Abstract
The roles of the cerebellum and basal ganglia have typically been confined in the literature to motor planning and control. However, mounting evidence suggests that these structures are involved in more cognitive domains such as language processing. In the current study, we looked at effective connectivity (the influence that one brain region has on another) of the cerebellum and basal ganglia with regions thought to be involved in phonological processing, i.e. left inferior frontal gyrus and left lateral temporal cortex. We analyzed functional magnetic resonance imaging data (fMRI) obtained during a rhyming judgment task in adults using dynamic causal modeling (DCM). The results showed that the cerebellum has reciprocal connections with both left inferior frontal gyrus and left lateral temporal cortex, whereas the putamen has unidirectional connections into these two brain regions. Furthermore, the connections between cerebellum and these phonological processing areas were stronger than the connections between putamen and these areas. This pattern of results suggests that the putamen and cerebellum may have distinct roles in language processing. Based on research in the motor planning and control literature, we argue that the putamen engages in cortical initiation while the cerebellum amplifies and refines this signal to facilitate correct decision making.
Keywords: Cerebellum, basal ganglia, putamen, language, phonological, dynamic causal modeling, effective connectivity, initiation, amplification, refinement
Introduction
Houk proposed that the basal ganglia is involved in the embodiment (i.e. selection and/or initiation) of cortical patterns of activation for both planned behaviors and for thoughts (Houk, 2005). In contrast, he proposed that the cerebellum engages in online amplification and refinement of behaviors or thoughts as they are occurring, which provides an error correction mechanism for performance of the task. Cerebellar regulation of motor planning and control is well documented, particularly for reaching and acquiring targets (Thach, 1998). A coherent limb movement can be broken down into smaller component sub-movements, which include online error corrections (Barto et al., 1999; Fishbach et al., 2006; Ghez & Martin, 1982). Houk (2005) also proposed that the cerebellar role in refinement and amplification and the striatal role of embodiment could be involved in language processing. The current study is the first to look at effective connectivity of the cerebellum and basal ganglia during a language processing task. Both of these structures have connections with frontal regions and temporo-parietal regions thought to be involved in language processing in humans (Alexander et al., 1986; Clower et al., 2005; Dum & Strick, 2003; Middleton & Strick, 1994, 1996). In particular, the inferior frontal gyrus and lateral temporal cortex have been implicated in phonological processing (Bitan et al., 2005; Booth et al., 2002a).
It is being increasingly recognized that the cerebellum is involved in many cognitive processes including language processing (Desmond & Fiez, 1998). Studies using rhyming tasks to visually presented words have shown activation in bilateral cerebellum (Fulbright et al., 1999). Although some studies show that superior portion of the cerebellum is interconnected with lateral temporal cortex (Brodal, 1978; Schmahmann & Pandya, 1991; Vaudano et al., 1991), studies in primates show that the superior portion of the cerebellar hemisphere is predominantly interconnected with inferior frontal cortex, whereas the inferior portion is predominantly interconnected with parietal cortex (Brodal, 1978; Schmahmann & Pandya, 1997). Based on the role of the inferior frontal cortex in articulation and the parietal cortex in phonological short-term memory, Desmond and colleagues proposed that superior portion of right cerebellum (VI/Crus I) is involved in articulatory control, whereas the inferior portion (VII) of right cerebellum is involved in phonological working memory (Desmond et al., 1997). This was supported by their finding that superior cerebellum showed activation in articulation, rehearsal and verbal working memory, whereas inferior cerebellum only showed activation in verbal working memory (Chen & Desmond, 2005a; Desmond et al., 1997). In a subsequent event-related study that allowed an examination of the time course of activation, they showed that both encoding and maintenance was associated with activation in superior cerebellum (VI/Crus I), but that only maintenance was associated with activation in inferior cerebellum (VII/VIII) (Chen & Desmond, 2005b). They interpreted the superior activation as due to the need to rapidly translate the consonant string into an articulatory trajectory.
Several studies have shown that the basal ganglia is involved in various reading and language tasks. Greater accuracy of the detection of phonological anomalies is correlated with greater activation in left caudate nucleus and faster phonological processing is correlated with greater activation in left putamen (Tettamanti et al., 2005b). Detecting syntactical anomalies has also been associated with greater activation in left caudate nucleus (Moro et al., 2001). Abdullaev and Melnichuk (1997) placed depth electrodes in the head of the caudate nucleus in Parkinson’s patients to measure population neuronal firing rates when these patients were performing a variety of cognitive tasks (Abdullaev & Melnichuk, 1997). They showed increased firing within 400–600 ms after stimulus onset during semantic processing by comparing lexical decision to words versus pseudo-words and increased firing within 1000–1200 ms after stimulus onset during phonological processing by comparing lexical decision to pseudo-words to non-words. They also showed that early neuronal firing in semantic processing was replicated in a categorization task (concrete versus abstract judgment) and that this activation was not associated with motor output. This study provides provocative evidence that the basal ganglia is involved in language processing. Comparing this study to an earlier report from the same group suggests that activation in the caudate nucleus seems to lag behind activation in left inferior frontal gyrus (Bechtereva et al., 1991). In his procedural/declarative model of language learning, Ullman (2001) proposes that the basal ganglia is part of a procedural system that is involved in the assembly of phonemes into words (Ullman, 2001).
Functional neuroimaging studies aim to identify network components that are selectively engaged by cognitive tasks. However, a network could shift from one behavioral goal to another not because of differences in the distribution of activations, but because of differences in the interactions among its components (Damasio, 1989; McIntosh, 2000; M. M. Mesulam, 1981; M.M. Mesulam, 1998). Analyses of effective connectivity (the modulatory influence that one brain region exerts upon another), and its non-directional counterpart known as functional connectivity (based on correlation of brain activation between regions), have, in fact, shown that network components can display task-dependent alterations in their interactions (Chaminade & Fonlupt, 2003; Homae et al., 2003; Horwitz et al., 1998; McIntosh et al., 1994; K. R. Pugh et al., 2000). Components of distributed networks serve multiple roles including the integration of convergent inputs, the binding of distributed information, the relay of information from one region to another, and the control of neural activity within other network components (M.M. Mesulam, 1998). Some studies have examined effective connectivity of the basal ganglia and cerebellum with cortical regions, but none have examined language processing. The cerebellum has been shown to influence parietal cortex during visual/motor imagery (Solodkin et al., 2004) and prefrontal cortex during recognition tasks (Nyberg et al., 1996). The basal ganglia has been shown to influence prefrontal regions during classification learning (Poldrack & Rodriguez, 2004) and object location learning (Honey et al., 2003).
In a previous study with adults, we used dynamic causal modeling to show that the cognitive demands of the reading task affect patterns of effective connectivity (Bitan et al., 2005). A spelling task was marked by converging influence from other brain regions on the intraparietal sulcus, whereas a rhyming task was marked by converging influence on the lateral temporal cortex, suggesting that these regions are sites of integration for processing task-selective information. In both tasks, modulating influences also converge on inferior frontal gyrus. We proposed that inferior frontal gyrus is involved in top-down modulation of task-selective regions in a way that differentially enhances their sensitivity to task relevant information. The goal of the current study was to examine the role of the basal ganglia and cerebellum in modulating cortical regions thought to be involved in phonological processing, i.e. left inferior frontal cortex and left lateral temporal cortex. Adults made rhyming judgments to words while undergoing functional magnetic resonance imaging (fMRI). In this task, three visual words were presented one after the other (e.g. hold-milk-cold, door-hope-soap, house-press-list) and the participant had to press a button indicating whether the final word rhymed with either of the previous two. Activation during rhyming judgment blocks was compared to line judgment blocks (e.g.//-\\-//) in which participants had to determine whether the final group of lines was the same as either of the previous two. We chose to use a rhyming task because several previous studies have consistently implicated left inferior frontal gyrus and left superior temporal gyrus in phonological processing (Crosson et al., 1999; Kareken et al., 2000; Lurito et al., 2000; Paulesu et al., 1996; Kenneth R. Pugh et al., 1996; Rumsey et al., 1992; Xu et al., 2001). Rhyming judgment in the visual modality is a relatively complex task that involves decoding the orthographic stimuli, holding the phonological/articulatory information in working memory, and making an explicit determination of whether words rhyme. In order to examine the effective connectivity between regions, we used Dynamic Causal Modeling (Friston et al., 2003). If, as proposed by Houk (2005), the basal ganglia is involved in initiating cortical activation related to phonological processing, we expected the basal ganglia to have stronger input to inferior frontal gyrus and lateral temporal cortex than their reciprocal output. If, as proposed by Houk (2005), the cerebellum is involved in amplifying and refining cortical activation related to phonological processing, we expected it to have significant input and reciprocal output.
Results
The mean accuracy of performance was 97% for the rhyming trials and 97% for the control trials. The mean reaction time was 954 ms for the rhyming trials, and 805 ms for the control trials. Reaction time was slower for the rhyming trials compared to the control trials (t(13) = 6.5, p < 0.001), but there were no significant differences in accuracy (t(13) = .88, p = .40).
Conventional Analysis
Figure 1 and Table 1 present the patterns of activation in the rhyming trials compared to control trials. The group maxima of clusters (uncorrected p<0.001, extent threshold: 45 voxels) were used as reference for choosing the individual regions of interest (ROIs, see Figure 2). These clusters included left middle/inferior frontal gyri (IFG: BA 46/45/9), left fusiform gyrus (FG: BA 19/37), left superior/middle temporal gyri (LTC: BA 21/22), cerebellum (VI/Crus I) and putamen.
Figure 1.
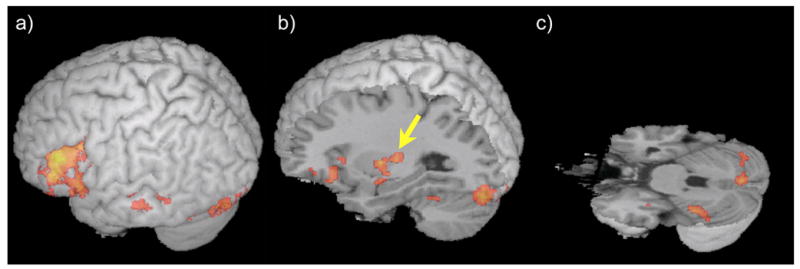
Group mean activation for words-lines condition in (a) IFG, LTC and FG (b) putamen (indicated by arrow) and (c) cerebellum (p < .001 uncorrected, > 45 voxels).
Table 1.
Group mean activation for the rhyming task
| z | |||||||
|---|---|---|---|---|---|---|---|
| Region | BA | H | score | voxels | x | y | z |
| Inferior/Middle Frontal Gyri | 46/45/9 | L | 5.15 | 850 | −51 | 30 | 21 |
| Fusiform Gyrus | 19/37 | L | 4.72 | 321 | −45 | −60 | −21 |
| Superior/Middle Temporal Gyri | 21/22 | L | 3.71 | 47 | −66 | −36 | −3 |
| Putamen | -- | L | 4.86 | 140 | −30 | −15 | −6 |
| Cerebellum | -- | R | 4.25 | 73 | 12 | −75 | −30 |
Note. BA – Brodmann’s Area; L: left hemisphere; R: right hemisphere. Significance level was set at p < .001 uncorrected, > 45 voxels.
Figure 2.
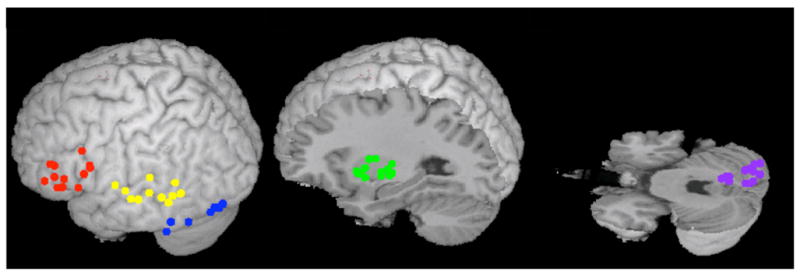
Center of ROIs in individual participants. Red - IFG, Yellow -LTC, Blue - FG, Green - putamen, Purple - cerebellum.
Effective Connectivity Analysis
Intrinsic connections
Figure 3 shows that all intrinsic influences among regions (i.e., connections that are independent of the task) were significant, except for the connection from cerebellum to FG.
Figure 3.
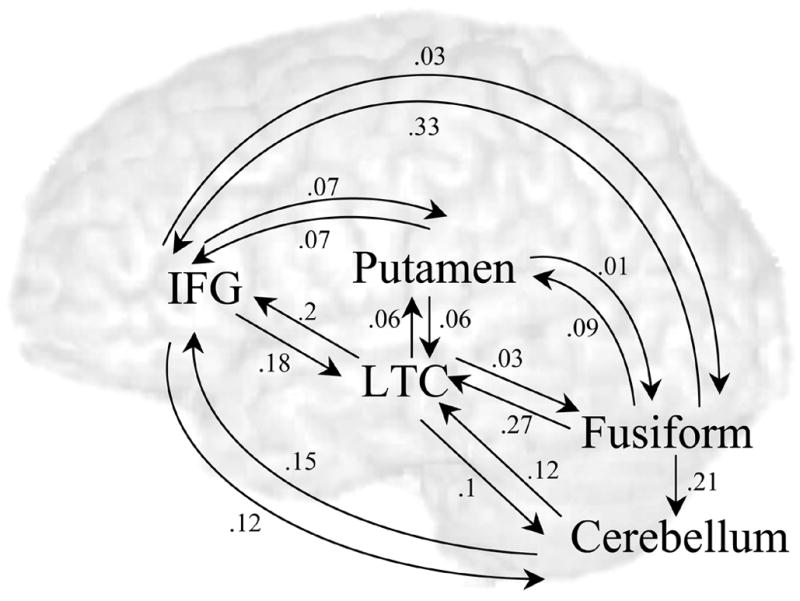
Intrinsic connections. Averaged strengths of influences across individuals are presented. Strengths with p-value of < .05 (corrected for 18 comparisons) are displayed.
Modulatory effects
Modulatory effects between regions are depicted in Figure 4. The modulatory effects between IFG and LTC were both significant, suggesting that these areas form a loop involved in phonological processing (into IFG: t(13) = 7.11, p < .001; out to LTC: t(13) = 5.92, p < .001). However, the focus of this paper is on connections into and out of the cerebellum and putamen.
Figure 4.
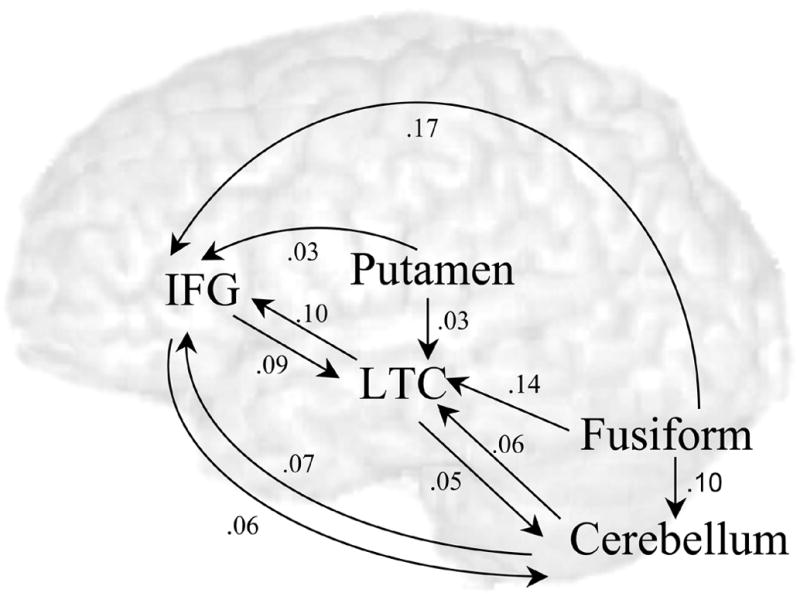
Modulatory effects of the rhyming task on the language network. Averaged strengths of effects across individuals are presented. Strengths with p-value of < .05 (corrected for 18 comparisons) are displayed.
The first main finding is that cerebellum had reciprocal modulatory effects with the phonological processing areas (into LTC: t(13) = 6.08, p < .001; out from LTC: (t(13) = 7.45, p < .001; into IFG: t(13) = 8.87, p < .001; out from IFG (t(13) = 8.50, p < .001)). There were no significant differences in modulatory input from IFG and LTC to cerebellum (t(13) = 2.34, p = .036) or modulatory output to these regions (t(13) = 1.70, p = .113). Modulatory output from cerebellum to IFG was not significantly different than its reciprocal input (t(13) = 2.93, p = .012) and modulatory output from cerebellum to LTC was not significantly different than its reciprocal input (t(13) = .61, p = .555).
The second main finding was that the putamen had significant modulatory output to the areas involved in phonological processing (IFG: t(13) = 6.45, p < .001 ; LTC: t(13) = 4.56, p = .001). Modulatory output to the IFG and LTC was not significantly different (t(13) = 1.14, p = .276). Modulatory input from these areas to putamen was not significant (IFG: t(13) = 1.89, p = .08; LTC: t(13) = 1.88, p = .08). It should be noted that there were significant intrinsic connections into the putamen from the IFG and LTC, but that these connections were not modulated differently for the rhyming compared to the control trials.
The third main finding was that the coupling with phonological processing areas was stronger for cerebellum than for putamen. Modulatory output from cerebellum to IFG was stronger than modulatory output from putamen to IFG (t(13) = 5.58, p < .001). Modulatory output from cerebellum to LTC was stronger than modulatory output from putamen to LTC (t(13) = 5.30, p < .001). Modulatory input to cerebellum from IFG was stronger than modulatory input to putamen from IFG (t(13) = 4.37, p = .001). Modulatory input to cerebellum from LTC was stronger than modulatory input to putamen from LTC (t(13) = 4.52, p = .001).
The fourth main finding was that modulatory input from FG to the putamen was not significant, (t(13) = 3.30, p = .006), but modulatory input from FG to the cerebellum was significant (t(13) = 8.83, p < .001).
We performed an exploratory analysis that examined correlations between the significant modulatory effects (p < .05, corrected for 52 correlations). Figure 5 presents these correlations. All of the significant correlations involved input to or output from the cerebellum. Output from cerebellum to LTC was correlated with its reciprocal input (r(13) = 0.869, p < .001) and output from cerebellum to IFG was correlated with its reciprocal input (r(13) = 0.901, p < .001). Output from the cerebellum to LTC was correlated with output from IFG to LTC (r(13) = 0.936, p < .001) and also with output from putamen to LTC (r(13) = 0.883, p < .001). Output from LTC to cerebellum was correlated with output from IFG to cerebellum (r(13) = 0.850, p < .001) and also with output from IFG to LTC (r(13) = 0.785, p < .001).
Figure 5.
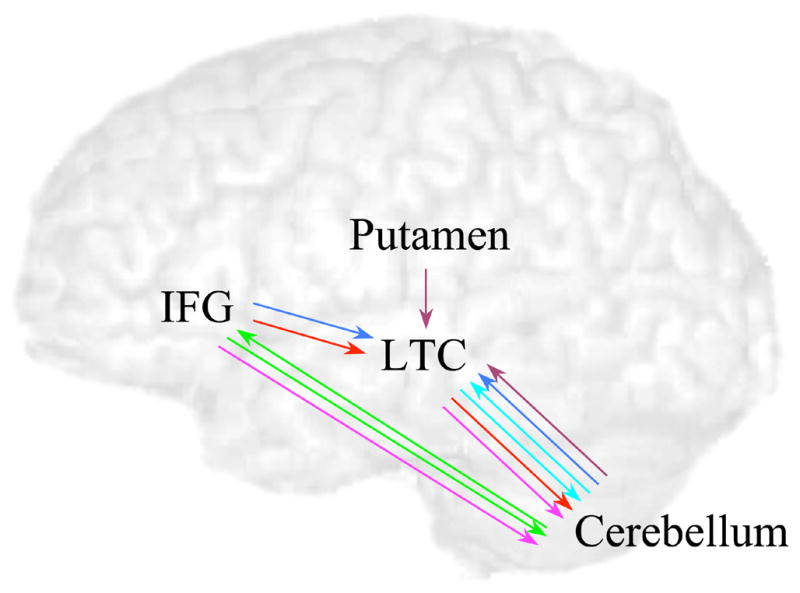
Correlations between modulatory effects. Arrows of the same color indicate that these modulatory connections were significantly correlated (p < .05, corrected for 52 comparisons).
Discussion
This study examined the role of the cerebellum and basal ganglia in language processing during a rhyming task presented in the visual modality. We used Dynamic Causal Modeling (DCM) to look at the directional influence of one brain region on another. We found that the cerebellum had reciprocal modulatory influences with brain regions involved in phonological processing (i.e. inferior frontal gyrus and lateral temporal cortex), whereas putamen only had unidirectional modulatory influences to these regions. Modulatory influences between cerebellum and these phonological processing areas were stronger than modulatory influences between putamen and these areas. Furthermore, correlations between modulatory influences all involved the cerebellum’s connections with these phonological processing areas. Finally, fusiform gyrus had strongest output to cerebellum and no output to putamen.
The finding of significant activation in and effective connectivity with the basal ganglia and cerebellum is consistent with other studies that have found activation in the cerebellum and basal ganglia in reading and language tasks (Abdullaev & Melnichuk, 1997; Chen & Desmond, 2005a, 2005b; Desmond et al., 1997; Moro et al., 2001; Tettamanti et al., 2005b). Our region of interest was in lobe VI/Crus I of the cerebellum. Based on a series of functional imaging studies, Desmond and colleagues have suggested that this region of the cerebellum is involved in articulatory control (Chen & Desmond, 2005a, 2005b; Desmond et al., 1997). Interestingly, we did not find activation in the caudate region of the basal ganglia, but rather only the putamen. Tettamanti et. al (2005) showed that speed of phonological processing is correlated with tracer binding potential in left putamen (Tettamanti et al., 2005b). A study using diffusion tensor imaging recently showed that the putamen is primarily connected to motor and pre-motor regions as well as posterior regions of the prefrontal cortex (Lehericy et al., 2004). These posterior regions of the frontal cortex have been associated with phonological segmentation and articulatory control (Bitan et al., submitted; Poldrack et al., 1999). Both the cerebellum and the basal ganglia may be involved in modulation of articulatory or phonological output representations in order to perform our rhyming task. Indeed, the basal ganglia may part of a procedural system that is involved in the assembly of phonemes into higher order structures such as words (Ullman, 2001).
The motor theory of speech argues that both perception and production of speech rely on articulatory gestures in that auditory and motor representations are already phonetic (Alvin M. Liberman & Mattingly, 1985; A. M. Liberman & Whalen, 2000). This theory further argues that letters also correspond to these articulatory gestures in that reading involves that activation of these representations. The motor theory of speech is consistent with studies showing that monkeys discharge the same neurons in motor cortex both when a sound is perceived and when it is executed (Kohler et al., 2002) and with studies showing that humans activate the same brain regions in motor cortex both when observing actions and when listening to action sentences (Tettamanti et al., 2005a). In their DIVA model speech acquisition and production, Guenther and colleagues have also argued that auditory cortex is not only involved in speech perception, but is also involved in planning movement trajectories based on acoustic and orosensory feedback as well as an efference copy of the motor command (Guenther et al., 1998; Nieto-Castanon et al., 2005). Based on this research, one would predict that both the superior temporal cortex and inferior frontal gyrus are involved articulatory planning and control. Thus, it is possible that not only the connections of the cerebellum and basal ganglia with inferior frontal gyrus are involved in articulatory processing, but also the connections of these regions with superior temporal gyrus are involved in articulatory processing.
Our study also showed that the cerebellum had bidirectional connections with left inferior frontal gyrus and left lateral temporal cortex, but that the basal ganglia had unidirectional connections into these cortical regions. Both left lateral temporal cortex and left inferior frontal gyrus are involved in phonological processing (Booth et al., 2002a, 2002b; Poldrack et al., 1999), so the cerebellum may be involved in amplifying and refining the patterns of activation in these regions through recurrent loops (Houk, 2005). The amplification is caused by positive feedback in the loop between cortex and the cerebellar nucleus. Purkinje cells in the cerebellar cortex burst or pause to shape the spatial pattern of activity – the bursts inhibit positive feedback whereas the pauses allow it to become stronger (Houk & Mugnaini, 2003). During limb movements (or cognitive processing), the majority of Purkinje cells burst while a significant minority pause (Miller et al., 2002). In essence, the bursts are preventing erroneous cortical activity whereas the pauses are allowing specific cortical neurons to become very active. Bidirectional communication between cortex and cerebellum is required for these operations.
In contrast to the bi-directional connections involving the cerebellum, the basal ganglia (putamen) showed only outgoing unidirectional connections into left inferior frontal gyrus and left lateral temporal cortex. This is consistent with the hypothesis that the basal ganglia may be involved in cortical initiation of phonological representations in these structures. Indeed, Houk and colleagues (in press) have argued that the basal ganglia is involved in generating a ‘ballpark’ estimate of motor programs that are amplified/refined by the cerebellum (Houk et al., in press). Houk et al’s (in press) model is consistent with the obervation that Parkinson patients with degeneration of the basal ganglia show motor initiation deficits (Liu et al., 2006; Rosin et al., 1997), where patients with cerebellar lesions exhibit dysmetria which is a disorder in the ability to fine-tune motor and cognitive processes (Botzel et al., 1993; Schmahmann, 2004). It is important to clarify that our results do not show a lack of feedback from cortex to putamen during the task, because the intrinsic connections from these cortical regions to the putamen were significant. The lack of significant modulatory effects into putamen shows that input was not differentially modulated in the rhyming task compared to the control task (line judgment).
Our suggestion that the cerebellum is involved in amplification and refinement, whereas the basal ganglia is involved cortical initiation of phonological representations is consistent with two of our findings. Our first finding was that the connections of the cerebellum with left inferior frontal gyrus and lateral temporal cortex are stronger than the connections of the putamen with these regions. Presumably, amplifying and refining phonological representations would require greater connectivity than aiding in a ‘ball-park’ estimate of these representations. This hypothesis is consistent with the Houk (2005) model of limb movement control. The loop through the basal ganglia selects a small focus of cortical activity that is subsequently amplified and refined by the dual-loop through the cerebellum in order to generate a strong and accurate composite motor command. Our second finding was that correlations between modulatory effects all involved the cerebellum. For example, if an individual showed a strong modulatory effect out from cerebellum to regions involved in phonological processing, s/he tended to show strong modulatory input from these regions. This finding is consistent with the hypothesis that the cerebellum forms recriprocal loops with cortical regions and is involved in amplification and refinement of representations.
Because the rhyming task was presented in the visual modality, we specified input to the model into the fusiform gyrus. The putamen did not have significant modulatory connections with the fusiform gyrus. However, the cerebellum had significant input from the fusiform gyrus and this input was stronger from the fusiform gyrus than input from any other region, suggesting that the cerebellum is involved in processing orthographic representations. This could happen in at least two ways. Either the cerebellum is involved in mapping orthographic information in the fusiform gyrus to phonological information in left inferior frontal gyrus and left lateral cortex or the cerebellum is actually using that information in the amplification and refinement process. Our study design does not allow us to distinguish between these two possibilities.
Our results are consistent with the mounting evidence that the basal ganglia and cerebellum are involved in reading and language processing in normal populations. They are also consistent with studies that have found abnormal cerebellar morphology or activity in patients with developmental dyslexia and language impairment. Structural MRI studies have shown that the right anterior lobe of the cerebellum is smaller in children with developmental dyslexia and is correlated with reading performance (Eckert et al., 2003). Another structural MRI study reported smaller cerebellar grey matter asymmetry in adults with developmental dyslexia and that smaller asymmetry was correlated with poorer reading performance (Rae et al., 2002). fMRI studies have shown less activation in the right cerebellum in adults with developmental dyslexia when executing pre-learned and new motor sequences (Nicolson et al., 1999) and in word and pseudoword reading tasks relying on phonological processing (Brunswick et al., 1999; Paulesu et al., 1996). Functional connectivity studies show less connectivity of the cerebellum with angular gyrus and inferior frontal gyrus during reading tasks in adults with developmental dyslexia (Horwitz et al., 1998; Stanberry et al., 2006). Less research has shown cerebellar abnormalities in patients with language impairment, but inherited verbal and orofacial dyspraxia has been reported to be associated with reduced grey matter density in bilateral cerebellum (Belton et al., 2003; Vargha-Khadem et al., 1998; Watkins et al., 2002). Studies on patients with developmental dyslexia and language impairment have also shown abnormalities in the basal ganglia. Adults with developmental dyslexia have been reported to show less activation in word and pseudoword reading tasks relying on phonological processing (Brunswick et al., 1999; Paulesu et al., 1996). Inherited verbal and orofacial dyspraxia is associated with more grey matter in bilateral putamen (Watkins et al., 2002) and underactive bilateral putamen (Liegeois et al., 2003).
In conclusion, the strong bi-directional connections in and out of the cerebellum with areas believed to be involved in phonological processing (i.e. left inferior frontal gyrus and left lateral temporal cortex) is consistent with the hypothesis that the cerebellum is involved in amplification and refinement of these representations. The weaker unidirectional connections out from putamen to these phonological processing areas is consistent with the hypothesis that this region is involved in the cortical initiation of these representations. These results are consistent with Houk’s (2005) model of the cerebellum and basal ganglia in motor control and consistent with the growing body of literature from normal and patient populations suggesting the integral role of these subcortical brain regions in reading and language.
Experimental Procedure
Participants
Fourteen adults, 5 males and 9 females, ages 21–36, (mean age 26) participated in this study. A subset of these participants were included in Bitan et al (2005). All participants were right-handed, native English speaking university students with no diagnosed neurological/psychiatric disorders or language/reading disabilities.
Stimuli and Task
Participants were scanned while performing a rhyming task in which a series of three target words appeared sequentially. Sixty percent of the trials contained a final word that rhymed with one of the preceding words. Half of these trials contained a target word that rhymed and was orthographically similar to one of the preceding two words (i.e., had the same rime, e.g. hold-cold). The other half contained a target word that rhymed but was orthographically dissimilar to one of the preceding two words (e.g. hope-soap). The experimental set-up for the control trials was exactly the same as for the word trials, except the three stimuli were abstract, non-linguistic symbols consisting of straight lines (e.g. \ \). For the rhyming and control trials, half involved a match to the first stimulus and half involved a match to the second stimulus. Participants were asked to determine whether the final stimulus rhymed/matched either of the first two stimuli by pressing one of two buttons.
Experimental Procedure
The task was administered over a 9-minute run that consisted of 10 blocks of 54 seconds in which 5 rhyming blocks alternated with 5 control blocks. In each trial for the rhyming and control blocks, three consecutive stimuli were presented, each for 800ms followed by a 200ms blank interval. Participants had 2000ms to respond after the presentation of the three stimuli. Each trial lasted a total of 5000ms. Each block began with a 4 seconds instruction (rhyming, lines) followed by 10 trials.
fMRI Data Acquisition
Images were acquired using a 1.5 Tesla General Electric (GE) scanner with ecoplanar imaging (EPI) method. The scanning parameters were: Time of repetition (TR) = 3000 ms, time of echo (TE) = 40 ms, flip angle = 90°, matrix size = 64 × 64, field of view = 22 cm, slice thickness = 4 mm, number of slices = 32. These scanning parameters resulted in a 3.437 × 3.437 × 4mm voxel size and 180 whole brain volumes. A high resolution, T1 weighted 3D image was also acquired (SPGR, TR = 21 ms, TE = 8 ms, flip angle = 20°, matrix size=256 × 256, field of view = 22 cm, slice thickness = 1 mm, number of slices = 124). Placement of slices began at the most superior portion of the cortex, so there was incomplete coverage of the most inferior portion of the cerebellum (below Crus II or the inferior semilunar lobule).
Image Data Analysis
Data analysis was performed using Statistical Parametric Mapping (SPM2, http://www.fil.ion.ucl.ac.uk/spm). The images were spatially realigned to the first volume to correct for head movements. No individual runs had more than 2.5 mm maximum displacement. Sinc interpolation was used to minimize timing-errors between slices. The functional images were coregistered with the anatomical image, and normalized to the standard T1 template volume (MNI). The data was then smoothed with a 7mm isotropic Gaussian kernel.
Statistical analyses at the first level were calculated using an epoch-based design, with the rhyming and control blocks as conditions of interest. A high pass filter with a cutoff period of 256 seconds was applied. Group results were obtained using random-effects analyses by combining subject-specific summary statistics across the group. The group results were then used for choosing the regions of interest for the effective connectivity analysis. In order to identify the most robustly activated regions of interest, only clusters larger than 45 voxels were included. In order to simplify the network, and due to the strong asymmetry in the activation clusters and well documented laterality of language processes, only left hemisphere neocortical clusters were included. This process resulted in three ROIs in left neocortex: fusiform gyrus (FG), inferior frontal gyrus (IFG), and lateral temporal cortex (LTC). We also included two subcortical regions: left putamen and right cerebellum. Within the cerebellum, lobe VI/Crus I were activated by the rhyming task (see Table 1 and Figure 1).
Effective Connectivity
Five regions of interest were specified for each individual: FG, IFG, LTC, putamen and cerebellum. Regional responses were summarized as the principal eigenvariates of responses within a 6 mm sphere centered on the most significant voxel for each subject. For the putamen ROI, a mask was used including only the putamen to ensure that activation was confined to this anatomically distinct area. Subject-specific maxima were defined operationally as the most significant voxels within 22mm of the group maximum in the appropriate statistical parametric map. Figure 2 shows the ROI’s for each subject.
Effective connectivity analysis was performed using the Dynamic Causal Modeling (DCM) tool in SPM2 (Friston et al., 2003; Penny et al., 2004). Dynamic causal modeling (DCM) is a nonlinear systems identification procedure that uses Bayesian estimation of parameters to make inferences about effective connectivity between neural systems and how this connectivity is affected by experimental conditions. In DCM, three sets of parameters are estimated: the direct influence of stimuli on regional activity; the intrinsic or latent connections between regions (i.e., the interregional influences in the absence of modulating experimental effects); and the changes in the intrinsic connectivity between regions induced by the experimental design (modulatory effects) (Mechelli et al., 2003). Since ‘connectivity’ in DCM is measured through the coupling of changes in imaging signals, rather than anatomically, a significant unidirectional modulatory influence of one brain region upon another does not necessarily reflect the presence of a direct and unidirectional anatomical connection. Instead, the connectivity revealed by DCM reflects the inferred direction of neural influences that are specific to the imaging conditions and that may be mediated through inter-neurons or other brain regions not explicitly included in the model.
The analysis adopted a two-stage procedure that is formally identical to the summary statistic approach used in random effects analysis of neuroimaging data. The parameters from the subject-specific, first level DCM models were taken to a second, between-subject level using the random effects approach (Bitan et al., 2005). Subject-specific DCMs were assumed to be fully connected with the exception of connection between the putamen and the cerebellum, resulting in 18 connections. The modulatory (bilinear) effects of the rhyming task were specified on the connections among all regions. Input was specified into FG.
One-sample t-tests (p < .05, corrected for 18 comparisons) were used to test for the intrinsic or modulatory effects. We also had planned comparisons on output from versus input to cerebellum or basal ganglia (4 comparisons), on output from the cerebellum versus basal ganglia to LTC or IFG (2 comparisons) and on input to cerebellum versus basal ganglia from LTC or IFG (2 comparisons). Paired t-tests were used to test for significant differences between modulatory effects (p < .05, corrected for 8 comparisons).
Acknowledgments
This research was supported by grants from the National Institute of Child Health and Human Development (HD042049) and by the National Institute of Deafness and Other Communication Disorders (DC06149) to James R. Booth and by grants from the National Institute of Neurological Diseases and Stroke (NS44383 and NS44837) to James C. Houk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullaev YG, Melnichuk KV. Cognitive operations in the human caudate nucleus. Neuroscience Letters. 1997;234(2–3):151–155. doi: 10.1016/s0304-3940(97)00680-0. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Delong MR, Strick PI. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Barto AG, Fagg AH, Sitkoff N, Houk JC. A cerebellar model of timing and prediction in the control of reaching. Neural Computation. 1999;11(3):565–594. doi: 10.1162/089976699300016575. [DOI] [PubMed] [Google Scholar]
- Bechtereva NP, Abdullaev YG, Medvedev SV. Neuronal activity in frontal speech area 44 of the human cerebral cortex during word recognition. Neuroscience Letters. 1991;124(1):61–64. doi: 10.1016/0304-3940(91)90822-b. [DOI] [PubMed] [Google Scholar]
- Belton E, Salmond CH, Watkins KE, Vargha-Khadem F, Gadian DG. Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Human Brain Mapping. 2003;18(3):194–200. doi: 10.1002/hbm.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM. Shifts of effective connectivity within a language network during rhyming and spelling. Journal of Neuroscience. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam MM, et al. Developmental changes in the neural correlates of phonological processing. Journal of Cognitive Neuroscience submitted. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. NeuroImage. 2002a;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Modality independence of word comprehension. Human Brain Mapping. 2002b;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzel K, Rottach K, Buttner U. Normal and pathological saccadic dysmetria. Brain. 1993;116:337–353. doi: 10.1093/brain/116.2.337. Pt 2. [DOI] [PubMed] [Google Scholar]
- Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978;101(2):251–283. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics. Brain. 1999;122(10):1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Fonlupt P. Changes of effective connectivity between the lateral and medial parts of the prefrontal cortex during a visual task. European Journal of Neuroscience. 2003;18(3):675–679. doi: 10.1046/j.1460-9568.2003.02787.x. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005a;24(2):332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005b;43(9):1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to 'aip'. Cerebral Cortex. 2005;15(7):913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Crosson B, Rao SM, Woodley SJ, Rosen AC, Bobholz JA, Mayer A, et al. Mapping of semantic, phonological, and orthographic verbal working memory in normal adults with functional magnetic resonance imaging. Neuropsychology. 1999;13(2):171–187. doi: 10.1037//0894-4105.13.2.171. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The brain binds entities and events by multi-regional activation from convergence zones. Neural Computation. 1989;1:123–132. [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: Language, leaning and memory. Trends in Cognitive Sciences. 1998;2:355–361. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional mri. Journal of Neuroscience. 1997;17(24):9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. Journal of Neurophysiology. 2003;89(1):634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Fishbach A, Roy SA, Bastianen C, Miller LE, Houk JC. Deciding when and how to correct a movement: Discrete submovements as a decision making process. Experimental Brain Research. 2006 doi: 10.1007/s00221-006-0652-y. DOI 10.1007/s00221-00006-00652-y. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Jenner AR, Mencl WE, Pugh KR, Shaywitz BA, Shaywitz SE, et al. The cerebellum's role in reading: A functional mr imaging study. Ajnr: American Journal of Neuroradiology. 1999;20(10):1925–1930. [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Martin JH. The control of rapid limb movement in the cat. Iii. Agonist - antagonist coupling. Experimental Brain Research. 1982;45(1–2):115–125. doi: 10.1007/BF00235770. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Hampson M, Johnson D. A theoretical investigation of reference frames for the planning of speech movements. Psychological Review. 1998;105(4):611–633. doi: 10.1037/0033-295x.105.4.611-633. [DOI] [PubMed] [Google Scholar]
- Homae F, Yahata N, Sakai KL. Selective enhancement of functional connectivity in the left prefrontal cortex during sentence processing. Neuroimage. 2003;20(1):578–586. doi: 10.1016/s1053-8119(03)00272-6. [DOI] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, et al. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–1781. doi: 10.1093/brain/awg184. Pt 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC. Agents of the mind. Biological Cybernetics. 2005;92:427–437. doi: 10.1007/s00422-005-0569-8. [DOI] [PubMed] [Google Scholar]
- Houk JC, Bastianen C, Fansler D, Fishbach A, Fraser D, Reber PJ, et al. Action selection and refinement in subcortical loops through basal ganglia and cerebellum. Philosophical Transactions of the Royal Society - B. doi: 10.1098/rstb.2007.2063. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC, Mugnaini E. Cerebellum. In: Squire LR, Bloom FE, Roberts JL, editors. Fundamental neuroscience. Academic Press; 2003. pp. 841–872. [Google Scholar]
- Kareken DA, Lowe M, Chen SHA, Lurito J, Mathews V. Word rhyming as a probe of hemispheric language dominance with functional magnetic resonance imaging. Neuropsychiatry Neuropsychology & Behavioral Neurology. 2000;13(4):264–270. [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: Action representation in mirror neurons. Science. 2002;297(5582):846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology. 2004;55(4):522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG. The motor theory of speech perception revised. Cognition. 1985;21(1):1–36. doi: 10.1016/0010-0277(85)90021-6. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Whalen DH. On the relation of speech to language. Trends in Cognitive Sciences. 2000;4(5):187–196. doi: 10.1016/s1364-6613(00)01471-6. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Khadem F. Language fmri abnormalities associated with foxp2 gene mutation. Nature Neuroscience. 2003;6(11):1230–1237. doi: 10.1038/nn1138. [DOI] [PubMed] [Google Scholar]
- Liu W, McIntire K, Kim SH, Zhang J, Dascalos S, Lyons KE, et al. Bilateral subthalamic stimulation improves gait initiation in patients with parkinson's disease. Gait & Posture. 2006;23(4):492–498. doi: 10.1016/j.gaitpost.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SHA, Mathews VP. Comparison of rhyming and word generation with fmri. Human Brain Mapping. 2000;10(3):99–106. doi: 10.1002/1097-0193(200007)10:3<99::AID-HBM10>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Networks. 2000;13(8–9):861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Ungerleider LG, Haxby JV, et al. Network analysis of cortical visual pathways mapped with pet. Journal of Neuroscience. 1994;14(2):655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ. A dynamic causal modeling study on category effects: Bottom-up or top-down mediation?[see comment] Journal of Cognitive Neuroscience. 2003;15(7):925–934. doi: 10.1162/089892903770007317. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(16):8683–8687. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, Holdefer RN, Houk JC. The role of the cerebellum in modulating voluntary limb movement commands. Archives Italiennes de Biologie. 2002;140(3):175–183. [PubMed] [Google Scholar]
- Moro A, Tettamanti M, Perani D, Donati C, Cappa SF, Fazio F. Syntax and the brain: Disentangling grammar by selective anomalies. Neuroimage. 2001;13(1):110–118. doi: 10.1006/nimg.2000.0668. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet. 1999;353(9165):1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Nieto-Castanon A, Guenther FH, Perkell JS, Curtin HD. A modeling investigation of articulatory variability and acoustic stability during american english/r/production. Journal of the Acoustical Society of America. 2005;117(5):3196–3212. doi: 10.1121/1.1893271. [DOI] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Cabeza R, Nilsson LG, Houle S, Habib R, et al. Network analysis of positron emission tomography regional cerebral blood flow data: Ensemble inhibition during episodic memory retrieval. Journal of Neuroscience. 1996;16(11):3753–3759. doi: 10.1523/JNEUROSCI.16-11-03753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, et al. Is developmental dyslexia a disconnection syndrome? Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. Neuroimage. 2004;22(3):1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Rodriguez P. How do memory systems interact? Evidence from human classification learning. Neurobiology of Learning & Memory. 2004;82(3):324–332. doi: 10.1016/j.nlm.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl E, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, et al. The angular gyrus in developmental dyslexia: Task specific differences in functional connectivity within posterior cortex. Psychological Science. 2000;11(1):51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain. 1996;119(4):1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Rae C, Harasty JA, Dzendrowskyj TE, Talcott JB, Simpson JM, Blamire AM, et al. Cerebellar morphology in developmental dyslexia. Neuropsychologia. 2002;40(8):1285–1292. doi: 10.1016/s0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Rosin R, Topka H, Dichgans J. Gait initiation in parkinson's disease. Movement Disorders. 1997;12(5):682–690. doi: 10.1002/mds.870120509. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, et al. Failure to activate the left tempoparietal cortex in dyslexia. Archives of Neurology. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Journal of Neuropsychiatry & Clinical Neurosciences. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. Journal of Comparative Neurology. 1991;308(2):224–248. doi: 10.1002/cne.903080209. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. Journal of Neuroscience. 1997;17(1):438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cerebral Cortex. 2004;14(11):1246–1255. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Stanberry LI, Richards TL, Berninger V, Nandy RR, Aylward EH, Maravilla KR, et al. Low-frequency signal changes reflect differences in functional connectivity between good readers and dyslexics during continuous phoneme mapping. Magnetic Resonance Imaging. 2006;24:217–229. doi: 10.1016/j.mri.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, et al. Listening to action-related sentences activates fronto-parietal motor circuits. Journal of Cognitive Neuroscience. 2005a;17(2):273–281. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, et al. Basal ganglia and language: Phonology modulates dopaminergic release. Neuroreport. 2005b;16(4):397–401. doi: 10.1097/00001756-200503150-00018. [DOI] [PubMed] [Google Scholar]
- Thach WT. What is the role of the cerebellum in motor learning and cognition. Trends in Cognitive Sciences. 1998;2:331–337. doi: 10.1016/s1364-6613(98)01223-6. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience. 2001;2(10):717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, et al. Neural basis of an inherited speech and language disorder. Proceedings of the National Academy of Sciences. 1998;95:12695–12700. doi: 10.1073/pnas.95.21.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudano E, Legg CR, Glickstein M. Afferent and efferent connections of temporal association cortex in rat: A horseradish peroxidase study. European Journal of Neuroscience. 1991;3:317–330. doi: 10.1111/j.1460-9568.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Vargha-Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, et al. Mri analysis of an inherited speech and language disorder: Structural brain abnormalities.[see comment] Brain. 2002;125:465–478. doi: 10.1093/brain/awf057. Pt 3. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, et al. Conjoint and extended neural networks for the computation of speech codes: The neural basis of selective impairment in reading words and pseudowords. Cerebral Cortex. 2001;11(3):267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]


