vanderschueren D, Geusens P. Musculoskeletal effects of the recombinant human IGF-I/IGF binding protein-3 complex in osteoporotic patients with proximal femoral fracture: A double-blind, placebo-controlled pilot study. J Clin Endocrinol Metab. 2002;87:1593–1599. doi: 10.1210/jcem.87.4.8426. [DOI] [PubMed] [Google Scholar]
- 56.Kemp SF, Thrailkill KM. SomatoKine: Is there a use in treating growth disorders? Curr Opin Investig Drugs. 2005;6:373–377. [PubMed] [Google Scholar]
- 57.Thrailkill KM. Insulin-like growth factor-I in diabetes mellitus: Its physiology, metabolic effects, and potential clinical utility. Diabetes Technol Ther. 2000;2:69–80. doi: 10.1089/152091599316775. [DOI] [PubMed] [Google Scholar]
- 58.Kaaks R. Nutrition, insulin, IGF-1 metabolism and cancer risk: A summary of epidemiological evidence. Novartis Found Symp. 2004;262:247–268. [PubMed] [Google Scholar]


